1. Introduction
An estimated 295,000 women die each year in childbirth [
1], with long-term consequences for families and offspring. The majority of maternal deaths (94%) occur in low- and middle-income countries (LMICs), particularly in sub-Saharan Africa and South Asia. The leading direct cause of maternal death worldwide is hemorrhage, with the vast majority of these occurring during the postpartum period [
2]. As per WHO, active management of the third stage of labor (AMTSL)—defined as intramuscular (IM) administration of 10 international units (IU) of oxytocin and controlled cord traction performed by a skilled birth attendant (SBA)—substantially reduces the risk of postpartum hemorrhage (PPH) [
3].
Considering the severity of mortality and morbidity caused by PPH in low-resource settings (LRS), it is likely that a dosage form that is safe, easy to administer and provides a rapid and effective response could be more effective in saving lives in these settings. Oral is the most desirable route of administration due to accessibility and simplicity of the dosage form. However, for peptide drugs such as oxytocin, oral delivery displays low systemic availability and diminished efficacy due to the physiology of this route where high proteolytic degradation in the stomach and intestinal region degrades the peptide molecules in addition to the chemical degradation by the harsh pH environment encountered in the stomach region. Furthermore, the characteristics of injectable oxytocin, including its reliance on the cold chain, limit its reach in terms of effective coverage [
4].
A heat-stable oxytocin in a fast-dissolving tablet (FDT) format for sublingual (under-the-tongue) delivery, offers easy administration of oxytocin with uncompromised quality (maintaining drug content), eliminating the need for injection and paving the way for community distribution in areas with limited health infrastructure, including potentially at-home births. In addition, heat-stable oxytocin as a SL FDT offers the favorable side effect profile of the WHO uterotonic drug of choice with the added advantage of being indicated potentially for both prevention and management of PPH.
The sublingual (SL) route offers a rapid onset of action [
5] and direct delivery into the systemic circulation, bypassing oral gastrointestinal degradation and the first-pass metabolism in the liver [
6]. This route is non-invasive and easily accessible, for administration by the caregiver, not requiring a trained health worker or a hospital setting as needed for parenteral (intramuscular (IM) or intravenous (IV)) administration of oxytocin. Furthermore, with the addition of excipients to impart a sweet taste to the product to improve palatability, this administration route anticipates better patient acceptability and compliance because the route does not involve conventional swallowing of the dosage form which may be difficult for woman undergoing PPH. Despite the obvious benefits, the sublingual route of administration has not been explored for delivery of oxytocin for the treatment of PPH.
Results from an initial feasibility study in minipigs reported previously by the authors show successful absorption of oxytocin via the SL route, along with improved thermostability of oxytocin in a fast-dissolving tablet form [
7]. Considering the significance of the SL route, the objective of this study was to confirm the findings of the feasibility study by conducting a toxicokinetic study in an anesthetized rabbit model using an optimized formulation containing excipients safe for human use. Building upon the previous findings, we hypothesized that presentation of oxytocin as a heat-stable, sublingual fast-dissolving tablet (FDT) will be safe and non-reactogenic and will result in rapid absorption of oxytocin and generate both sufficient oxytocin levels in plasma and a long product shelf life under elevated temperature conditions as needed in areas with no or low cold storage facilities. Confirmation of these factors was considered to be a prerequisite for advancing a heat-stable formulation of oxytocin in a sublingual fast dissolving tablet into an exploratory clinical study.
2. Materials and Methods
2.1. Lyophilization Cycle for Production of Sublingual Oxytocin Fast-Dissolving Tablets
Heat-stable SL oxytocin tablets were produced using the lyophilization cycle used previously [
7] as shown in
Table 1. The formulation composition was similar to the previous formulation with one exception being replacement of carbomer with 0.25 wt% chitosan. To formulate the tablets, we used pharma grade Sucrose and Mannitol purchased from J. T. Baker (Phillipsburg, NJ, USA), Dextran-40 purchased from Pharmacosmos (Holbæk, Denmark) and pharma grade Chitosan purchased from Glentham Life Sciences (Corsham, UK). The size of the sublingual tablets were 8–10 mm diameter and 2–3 mm in thickness.
2.2. Sublingual Toxicokinetic Study Design
The toxicokinetic study in rabbits (
Table 2) was conducted at a contract animal facility (Noble Life Sciences, Sykesville, MD, USA) under approval from Noble Life Sciences IACUC (approval code: NLS-589, date: 13 May 2020) and in compliance with the U.S. Food and Drug Administration Good Laboratory Practice Regulations for Nonclinical Laboratory Studies (21 CFR Part 58).
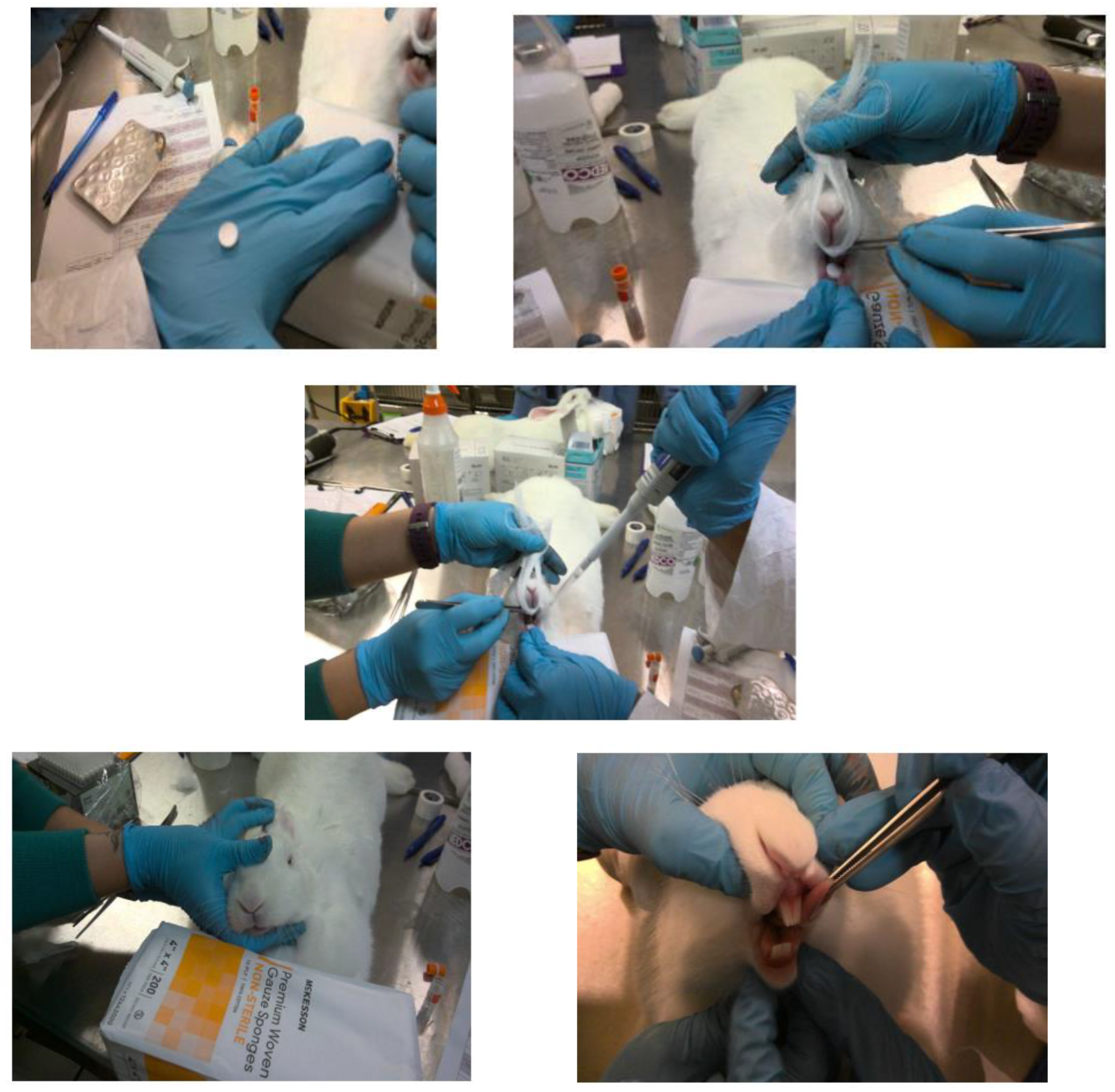
For SL placement of fast dissolving tablets, the rabbit was anesthetized and positioned on a flat table, with the lower jaw strongly supported in a horizontal level on the table. Using rounded tip stainless tweezers, the rabbit’s tongue was carefully raised, and the sublingual tablet was placed beneath the tongue (see
Figure 1). Anesthesia was maintained for 3 h after drug administration to keep the tablet in sublingual position and prevent entering the gastrointestinal tract.
2.3. Administration Site Observations
A “Draize-like” scoring paradigm for injection site monitoring was used to grade the presence of erythema (and/or edema according to the reported method) [
8]. In addition, any other change at the administration site (such as eschar, abscesses, necrosis, suppuration) was recorded when observed. Additionally, ulceration, blood, mucus, scars, and lesions were observed one hour before dosing, 3 h post-dose, and at euthanasia.
2.4. Blood Collection
Animals were not fasted prior to blood collection. Blood was collected at the time points of 0 min (pre-dose) and 5, 10, 15, 20, 30, 60, 120, and 180 min post-dose and at euthanasia only the first four animals for SL group. All animals were anesthetized pre-dose, prior to dosing. All animals were anesthetized with ketamine (35–50 mg/kg) and xylazine (3–5 mg/kg) via a subcutaneous or intramuscular (IM) route followed by maintenance anesthesia with additional half doses if needed or via inhaled isoflurane. At the selected time points, whole blood was collected aseptically from the auricular artery and dispensed into the appropriate collection tubes in any order: (1) K3 EDTA for hematology determinations; (2) 0.129 M, 3.8% sodium citrate for coagulation analyses (plasma); (3) serum separator tube for serum chemistry and C-reactive protein; and (4) aprotinin-spiked (K2EDTA) tubes for plasma oxytocin determinations. Tubes containing hematology specimens were placed at room temperature on a rocker for at least 30 min prior to shipment. Tubes containing whole blood for serum isolation were maintained for at least 30 min and no more than 4 h at room temperature before being processed to serum. Tubes containing aprotinin-spiked (K2EDTA) specimens for oxytocin determinations were maintained on wet ice or cold packs before use and after collection. They were also maintained on a rocker for at least 30 min prior to processing.
2.5. Plasma and Serum Processing
Blood samples for oxytocin determination were collected into the Aprotinin spiked (K2EDTA) vacutainer tubes and processed for collection of plasma. The samples were processed by centrifuging for approximately 15 min at approximately 3000 rpm and approximately 4 °C; they were aliquoted into cryovials without anticoagulant and were immediately placed on dry ice. At termination, blood was collected from the SL group for serum chemistry, C-reactive protein, hematology, and coagulation. Blood collected for coagulation analyses was collected into tubes containing 3.8% sodium citrate and was processed for plasma per the Noble protocol. All serum and plasma samples were processed aseptically.
2.6. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
Each plasma sample was aliquoted into two vials of an approximately equal volume. All the collected plasma was analyzed for oxytocin levels by the LC-MS/MS method.
The bioanalytical method for quantification of oxytocin in rabbit plasma (K
2EDTA) was validated following the FDA guidelines [
9] for a bioanalytical method validation at NorthEast BioAnalytical Laboratory, LLC (NEBA) (Hamden, CT, USA), via reverse phase liquid chromatography separation (Column: Zorbax SB C18 (Agilent, Santa Clara, CA, USA) 3.5 μm 150 × 4.6 mm, Security/Guard Cartridge Gemini C18 4 × 2.0 mm) and detection by tandem mass spectrometry (LC/MS/MS) using Sciex API-5000 Mass Spectrometer. Analysis of Oxytocin was performed using stable label internal standard, Oxytocin-d5. Oxytocin was received as an acetate salt and Oxytocin-d5 was received as Trifluoroacetate salt.
For quantitation, the LC/MS/MS data were acquired using Analyst
® v1.6 software (Sciex, Framingham, MA, USA). The calibration curves for Oxytocin were constructed as a non-linear function of the weighted (1/x
2) linear regression of the theoretical concentration versus response (where response = ratio of analyte peak area to internal standard peak area) using the following equation:
where:
Accuracy and precision were calculated using the following formulas
Precision:
Accuracy was calculated using theoretical concentrations. Accuracy and precision were reported with one (1) decimal place. All concentration data are reported to two (2) decimal places. Excel® was used for formatting data tables and computing summary statistics.
2.7. Pharmacokinetic Analysis
A sublingual FDT containing 400 IU oxytocin was administered in rabbits. A 10 IU dose of oxytocin in the form of IM injection was used as a control. The serum peak concentration (Cmax) and time-to-peak concentration (Tmax) were taken from the plasma concentration time profiles. Plasma concentration (μg) versus time (h) profiles were prepared and peak plasma concentration (Cmax) and time of its occurrence (tmax) were read directly from the respective profiles. The area under the concentration time curve (AUC0→t) was calculated according to linear trapezoidal method using GraphPad Prism version 4.
2.8. Good Laboratory Practice Sublingual Reactogenicity/Toxicity Study
On Day 1, after the sample collection, all animals were euthanized and the first four animals in the SL group were subjected to a necropsy. Protocol-required tissues were processed, embedded in paraffin, sectioned, and stained with hemotoxylin and eosin (H&E) at Histo-Scientific Research Laboratories (HSRL) (Mount Jackson, VA, USA). The following tissues from the SL group were preserved in 10% neutral buffered formalin.
- 1.
Appendix
- 2.
Cecum
- 3.
Colon
- 4.
Duodenum
- 5.
Esophagus
- 6.
Ileum
- 7.
Jejunum
- 8.
Rectum
- 9.
Sacculus rotundus
- 10.
Sublingual tissue
4. Discussion
Our goal was to validate the findings from the previous feasibility study [
7] by using a preclinical rabbit model and a formulation that was optimized for potential use in humans by using excipients which are Generally Recognized As Safe (GRAS), with low or minimal toxicity or reactogenicity. We also assessed the optimized formulation for thermostability of oxytocin and pharmacokinetic behavior when administered via the sublingual route. In the previous feasibility study, we used carbomer as the mucoadhesive agent and sodium taurocholate as the permeability enhancer for oxytocin (by potentially altering the lipid bilayer fluidity, modulating the tight junction permeability to help oxytocin cross the epithelium). In this study, we attempted two approaches to optimize the previously reported formulation: (1) was replaced sodium taurocholate with pharmaceutical grade sodium cholate [
10,
11], as a permeation enhancer; and (2) we replaced carbomer and sodium taurocholate with chitosan [
12,
13,
14], which has both mucoadhesive and permeation-enhancing properties. During formulation preparation, we observed that carbomer is light and fluffy and tends to float on water, requiring a good vortex to become thoroughly wet. It requires great accuracy during mixing in order to avoid irregular dispersion of the powder, leading to the formation of lumps and heterogeneous samples. In addition, due to the acrylic acid backbone, when hydrated, this polymer forms acidic solutions that become increasingly viscous and gel-like as the pH is adjusted to above 4 (as needed for oxytocin). This poses a difficulty, especially during formulation preparation and scale-up. The carbomer formulation also generated residue in the blister sheet upon removal of the tablets. Due to these undesirable formulation attributes, the formulation containing carbomer was not selected as the lead formulation in this study.
Chitosan, a natural cationic polysaccharide, has received a great deal of attention in the past few years for its mucoadhesive character [
15], permeation enhancing properties, and controlled release of drugs. It is recognized by the Food and Drug Administration as GRAS [
16,
17], as listed in the Code of Federal Regulations (CFR 21). Since the formulation was aimed at developing a dosage form, that was robust, heat stable, rapid dissolving, and which could provide quicker onset of action, the developed sublingual formulation met all the criteria. In addition, there were no test article-related adverse changes in mortality, clinical observations, injection site scores, body weights, food consumption, fecal output, heart rate, blood pressure, or body temperature. The necropsy at 24-h post dosing and histopathology in sublingual and alimentary tract tissues were normal. This is important as during sublingual administration, there is an involuntary swallowing via the gastro-intestinal route. However as noted, the formulation showed no apparent test article-related microscopic findings in the sublingual tissue or the alimentary tract of rabbits who underwent necropsy. There were no abnormal findings in the clinical pathology data that could be attributed to the sublingual administration of oxytocin containing FDT formulation.
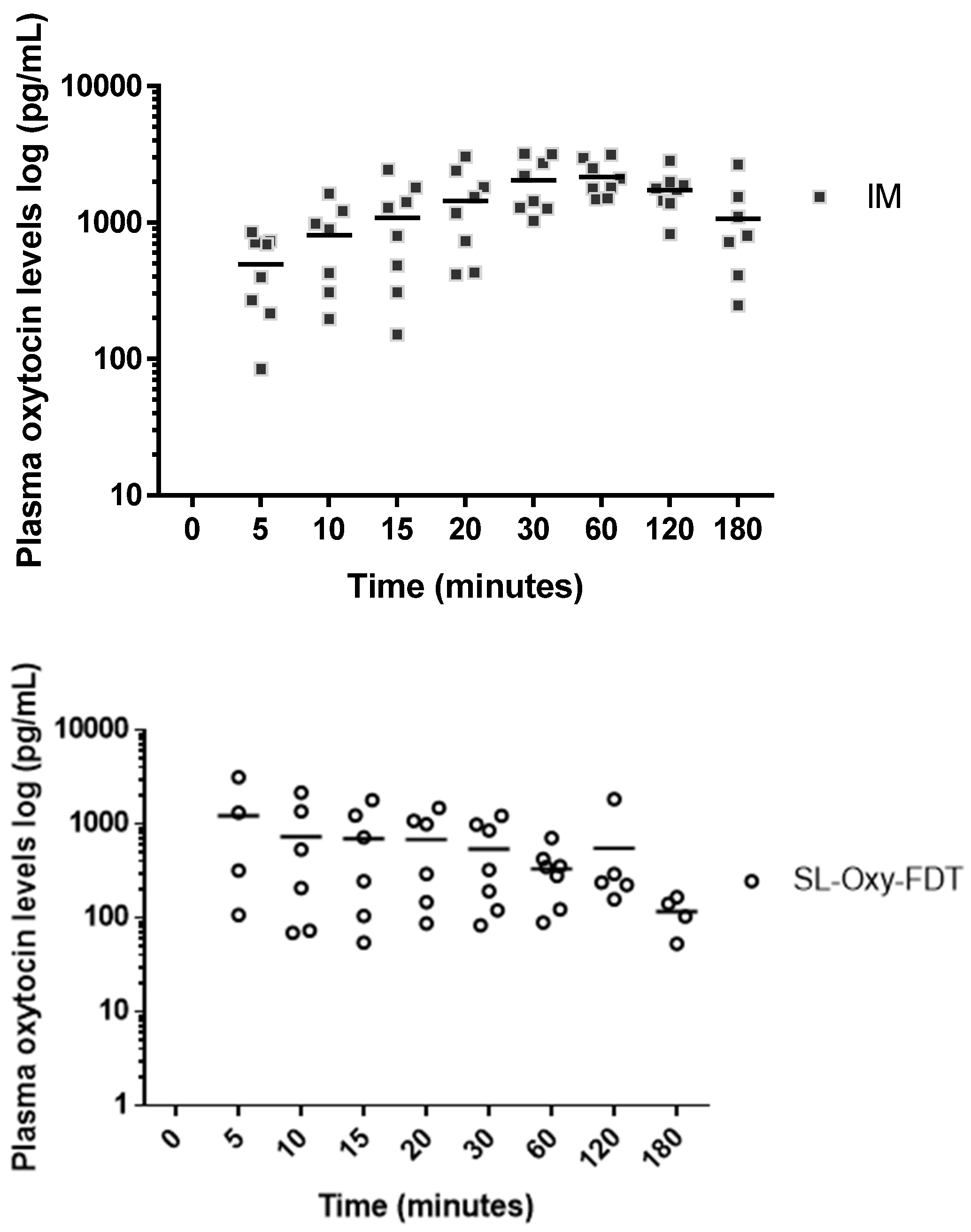
It is important to highlight that in the pharmacokinetic study, with SL administration, the absorption of oxytocin was rapid, with a mean absorption time within about 5 min of administration. Rapid absorption is a great advantage for the SL route because it is legitimate to assume that, if Cmax is obtained quickly, the biological effect of uterus contraction as needed to reduce bleeding (during PPH) would also be obtained quickly. As per the World Health Organization guidelines, oxytocin is administered either via IM or IV, and each route has potential advantages. The IV administration has a clinical advantage, as it leads to a faster response and a higher peak in plasma oxytocin levels; however, IM injection confers practical advantages, requiring fewer skills and less equipment to administer, making it a more serviceable option in a wider array of settings. Considering the rich vasculature in the SL region, where the blood vessels under the tongue drain into the jugular vein, rapid absorption of oxytocin into the blood circulatory system via the SL route is expected and in principle would mimic IV injection by introducing a drug directly into the systemic circulation.
The results from the pharmacokinetic study in rabbits showed that, although the SL route appeared to be associated with rapid absorption of oxytocin in plasma, the overall bioavailability was significantly lower with a higher variability among animals within the SL-dosed group as compared to the IM control group. This means that, despite the high sublingual dose of 400 IU, only a fraction of oxytocin was being absorbed via the sublingual route. The maximum concentration of oxytocin obtained via the SL route was 1164 pg/mL; for the control group (IM administration to anesthetized rabbits), the maximum oxytocin concentration was 2662 pg/mL. Specifically, for SL formulations, the plasma levels (C
max) pg/mL obtained were about 30% to 45% of the oxytocin levels obtained via the IM route (control). In a study conducted in 2004 by Carvalho et al. [
18,
19], the authors stated the minimum effective initial dose of oxytocin to minimize blood loss and to prevent PPH in elective caesarean deliveries to be 0.35 IU, which was far less than the previous conventional dosages ranging from 5 to 10 IU, suggesting the necessity of further research into the administration of oxytonics in a rational and judicious manner to minimize the side effects while maintaining efficacy. Similarly, another clinical study suggested that excessive doses of oxytocin to achieve an adequate uterine tone needs re-evaluation and observed that the adequate uterine tone can be achieved with small bolus doses like 0.5–3 IU of oxytocin. In view of these observations, it is possible that even the low plasma oxytocin levels obtained via the SL route in this study may still be therapeutically relevant in treating PPH; however, this can only be confirmed by testing in a relevant PPH preclinical model.
In our study, it is difficult to ascertain whether the lower oxytocin levels were due to the effect of anesthesia, which may have impacted available saliva, resulting in improper or incomplete absorption of the dose, or if they were due to the structural constraints imposed by oxytocin, limiting the absorption via passive diffusion across the sublingual mucosa. The effect of anesthesia has been previously noted by the authors where by administering the sublingual tablet in unanesthetized animals, the plasma levels of oxytocin obtained were higher, although they were inconsistent due to chewing (oral swallowing) of the tablets by the awake animals (PATH internal data), indicating the importance of a correct administration approach with the SL route of delivery.
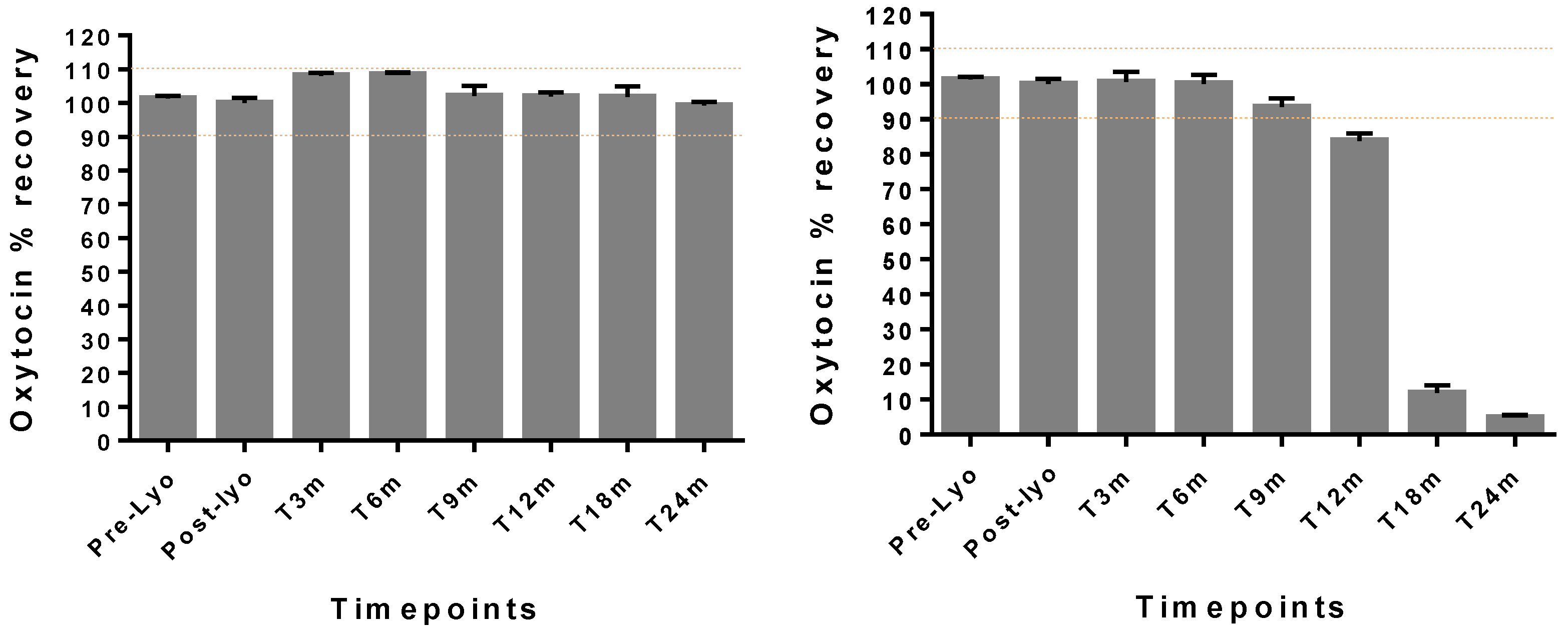
Lastly, the optimized formulation containing chitosan was robust, with good handling properties. This formulationmaintained oxytocin drug content in the range of 90–110% drug content as per the USP monograph acceptable potency criteria for oxytocin [
20,
21] under ambient conditions (30 °C/65%RH) with a high humidity for up to 2 years. The WHO guidelines on stability testing of pharmaceutical products require that manufacturers of products to be stored outside the refrigerator and intended for sale in hot climatic regions (i.e., climatic zones III and IV) should demonstrate adequate stability at 30 °C (not 25 °C) over the intended shelf-life of the product. In this study, even under extreme storage temperatures of 40 °C, oxytocin was stable for at least 9 months, suggesting the protection offered by the stabilizing excipients present in the optimized formulation [
22].
The present study, as is obvious, has some limitations as correlated with the pharmacokinetic study. First, the rabbits had to be anesthetized for SL administration and therefore, there was limited saliva volume available for tablet disintegration, requiring external administration of fluid 0.25 mL to assist with tablet disintegration. Although the smaller volume had a lower chance of inducing swallowing and/or loss of oxytocin, in an anesthetized animal, it was difficult to “hold” the fluid in place. This may have contributed to involuntary loss in dose, thus contributing to a lower available dose and lower plasma levels of oxytocin, despite the high SL dose in the formulation.