Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of PAG
2.3. Chemical Composition of PAG
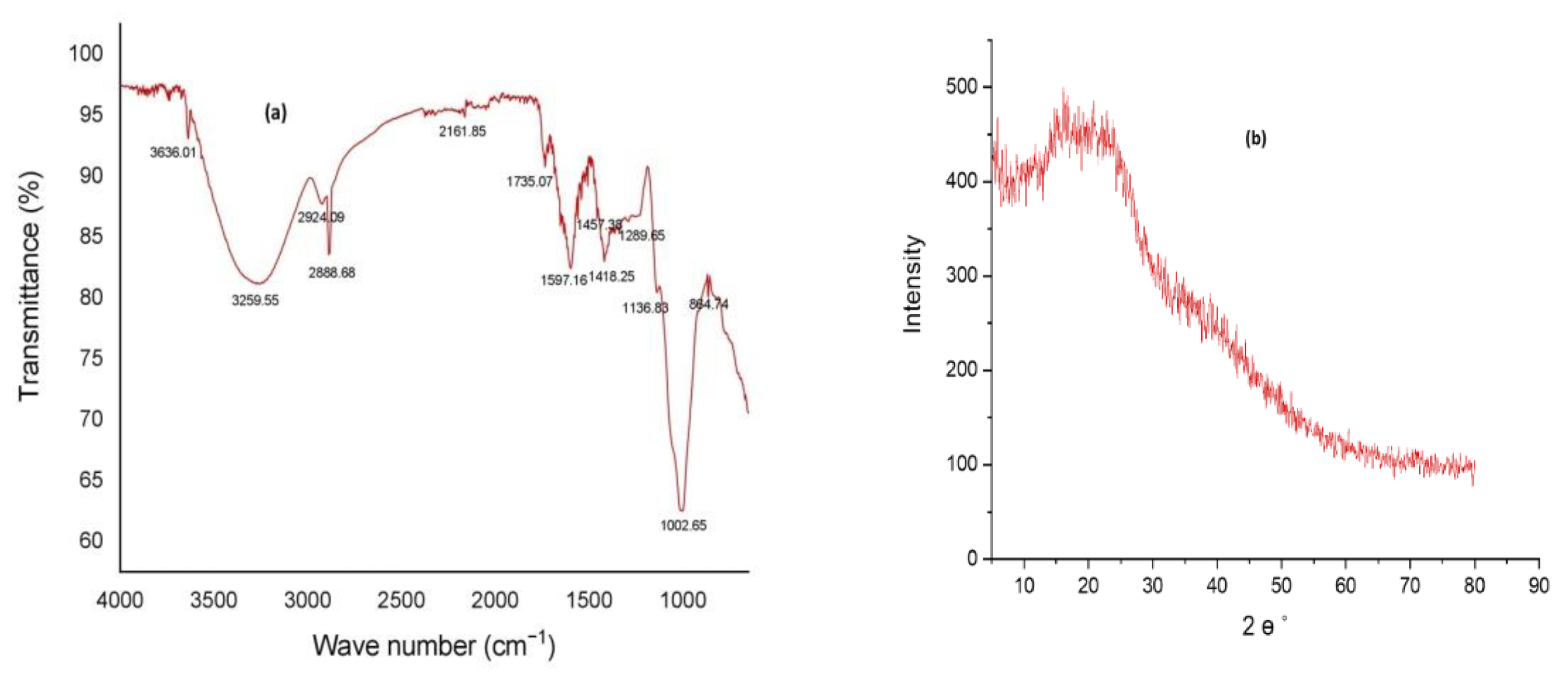
2.4. Structural Analysis of PAG
2.5. Thermal Analysis
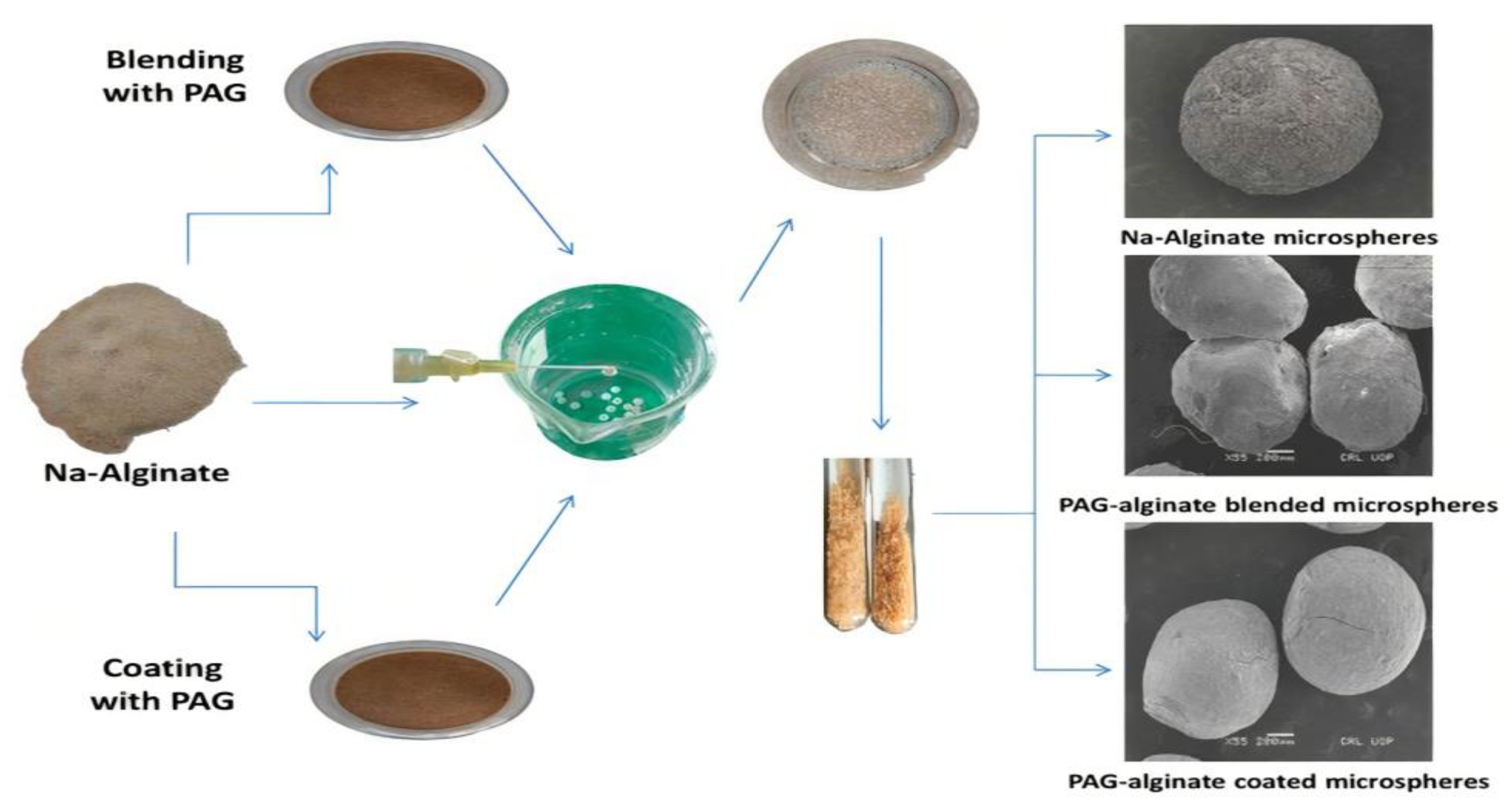
2.6. Experimental Design for Preparation of Microspheres
2.7. Preparation of Microspheres
2.8. Particle Size
2.9. Drug Encapsulation Efficiency
2.10. Swelling and Degradation Studies
2.11. In Vitro Drug Release Studies
2.12. Drug Release Kinetic Analysis
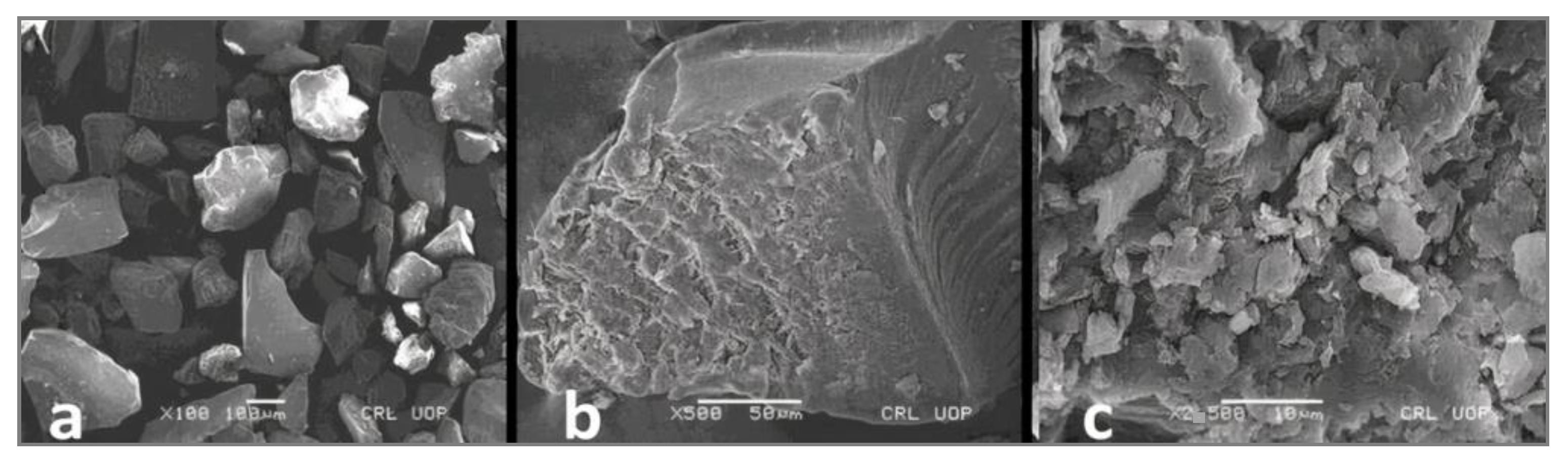
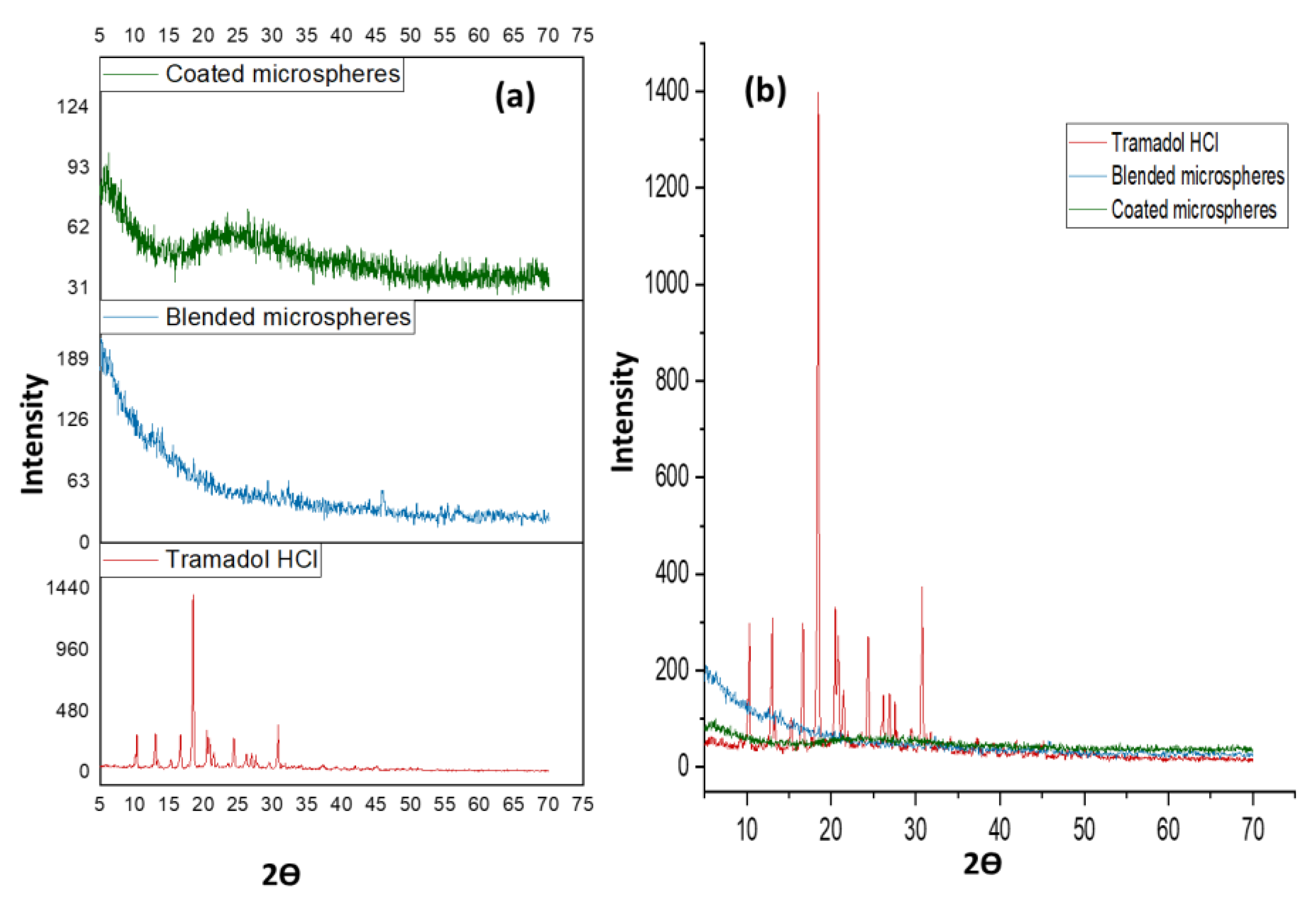
2.13. Structural Analysis of Microspheres
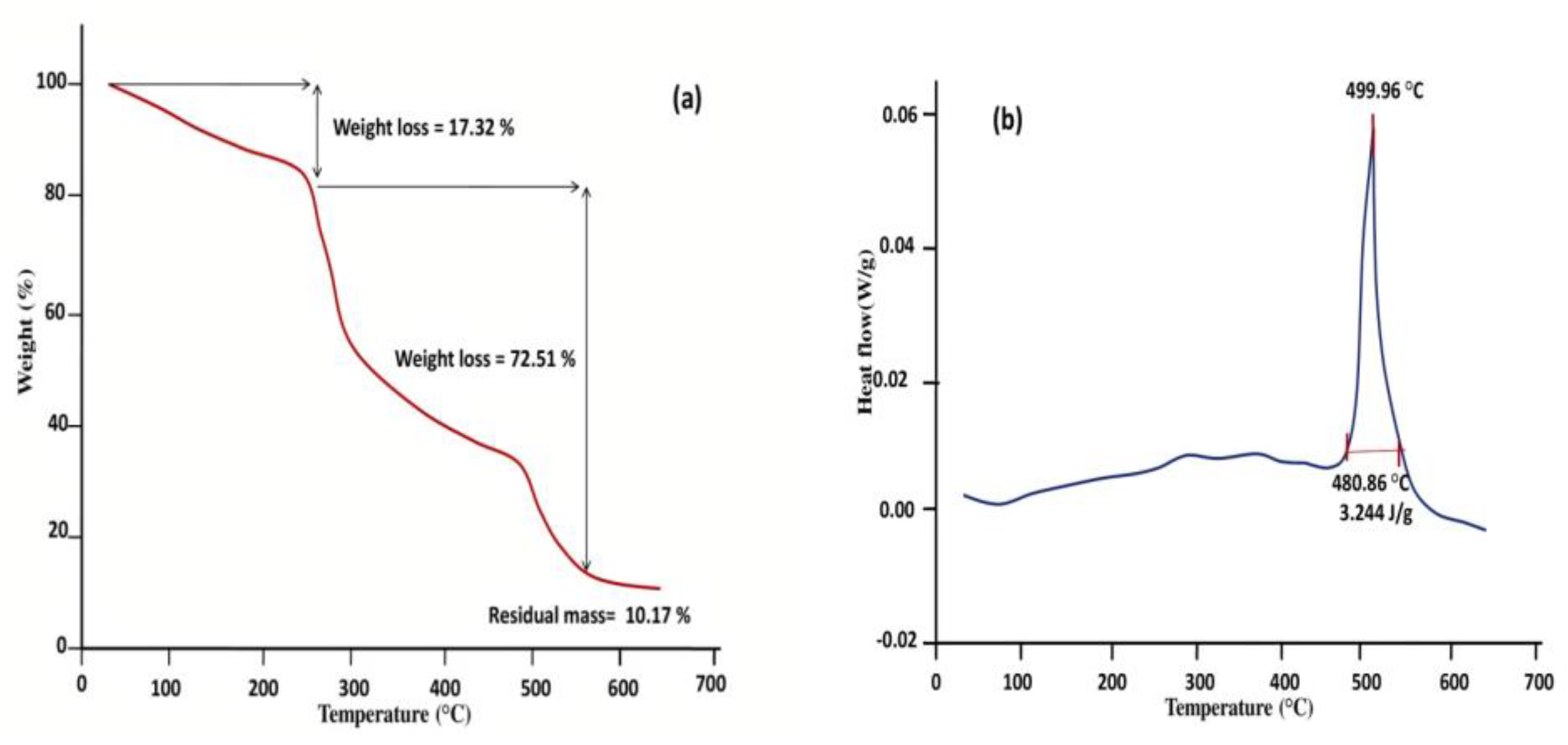
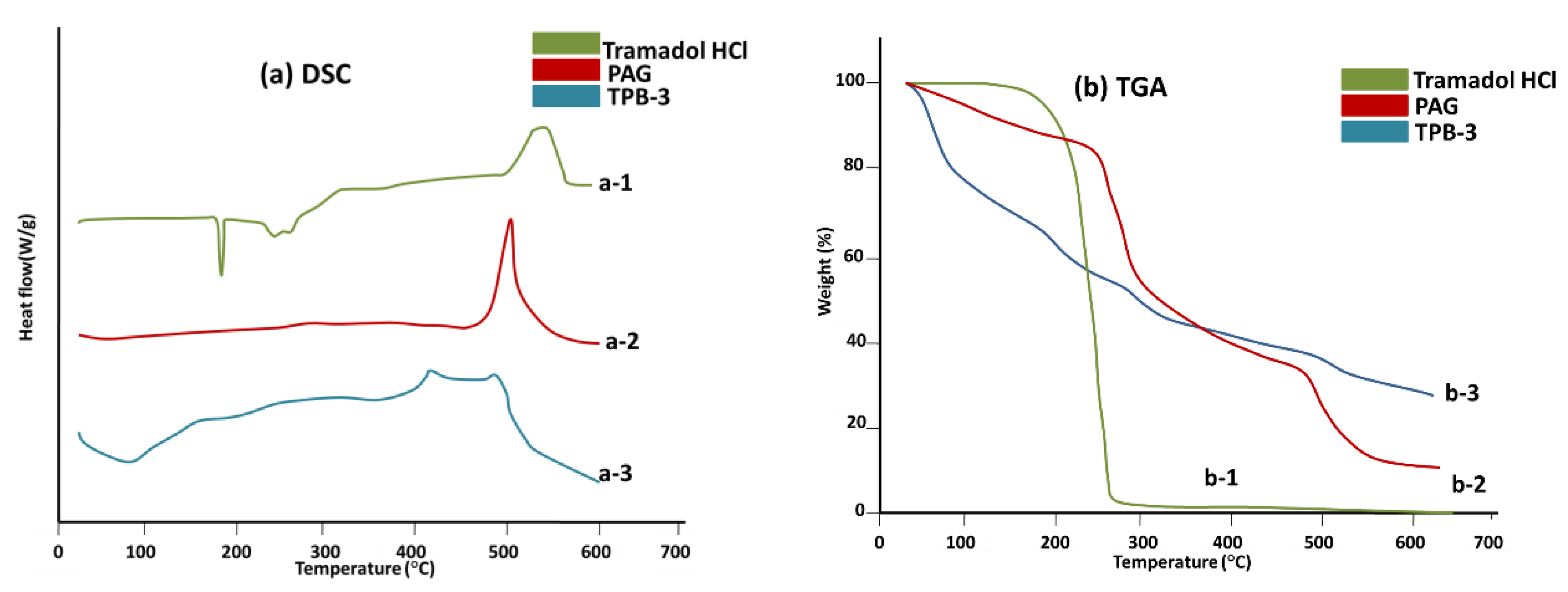
2.14. Thermal Analysis
2.15. Acute Toxicology Study
2.16. Analgesic Activity
Writhing Test
2.17. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of PAG
3.2. Structural Analysis of PAG
3.3. Thermal Analysis
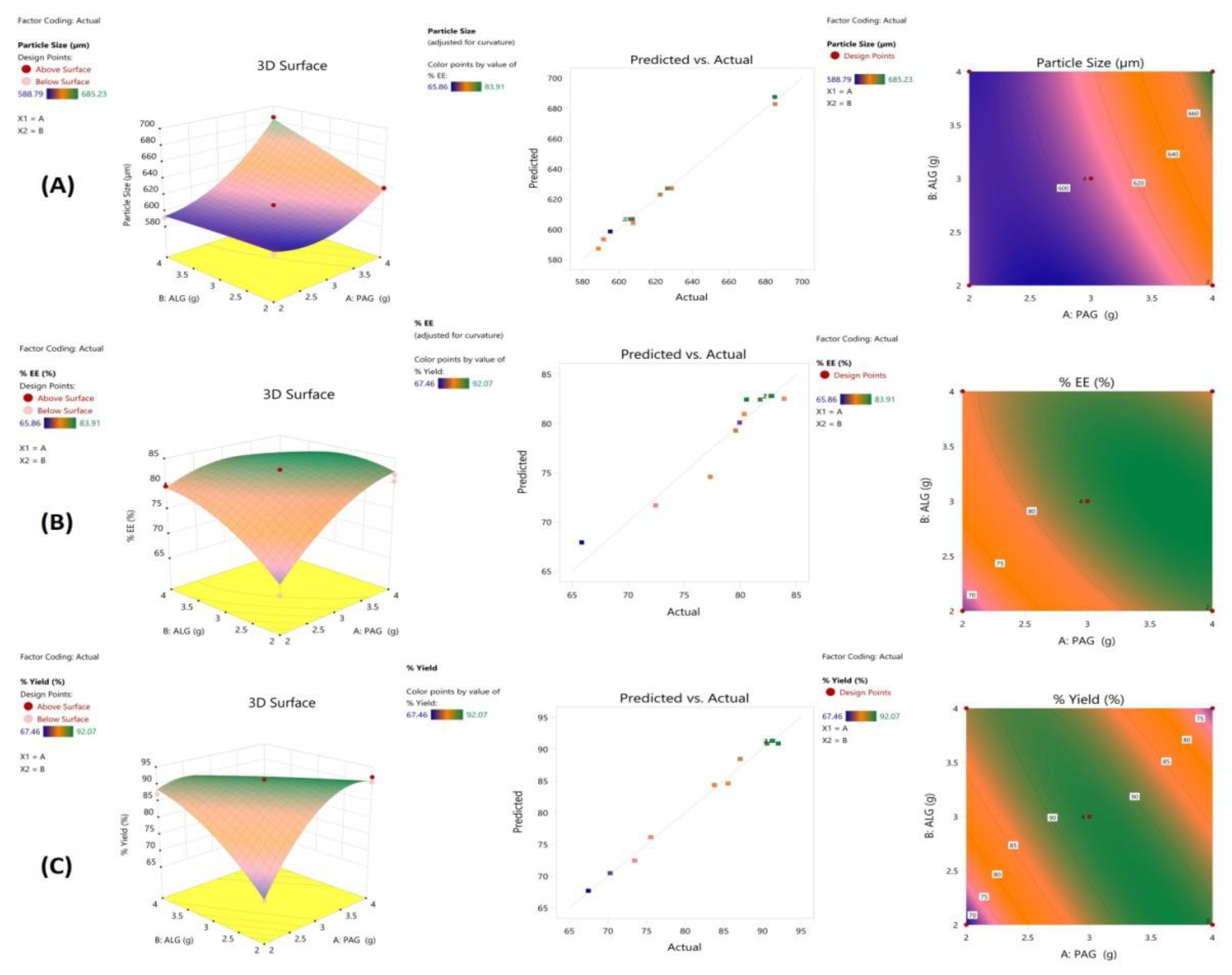
3.4. Experimental Design and Response Surface Methodology
3.5. Formulation Optimization
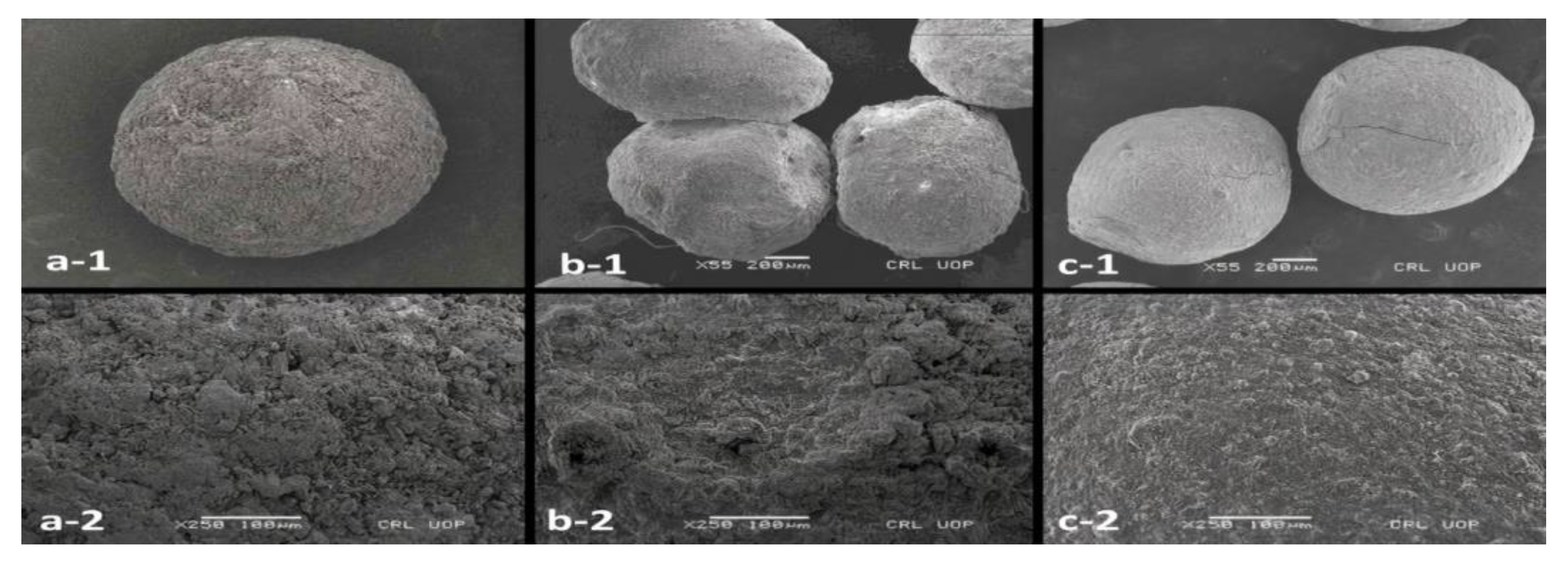
3.6. Preparation of Microspheres
3.7. Particle Size
3.8. Drug Encapsulation Efficiency
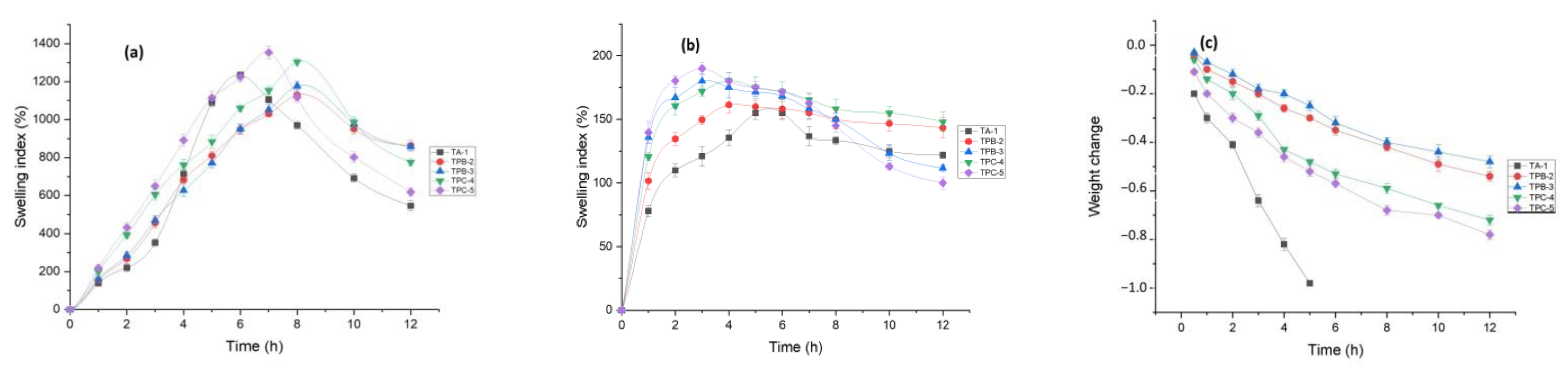
3.9. Swelling and Degradation Studies
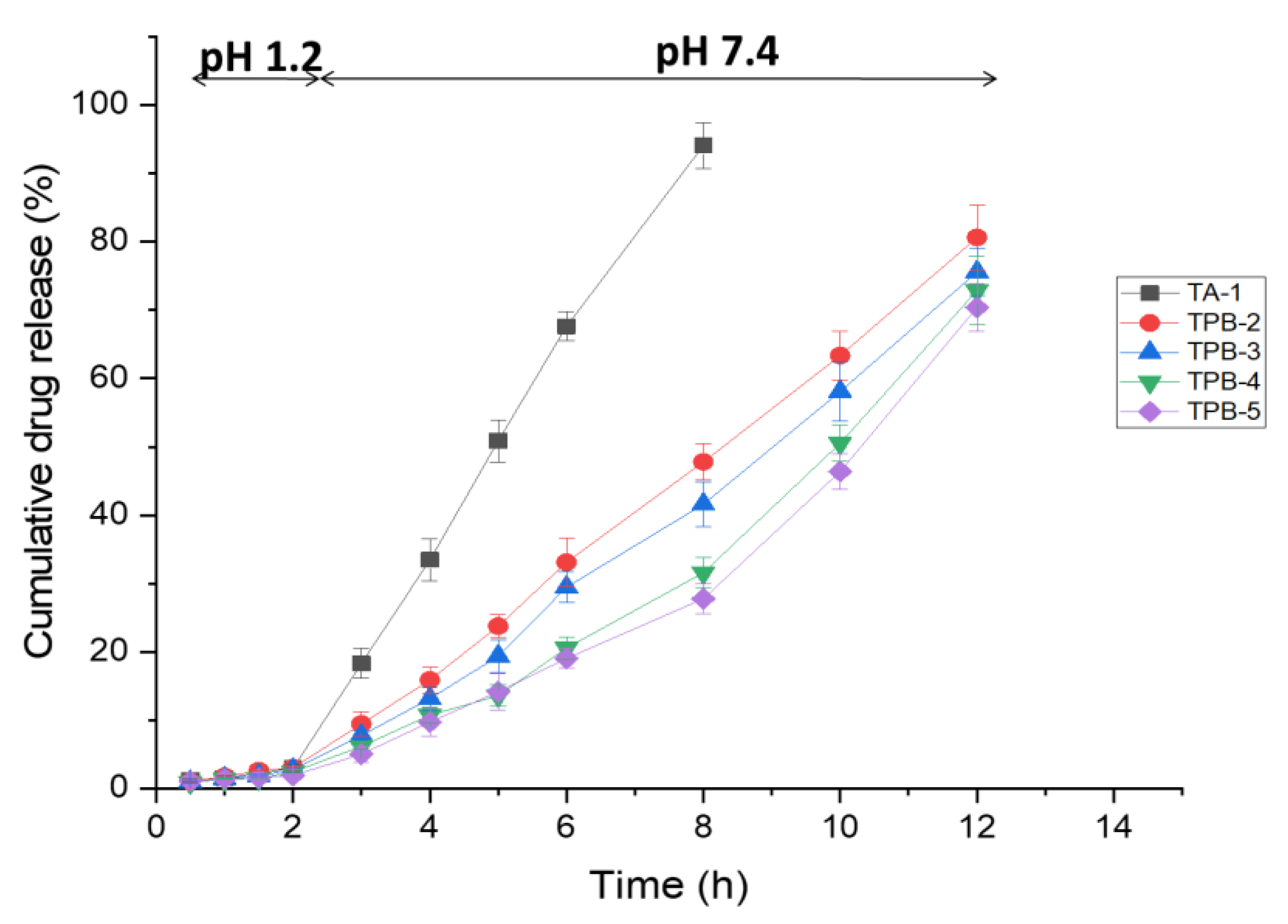
3.10. In Vitro Drug Release Studies
3.11. Drug Release Kinetic Analysis
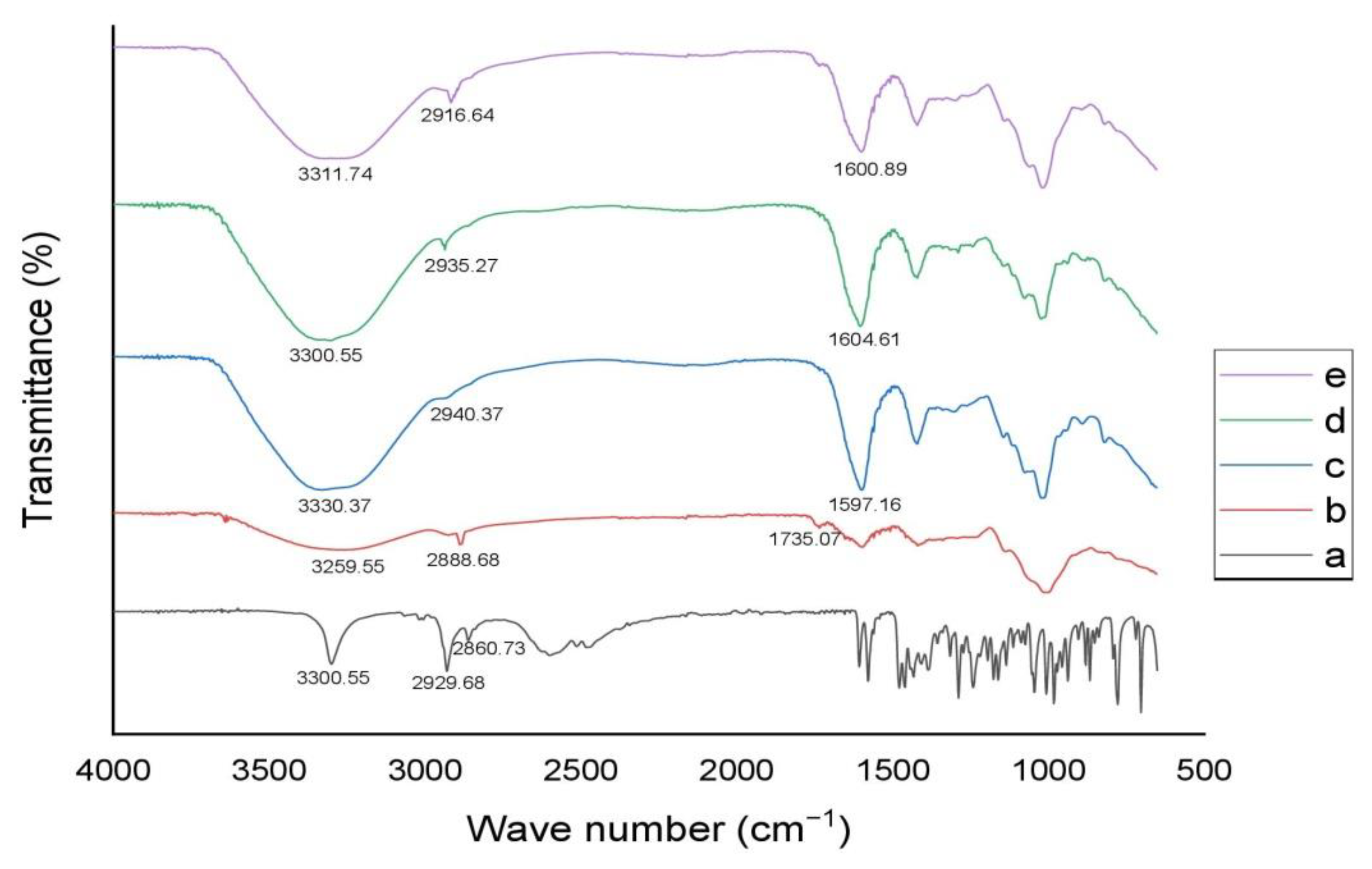
3.12. Structural Analysis of Microspheres
3.13. Thermal Analysis
3.14. Acute Toxicology Study
3.14.1. General Observations
3.14.2. Biochemical Analysis
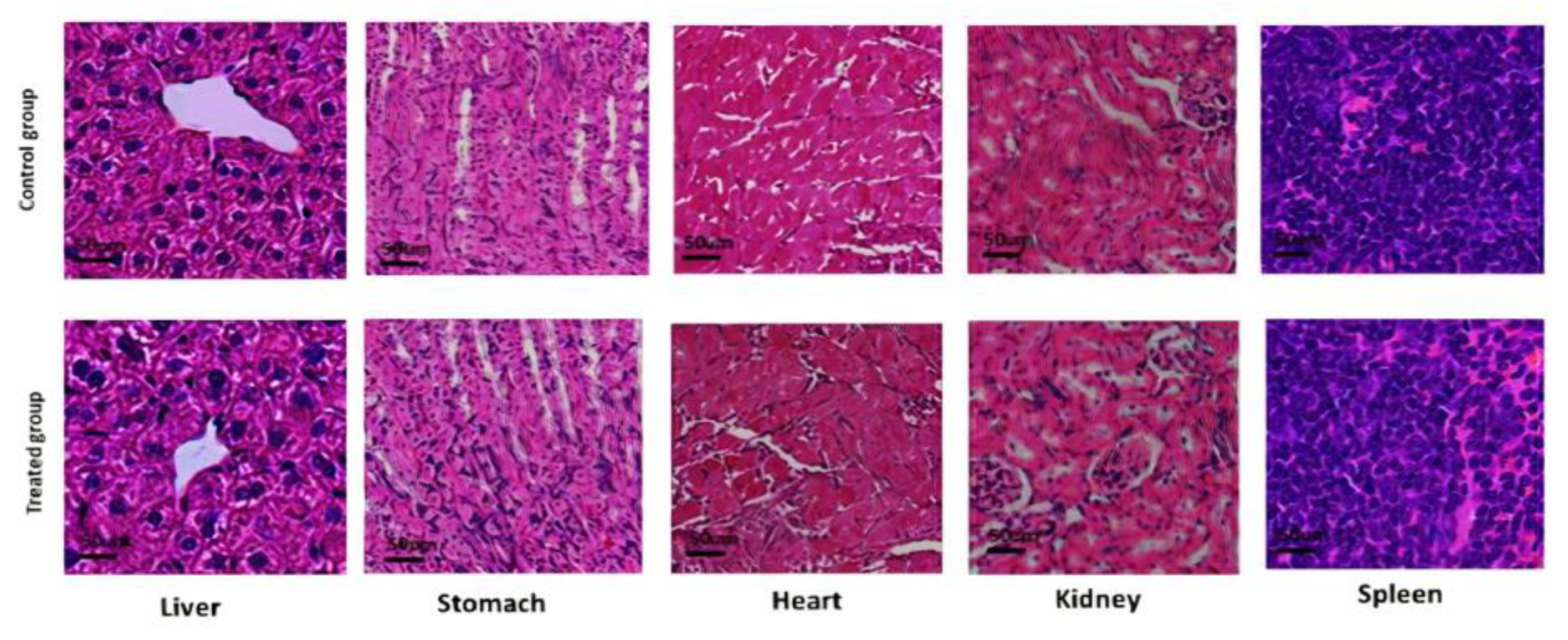
3.14.3. Histological Analysis
3.15. Analgesic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, A.K.; Ara, T.J.; Hasnain, M.S.; Hoda, N. Okra gum–alginate composites for controlled releasing drug delivery. Appl. Nanocomposite Mater. Drug Deliv. 2018, 761–785. [Google Scholar] [CrossRef]
- Bokov, O.D.; Sokurenko, S.M.; Malinkin, D.A.; Khromchenkova, P.E.; Shevyakova, V.L.; Bessonov, V.V. Physiochemical Features, Qualitative and Quantitative Analysis, Present Status and Application Prospects of Polysaccharide Gums. Int. J. Pharm. Qual. Assur. 2020, 11, 151–159. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Seelan, T.V.; Kumari, H.L.J.; Kishore, N.; Selvamani, P.; Lalhlenmawia, H.; Thanzami, K.; Pachuau, L.; Ruckmani, K. Exploitation of novel gum Prunus cerasoides as mucoadhesive beads for a controlled-release drug delivery. Int. J. Biol. Macromol. 2016, 85, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Prakash, D.; Ramesh, B.; Singh, N.; Mani, T. Biodegradable polymeric microspheres as drug carriers; A review. Indian J. Nov. Drug Deliv. 2011, 3, 70–82. [Google Scholar]
- Boni, F.I.; Prezotti, F.G.; Cury, B.S.F. Gellan gum microspheres crosslinked with trivalent ion: Effect of polymer and crosslinker concentrations on drug release and mucoadhesive properties. Drug Dev. Ind. Pharm. 2016, 42, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Shahidulla, S.; Fatima, S. Formulation and In-vitro Evaluation of Ciprofloxacin Hydrochloride Mucoadhesive Microspheres by Ionic Gelation Method. Int. J. Pharma Res. Health Sci. 2019, 7, 3032–3038. [Google Scholar] [CrossRef]
- Kar, A.K.; Samanta, P.; Kar, B.; Majumdar, R. Cross-linked natural gum microspheres: A feasible attitude for boosting the delivery of dicyclomine hydrochloride. Pharma Innov. J. 2020, 9, 278–284. [Google Scholar]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Ghumman, S.A.; Bashir, S.; Ahmad, J.; Hameed, H.; Khan, I.U. Colocasia esculenta corms mucilage-alginate microspheres of oxcarbazepine: Design, optimization and evaluation. Acta Pol. Pharm. 2017, 74, 505–517. [Google Scholar]
- Hameed, H.; Rehman, K.; Hameed, A.; Qayuum, A. Preparation and characterization of pH-responsive ionic crosslinked microparticles of mercaptopurine to target ulcerative colitis. Polym. Polym. Compos. 2021, 29, S1248–S1256. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ansari, M.T.; Sami, F.; Singh, H.K.B.; Hasnain, M.S. Alginates as drug delivery excipients. In Alginates in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–39. [Google Scholar]
- Benfattoum, K.; Haddadine, N.; Bouslah, N.; Benaboura, A.; Maincent, P.; Barillé, R.; Sapin-Minet, A.; El-Shall, M.S. Formulation characterization and in vitro evaluation of acacia gum–calcium alginate beads for oral drug delivery systems. Polym. Adv. Technol. 2018, 29, 884–895. [Google Scholar] [CrossRef]
- Saha, S.; Vaseem, M.; Ali, M. Formulation of Sustained Release Aceclofenac Matrix tablets using Prunus Armenica L. Gum as a release retardant. J. Adv. Pharm. Educ. Res. 2013, 3, 206–216. [Google Scholar]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Malik, M.Z. Taro-corms mucilage-alginate microspheres for the sustained release of pregabalin: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2019, 139, 1191–1202. [Google Scholar]
- Lee, D.; Hwang, S.; Park, J.; Park, H.J. Preparation and release characteristics of polymer-coated and blended alginate microspheres. J. Microencapsul. 2003, 20, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Simas, F.F.; Gorin, P.A.; Wagner, R.; Sassaki, G.L.; Bonkerner, A.; Iacomini, M. Comparison of structure of gum exudate polysaccharides from the trunk and fruit of the peach tree (Prunus persica). Carbohydr. Polym. 2008, 71, 218–228. [Google Scholar] [CrossRef]
- Malsawmtluangi, C.; Thanzami, K.; Lalhlenmawia, H.; Selvan, V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Physicochemical characteristics and antioxidant activity of Prunus cerasoides D. Don gum exudates. Int. J. Biol. Macromol. 2014, 69, 192–199. [Google Scholar] [CrossRef]
- Fathi, M.; Mohebbi, M.; Koocheki, A. Some physico-chemical properties of Prunus armeniaca L. gum exudates. Int. J. Biol. Macromol. 2016, 82, 744–750. [Google Scholar] [CrossRef]
- Sharma, A.; Bhushette, P.R.; Annapure, U.S. Purification and physicochemical characterization of Prunus domestica exudate gum polysaccharide. Carbohydr. Polym. Technol. Appl. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Fathi, M.; Mohebbi, M.; Koocheki, A.; Hesarinejad, M.A. Dilute solution properties of Prunus armeniaca gum exudates: Influence of temperature, salt, and sugar. Int. J. Biol. Macromol. 2017, 96, 501–506. [Google Scholar] [CrossRef]
- Azam Khan, M.; Ahmad, W.; Khan, S.; Rahim, H.; Roohullah, R.; Amin, F. Formulation and evaluation of sustained release tablets using Prunus armeniaca (L.) and Prunus domestica (L.) gums. Iran. J. Pharm. Sci. 2012, 8, 233–240. [Google Scholar]
- Rahim, H.; Sadiq, A.; Khan, S.; Khan, M.A.; Amin, F.; Jan, N.U.; Shahid, M.; Kifayatullah, M.; Ali, N.; Chishti, K.A. Prunus armeniaca and Prunus domestica gums: Exploring their synergistic binding potential in tablets. Lat. Am. J. Pharm. 2018, 37, 1672–1683. [Google Scholar]
- Sivakumar, R. Assessment of Prunus Amygdalus Gum for Functionality of Tablet Excipient. Saudi J. Med. Pharm. Sci. 2021, 7, 200–204. [Google Scholar]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M. Gummy gold and silver nanoparticles of apricot (Prunus armeniaca) confer high stability and biological activity. Arab. J. Chem. 2019, 12, 3977–3992. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M.; Khan, H.; Muhammad, N.; Sultana, S. Medicinal plants used for the treatment of jaundice and hepatitis based on socio-economic documentation. Afr. J. Biotechnol. 2009, 8, 1643–1650. [Google Scholar]
- Bouaziz, F.; Koubaa, M.; Ghorbel, R.E.; Chaabouni, S.E. Recent advances in Rosaceae gum exudates: From synthesis to food and non-food applications. Int. J. Biol. Macromol. 2016, 86, 535–545. [Google Scholar] [CrossRef]
- Shamsara, O.; Jafari, S.M.; Muhidinov, Z.K. Fabrication, characterization and stability of oil in water nano-emulsions produced by apricot gum-pectin complexes. Int. J. Biol. Macromol. 2017, 103, 1285–1293. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Raeisi, S. Evaluation of antifungal and antioxidant properties of edible coating based on apricot (Prunus armeniaca) gum containing Satureja intermedia extract in fresh wild almond (Amygdalus scoparia) kernels. J. Food Meas. Charact. 2018, 12, 362–369. [Google Scholar] [CrossRef]
- Salarbashi, D.; Jahanbin, K.; Tafaghodi, M.; Fahmideh-Rad, E. Prunus armeniaca gum exudates: An overview on purification, structure, physicochemical properties, and applications. Food Sci. Nutr. 2021, 9, 1240–1255. [Google Scholar] [CrossRef]
- Şensoy, D.; Cevher, E.; Altinkurt, T. Characterization of apricot gum as a binder in tablet formulations. Acta Pharm. Sci. 2006, 48, 35–43. [Google Scholar]
- Malana, M.A.; Zohra, R. The release behavior and kinetic evaluation of tramadol HCl from chemically cross linked Ter polymeric hydrogels. DARU J. Pharm. Sci. 2013, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Dhanya, K.; Cinu, T.; Aleykutty, N. Multiparticulate system for colon targeted delivery of ondansetron. Indian J. Pharm. Sci. 2010, 72, 58. [Google Scholar] [CrossRef] [Green Version]
- Cerciello, A.; Del Gaudio, P.; Granata, V.; Sala, M.; Aquino, R.P.; Russo, P. Synergistic effect of divalent cations in improving technological properties of cross-linked alginate beads. Int. J. Biol. Macromol. 2017, 101, 100–106. [Google Scholar] [CrossRef]
- AOAC International. Association of Official Analytical Chemists; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Sadasivam, S. Biochemical Methods; New Age International: Delhi, India, 1996. [Google Scholar]
- Brummer, Y.; Cui, S.W. Understanding carbohydrate analysis. In Food Carbohydrates: Chemistry, Physical Properties and Applications; Taylor & Francis: Abingdon, UK, 2005; pp. 1–38. [Google Scholar]
- Rivera-Corona, J.L.; Rodríguez-González, F.; Rendón-Villalobos, R.; García-Hernández, E.; Solorza-Feria, J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT-Food Sci. Technol. 2014, 59, 806–812. [Google Scholar] [CrossRef]
- Sinha, P.; Ubaidulla, U.; Hasnain, M.S.; Nayak, A.K.; Rama, B. Alginate-okra gum blend beads of diclofenac sodium from aqueous template using ZnSO4 as a cross-linker. Int. J. Biol. Macromol. 2015, 79, 555–563. [Google Scholar] [CrossRef]
- Vinod, V.; Sashidhar, R.; Suresh, K.; Rao, B.R.; Saradhi, U.V.; Rao, T.P. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S. In vivo, in vitro evaluation of linseed mucilage based buccal mucoadhesive microspheres of venlafaxine. Drug Deliv. 2011, 18, 111–121. [Google Scholar] [CrossRef]
- Tesfay, D.; Abrha, S.; Yilma, Z.; Woldu, G.; Molla, F. Preparation, optimization, and evaluation of epichlorohydrin cross-linked enset (Ensete ventricosum (Welw.) Cheeseman) starch as drug release sustaining excipient in microsphere formulation. BioMed Res. Int. 2020, 2020, 2147971. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Bar, H.M.; Abd el Basset Sanad, R. Endocytic pathways of optimized resveratrol cubosomes capturing into human hepatoma cells. Biomed. Pharmacother. 2017, 93, 561–569. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Taher, M.; Mohamed, F.; Octavianti, F.; Lestari, W.; Mukti, A.G.; Nirwandar, S.; Hamad Almansori, B.B. Optimization and formulation of fucoxanthin-loaded microsphere (F-LM) using response surface methodology (RSM) and analysis of its fucoxanthin release profile. Molecules 2019, 24, 947. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Nayak, A.K. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: In vitro–in vivo evaluation. Drug Deliv. 2012, 19, 123–131. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Swelling and drug release behavior of metformin HCl-loaded tamarind seed polysaccharide-alginate beads. Int. J. Biol. Macromol. 2016, 82, 1023–1027. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly (vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Sharma, H.K.; Sarangi, B.; Pradhan, S.P. Preparation and in-vitro evaluation of mucoadhesive microbeads containing Timolol Maleate using mucoadhesive substances of Dillenia indica L. Arch. Pharm. Sci. Res. 2009, 1, 181–188. [Google Scholar]
- Nayak, A.K.; Pal, D.; Das, S. Calcium pectinate-fenugreek seed mucilage mucoadhesive beads for controlled delivery of metformin HCl. Carbohydr. Polym. 2013, 96, 349–357. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Tamarind seed polysaccharide–gellan mucoadhesive beads for controlled release of metformin HCl. Carbohydr. Polym. 2014, 103, 154–163. [Google Scholar] [CrossRef]
- Tan, L.; Xu, X.; Song, J.; Luo, F.; Qian, Z. Synthesis, characterization, and acute oral toxicity evaluation of pH-sensitive hydrogel based on MPEG, poly (ε-caprolactone), and itaconic acid. BioMed Res. Int. 2013, 2013, 239838. [Google Scholar] [CrossRef] [Green Version]
- Gawade, S. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Mohebbi, M.; Koocheki, A. Introducing Prunus cerasus gum exudates: Chemical structure, molecular weight, and rheological properties. Food Hydrocoll. 2016, 61, 946–955. [Google Scholar] [CrossRef]
- Keshani-Dokht, S.; Emam-Djomeh, Z.; Yarmand, M.-S.; Fathi, M. Extraction, chemical composition, rheological behavior, antioxidant activity and functional properties of Cordia myxa mucilage. Int. J. Biol. Macromol. 2018, 118, 485–493. [Google Scholar] [CrossRef]
- Bhushette, P.R.; Annapure, U.S. Physicochemical, functional and rheological investigation of Soymida febrifuga exudate gum. Int. J. Biol. Macromol. 2018, 111, 1116–1123. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Chouaibi, M.; Donsi, F.; Ferrari, G.; Hamdi, S. Chemical composition and functional properties of gum exudates from the trunk of the almond tree (Prunus dulcis). Food Sci. Technol. Int. 2012, 18, 241–250. [Google Scholar] [CrossRef]
- Pachuau, L.; Lalhlenmawia, H.; Mazumder, B. Characteristics and composition of Albizia procera (Roxb.) Benth gum. Ind. Crops Prod. 2012, 40, 90–95. [Google Scholar] [CrossRef]
- Uhniat, M.; Sudoł, M.; Kudła, S. Stabilisation of LDPE cross-linked in the presence of peroxidesII. FTIR study of chemical changes taking place in the LDPE–dicumyl peroxide–Irganox 1081 system. Polym. Degrad. Stab. 2000, 71, 75–82. [Google Scholar] [CrossRef]
- Faccio, C.; Machado, R.A.; de Souza, L.M.; Zoldan, S.R.; Quadri, M.G. Characterization of the mucilage extracted from jaracatiá (Carica quercifolia (A. St. Hil.) Hieron). Carbohydr. Polym. 2015, 131, 370–376. [Google Scholar] [CrossRef]
- Fadavi, G.; Mohammadifar, M.A.; Zargarran, A.; Mortazavian, A.M.; Komeili, R. Composition and physicochemical properties of Zedo gum exudates from Amygdalus scoparia. Carbohydr. Polym. 2014, 101, 1074–1080. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Tavanai, H. Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydrocoll. 2016, 60, 461–469. [Google Scholar] [CrossRef]
- Sharma, A.; Bhushette, P.R.; Annapure, U.S. Physicochemical and rheological properties of Acacia Catechu exudate gum. Carbohydr. Polym. Technol. Appl. 2021, 2, 100127. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.-Y.; Ahmad, A. Linum usitatissimum L. seeds: Flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef]
- Vishwa, B.; Moin, A.; Gowda, D.; Rizvi, S.; Hegazy, W.A.; Abu Lila, A.S.; Khafagy, E.-S.; Allam, A.N. Pulmonary targeting of inhalable moxifloxacin microspheres for effective management of tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef]
- Hazra, M.; Mandal, D.D.; Mandal, T.; Rao, B.P.; Samaddar, S. Optimization and characterization of hepatoprotective andrographolide microspheres in the necrotic rat model. J. Drug Deliv. Sci. Technol. 2021, 65, 102679. [Google Scholar] [CrossRef]
- Telange, D.R.; Pandharinath, R.R.; Pethe, A.M.; Jain, S.P.; Pingale, P.L. Calcium Ion-Sodium Alginate-Piperine-Based Microspheres: Evidence of Enhanced Encapsulation Efficiency, Bio-Adhesion, Controlled Delivery, and Oral Bioavailability of Isoniazid. AAPS PharmSciTech 2022, 23, 1–18. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, S.S.; Krishnamoorthy, B.; Basu, S.K. Development and evaluation of calcium alginate beads prepared by sequential and simultaneous methods. Braz. J. Pharm. Sci. 2010, 46, 785–793. [Google Scholar] [CrossRef]
- Bera, H.; Kandukuri, S.G.; Nayak, A.K.; Boddupalli, S. Alginate–sterculia gum gel-coated oil-entrapped alginate beads for gastroretentive risperidone delivery. Carbohydr. Polym. 2015, 120, 74–84. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B.; Maji, R. Calcium alginate/gum Arabic beads containing glibenclamide: Development and in vitro characterization. Int. J. Biol. Macromol. 2012, 51, 1070–1078. [Google Scholar] [CrossRef]
- Ghumman, S.A.; Noreen, S.; Muntaha, S.T. Linum usitatissimum seed mucilage-alginate mucoadhesive microspheres of metformin HCl: Fabrication, characterization and evaluation. Int. J. Biol. Macromol. 2020, 155, 358–368. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Beg, S.; Alam, M.I. Mucoadhesive beads of gliclazide: Design, development, and evaluation. Sci Asia 2010, 36, 319–325. [Google Scholar] [CrossRef]
- Freitas, E.D.; Freitas, V.M.; Rosa, P.C.; da Silva, M.G.; Vieira, M.G. Development and evaluation of naproxen-loaded sericin/alginate beads for delayed and extended drug release using different covalent crosslinking agents. Mater. Sci. Eng. C 2021, 118, 111412. [Google Scholar] [CrossRef]
- Sinha, P.; Ubaidulla, U.; Nayak, A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015, 72, 1069–1075. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, S.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, H.H.; Mohammed, F.A.; El-Sayed, A.M.; Soliman, G.M. Colon-targeting of progesterone using hybrid polymeric microspheres improves its bioavailability and in vivo biological efficacy. Int. J. Pharm. 2020, 577, 119070. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Malviya, R.; Sharma, P.K. Design and development of multi particulate system for targeted drug delivery using natural polymer. Pharm. Anal. Acta 2015, 6, 366. [Google Scholar]
- Andishmand, H.; Tabibiazar, M.; Mohammadifar, M.A.; Hamishehkar, H. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int. J. Biol. Macromol. 2017, 97, 16–22. [Google Scholar] [CrossRef]
- Kajale, A.D.; Chandewar, A. Formulation and evaluation of oral floating beads of tramodol hydrochloride. J. Drug Deliv. Ther. 2016, 6, 7–16. [Google Scholar] [CrossRef]
- Ammar, H.; Ghorab, M.; El-Nahhas, S.; Kamel, R. Polymeric matrix system for prolonged delivery of tramadol hydrochloride, part I: Physicochemical evaluation. Aaps. Pharmscitech. 2009, 10, 7–20. [Google Scholar] [CrossRef]
- Shetty, A.; Azharuddin, M.; Shabaraya, A. Development and evaluation of tramadol hydrochloride microspheres as colon targeted drug delivery system. Int. J. Innov. Drug Discov. 2015, 5, 7–13. [Google Scholar]
- Kamel, R.; Abbas, H. Self-assembled carbohydrate hydrogels for prolonged pain management. Pharm. Dev. Technol. 2013, 18, 990–1004. [Google Scholar] [CrossRef]
- Dash, A. Determination of the physical state of drug in microcapsule and microsphere formulations. J. Microencapsul. 1997, 14, 101–112. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Heras, A. Chitosan-genipin microspheres for the controlled release of drugs: Clarithromycin, tramadol and heparin. Mar. Drugs 2010, 8, 1750–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, S.; Chowdhury, M.; Chakraborty, A.; Ray, S.; Sa, B. Sulfated locust bean gum hydrogel beads for immediate analgesic effect of tramadol hydrochloride. J. Sci. Ind. Res. 2014, 73, 21–28. [Google Scholar]
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of ionotropic cross-linked chitosan/gelatin B microspheres of tramadol hydrochloride. Aaps. Pharmscitech. 2011, 12, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, R.; Mahmoud, A.; El-Feky, G. Double-phase hydrogel for buccal delivery of tramadol. Drug Dev. Ind. Pharm. 2012, 38, 468–483. [Google Scholar] [CrossRef]
- Kaur, P.; Ghildiyal, S.; Soni, S. Development and Evaluation of Hydrodynamically Balanced System of Tramadol Hydrochloride by Using Chitosan and Locust Bean Gum. J. Intern. Med. Emerg. Res. 2020, 1, 1–20. [Google Scholar]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Riffat, S.; Abbas, M. Polymeric microspheres of okra mucilage and alginate for the controlled release of oxcarbazepine: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2018, 111, 1156–1165. [Google Scholar] [PubMed]
| Formulation Code | Concentration (g) | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| PAG | ALG | PS (µm) | EE (%) | Yield (%) | ||||
| X1 | X2 | Y1 | Y2 | Y3 | ||||
| Exp. | Pred. * | Exp. | Pred. ** | Exp. | Pred. *** | |||
| 1 | 4 | 2 | 626.42 | 627.07 | 81.78 | 82.42 | 90.60 | 90.89 |
| 2 | 4.4 | 3 | 685.00 | 687.52 | 83.91 | 82.50 | 75.54 | 76.13 |
| 3 | 2 | 4 | 591.54 | 593.42 | 79.59 | 79.27 | 87.13 | 88.45 |
| 4 | 3 | 4.4 | 622.45 | 623.01 | 80.36 | 80.94 | 85.56 | 84.58 |
| 5 | 3 | 3 | 607.34 | 606.80 | 82.74 | 82.79 | 91.27 | 91.31 |
| 6 | 4 | 2 | 628.62 | 627.07 | 80.56 | 82.42 | 92.07 | 90.89 |
| 7 | 4 | 4 | 685.23 | 682.84 | 79.95 | 80.08 | 70.28 | 70.47 |
| 8 | 3 | 3 | 606.25 | 606.80 | 82.83 | 82.79 | 91.34 | 91.31 |
| 9 | 1.5 | 3 | 607.67 | 604.16 | 72.45 | 71.67 | 73.45 | 72.44 |
| 10 | 3 | 1.5 | 588.79 | 587.24 | 77.33 | 74.56 | 83.78 | 84.34 |
| 11 | 2 | 2 | 595.23 | 598.60 | 65.86 | 67.91 | 67.46 | 67.69 |
| 12 | 3 | 3 | 607.34 | 606.80 | 82.74 | 82.79 | 91.27 | 91.31 |
| 13 | 3 | 3 | 606.25 | 606.80 | 82.83 | 82.79 | 91.34 | 91.31 |
| Component | Quantity (%) |
|---|---|
| Moisture | 8.45 ± 0.29 |
| Ash | 3.86 ± 0.06 |
| Fat | 1.26 ± 0.09 |
| Protein | 2.53 ± 0.05 |
| Total sugars | 71.32 ± 0.78 |
| Uronic acid | 9.64 ± 0.21 |
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | PRESS | Remarks | ||
| Mean Particle Size (µm) | Linear | 0.002 | <0.0001 | 0.632 | 0.395 | 7176.4 | -------- | ||
| 2FI | 0.104 | <0.0001 | 0.7 | 0.542 | 5418.13 | -------- | |||
| Quadratic | <0.0001 | 0.0112 | 0.993 | 0.975 | 285.09 | Suggested | |||
| Cubic | 0.006 | 0.1667 | 0.998 | 0.987 | 146.13 | Aliased | |||
| Encapsulation efficiency (%) | Linear | 0.022 | 0.0002 | 0.439 | 0.131 | 269.40 | -------- | ||
| 2FI | 0.067 | 0.0003 | 0.579 | 0.244 | 234.34 | -------- | |||
| Quadratic | 0.003 | 0.003 | 0.862 | 0.684 | 97.96 | Suggested | |||
| Cubic | 0.126 | 0.0032 | 0.936 | −0.276 | 396.03 | Aliased | |||
| Yield (%) | Linear | 0.828 | <0.0001 | −0.155 | −0.829 | 1791.02 | -------- | ||
| 2FI | 0.019 | <0.0001 | 0.320 | −0.054 | 1032.84 | -------- | |||
| Quadratic | <0.0001 | 0.0584 | 0.989 | 0.965 | 33.75 | Suggested | |||
| Cubic | 0.042 | 0.2106 | 0.995 | 0.963 | 35.56 | Aliased | |||
| B | |||||||||
| Terms | Responses | ||||||||
| P.S. | EE (%) | Yield (%) | |||||||
| Source | Sum of Squares | F-Value | p-Value | Sum of Squares | F-Value | p-Value | Sum of Squares | F-Value | p-Value |
| Model | 11,803.49 | 359.13 | <0.0001 | 291.5 | 21.74 | 0.0004 | 973.09 | 228.89 | <0.0001 |
| X1-Alginate | 7528.63 | 1145.33 | <0.0001 | 127.03 | 47.36 | 0.0002 | 14.73 | 17.33 | 0.0042 |
| X2-PAG | 1386.19 | 210.88 | <0.0001 | 44.09 | 16.44 | 0.0048 | 0.06 | 0.07 | 0.7932 |
| X1X2 | 1097.69 | 166.99 | <0.0001 | 55.46 | 20.68 | 0.0026 | 500.90 | 589.11 | <0.0001 |
| X12 | 2477.60 | 376.92 | <0.0001 | 52.73 | 19.66 | 0.003 | 470.70 | 553.59 | <0.0001 |
| X22 | 4.52 | 0.6877 | 0.4343 | 41.13 | 15.34 | 0.005 | 76.12 | 89.53 | <0.0001 |
| Lack of Fit | 42.41 | 15.67 | 0.0112 | 18.03 | 31.94 | 0.003 | 1.62 | 5.98 | 0.05 |
| Formulations | Components | D-P Ratio (g) | CaCl2 W/V (%) | Yield (%) Mean ± SD | Diameter (µm) Mean ± SD | EE (%) Mean ± SD |
|---|---|---|---|---|---|---|
| TA-1 | TRHC-ALG | 1:6 | 10 | 88.34 ± 0.96 | 657.67 ± 08.74 | 77.47 ± 0.69 |
| TPB-2 | TRHC-ALG-PAG | 1:4:2 | 10 | 86.60 ± 1.14 | 633.42 ± 10.59 | 81.78 ± 0.42 |
| TPB-3 | TRHC-ALG-PAG | 1:3:3 | 10 | 91.27 ± 0.85 | 607.34 ± 06.02 | 83.74 ± 0.79 |
| TPC-4 | TRHC-ALG-PAG | 1:4:2 | 10 | 82.13 ± 1.54 | 591.54 ± 09.01 | 78.59 ± 0.67 |
| TPC-5 | TRHC-ALG-PAG | 1:3:3 | 10 | 80.46 ± 1.08 | 579.23 ± 07.09 | 65.86 ± 0.26 |
| Formulation | Correlation Coefficient (R2) | ||||
|---|---|---|---|---|---|
| TA-1 | TPB-2 | TPB-3 | TPC-4 | TPC-5 | |
| Zero-order | 0.8953 | 0.9451 | 0.9285 | 0.8569 | 0.8467 |
| First-order | 0.7762 | 0.8595 | 0.8476 | 0.7727 | 0.7689 |
| Higuchi | 0.5985 | 0.6659 | 0.6411 | 0.5608 | 0.5519 |
| Hixson-Crowell | 0.8139 | 0.8886 | 0.8746 | 0.7991 | 0.7934 |
| Korsmeyer-Peppas | 0.9803 | 0.9954 | 0.9966 | 0.9956 | 0.9917 |
| Release exponent (n) | 1.604 | 1.434 | 1.537 | 1.985 | 2.041 |
| Parameters to Observe | Control Group | Treated Group |
|---|---|---|
| Mortality | Nil | Nil |
| Sign of illness | Nil | Nil |
| Food intake (g) | ||
| Pre-treatment | 2.8 ± 1.4 | 2.4 ± 1.2 |
| Day 1 | 2.9 ± 1.6 | 2.7 ± 1.4 |
| Day 3 | 3.2 ± 1.4 | 3.0 ± 1.6 |
| Day 7 | 3.8 ± 1.0 | 3.6 ± 1.2 |
| Day 10 | 4.0 ± 1.4 | 3.8 ± 1.4 |
| Day 14 | 4.4 ± 1.2 | 4.2 ± 1.6 |
| Water intake (mL) | ||
| Pre-treatment | 6.8 ± 1.4 | 6.4 ± 1.0 |
| Day 1 | 7.4 ± 1.2 | 7.2 ± 1.4 |
| Day 3 | 8.2 ± 1.6 | 7.8 ± 1.6 |
| Day 7 | 10.2 ± 0.9 | 10.4 ± 1.2 |
| Day 10 | 10.6 ± 2.0 | 10.9 ± 1.8 |
| Day 14 | 12.2 ± 1.0 | 12.6 ± 0.9 |
| Bodyweight (g) | ||
| Pre-treatment | 22.5 ± 1.4 | 22.8 ± 1.2 |
| Day 1 | 23.8 ± 1.2 | 24.2 ± 1.4 |
| Day 3 | 24.5 ± 1.6 | 24.9 ± 1.2 |
| Day 7 | 27.5 ± 1.2 | 27.9 ± 1.6 |
| Day 10 | 29.8 ± 1.4 | 30.2 ± 1.2 |
| Day 14 | 31.5 ± 1.6 | 31.8 ± 1.4 |
| Plasma Analysis | Control Group | Treated Group |
|---|---|---|
| ALT (IU/L) | 62 ± 1.9 | 58 ± 2.3 |
| AST (IU/L) | 136 ± 5.8 | 124 ± 6.3 |
| Cholesterol (mg/dL) | 127 ± 4.3 | 122 ± 4.2 |
| Triglyceride (mg/dL) | 126 ± 3.8 | 116 ± 3.4 |
| Creatinine (mg/dL) | 0.33 ± 1.1 | 0.36 ± 0.9 |
| Urea (mg/dL) | 60 ± 2.2 | 58 ± 2.4 |
| Uric acid (mg/dL) | 5.4 ± 1.6 | 4.8 ± 1.8 |
| Group | Liver | Stomach | Heart | Kidney | Spleen |
|---|---|---|---|---|---|
| Control | 5.86 ± 0.16 | 1.56 ± 0.44 | 0.58 ± 0.03 | 0.86 ± 0.03 | 0.62 ± 0.01 |
| Treated | 5.66 ± 0.18 | 1.62 ± 0.64 | 0.52 ± 0.05 | 0.79 ± 0.04 | 0.58 ± 0.02 |
| Groups | Number of Writhes (Mean ± SEM) | Reduction in Writhes Count (%) |
|---|---|---|
| Control (2.5 h prior administration of non-drug loaded PAG-alginate microspheres) + (1% acetic acid) | 62.4 ± 5.8 | 0% |
| Treated (2.5 h prior administration of TRHC drug-loaded PAG-alginate microspheres) + (1% acetic acid) | 30.8 ** ± 4.2 ** p < 0.01 (n = 6) | 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noureen, S.; Noreen, S.; Ghumman, S.A.; Batool, F.; Hameed, H.; Hasan, S.; Noreen, F.; Elsherif, M.A.; Bukhari, S.N.A. Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation. Pharmaceutics 2022, 14, 916. https://doi.org/10.3390/pharmaceutics14050916
Noureen S, Noreen S, Ghumman SA, Batool F, Hameed H, Hasan S, Noreen F, Elsherif MA, Bukhari SNA. Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation. Pharmaceutics. 2022; 14(5):916. https://doi.org/10.3390/pharmaceutics14050916
Chicago/Turabian StyleNoureen, Shazia, Sobia Noreen, Shazia Akram Ghumman, Fozia Batool, Huma Hameed, Sara Hasan, Fozia Noreen, Mervat A. Elsherif, and Syed Nasir Abbas Bukhari. 2022. "Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation" Pharmaceutics 14, no. 5: 916. https://doi.org/10.3390/pharmaceutics14050916
APA StyleNoureen, S., Noreen, S., Ghumman, S. A., Batool, F., Hameed, H., Hasan, S., Noreen, F., Elsherif, M. A., & Bukhari, S. N. A. (2022). Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation. Pharmaceutics, 14(5), 916. https://doi.org/10.3390/pharmaceutics14050916







