Overcoming Hypoxia-Induced Drug Resistance via Promotion of Drug Uptake and Reoxygenation by Acousto–Mechanical Oxygen Delivery
Abstract
1. Introduction
2. Materials and Methods
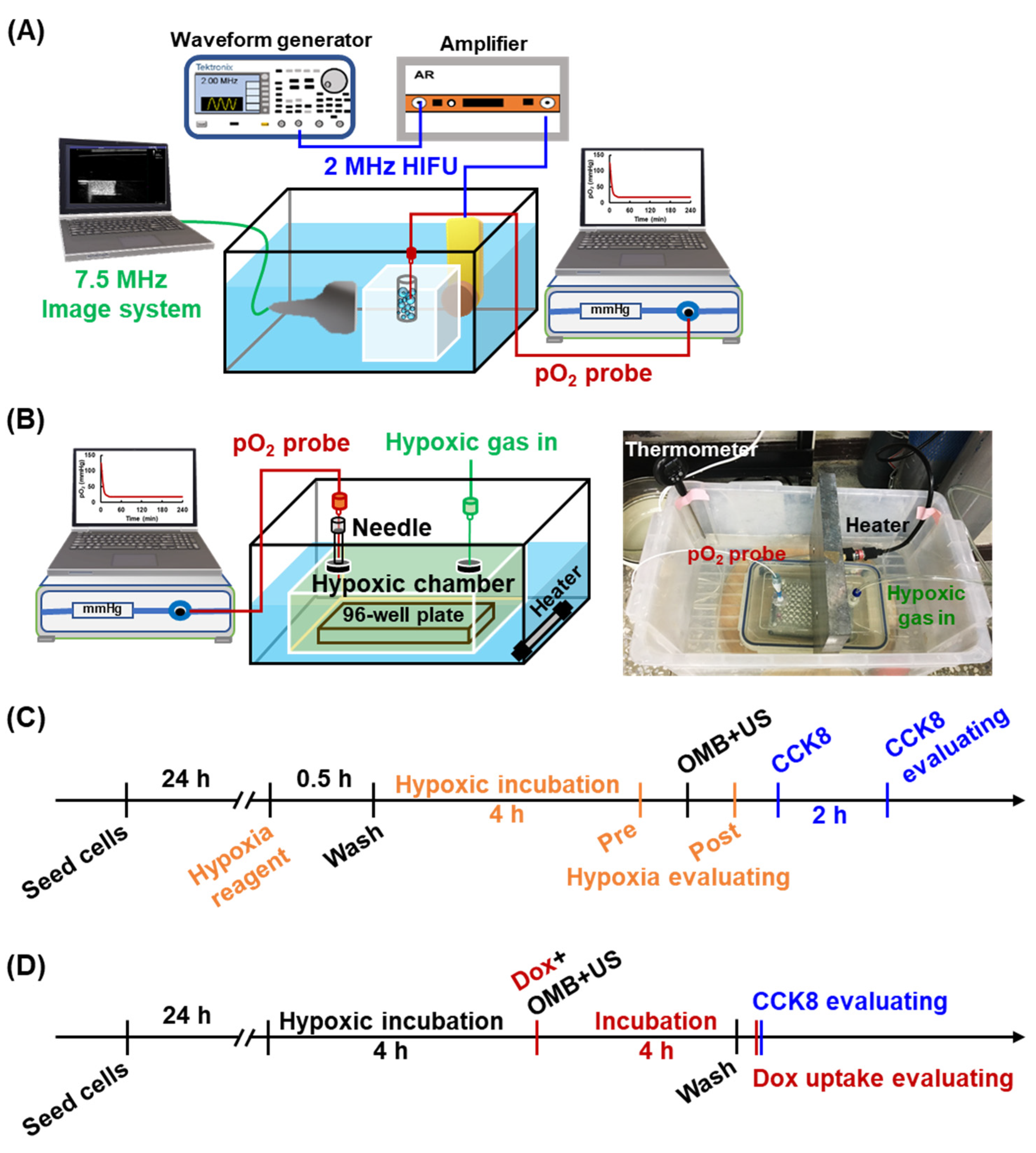
2.1. Fabrication of OMBs
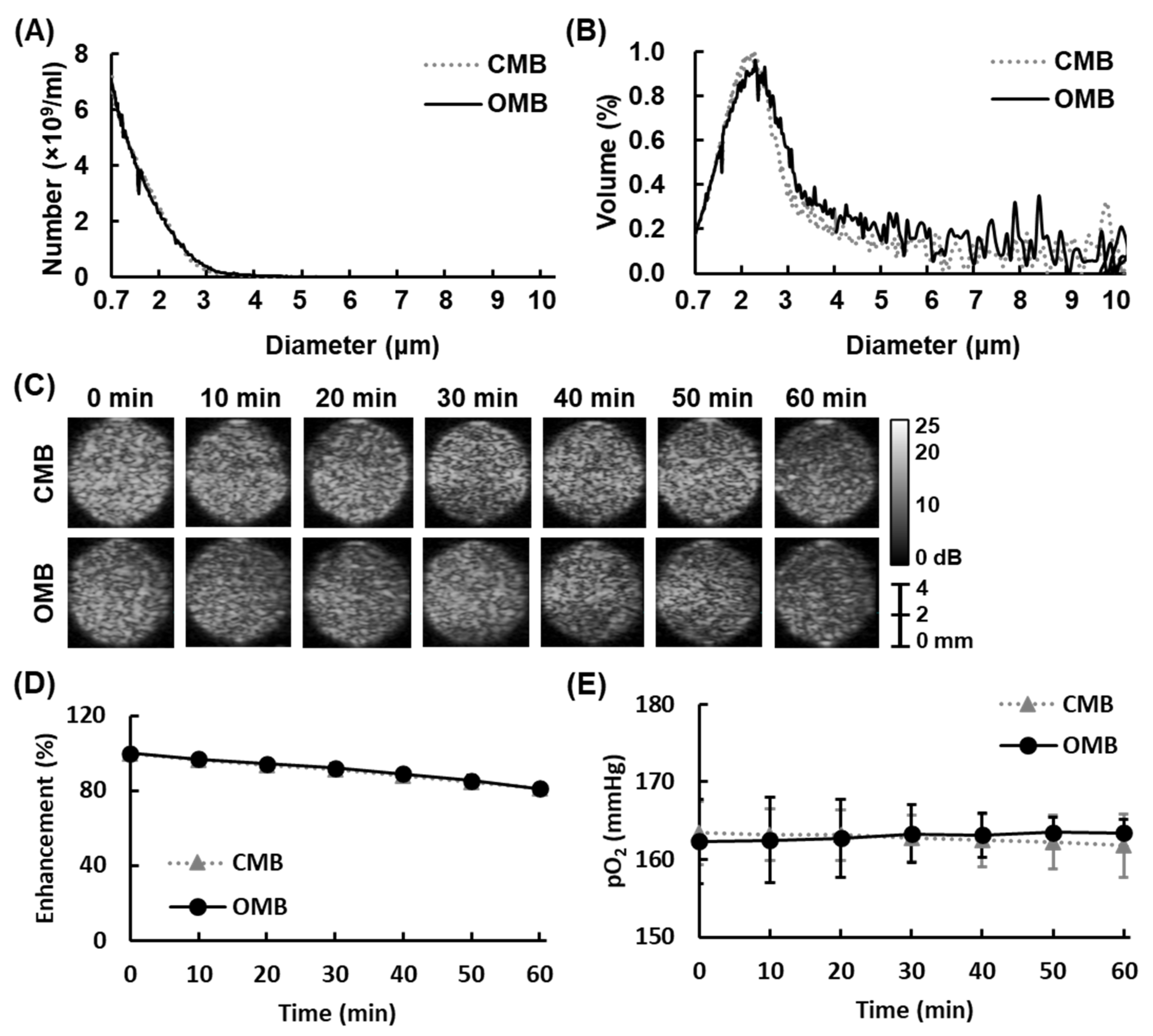
2.2. Evaluation of the In Vitro Stability of OMBs
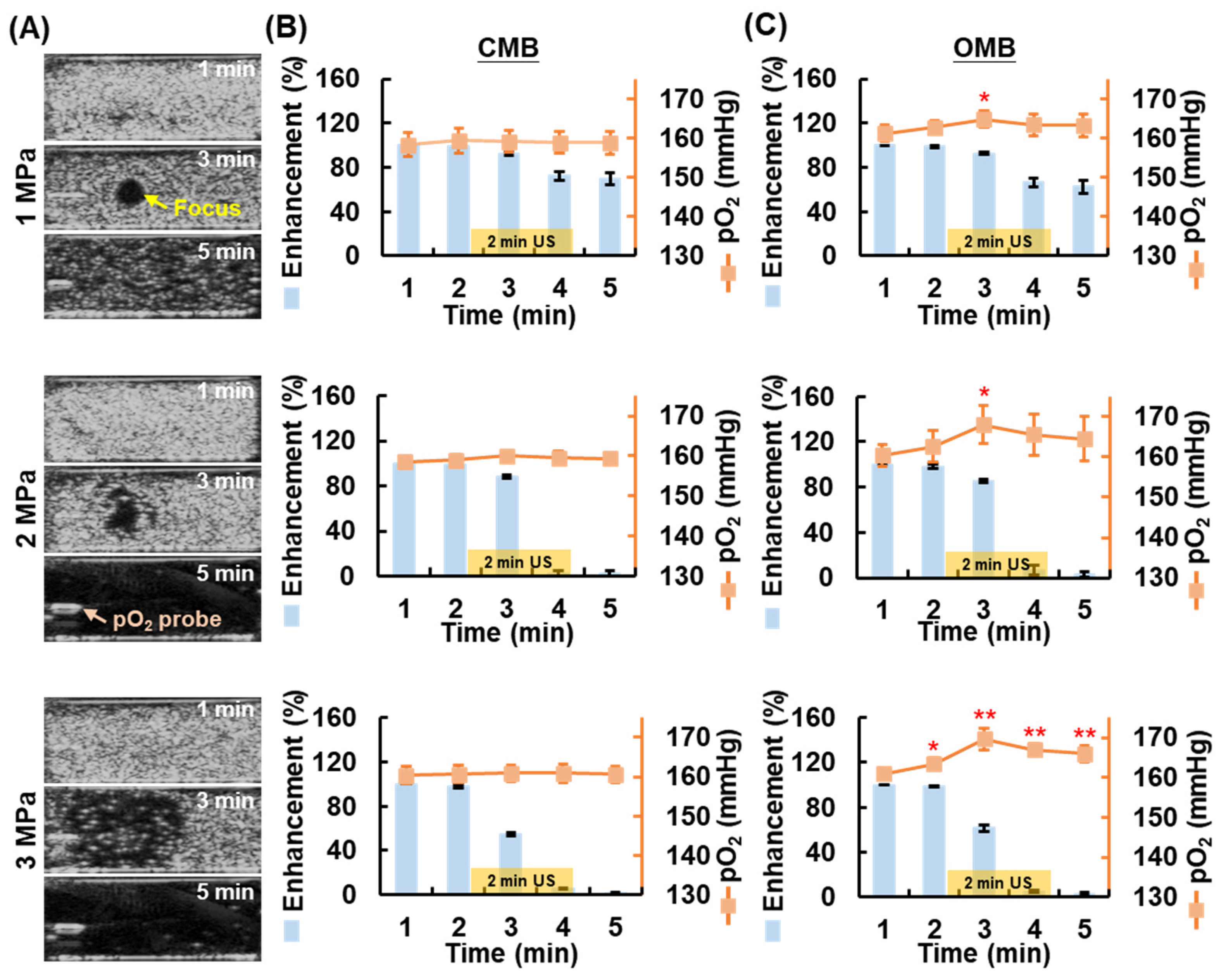
2.3. Local O2 Release from OMBs by HIFU Sonication
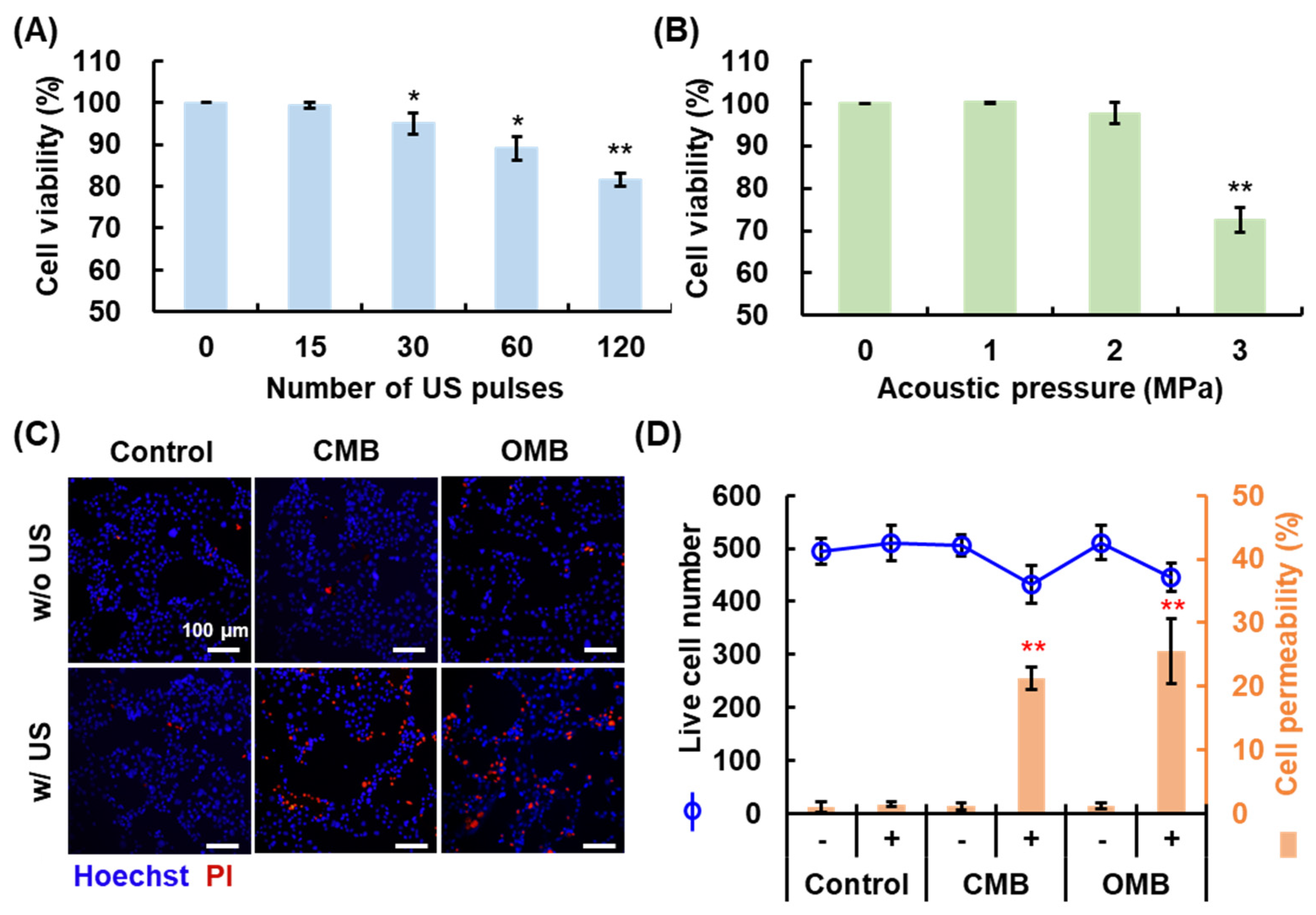
2.4. Cytotoxicity of Acoustic Parameters
2.5. Evaluation of Cell Viability and Permeability
2.6. HDR Cell Model and Reoxygenation
2.7. Cytotoxicity and Drug Uptake after OMB Treatment
2.8. Statistical Analysis
3. Results
3.1. Size Distribution and Stability of OMBs
3.2. Local O2 Release from OMBs Triggered by US
3.3. Optimal HIFU Parameters for Cell Sonication
3.4. Enhancement of Cell Permeability by OMB Treatment
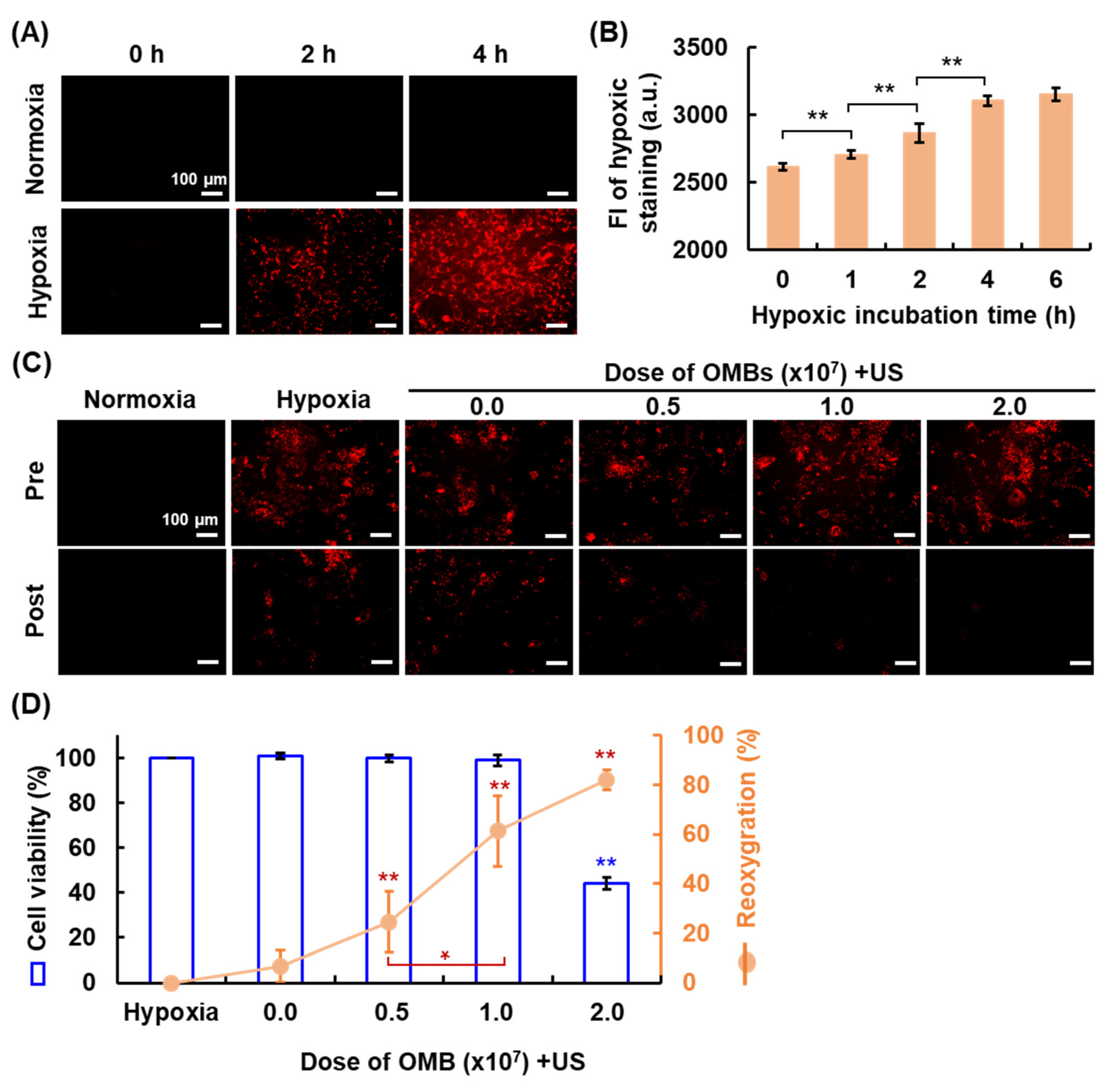
3.5. Optimal OMB Dose for HDR Cell Reoxygenation
3.6. Overcoming HDR by OMB Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9 (Suppl. 5), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, A.; Melillo, G. Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug Resist. Updates 2009, 12, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Exploiting the hypoxic cancer cell: Mechanisms and therapeutic strategies. Mol. Med. Today 2000, 6, 157–162. [Google Scholar] [CrossRef]
- Primeau, A.J.; Rendon, A.; Hedley, D.; Lilge, L.; Tannock, I.F. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 2005, 11, 8782–8788. [Google Scholar] [CrossRef]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tsuneyama, K.; Yen, M.H.; Lee, J.T.; Chen, J.L.; Huang, S.M. Hyperbaric oxygen suppressed tumor progression through the improvement of tumor hypoxia and induction of tumor apoptosis in A549-cell-transferred lung cancer. Sci. Rep. 2021, 11, 12033. [Google Scholar] [CrossRef]
- Hughes, V.S.; Wiggins, J.M.; Siemann, D.W. Tumor oxygenation and cancer therapy-then and now. Br. J. Radiol. 2018, 92, 20170955. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; de la Guardia, M.; Ahmadi, D.; Yousefi, B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J. Cell. Physiol. 2018, 233, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Huang, C.C.; Fan, C.H.; Liu, H.L.; Yeh, C.K. Ultrasonic technologies in imaging and drug delivery. Cell. Mol. Life Sci. 2021, 78, 6119–6141. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Develop. Ther. 2013, 7, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Chu, S.W.; Liao, E.C.; Fan, C.H.; Chan, H.L.; Wei, K.C.; Yeh, C.K. Normalization of Tumor Vasculature by Oxygen Microbubbles with Ultrasound. Theranostics 2019, 9, 7370–7383. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Seo, Y.; Shin, K.; Lee, K.; Park, C.; Choi, Y.; Hong, J.W.; Choi, J. Engineering oxygen nanobubbles for the effective reversal of hypoxia. Artif. Cells Nanomed. Biotechnol. 2018, 46, S318–S327. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Seo, Y.; Jeon, H.; Hong, J.W.; Choi, J. Anti-Tumor Drug-Loaded Oxygen Nanobubbles for the Degradation of HIF-1 alpha and the Upregulation of Reactive Oxygen Species in Tumor Cells. Cancers 2019, 11, 1464. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Shraim, R.; Liu, J.B.; Li, J.Z.; Stanczak, M.; Oeffinger, B.; Leeper, D.B.; Keith, S.W.; Jablonowski, L.J.; Forsberg, F.; et al. Sensitization of Hypoxic Tumors to Radiation Therapy Using Ultrasound-Sensitive Oxygen Microbubbles. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 88–96. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Ho, Y.J.; Chang, H.C.; Lin, C.W.; Fan, C.H.; Lin, Y.C.; Wei, K.C.; Yeh, C.K. Oscillatory behavior of microbubbles impacts efficacy of cellular drug delivery. J. Control Release 2021, 333, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Han, T.; Yu, A.C.H.; Xu, L. Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery. J. Control Release 2018, 272, 169–181. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; Errington, R.; Pors, K. Current challenges and opportunities in treating hypoxic prostate tumors. J. Cancer Metastasis Treat. 2018, 4, 11. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Lin, L.; Cheng, M.; Jin, L.; Du, L.; Han, T.; Xu, L.; Yu, A.C.H.; Qin, P. Effect of acoustic parameters on the cavitation behavior of SonoVue microbubbles induced by pulsed ultrasound. Ultrason. Sonochem. 2017, 35, 176–184. [Google Scholar] [CrossRef]
- Lai, C.Y.; Wu, C.H.; Chen, C.C.; Li, P.C. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound Med. Biol. 2006, 32, 1931–1941. [Google Scholar] [CrossRef]
- Wu, N.; Fan, C.H.; Yeh, C.K. Ultrasound-activated nanomaterials for sonodynamic cancer theranostics. Drug Discov. Today 2022. [Google Scholar] [CrossRef]
- Miller, D.L.; Gies, R.A. Enhancement of ultrasonically-induced hemolysis by perfluorocarbon-based compared to air-based echo-contrast agents. Ultrasound Med. Biol. 1998, 24, 285–292. [Google Scholar] [CrossRef]
- Ho, Y.J.; Wang, T.C.; Fan, C.H.; Yeh, C.K. Spatially Uniform Tumor Treatment and Drug Penetration by Regulating Ultrasound with Microbubbles. ACS Appl. Mater. Interfaces 2018, 10, 17784–17791. [Google Scholar] [CrossRef]
- McEwan, C.; Kamila, S.; Owen, J.; Nesbitt, H.; Callan, B.; Borden, M.; Nomikou, N.; Hamoudi, R.A.; Taylor, M.A.; Stride, E.; et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials 2016, 80, 20–32. [Google Scholar] [CrossRef]
- Fu, J.K.; Li, T.; Yang, Y.Z.; Jiang, L.P.; Wang, W.H.; Fu, L.J.; Zhu, Y.C.; Hao, Y.Q. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2021, 268, 120537. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Therapeutic oxygen delivery by perfluorocarbon-based colloids. Adv. Colloid Interface Sci. 2021, 294, 102407. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Hong, J.; Park, B.; Choi, Y.; Khan, M.S.; Hwang, J.; Tanaka, M.; Choi, J. Oxygen transport to mammalian cell and bacteria using nano-sized liposomes encapsulating oxygen moleculesY. J. Biosci. Bioeng. 2021, 132, 657–665. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Albala, L.; Kramer, M.R.; Daroshefski, N.; Brown, D.; Liu, J.B.; Stanczak, M.; O’Kane, P.; Forsberg, F.; Wheatley, M.A. Development of an ultrasound sensitive oxygen carrier for oxygen delivery to hypoxic tissue. Int. J. Pharm. 2015, 478, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Xiao, H.; Sun, Y.; Zhu, L.R.; Gao, Y.; Kwok, S.; Wang, Z.G.; Tang, Y. Lipid Microbubbles as Ultrasound-Stimulated Oxygen Carriers for Controllable Oxygen Release for Tumor Reoxygenation. Ultrasound Med. Biol. 2018, 44, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.T.; Forsberg, F.; Vaidyanathan, P.; Tornes, A.; Ostensen, J.; Goldberg, B.B. The influence of acoustic transmit parameters on the destruction of contrast microbubbles in vitro. Phys. Med. Biol. 2006, 51, 4031–4045. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Quddus, J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 10179–10184. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Broxterman, H.J.; Gotink, K.J.; Verheul, H.M. Understanding the causes of multidrug resistance in cancer: A comparison of doxorubicin and sunitinib. Drug Resist. Updates 2009, 12, 114–126. [Google Scholar] [CrossRef]
- Frederiksen, L.J.; Siemens, D.R.; Heaton, J.P.; Maxwell, L.R.; Adams, M.A.; Graham, C.H. Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J. Urol. 2003, 170, 1003–1007. [Google Scholar] [CrossRef]
- Sun, J.; Yin, M.; Zhu, S.; Liu, L.; Zhu, Y.; Wang, Z.; Xu, R.X.; Chang, S. Ultrasound-mediated destruction of oxygen and paclitaxel loaded lipid microbubbles for combination therapy in hypoxic ovarian cancer cells. Ultrason. Sonochem. 2016, 28, 319–326. [Google Scholar] [CrossRef]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control Release 2015, 203, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F. Tumor physiology and drug resistance. Cancer Metastasis Rev. 2001, 20, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kondo, T.; Zhao, Q.L.; Feril, L.B., Jr.; Kitagawa, H. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med. Biol. 2004, 30, 683–692. [Google Scholar] [CrossRef]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res. 2005, 68, 26–36. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.-J.; Thao, D.T.; Yeh, C.-K. Overcoming Hypoxia-Induced Drug Resistance via Promotion of Drug Uptake and Reoxygenation by Acousto–Mechanical Oxygen Delivery. Pharmaceutics 2022, 14, 902. https://doi.org/10.3390/pharmaceutics14050902
Ho Y-J, Thao DT, Yeh C-K. Overcoming Hypoxia-Induced Drug Resistance via Promotion of Drug Uptake and Reoxygenation by Acousto–Mechanical Oxygen Delivery. Pharmaceutics. 2022; 14(5):902. https://doi.org/10.3390/pharmaceutics14050902
Chicago/Turabian StyleHo, Yi-Ju, Dinh Thi Thao, and Chih-Kuang Yeh. 2022. "Overcoming Hypoxia-Induced Drug Resistance via Promotion of Drug Uptake and Reoxygenation by Acousto–Mechanical Oxygen Delivery" Pharmaceutics 14, no. 5: 902. https://doi.org/10.3390/pharmaceutics14050902
APA StyleHo, Y.-J., Thao, D. T., & Yeh, C.-K. (2022). Overcoming Hypoxia-Induced Drug Resistance via Promotion of Drug Uptake and Reoxygenation by Acousto–Mechanical Oxygen Delivery. Pharmaceutics, 14(5), 902. https://doi.org/10.3390/pharmaceutics14050902







