Anti-Cancerous Potential of Polysaccharides Derived from Wheat Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cell Culture, Polysaccharides Purification and Separation
2.2. Characterization of Polysaccharide Fractions, Monosaccharide Content Determination
2.3. Cell Lines
2.4. In Vitro Cytotoxicity Assays
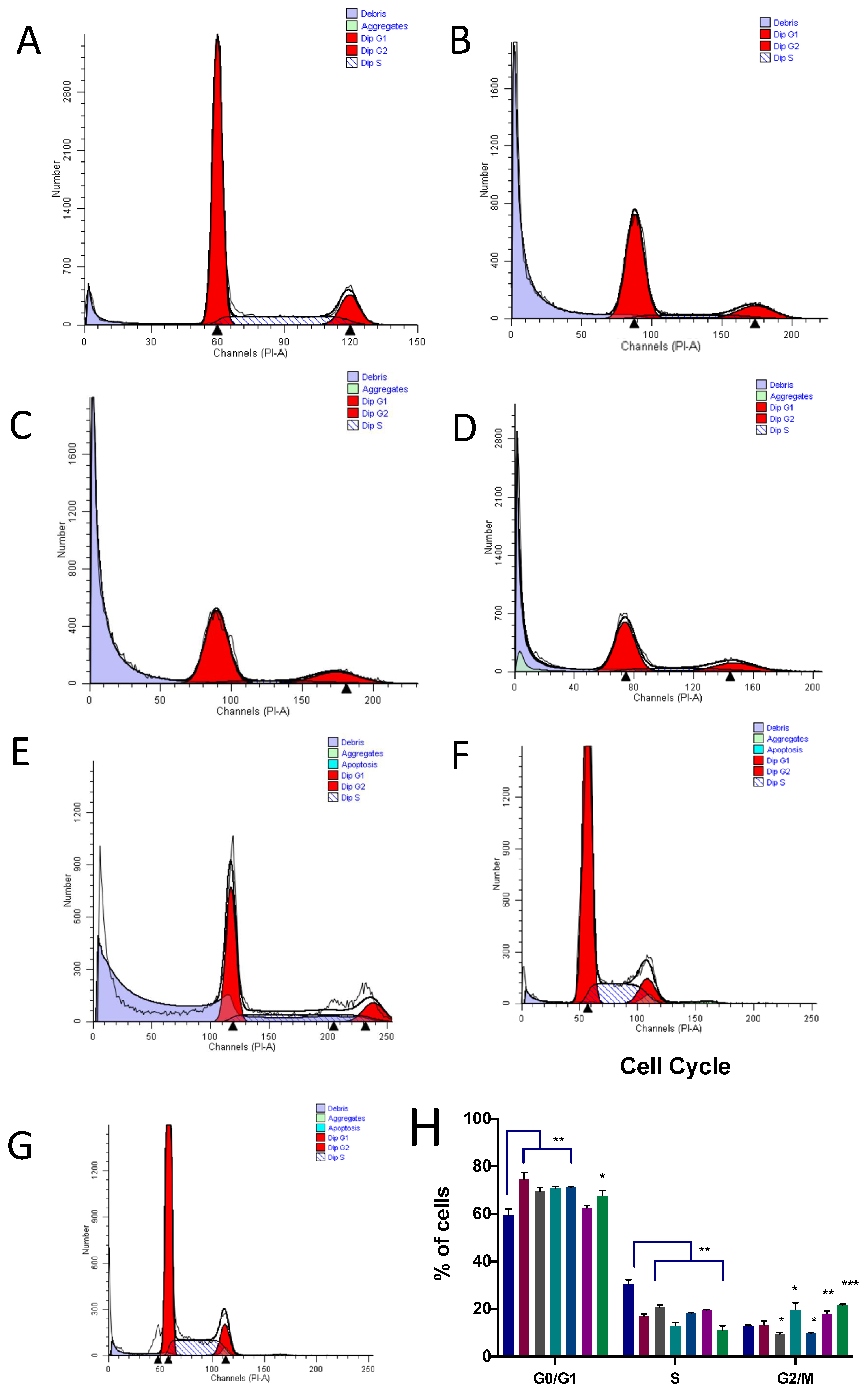
2.5. Apoptosis and Cell Cycle Assays
2.6. Electron Microscopy
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Monosaccharide Composition of WCCPSs
3.2. Wheat Cell Culture Polysaccharides Have an Antiproliferative and Selective Inhibitory Effect on Colon Cancer Cell Lines
3.3. Induction of Apoptosis and Cell Cycle Arrest
3.4. Electron Microscopy of HCT-116 Cells under the Influence of WCCPSs
3.4.1. Transmission Electron Microscopy (TEM)
3.4.2. Scanning Electron Microscopy (SEM)
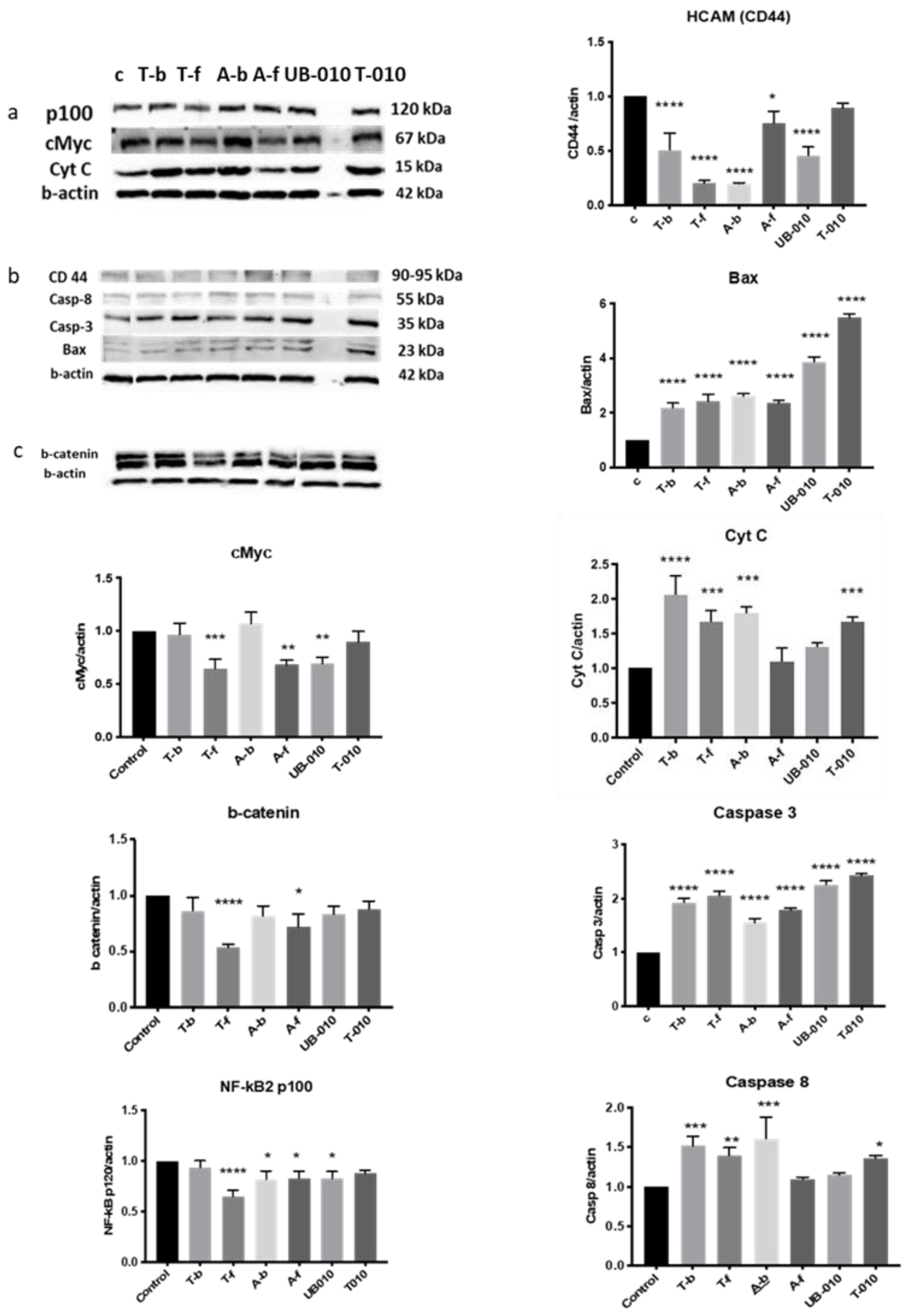
3.5. Mechanism of Anticancerous Action of WCCPS Fractions
3.5.1. c-Myc
3.5.2. Beta-Catenin
3.5.3. NF-kB2
3.5.4. HCAM (CD44)
3.5.5. Bax
3.5.6. Cytochrome c
3.5.7. Caspase 3
3.5.8. Caspase 8
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WCCPSs | Wheat cell culture polysaccharides |
| CRC | Colorectal cancer |
| APC | Adenomatous polyposis coli |
| EMT | Endothelial mesenchymal transition |
References
- Diegues, G.; Ferro, C.; Pyenson, B. A Multi-Year Look at the Cost Burden of Cancer Care; Millian Inc.: New York, NY, USA, 2017; pp. 1–4. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2020, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Wild, C.P. (Eds.) World Cancer Report 2014; International Agency for Research on Cancer (IARC): Lyon, France, 2014; pp. 6–8. [Google Scholar]
- Brody, H. Colorectal cancer. Nature 2015, 521, S1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X. Polysaccharide, a Potential Anticancer Drug with High Efficacy and Safety. J. Oncol. Res. Treat. 2016, 1, 2. [Google Scholar]
- Pang, G.; Wang, F.; Zhang, L.W. Dose matters: Direct killing or immunoregulatory effects of natural polysaccharides in cancer treatment. Carbohydr. Polym. 2018, 195, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Liu, M.-M.; Zeng, P.; Li, X.-T.; Shi, L.-G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Ramawat, K.G.; Merillon, J.-M. Polysaccharides Bioactivity and Biotechnology, 1st ed.; Springer International Publishing: New York, NY, USA, 2015; Chapter 72; pp. 2179–2215. [Google Scholar]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Kozlova, L.V.; Mikshina, P.V. Spatial structure of plant cell wall polysaccharides and its functional significance. Biochemistry 2013, 78, 836–853. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes versicolor in Women with Breast Cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT00269555?term=polysaccharide&cond=cancer&draw=2&rank=4 (accessed on 3 May 2022).
- Muralikrishna, G.; Rao, M.V.S. Cereal Non-Cellulosic Polysaccharides: Structure and Function Relationship—An Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 599–610. [Google Scholar] [CrossRef]
- Steve, W.C.; Wang, Q. Cell wall polysaccharides in cereals: Chemical structures and functional properties. Struct. Chem. 2009, 20, 291–297. [Google Scholar]
- Wang, L.; Li, Y.; Zhu, L.; Yin, R.; Wang, R.; Luo, X.; Li, Y.; Li, Y.; Chen, Z. Antitumor activities and immunomodulatory of rice bran polysaccharides and its sulfates in vitro. Int. J. Biol. Macromol. 2016, 88, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Wheat Bran Polysaccharide with Antitumor and Immune Regulation Activities and Extraction Method Thereof. Patent CN101705268B. Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences. Application Granted 7 December 2011. Available online: https://patents.google.com/patent/CN101705268B/en (accessed on 20 January 2019).
- Gunter, E.A.; Ovodov, Y.S. Production of Polysaccharides by Silene vulgaris Callus Culture Depending on Carbohydrates of the Medium. Biochemistry 2003, 68, 882–889. [Google Scholar] [PubMed]
- Mahmoudifar, N.; Chai, E.; Dunstan, D.; Lane, A. Cooperative Research Centre for Industrial Plant Biopolymers Production and applications of novel plant cell culture polysaccharides. Hydrocolloids 2000, 135–138. [Google Scholar] [CrossRef]
- Gunter, E.A.; Ovodov, Y.S. Khimija Rastitel’nogo Syr’ja. Chem. Plant Raw Mater. 2001, 2, 57–62. (In Russian) [Google Scholar]
- Ochoa-Villarreal, M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Ngel, M. Plant Cell Wall Polymers: Function, Structure and Biological Activity of Their Derivatives. In Polymerization; InTech: Rijeka, Croatia, 2012; pp. 63–74. [Google Scholar]
- Kieran, P.M. Bioreactor design for plant cell suspension cultures. Chapter 14. In Multiphase Bioreactor Design; Cabral, J.M.S., Ed.; Francis and Taylor: London, UK, 2001; pp. 417–422. [Google Scholar]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Bishimbayeva, N.K. Cytophysiological Bases of Biotechnology of Long-term Plant Regeneration in the Tissue Culture of Cereals: Abstract of the Dissertation for the Degree of Doctor of Biological Sciences; Massaget Press: Almaty, Kazakhstan, 2007; p. 37. (In Russian) [Google Scholar]
- Bishimbayeva, N.K.; Sartbayeva, I.A.; Murtazina, A.S.; Gunter, E.A. Chemical composition of polysaccharides from wheat cell culture. Int. J. Biol. Chem. 2015, 8, 13–17. [Google Scholar] [CrossRef]
- Kazybekova, S.K.; Bishimbaeyva, N.K.; Murtazina, A.S.; Tazhibayeva, S.; Miller, R.; Al-Farabi Kazakh National University; Max Planck Institute of Colloids and Interfaces. Physico-chemical properties of physiologically active polysaccharides from wheat tissue culture. Int. J. Biol. Chem. 2015, 8, 18–22. [Google Scholar] [CrossRef]
- Bishimbayeva, N.K.; Murtazina, A.S.; McDougall, G.J. Influence of Phytohormones on Monosaccharide Composition of Polysaccharides from Wheat Suspension Culture. Eurasian Chem. J. 2017, 19, 231. [Google Scholar] [CrossRef]
- Bento, J.F.; Noleto, G.R.; Petkowicz, C. Isolation of an arabinogalactan from Endopleura uchi bark decoction and its effect on HeLa cells. Carbohydr. Polym. 2014, 101, 871–877. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Glucans and cancer: Comparison of commercially available beta-glucans—Part IV. Anticancer Res. 2018, 38, 1327–1333. [Google Scholar] [PubMed]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phisiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Parmar, S.S.; Sainger, M.; Chaudhary, D.; Jaiwal, P.K. Plant regeneration from mature embryo of commercial Indian bread wheat (Triticum aestivum L.) cultivars. Physiol. Mol. Biol. Plants 2012, 18, 177–183. [Google Scholar] [CrossRef][Green Version]
- Mehaboob, V.M.; Faizal, K.; Raja, P.; Thiagu, G.; Aslam, A.; Shajahan, A. Effect of nitrogen sources and 2, 4-D treatment on indirect regeneration of ginger (Zingiber officinale Rosc.) using leaf base explants. J. Plant Biotechnol. 2019, 46, 17–21. [Google Scholar] [CrossRef]
- Karimian, R.; Lahouti, M.; Davarpanah, S.J. Effects of Different Concentrations of 2, 4-D and Kinetin on Callogenesis of Taxus Brevifolia Nutt. J. Appl. Biotechnol. Rep. 2014, 1, 167–170. [Google Scholar]
- Markowski, M.; Alsoufi, A.S.M.; Szakiel, A.; Długosz, M. Effect of Ethylene and Abscisic Acid on Steroid and Triterpenoid Synthesis in Calendula officinalis Hairy Roots and Saponin Release to the Culture Medium. Plants 2022, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colometric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Austin, C.; Stewart, D.; Allwood, J.W.; McDougall, G.J. Extracts from the edible seaweed Ascophyllum nodosum inhibit lipase activity in vitro: Contributions of phenolic and polysaccharide components. Food Funct. 2018, 9, 502–510. [Google Scholar] [CrossRef]
- Ross, H.A.; Wright, K.M.; McDougall, G.J.; Roberts, A.G.; Chapman, S.N.; Morris, W.L.; Hancock, R.D.; Stewart, D.; Tucker, G.A.; James, E.K.; et al. Potato tuber pectin structure is influenced by pectin methyl esterase activity and impacts on cooked potato texture. J. Exp. Bot. 2011, 62, 371–381. [Google Scholar] [CrossRef]
- Ramírez, A.; Boulaiz, H.; Tarifa, C.M.; Perán, M.; Jiménez, G.; Ruiz, M.P.; Agil, A.; Cruz-López, O.; Conejo-García, A.; Campos, J.M.; et al. HER2-signaling pathway, JNK and ERKs kinases, and cancer stem-like cells are targets of Bozepinib. Oncotarget 2014, 5, 3590–3606. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.A.; Boulaiz, H.; Suárez, I.; Saniger, E.; Campos, J.; Carrillo, E.; Prados, J.; Gallo, M.A.; Espinosa, A.; Aránega, A. Growth inhibition, G1-arrest, and apoptosis in MCF-7 human breast cancer cells by novel highly lipophilic 5-fluorouracil derivatives. Investig. New Drugs 2004, 22, 379–389. [Google Scholar] [CrossRef]
- Boulaiz, H.; Prados, J.; Melguizo, C.; García, A.M.; Marchal, J.A.; Ramos, J.L.; Carrillo, E.; Vélez, C.; Aranega, A. Inhibition of growth and induction of apoptosis in human breast cancer by transfection of gef gene. Br. J. Cancer 2003, 89, 192–198. [Google Scholar] [CrossRef]
- Jiménez-Martínez, Y.; Griñán-Lisón, C.; Khaldy, H.; Martín, A.; Cambrils, A.; Grau, A.I.; Jiménez, G.; Marchal, J.A.; Boulaiz, H. LdrB Toxin with In Vitro and In Vivo Antitumor Activity as a Potential Tool for Cancer Gene Therapy. Cancers 2019, 11, 1016. [Google Scholar] [CrossRef]
- Cáceres, B.; Ramirez, A.; Carrillo, E.; Jimenez, G.; Griñán-Lisón, C.; López-Ruiz, E.; Jiménez-Martínez, Y.; Marchal, J.A.; Boulaiz, H. Deciphering the Mechanism of Action Involved in Enhanced Suicide Gene Colon Cancer Cell Killer Effect Mediated by Gef and Apoptin. Cancers 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef]
- Fagin, D. Toxicology: The learning curve. Nature 2012, 490, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Varret, C.; Beronius, A.; Bodin, L.; Bokkers, B.; Boon, P.; Burger, M.; De Wit-Bos, L.; Fischer, A.; Hanberg, A.; Litens-Karlsson, S.; et al. Evaluating the evidence for non-monotonic dose-response relationships: A systematic literature review and (re-)analysis of in vivo toxicity data in the area of food safety. Toxicol. Appl. Pharmacol. 2018, 339, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Bandani, A.R.; Sabahi, Q. Population statistics and biological traits of Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) affected by LC30 of thiamethoxam and pirimicarb. Arch. Phytopathol. Plant Prot. 2013, 46, 1839–1847. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Deshpande, A.; Dhadi, S.R.; Hager, E.J.; Ramakrishna, W. Anticancer Activity of Rice Callus Suspension Culture. Phytother. Res. 2012, 26, 1075–1081. [Google Scholar] [CrossRef]
- Baldi, A.; Srivastava, A.; Bisaria, V.S. Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. In Symbiotic Fungi Soil Biology; Varma, A., Kharkwal, A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 373–380. [Google Scholar] [CrossRef]
- Li, P.; Mou, Y.; Shan, T.; Xu, J.; Li, Y.; Lu, S.; Zhou, L. Effects of Polysaccharide Elicitors from Endophytic Fusarium oxysporium Dzf17 on Growth and Diosgenin Production in Cell Suspension Culture of Dioscorea zingiberensis. Molecules 2011, 16, 9003–9016. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Peč, J.; Fei, J.; Choi, Y.H.; Dušek, J.; Verpoorte, R. Elicitation studies in cell suspension cultures of Cannabis sativa L. J. Biotechnol. 2009, 143, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Wani, Z.A.; Sharma, S.; Mahajan, G.; Satti, N.K.; Adeyemi, O.O.; Mondhe, D.M.; Saxena, A.K. In Vitro and In Vivo Anticancer Activity of Root Extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid.-Based Complement. Altern. Med. 2015, 2015, 560404. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F.; Liu, H.-B.; Zhang, Q.-W.; Li, Z.-P.; Wong, T.-L.; Fung, H.-Y.; Zhang, J.-X.; Bai, S.-P.; Lu, A.-P.; Han, Q.-B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 6172. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-E.; Yi, Y.-J.; Guo, Y.-T.; Wang, R.-C.; Hu, Q.-L.; Xiong, X.-Y. Inhibition of migration and induction of apoptosis in LoVo human colon cancer cells by polysaccharides from Ganoderma lucidum. Mol. Med. Rep. 2015, 12, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Shen, J.; Xia, Y.M.; Zhang, J.; Park, H.S. The polysaccharides from Ganoderma lucidum: Are they always inhibitors on human hepatocarcinoma cells? Carbohydr. Polym. 2012, 90, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Feng, Y.; Xie, M. A novel polysaccharide from Ganoderma atrum exerts antitumor activity by activating mitochondria-mediated apoptotic pathway and boosting the immune system. J. Agric. Food Chem. 2014, 62, 1581–1589. [Google Scholar] [CrossRef]
- Mei, Y.-X.; Yang, W.; Zhu, P.-X.; Peng, N.; Zhu, H.; Liang, Y.-X. Isolation, Characterization, and Antitumor Activity of a Novel Heteroglycan from Cultured Mycelia of Cordyceps sinensis. Planta Med. 2014, 80, 1107–1112. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Luo, S.; Zheng, Y.; Luo, X.; Zhou, L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: An in vitro and in vivo study. Food Nutr. Res. 2017, 61, 1399770. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2019, 64, 103677. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, Y.; Guo, Y.; Wang, R.; Hu, Q.; Xiong, X. Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103–9116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.; Thakur, K.; Hu, F.; Zhang, J.G. Anti-cancerous potential of polysaccharide fractions extracted from peony seed dreg on various human cancer cell clones via cell cycle arrest and apoptosis. Front. Pharmacol. 2017, 8, 102–114. [Google Scholar] [PubMed]
- García, M.A.; Carrasco, E.; Ramírez, A.; Jiménez, G.; Elena, L.-R.; Perán, M.; Picón, M.; Campos, J.; Boulaiz, H.; Marchal, J.A. Chapter 5. Apoptosis as a therapeutic target in cancer and cancer stem cells: Novel strategies and futures perspectives. In Apoptosis and Medicine; Ntuli, T.M., Ed.; Intech: New York, NY, USA, 2012; pp. 111–117. [Google Scholar] [CrossRef][Green Version]
- Hanahan, D.; Weinberg, R. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Lin, L.; Cheng, K.; Xie, Z.; Chen, C.; Chen, L.; Huang, Y.; Liang, Z. Purification and characterization a polysaccharide from Hedyotis diffusa and its apoptosis inducing activity toward human lung cancer cell line A549. Int. J. Biol. Macromol. 2019, 122, 64–71. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Cao, Y.-Y.; Liao, B.-Y.; Zhang, J.-G.; Wei, Z.-J. Anticancerous potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua in Human cervical cancer Hela cells. Int. J. Biol. Macromol. 2020, 148, 843–850. [Google Scholar] [CrossRef]
- Murad, H.; Hawat, M.; Ekhtiar, A.; Aljapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. [Google Scholar] [CrossRef]
- Suo, H.; Song, J.-L.; Zhou, Y.; Liu, Z.; Yi, R.; Zhu, K.; Xie, J.; Zhao, X. Induction of apoptosis in HCT-116 colon cancer cells by polysaccharide of Larimichthys crocea swim bladder. Oncol. Lett. 2015, 9, 972–978. [Google Scholar] [CrossRef]
- Balvan, J.; Krizova, A.; Gumulec, J.; Raudenska, M.; Sladek, Z.; Sedackova, M.; Babula, P.; Sztalmachova, M.; Kizek, R.; Chmelik, R.; et al. Multimodal Holographic Microscopy: Distinction between Apoptosis and Oncosis. PLoS ONE 2015, 10, e0121674. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Y.; Na, K.; Wang, Y.; Wang, L.; Li, Z.; Guo, C.; Guo, D.; Wang, X. Autophagic flux disruption contributes to Ganoderma lucidum polysaccharide-induced apoptosis in human colorectal cancer cells via MAPK/ERK activation. Cell Death Dis. 2019, 10, 456. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Y.; Liu, H.; Chen, C.; Li, S.; Li, N.; Li, X.; Zhang, X.; Zhang, H.; Wang, W.; et al. Expression of CIAPIN1 in human colorectal cancer and its correlation with prognosis. BMC Cancer 2010, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Mayo, C.; Lloreta, J.; Real, F.X.; Mayol, X. In vitro differentiation of HT-29 M6 mucus-secreting colon cancer cells involves a trychostatin A and p27KIP1-inducible transcriptional program of gene expression. J. Cell. Physiol. 2007, 212, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Moal, V.L.-L.; Servin, A.L. Pathogenesis of Human Enterovirulent Bacteria: Lessons from Cultured, Fully Differentiated Human Colon Cancer Cell Lines. Microbiol. Mol. Biol. Rev. 2013, 77, 380–439. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.; Perkins, D.N. NF-κB and the cell cycle. Biochem. Soc. Trans. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration: Senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Xiong, W.; Li, J.; Jiang, R.; Li, D.; Liu, Z.; Chen, D. Research on the effect of ginseng polysaccharide on apoptosis and cell cycle of human leukemia cell line K562 and its molecular mechanisms. Exp. Ther. Med. 2017, 13, 924–934. [Google Scholar] [CrossRef]
- Hector, S.; Prehn, J. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: A review. Biochim. Biophys. Acta 2009, 1795, 117–129. [Google Scholar] [CrossRef]
- Zlobec, I.; Steele, R.; Terracciano, L.; Jass, J.R.; Lugli, A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J. Clin. Pathol. 2007, 60, 1112–1116. [Google Scholar] [CrossRef]
- Paik, S.S.; Jang, K.-S.; Song, Y.S.; Jang, S.-H.; Min, K.-W.; Han, H.X.; Na, W.; Lee, K.H.; Choi, D.; Jang, S.J. Reduced Expression of Apaf-1 in Colorectal Adenocarcinoma Correlates with Tumor Progression and Aggressive Phenotype. Ann. Surg. Oncol. 2007, 14, 3453–3459. [Google Scholar] [CrossRef]
- Endo, K.; Kohnoe, S.; Watanabe, A.; Tashiro, H.; Sakata, H.; Morita, M.; Kakeji, Y.; Maehara, Y. Clinical significance of Smac/DIABLO expression in colorectal cancer. Oncol. Rep. 2009, 21, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Strãter, J.; Herter, I.; Merkel, G.; Hinz, U.; Weitz, J.; Mãller, P. Expression and prognostic significance of APAF-1, caspase-8 and caspase-9 in stage II/III colon carcinoma: Caspase-8 and caspase-9 is associated with poor prognosis. Int. J. Cancer 2009, 127, 873–880. [Google Scholar] [CrossRef]
- Abraha, A.M.; Ketema, E.B. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J. Gastrointest. Oncol. 2016, 8, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Springs, S.L.; Diavolitsis, V.M.; Goodhouse, J.; McLendon, G.L. The kinetics of translocation of Smac/DIABLO from the mitochondria to the cytosol in HeLa cells. J. Biol. Chem. 2002, 277, 45715–45718. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi et al. Nat. Struct. Biol. 2002, 9, 436–441. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a008656. [Google Scholar] [CrossRef]
- Tetsu, O.; McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef]
- Saifo, M.S.; Rempinski, D.R.; Rustum, Y.M.; Azrak, R.G. Targeting the oncogenic protein beta-catenin to enhance chemotherapy outcome against solid human cancers. Mol. Cancer 2010, 9, 310. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; Da Costa, L.T.; Morin, P.J. Vogelstein B and Kinzler KW: Identification of c-MYC as a Target of the APC Pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Demeterco, C.; Itkin-Ansari, P.; Tyrberg, B.; Ford, L.P.; Jarvis, R.A.; Levine, F. c-Myc controls proliferation versus differentiation in human pancreatic endocrine cells. J. Clin. Endocrinol. Metab. 2002, 87, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Leu, W.-J.; Chang, H.-S.; Chan, S.-H.; Hsu, J.-L.; Yu, C.-C.; Hsu, L.-C.; Chen, I.-S.; Guh, J.-H. Reevesioside A, a Cardenolide Glycoside, Induces Anticancer Activity against Human Hormone-Refractory Prostate Cancers through Suppression of c-myc Expression and Induction of G1 Arrest of the Cell Cycle. PLoS ONE 2014, 9, e87323. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Schnitter, A. Cell cycle and differentiation. Curr. Opin. Plant Biol. 2004, 7, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Merga, Y.J.; O’Hara, A.; Burkitt, M.D.; Duckworth, C.A.; Probert, C.S.; Campbell, B.J.; Pritchard, D.M. Importance of the alternative NF-κB activation pathway in inflammation-associated gastrointestinal carcinogenesis. Am. J. Physiol. Liver Physiol. 2016, 310, G1081–G1090. [Google Scholar] [CrossRef]
- Weber, C.K.; Liptay, S.; Wirth, T.; Adler, G.; Schmid, R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000, 119, 1209–1218. [Google Scholar] [CrossRef]
- Horst, D.; Budczies, J.; Brabletz, T.; Kirchner, T.; Hlubek, F. Invasion associated up-regulation of nuclear factor kappaB target genes in colorectal cancer. Cancer 2009, 115, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-J.; Lai, H.-M.; Chang, Y.-W.; Chen, G.-Y.; Lee, J.-L. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011, 30, 3186–3199. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R. The role of CD44 in epithelial–mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar] [CrossRef]
- Pham, P.V.; Phan, N.L.; Nguyen, N.T.; Truong, N.H.; Duong, T.T.; Le, D.V.; Truong, K.D.; Phan, N.K. Differentiation of breast cancer stem cells by knockdown of CD44: Promising differentiation therapy. J. Transl. Med. 2011, 9, 209. [Google Scholar] [CrossRef]
- Chen, Y.J.; Shiao, M.S.; Lee, S.S.; Wang, S.Y. Effect of Cordicepssinensis on the proliferation and differentiation of human leukemic U937 cell. Life Sci. 1997, 60, 2349–2359. [Google Scholar] [CrossRef]
- Liao, H.-F.; Chen, Y.-Y.; Yang, Y.-C.; Wang, C.-S. Rice (Oryza sativa L.) inhibits growth and induces differentiation of human leukemic U937 cells through activation of peripheral blood mononuclear cells. Food Chem. Toxicol. 2006, 44, 1724–1729. [Google Scholar] [CrossRef]
- Hsu, J.-W.; Huang, H.-C.; Chen, S.-T.; Wong, C.-H.; Juan, H.-F. Ganoderma lucidumPolysaccharides Induce Macrophage-Like Differentiation in Human Leukemia THP-1 Cells via Caspase and p53 Activation. Evid.-Based Complement. Altern. Med. 2011, 2011, 358717. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, R.; Song, S.-D.; Hua, Z.-S.; Wang, J.-W.; Wang, Y.-P. Angelica sinensis polysaccharide induces erythroid differentiation of human chronic myelogenous leukemia k562 cells. Asian Pac. J. Cancer Prev. 2015, 16, 3715–3721. [Google Scholar] [CrossRef]






| T-010 | B-010 | UB-010 | A-b | A-f | T-b | T-f | |
|---|---|---|---|---|---|---|---|
| Ara | 9.1 | 32.6 | 8.7 | 1.4 | 7.35 | 7.9 | 16.2 |
| Gal | 10.2 | 20.9 | 5.9 | 0.76 | 3.3 | 15.1 | 9.6 |
| Xyl | 6.7 | 18.3 | 9.7 | 1.9 | 4.8 | 13.86 | 13.6 |
| Glc | 73.9 | 3.3 | 75.6 | 91.4 | 83.2 | 56.6 | 58.0 |
| GlcUA | 0 | 24.9 | 0 | 0.4 | 1.1 | 1.74 | 2.2 |
| GalUA | 0 | 0 | 0 | 0.6 | 0.13 | 0.2 | 0.3 |
| Man | 0 | 0 | 0 | 3.5 | 0.02 | 4.6 | 0 |
| Fraction | HCT-116 IC50 (µg/mL) | CCD-18CO IC50 (µg/mL) | Therapeutic Index (TI) |
|---|---|---|---|
| T-010 ** | 1600 | ND | NC * |
| B-010 | NM * | 2500 | NC |
| UB-010 *** | 1600 | 800 | 0.5 |
| T-b | 78 | ND | NC |
| A-b | 160 | 1487 | 9.25 |
| T-f | 1657 | ND | NC |
| A-f | 10 | 700 | 70 |
| Fraction | HCT-116 (%) | CCD-18CO (%) | Ratio |
|---|---|---|---|
| T-010 (1600 µg/mL) | 47 | 20 | 2.35 |
| T-b (100 µg/mL) | 86 | 30 | 2.86 |
| T-f (1600µg/mL) | 50 | 20 | 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtazina, A.; Ruiz Alcala, G.; Jimenez-Martinez, Y.; Marchal, J.A.; Tarabayeva, A.; Bitanova, E.; McDougall, G.; Bishimbayeva, N.; Boulaiz, H. Anti-Cancerous Potential of Polysaccharides Derived from Wheat Cell Culture. Pharmaceutics 2022, 14, 1100. https://doi.org/10.3390/pharmaceutics14051100
Murtazina A, Ruiz Alcala G, Jimenez-Martinez Y, Marchal JA, Tarabayeva A, Bitanova E, McDougall G, Bishimbayeva N, Boulaiz H. Anti-Cancerous Potential of Polysaccharides Derived from Wheat Cell Culture. Pharmaceutics. 2022; 14(5):1100. https://doi.org/10.3390/pharmaceutics14051100
Chicago/Turabian StyleMurtazina, Alima, Gloria Ruiz Alcala, Yaiza Jimenez-Martinez, Juan Antonio Marchal, Anel Tarabayeva, Elmira Bitanova, Gordon McDougall, Nazira Bishimbayeva, and Houria Boulaiz. 2022. "Anti-Cancerous Potential of Polysaccharides Derived from Wheat Cell Culture" Pharmaceutics 14, no. 5: 1100. https://doi.org/10.3390/pharmaceutics14051100
APA StyleMurtazina, A., Ruiz Alcala, G., Jimenez-Martinez, Y., Marchal, J. A., Tarabayeva, A., Bitanova, E., McDougall, G., Bishimbayeva, N., & Boulaiz, H. (2022). Anti-Cancerous Potential of Polysaccharides Derived from Wheat Cell Culture. Pharmaceutics, 14(5), 1100. https://doi.org/10.3390/pharmaceutics14051100










