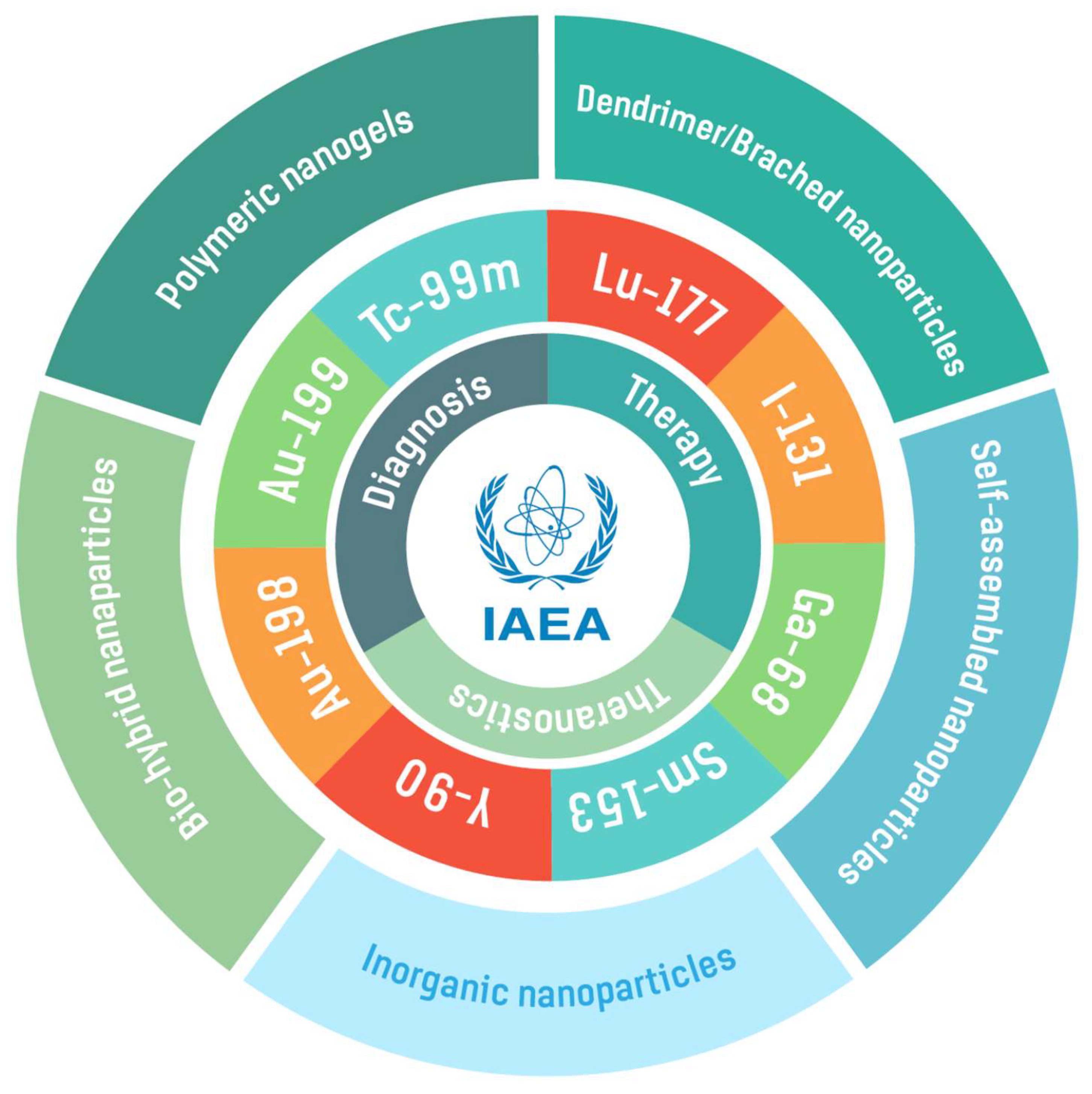
IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery
Abstract
:1. Introduction
2. Radionuclides for Radiolabeling Various Nanoparticles
| Radionuclide | Half-Life | Decay Mode (Energy, Intensity) | Applications | Type of Nanoparticle | Ref. |
|---|---|---|---|---|---|
| Ga-68 | 67.7 m | β+ (1.89 MeV, 88%) | PET imaging | 68Ga-DOTA-BN-TMC-MNPs | [43] |
| Tc-99m | 6 h | γ-ray (140 keV) | SPECT imaging | 99mTc-AuNPs 99mTc-AuNP-Tyr3-Octreotide 99mTc-DOX-AuNPs 99mTc-AFCuONPs 99mTc-SeNPs 99mTc-VitC-SeNPs 99mTc-MIONPs 99mTc-PAMAM-Tyr3-Octreotide | [44,45] [17,46] [22,47] [48] [49] [50] [51] [17,46] |
| I-131 | 8 d | β− (606 keV, 90%) γ-ray (364 keV, 81%) | Therapy and SPECT imaging | 131I-doped Ag-PEG NPs | [52] |
| Sm-153 | 46.3 h | β− (634 keV, 30%, 704 keV, 49% and 807 keV 20%) γ-ray (103 keV, 29%) | Therapy | 153Sm-CSNPs-PEI-folate | [53] |
| Lu-177 | 6.6 d | β− (496 keV, 80%) γ-ray (113 keV, 6% and 208 keV, 10%) | Theranostic | 177Lu-DOTA-DN(PTX)-BN 177Lu-BN-PLGA(PTX) 177Lu-DOTA-HA-PLGA(MTX) 177Lu-DN(AuNP)-folate-BN 177Lu-DN(C19)-CXCR4 | [29] [26] [54] [55] [31,56] |
| Au-198 | 2.7 d | β− (961 keV, 99%) γ-ray (411 keV, 96%) | Therapy | 198AuNPs 198AuNPs-BSA MGF-198AuNPs AX-encapsulated 198AuNPs | [57] [58] [38,42] [59] |
| Au-199 | 3.1 d | β− (243 keV, 22% and 293 keV, 72%) γ-ray (158 keV, 40%) | Therapy | 199AuNPs | [57] |
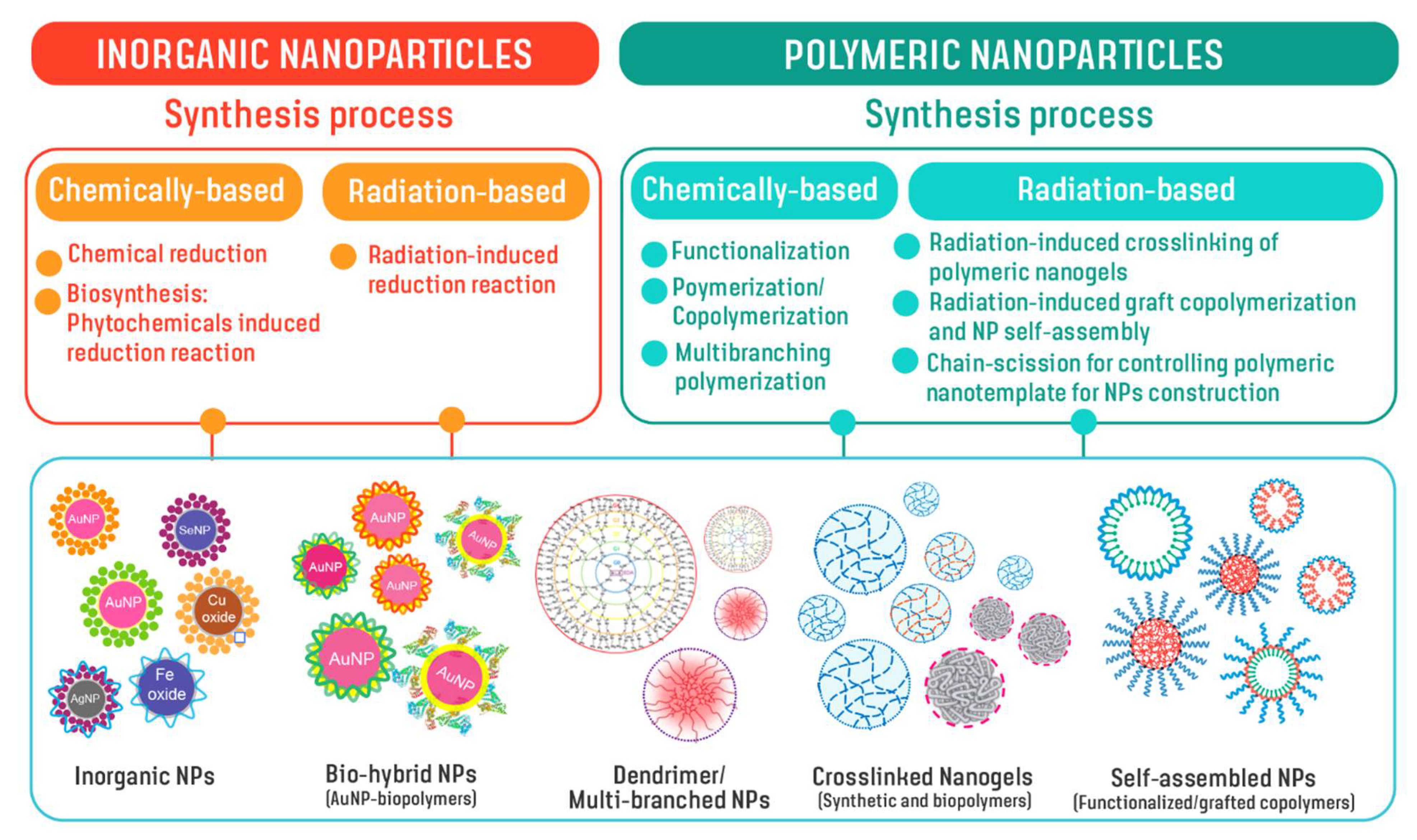
3. Design and Development of Nanoparticles
Chemical-Based Synthesis of Nanoparticles—General Aspects of Production
4. Radiation-Based Synthesis of Nanoparticles
| Categories of NPs | Name of NPs | Size (nm) | Information of NPs | Ref. |
|---|---|---|---|---|
| Polymeric | PAA nanogels | 30–200 a | Nanogels of poly(acrylic acid) synthesized by preparative pulse radiolysis and decorated with bombesin/DOTA, tested for radioisotope binding and subjected to preliminary tests on animal model (mice). | [73,74,75] |
| PAA-PEO IPC nanogels | ~100–240 a | Poly(acrylic acid)-poly(ethylene oxide) interpolymer (IPC) complex nanogels exhibiting pH- and temperature-responsive functions and possible for drug-controlled release. | [76] | |
| PVP-g-AA-Folate PVP-g-AA-Doxo PVP-g-AA-SiRNA | 20–60 a | Polyvinyl pyrrolidone based nanogels with acrylic acid grafts: (i) decorated with folic acid (FA) for preferential uptake by cells that overexpress folate receptors; (ii) conjugated, via a redox-cleavable linker, to either doxorubicin or a silencing RNA. | [82,83,84] | |
| PVP-g-AA-AntiMIR | ~50 a | Polyvinyl pyrrolidone-based nanogels with acrylic acid grafts conjugated to the amino-terminated AntimiR-31, that targets MiR-31, a microRNA overexpressed by primary and metastatic tissue colon cancer cells (CCR) and target of the E2F2 gene that plays a crucial role in the control of CCR progression. | [100] | |
| PVP-g-AA-Insulinl | ~80 a | Polyvinyl pyrrolidone-based nanogels with acrylic acid grafts, conjugated to insulin to be intranasally delivered, bypass the blood–brain barrier and target the brain. | [96,97] | |
| PVP-g-APMAM | 80–300 a | Polyvinyl pyrrolidone-based nanogels with (3-N-aminopropyl)methacrylamide hydrochloride grafts, conjugated to a monoclonal antibody which recognizes the αvβ3 integrin, a receptor important in tumor angiogenesis and metastasis. | [98,99] | |
| Protein NPs | 20–40 a | Albumin NPs prepared by a novel radiation-induced crosslinking method. Albumin preserve their original drug-carrier properties. | [77,78,79,80,81] | |
| CS-DC NPs | 50–100 b 30–50 b | Deoxycholate conjugated chitosan NPs containing hydrophobic core for water-insoluble drug encapsulation. NPs remaining -OH and -NH2 groups for further conjugating with chelating/peptide molecules. | [85,86,91] | |
| pPEGMA-CS-DC NPs | 70–130 b | Poly(ethylene glycol) monomethacrylate grafted chitosan deoxycholate amphiphilic NPs containing hydrophobic core for water-insoluble drug encapsulation (e.g., berberine). NPs remaining -OH and -NH2 groups for further conjugating with chelating/peptide molecules. | [87,91] | |
| pSMA-CS NPs | 50–140 b,c | Poly(stearyl methacylate) grafted chitosan NPs providing -OH and -NH2 groups for further conjugating with chelating/peptide molecules. NPs having hydrophobic core for water-insoluble drug encapsulation. | [87,91,102] | |
| pSMA-CS-PPD NPs | 50–100 b | Piperidine conjugate poly(stearylate)-grafted chitosan NPs exhibiting antioxidant function and remaining -OH and -NH2 groups for further conjugating with chelating/peptide molecules. | [88,91] | |
| WSCS nanocolloids | 49 ± 2.15 a 10–50 b | Water-soluble chitosan nanocolloids exhibiting antioxidant activities and reducing power. NPs remaining -OH and -NH2 groups for conjugating with chelating/peptide molecules. NPs enable use as biopolymer-template synthesis of Au-197 and Au-198 analogue. | [89,91] | |
| SF nanocolloids | ~40 b | Silk fibroin (SF) nanocolloids exhibit antioxidant activities and reducing power. NPs enable use as biopolymer-template synthesis of Au-197 and Au-198 analogue. | [101] | |
| WSCS-DOTA-BBN NPs | 86 ± 2.03 a | Water-soluble chitosan conjugated DOTA NPs chelator and BBN peptide enable use as one-pot synthesis of targeted AuNPs. | [91] | |
| Inorganic/hybrid | AuNPs-CS | 5–80 b | Protocol synthesis of AuNPs capped with chitosan enable use for one-pot synthesis of 198Au nanoradiotherapeutics. | [89,90] |
| AuNPs-WSCS | 5–25 b | Protocol synthesis of AuNPs capped with water-soluble chitosan enable use for one-pot synthesis of 198Au nanoradiotherapeutics. | [90] | |
| AuNPs-WSCS-DOTA-BBN | 62 ± 21 a 20 ± 7 b | AuNPs capped with water-soluble chitosan conjugated DOTA chelator and BBN peptide acting as targeted therapeutic agent for prostate cancer cells (i.e., PC-3, LNCaP). Protocol for a one-pot synthesis of the targeted 198Au nanoradiotherapeutics. | [91] | |
| AuNPs-WSCS-GA-DOTA-BBN | 40–60 a | BBN peptide conjugated water-soluble chitosan gallate as a new nanopharmaceutical architechture for the rapid one-pot synthesis of prostate tumor targeted AuNPs | [103] | |
| bioHNPs | 77 ± 7 a | AuNPs coated with human albumin multilayer and further decorated with DOTA chelator and BBN peptide acting as targeted therapeutic agent for prostate cancer cells (i.e., PC-3). | [92,93,94,95] |
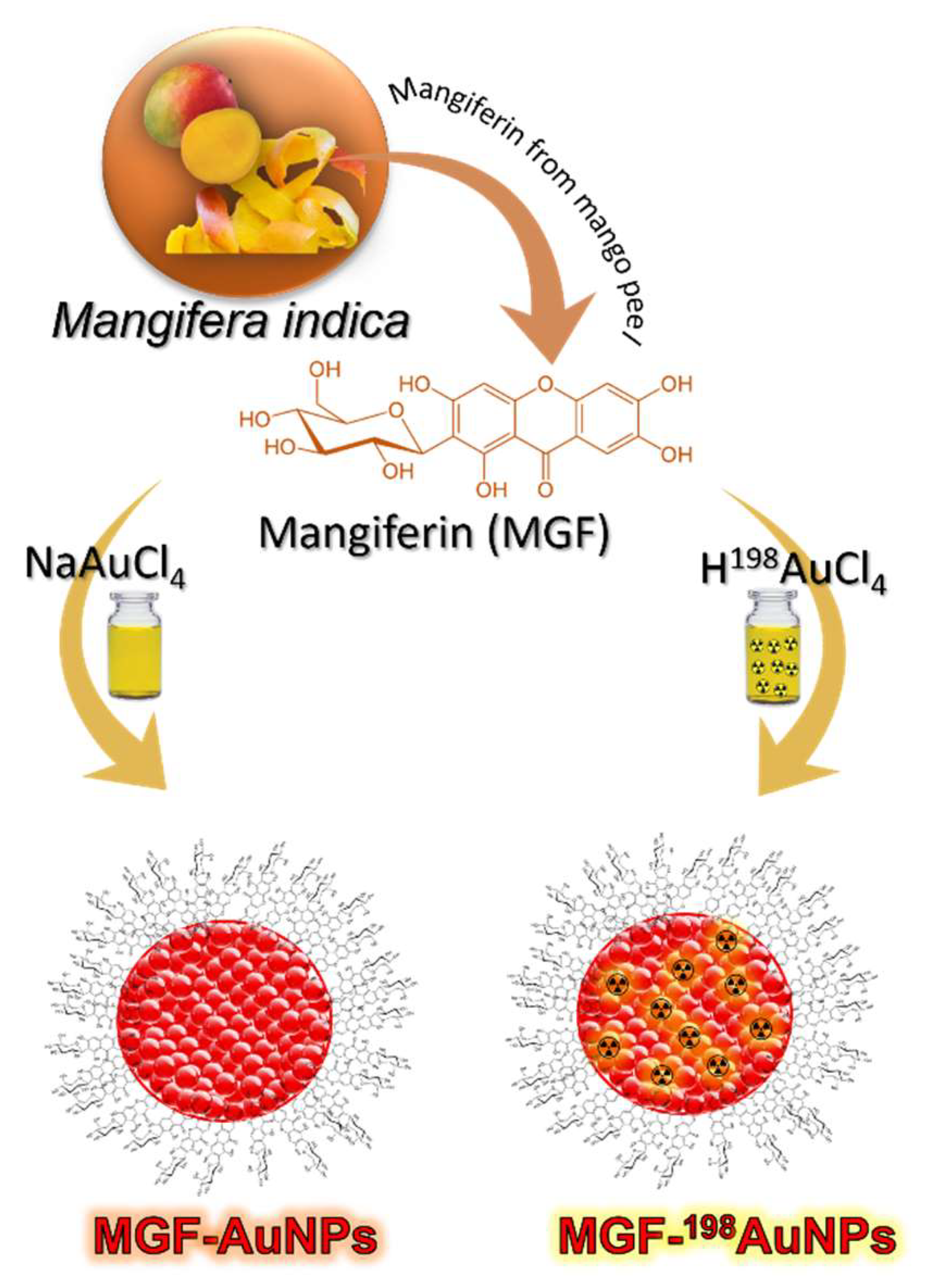
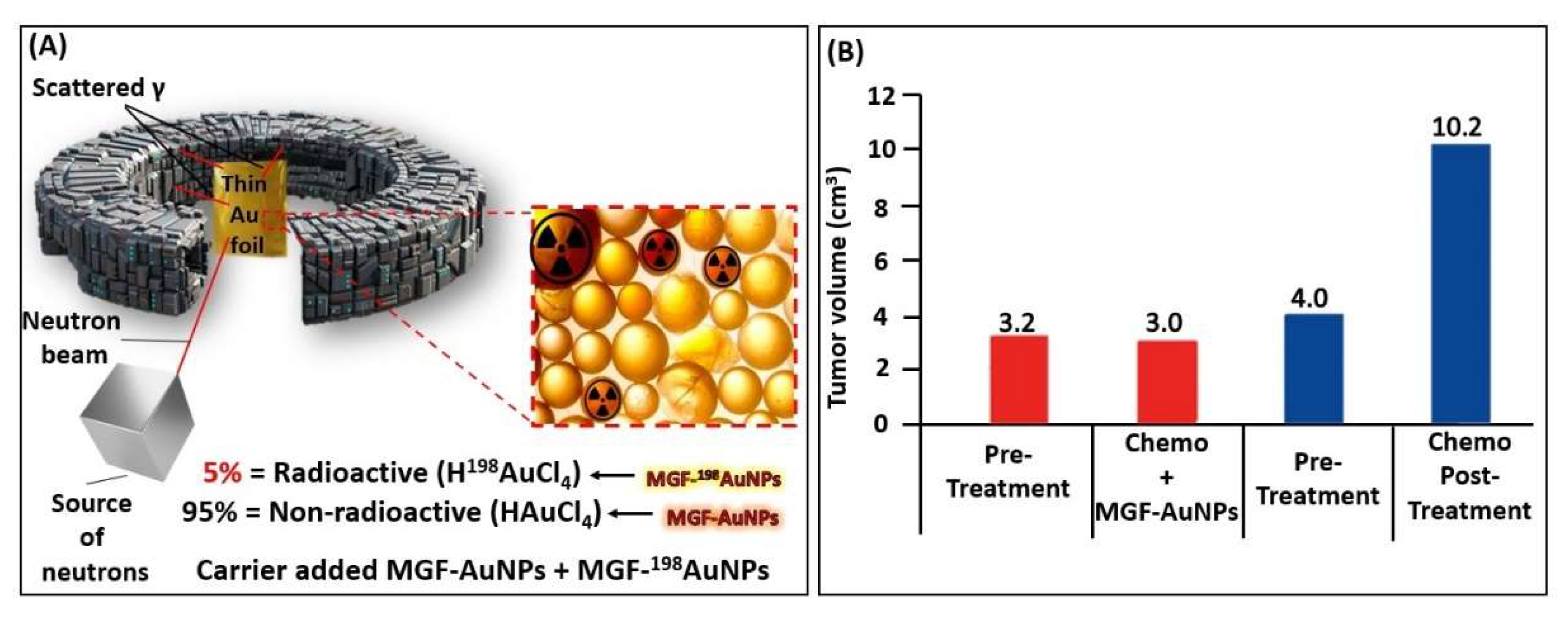
5. Production, Characterization, and Pre-Clinical/Clinical Investigations of Radioactive MGF-198AuNPs
5.1. Production, and Characterization of Radioactive MGF-198AuNPs
5.2. Preparation of ‘Premix’
5.3. Production of Radioactive MGF-198AuNPs
5.4. Characterization
5.5. Laminin 67 Receptor-Mediated Cellular Internalization of MGF-AuNPs
5.6. Pre-Clinical In Vivo Tumor Retention and Therapeutic Efficacy Investigations of MGF-AuNPs
5.7. Therapeutic Efficacy of MGF-198AuNPs Nanoradiotherapeutic Agent
5.8. Clinical Trials of MGF-AuNPs in Human Cancer Patients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 131I-doped Ag-PEG NPs | Polyethylene glycol capped silver NPs doped with I-131 radionuclide |
| AFCuONPs | Aspergillus flavus synthesized copper oxide NPs |
| AuNPs | Gold nanoparticles |
| AuNPs-BSA | Gold nanoparticle capped with bovine serum albumin |
| AuNP-Tyr3-Octreotide | Gold nanoparticles functionalized with Tyr(3)-octreotide peptide |
| AX-encapsulated 198AuNPs | Arabinoxylan-encapsulated functionalized radioactive gold nanoparticles |
| BN-PLGA(PTX) | Poly lactic-co-glycolic acid nanoparticles loaded with paclitaxel (PTX) and functionalized with bombesin |
| CSNPs-PEI-folate | Chitosan nanoparticles functionalized with folate and polyethyleneimine |
| DN(AuNP)-folate-BN | Dendrimer(PAMAM-G4)-folate-bombesin with gold nanoparticles (AuNPs) in the dendritic cavity |
| DN(C19)-CXCR4 | Dendrimer encapsulating C19 and functionalized to target CXCR4 |
| DOTA-BN-TMC-MNPs | Trimethyl chitosan superparamagnetic nanoparticles functionalized with DOTA-bombesin |
| DOTA-DN(PTX)-BN | Polyamidoamine dendrimer (DN) loaded with paclitaxel (PTX) and functionalized with DOTA-bombesin |
| DOTA-HA-PLGA(MTX) | Poly lactic-co-glycolic acid nanoparticles loaded with methotrexate and functionalized with hyaluronic acid (HA) and DOTA |
| DOX-AuNPs | Gold nanoparticles loaded with doxorubicin |
| MGF-AuNPs | Mangiferin functionalized radioactive gold nanoparticles |
| MIONPs | Magnetic iron oxide nanoparticles platform |
| PAMAM-Tyr3-Octreotide | Polyamidoamine (PAMAM) dendrimers functionalized with Tyr(3)-Octreotide peptide |
| SeNPs | Selenium nanoparticles |
| VitC-SeNPs | Selenium nanoparticles coated with vitamin C |
| NP | Nanoparticle |
| RGD | arginine-glycine-aspartic acid |
| PMMA | Poly(methyl methacrylate) |
| PLGA | Polylactic-co-glycolic acid |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| TEM | Transmission electron microscopy |
| AFM | Atomic force microscopy |
| BBN | Bombesin |
| PAA | poly(acrylic acid) |
| PAA-PEO IPC | Poly(acrylic acid)-poly(ethylene oxide) (PAA-PEO) interpolymer complexes |
| PVP | Polyvinylpyrrolidone |
| PVP-g-AA-Folate | Polyvinyl pyrrolidone with acrylic acid grafts decorated with folic acid |
| PVP-AA_Doxo | Polyvinyl pyrrolidone with acrylic acid grafts decorated with Doxorubicin |
| PVP-g-AA-SiRNA | Polyvinyl pyrrolidone with acrylic acid grafts decorated with SilencingRNA |
| PVP-g-AA-AntiMIR | Polyvinyl pyrrolidone-based nanogels with acrylic acid grafts conjugated to the amino-terminated AntimiR-31 |
| PVP-g-AA-Insulinl | Polyvinyl pyrrolidone- nanogels with acrylic acid grafts, conjugated to insulin |
| PVP-g-APMAM | Polyvinyl pyrrolidone-based nanogels with (3-N-aminopropyl)methacrylamide |
| CS-DC NPs | Deoxycholate conjugated chitosan Nanoparticles |
| pPEGMA-CS-DC NPs | Poly(ethylene glycol) monomethacrylate grafted chitosan deoxycholate amphiphilic Nanoparticles |
| pSMA-CS NPs | Poly(stearyl methacylate) grafted chitosan Nanoparticles |
| pSMA-CS-PPD NPs | Piperidine conjugate poly(stearylate)-grafted chitosan Nanoparticles |
| WSCS nanocolloids | Water-soluble chitosan nanocolloids |
| SF nanocolloids | Silk fibrin nanocolloids |
| WSCS-DOTA-BBN NPs | Water-soluble chitosan conjugated DOTA Nanoparticles and Bombesin peptide |
| AuNPs-CS | Gold Nanoparticles capped with Chitosan |
| AuNPs-WSCS | Gold nanoparticles capped with water-soluble chitosan |
| AuNPs-WSCS-DOTA-BBN | Gold nanoparticles capped with water-soluble chitosan conjugated DOTA chelator and BBN peptide |
| AuNPs-WSCS-GA-DOTA-BBN | Gold nanoparticles, stabilized with Gum Arabic, and capped with water-soluble chitosan conjugated DOTA chelator and BBN peptide |
| bioHNPs | AuNPs coated with human albumin multilayers |
| SCID | Severe combined immunodeficient mice |
References
- Kumar, P.P.P.; Lim, D.-K. Gold-Polymer Nanocomposites for Future Therapeutic and Tissue Engineering Applications. Pharmaceutics 2022, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Palombarini, F.; Masciarelli, S.; Incocciati, A.; Liccardo, F.; Di Fabio, E.; Iazzetti, A.; Fabrizi, G.; Fazi, F.; Macone, A.; Bonamore, A. Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J. Nanobiotechnol. 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-coated gold nanoparticles for efficient folate-targeted mRNA delivery in vitro. Pharmaceutics 2021, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-Tagged Folate-Targeted Gold Nanoparticles for Enhanced Transgene Expression in Breast Cancer Cells In Vitro. Pharmaceutics 2022, 14, 53. [Google Scholar] [CrossRef]
- Fan, L.; Wang, W.; Wang, Z.; Zhao, M. Gold nanoparticles enhance antibody effect through direct cancer cell cytotoxicity by differential regulation of phagocytosis. Nat. Commun. 2021, 12, 6371. [Google Scholar] [CrossRef]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Zhang, C.; Liu, H.; Hao, M.; Kan, S.; Liu, D.; Liu, W. The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front. Oncol. 2022, 11, 819329. [Google Scholar] [CrossRef]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Aghanejad, A.; Safari, B.; Barar, J.; Rasta, S.H.; Davaran, S. Aptamer-conjugated gold nanoparticles for targeted paclitaxel delivery and photothermal therapy in breast cancer. J. Drug Deliv. Sci. Technol. 2022, 67, 102954. [Google Scholar] [CrossRef]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [Green Version]
- Ferro-Flores, G. Targeted Nanomedicines: In the Right Route Towards Improved Therapies. Curr. Cancer Ther. Rev. 2020, 16, 3–4. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, M.; Morales-Avila, E.; Cruz-Nova, P.; Katti, K.V.; Ocampo-García, B. Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications. Pharmaceutics 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Zampella, E.; Nappi, C.; Nicolai, E.; Ambrosio, R.; Califaretti, E.; Lamartina, L.; Schlumberger, M.; Deandreis, D.; Salvatore, D. Advances in Functional Imaging of Differentiated Thyroid Cancer. Cancers 2021, 13, 4748. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J. Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. Int. J. Mol. Sci. 2019, 20, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghevlian, S.; Cai, Z.L.; Lu, Y.J.; Hedley, D.W.; Winnik, M.A.; Reilly, R.M. Radioimmunotherapy of PANC-1 Human Pancreatic Cancer Xenografts in NRG Mice with Panitumumab Modified with Metal-Chelating Polymers Complexed to Lu-177. Mol. Pharm. 2019, 16, 768–778. [Google Scholar] [CrossRef]
- Ocampo-Garcia, B.E.; Ramirez, F.D.; Ferro-Flores, G.; De Leon-Rodriguez, L.M.; Santos-Cuevas, C.L.; Morales-Avila, E.; de Murphy, C.A.; Pedraza-Lopez, M.; Medina, L.A.; Camacho-Lopez, M.A. Tc-99m-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection. Nucl. Med. Biol. 2011, 38, 1–11. [Google Scholar] [CrossRef]
- Orocio-Rodriguez, E.; Ferro-Flores, G.; Santos-Cuevas, C.L.; Ramirez, F.D.M.; Ocampo-Garcia, B.E.; Azorin-Vega, E.; Sanchez-Garcia, F.M. Two Novel Nanosized Radiolabeled Analogues of Somatostatin for Neuroendocrine Tumor Imaging. J. Nanosci. Nanotechnol. 2015, 15, 4159–4169. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.N.; Ferro-Flores, G.; Ocampo-García, B.E.; Morales-Avila, E.; Ramírez, F.d.M.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderón, E.L.; Camacho-López, M.A. Lys3-Bombesin Conjugated to99mTc-Labelled Gold Nanoparticles for In Vivo Gastrin Releasing Peptide-Receptor Imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef]
- Ocampo-García, B.; Ferro-Flores, G.; Morales-Avila, E.; de María Ramírez, F. Kit for preparation of multimeric receptor-specific 99mTc-radiopharmaceuticals based on gold nanoparticles. Nucl. Med. Commun. 2011, 32, 1095–1104. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; García-Becerra, R.; Medina, L.A.; Gomez-Olivan, L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c [RGDfK (C)] for molecular imaging of tumor α (v) β (3) expression. Bioconjug Chem. 2011, 22, 913–922. [Google Scholar] [CrossRef]
- Isaac-Olive, K.; Ocampo-Garcia, B.E.; Aranda-Lara, L.; Santos-Cuevas, C.L.; Jimenez-Mancilla, N.P.; Luna-Gutierrez, M.A.; Medina, L.A.; Nagarajan, B.; Sabnis, N.; Raut, S.; et al. Tc-99m-HYNIC-N-dodecylamide: A new hydro-phobic tracer for labelling reconstituted high-density lipoproteins (rHDL) for radioimaging. Nanoscale 2019, 11, 541–551. [Google Scholar] [CrossRef] [PubMed]
- El-Ghareb, W.I.; Swidan, M.M.; Ibrahim, I.T.; Abd El-Bary, A.; Tadros, M.I.; Sakr, T.M. Tc-99m-doxorubicin-loaded gallic acid-gold nanoparticles (Tc-99m-DOX-loaded GA-Au NPs) as a multifunctional theranostic agent. Int. J. Pharm. 2020, 586, 119514. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; Morsy, S.A.; Mahmoud, N.A.; Rashed, H.M.; Abd El-Rehim, H.; Khoobchandani, M.; Katti, K.K.; Katti, K.V. Preparation, characterization, cytotoxicity and biological evaluation of 99mTc-Doxorubicin-Epigallocatechingallate functionalized gold nanoparticles as a new generation of theranostic radiopharmaceutical. Preprints 2018, 2018, 2018080331. [Google Scholar]
- Jimenez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-Garcia, B.; Luna-Gutierrez, M.; Azorin-Vega, E.; Isaac-Olive, K.; Camacho-Lopez, M.; Torres-Garcia, E. Multifunctional targeted therapy system based on Tc-99m/Lu-177-labeled gold nanoparticles-Tat(49-57)-Lys(3)-bombesin internalized in nuclei of prostate cancer cells. J. Label. Compd. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Santos-Cuevas, C.L.; Ferro-Flores, G.; de Murphy, C.A.; Ramirez, F.D.; Luna-Gutierrez, M.A.; Pedraza-Lopez, M.; Garcia-Becerra, R.; Ordaz-Rosado, D. Design, preparation, in vitro and in vivo evaluation of Tc-99m-N2S2-Tat(49-57)-bombesin: A target-specific hybrid radiopharmaceutical. Int. J. Pharm. 2009, 375, 75–83. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of Lu-177 for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Luna-Gutierrez, M.; Ferro-Flores, G.; Ocampo-Garcia, B.E.; Santos-Cuevas, C.L.; Jimenez-Mancilla, N.; De Leon-Rodriguez, L.M.; Azorin-Vega, E.; Isaac-Olive, K. A Therapeutic System of Lu-177-labeled Gold Nanoparticles-RGD Internalized in Breast Cancer Cells. J. Mex. Chem. 2013, 57, 212–219. [Google Scholar]
- Vilchis-Juarez, A.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Ocampo-Garcia, B.; Diaz-Nieto, L.; Luna-Gutierrez, M.; Jimenez-Mancilla, N.; Pedraza-Lopez, M.; Gomez-Olivan, L. Molecular Targeting Radiotherapy with Cyclo-RGDfK(C) Peptides Conjugated to Lu-177-Labeled Gold Nanoparticles in Tumor-Bearing Mice. J. Biomed. Nanotech. 2014, 10, 393–404. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Luna-Gutierrez, M.; Ramirez-Nava, G.; Ocampo-Garcia, B. Synthesis and Evaluation of Lu-177-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug-Therapeutic Effect on Breast Cancer Cells. Polymers 2019, 11, 1572. [Google Scholar] [CrossRef] [Green Version]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Melendez-Alafort, L.; Trujillo-Nolasco, M.; Ocampo-Garcia, B. Lu-177-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110043. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, M.; Cruz-Nova, P.; Ferro-Flores, G.; Gibbens-Bandala, B.; Morales-Avila, E.; Aranda-Lara, L.; Vargas, M.; Ocampo-Garcia, B. Development of Lu-177-DN(C19)-CXCR4 Ligand Nanosystem for Combinatorial Therapy in Pancreatic Cancer. J. Biomed. Nanotech. 2021, 17, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ruiz, A.; Ferro-Flores, G.; Jimenez-Mancilla, N.; Escudero-Castellanos, A.; Ocampo-Garcia, B.; Luna-Gutierrez, M.; Santos-Cuevas, C.; Morales-Avila, E.; Isaac-Olive, K. In vitro and in vivo synergistic effect of radiotherapy and plasmonic photothermal therapy on the viability of cancer cells using Lu-177-Au-NLS-RGD-Aptamer nanoparticles under laser irradiation. J. Radioanal. Nucl. Chem. 2018, 318, 1913–1921. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramirez, F.D.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Azorin-Vega, E.; Jimenez-Mancilla, N.; Luna-Gutierrez, M.; Isaac-Olive, K. Fluorescent, Plasmonic, and Radiotherapeutic Properties of the Lu-177-Dendrimer-AuNP-Folate-Bombesin Nanoprobe Located Inside Cancer Cells. Mol. Imaging. 2017, 16, 1536012117704768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancira-Cortez, A.; Ferro-Flores, G.; Jimenez-Mancilla, N.; Morales-Avila, E.; Trujillo-Benitez, D.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutierrez, M. Synthesis, chemical and biochemical characterization of Lu2O3 -iPSMA nanoparticles activated by neutron irradiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111335. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Ehlerding, E.B.; Cai, W.B. Intrinsically Radiolabeled Nanoparticles: An Emerging Paradigm. Small 2014, 10, 3825–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo-Benitez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jimenez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutierrez, M.; Azorin-Vega, E. Synthesis and Biochemical Evaluation of Samarium-153 Oxide Nanoparticles Functionalized with iPSMA-Bombesin Heterodimeric Peptide. J. Biomed. Nanotech. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- Ancira-Cortez, A.; Trujillo-Benítez, D.; Jiménez-Mancilla, N.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Synthesis and physicochemical characterization of Lu and Sm sesquioxide nanoparticles by precipitation-calcination and pulsed laser ablation in liquids. Mater. Chem. Phys. 2022, 275, 125229. [Google Scholar] [CrossRef]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugao, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-(198)AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-(AuNP)-Au-198 nanoconstruct in prostate tumor-bearing mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef]
- Cutler, C.; Al-Yasiri, A.; Kuchuk, M.; Loyalka, S.; Watkinson, L.; Carmack, T.; Smith, C.; Katti, K. Comparison of in vivo uptake of radioactive gold nanoparticles formulated using phytochemicals. J. Nucl. Med. 2015, 56, 1267. [Google Scholar]
- Katti, K.V. Renaissance of nuclear medicine through green nanotechnology: Functionalized radioactive gold nanoparticles in cancer therapy-my journey from chemistry to saving human lives. J. Radioanal. Nucl. Chem. 2016, 309, 5–14. [Google Scholar] [CrossRef]
- Katti, K.V.; Khoobchandani, M.; Thipe, V.C.; Al-Yasiri, A.Y.; Katti, K.K.; Loyalka, S.K.; Sakr, T.M.; Lugao, A.B. Prostate tumor therapy advances in nuclear medicine: Green nanotechnology toward the design of tumor specific radioactive gold nanoparticles. J. Radioanal. Nucl. Chem. 2018, 318, 1737–1747. [Google Scholar] [CrossRef]
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Akhlaghi, M.; Geramifar, P.; Jalilian, A.R.; Mazidi, S.M.; Yousefnia, H.; Shahhosseini, S.; Beiki, D. Ga-68-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: Preparation, characterization and biological evaluation. Int. J. Nanomed. 2019, 14, 2591–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, B.M.; El-Mohty, A.A.; El-Hashash, M.A.; Sakr, T.M. Tc-99m-citrate-gold nanoparticles as a tumor tracer: Synthesis, characterization, radiolabeling and in-vivo studies. Radiochim. Acta 2020, 108, 809–819. [Google Scholar] [CrossRef]
- Sakr, T.M.; El-Hashash, M.A.; El-Mohty, A.A.; Essa, B.M. Tc-99m-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl. Radiat. Isot. 2020, 164, 109269. [Google Scholar] [CrossRef]
- Ocampo-Garcia, B.; Gibbens-Bandala, B.; Morales-Avila, E.; Melendez-Alafort, L.; Khoobchandani, M.; Trujillo-Nolasco, M.; Katti, K.K. Dual-Targeted Therapy and Molecular Imaging with Radiolabeled Nanoparticles. In Biotechnology Products in Everyday Life; Springer: Cham, Switzerland, 2019. [Google Scholar]
- El-Safoury, D.M.; Ibrahim, A.B.; El-Setouhy, D.A.; Khowessah, O.M.; Motaleb, M.A.; Sakr, T.M. Gold nanoparticles for Tc-99m-doxorubicin delivery: Formulation, in vitro characterization, comparative studies in vivo stability and biodistribution. J. Radioanal. Nucl. Chem. 2021, 328, 325–338. [Google Scholar] [CrossRef]
- Amin, M.A.; El-Aasser, M.M.; Ayoub, S.M.; Shiekh, H.E.H.; Sakr, T.M. Exploitation of Aspergillus flavus synthesized copper oxide nanoparticles as a novel medical agent. J. Radioanal. Nucl. Chem. 2021, 328, 299–313. [Google Scholar] [CrossRef]
- Korany, M.; Marzook, F.; Mahmoud, B.; Ahmed, S.A.; Ayoub, S.M.; Sakr, T.M. Exhibiting the diagnostic face of selenium nanoparticles as a radio-platform for tumor imaging. Bioorg. Chem. 2020, 100, 103910. [Google Scholar] [CrossRef]
- Korany, M.; Mahmoud, B.; Ayoub, S.M.; Sakr, T.M.; Ahmed, S.A. Synthesis and radiolabeling of vitamin C-stabilized selenium nanoparticles as a promising approach in diagnosis of solid tumors. J. Radioanal. Nucl. Chem. 2020, 325, 237–244. [Google Scholar] [CrossRef]
- Swidan, M.M.; Khowessah, O.M.; Abd El-Motaleb, M.; Abd El-Bary, A.; El-Kolaly, M.T.; Sakr, T.M. Iron oxide nanoparticulate system as a cornerstone in the effective delivery of Tc-99m radionuclide: A potential molecular imaging probe for tumor diagnosis. DARU J. Pharm. Sci. 2019, 27, 49–58. [Google Scholar] [CrossRef]
- Sakr, T.M.; Khowessah, O.; Motaleb, M.; Abd El-Bary, A.; El-Kolaly, M.; Swidan, M.M. I-131 doping of silver nanoparticles platform for tumor theranosis guided drug delivery. Eur. J. Pharm. Sci. 2018, 122, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mollarazi, E.; Jalilian, A.R.; Johari-daha, F.; Atyabi, F. Development of Sm-153-folate-polyethyleneimine-conjugated chitosan nanoparticles for targeted therapy. J. Label. Compd. Radiopharm. 2015, 58, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-Garcia, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109766. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramirez, F.D.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorin-Vega, E.; Morales-Avila, E.; Isaac-Olive, K. Lu-177-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomater. 2016, 2016, 1039258. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Lara, L.; Ocampo-Garcia, B.; Isaac-Olivé, K.; Ferro-Flores, G.; Meléndez-Alafort, L.; Morales-Avila, E. Drug Delivery Systems-Based Dendrimers and Polymer Micelles for Nuclear Diagnosis and Therapy. Macromol. Biosci. 2021, 21, 2000362. [Google Scholar] [CrossRef]
- Al-Yasiri, A.Y.; White, N.E.; Katti, K.V.; Loyalka, S.K. Estimation of tumor and local tissue dose in gold nanoparticles radiotherapy for prostate cancer. Rep. Pract. Oncol. Radiother. 2019, 24, 288–293. [Google Scholar] [CrossRef]
- Santos, J.J.; Leal, J.; Dias, L.A.P.; Toma, S.H.; Corio, P.; Genezini, F.A.; Katti, K.V.; Araki, K.; Lugao, A.B. Bovine Serum Albumin Conjugated Gold-198 Nanoparticles as Model To Evaluate Damage Caused by Ionizing Radiation to Biomolecules. ACS Appl. Nano Mater. 2018, 1, 5062–5070. [Google Scholar] [CrossRef]
- Iram, F.; Iqbal, M.S.; Khan, I.U.; Rasheed, R.; Khalid, A.; Khalid, M.; Aftab, S.; Shakoori, A.R. Synthesis and Biodistribution Study of Biocompatible Au-198 Nanoparticles by use of Arabinoxylan as Reducing and Stabilizing Agent. Biol. Trace Elem. Res. 2020, 193, 282–293. [Google Scholar] [CrossRef]
- El-Safoury, D.; Ibrahim, A.B.; El-Setouhy, D.; Khowessah, O.; Motaleb, M.; Sakr, T.M. Amelioration of Tumor Targeting and In Vivo Biodistribution of 99mTc-Methotrexate-Gold Nanoparticles (99mTc-Mex-AuNPs). J. Pharm. Sci. 2021, 110, 2955–2965. [Google Scholar] [CrossRef]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-García, B.E.; Medina, L.A.; López-Téllez, G.; Gibbens-Bandala, B.V.; Izquierdo-Sánchez, V. Biodegradable poly (D, L-lactide-co-glycolide)/poly (L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 743–751. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.V.; Ocampo-García, B.E.; Ferro-Flores, G.; Morales-Avila, E.; Ancira-Cortez, A.; Jaimes-Aguirre, L. Multimeric System of RGD-Grafted PMMA-Nanoparticles as a Targeted Drug-Delivery System for Paclitaxel. Curr. Pharm. Des. 2017, 23, 3415–3422. [Google Scholar] [PubMed]
- Abbasi, A.; Khojasteh, H.; Hamadanian, M.; Salavati-Niasari, M. Synthesis of CoFe2O4 nanoparticles and investigation of the temperature, surfactant, capping agent and time effects on the size and magnetic properties. J. Mater. Sci. Mater. 2016, 27, 4972–4980. [Google Scholar] [CrossRef]
- Feynman, R. There is plenty of room at the bottom. California Institute of Technology. Int. J. Eng. Sci. 1960, 4, 23–36. [Google Scholar]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials—A review. Anal. Chim. Acta 2019, 1069, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Majidi, R.F.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Mikrochim. Acta 2019, 186, 49. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, characterization, and applications of metal nanoparticles. In Biomaterials and Bionanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 527–612. [Google Scholar]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Sakr, T.M.; Korany, M.; Katti, K.V. Selenium nanomaterials in biomedicine—An overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Technol. 2018, 46, 223–233. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of poly (acrylic acid) nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Rosiak, J.M. Nanogels synthesized by radiation-induced intramolecular crosslinking of water-soluble polymers. Radiat. Phys. Chem. 2020, 169, 108099. [Google Scholar] [CrossRef]
- Rurarz, B.P.; Gibka, N.; Bukowczyk, M.; Kadłubowski, S.; Ulański, P. Radiation synthesis of poly(acrylic acid) nanogels for drug delivery applications—Postsynthesis product stability. Nukleonika, 2021; in press. [Google Scholar]
- Rattanawongwiboon, T.; Ghaffarlou, M.; Sütekin, S.D.; Pasanphan, W.; Güven, O. Preparation of multifunctional poly (acrylic acid)-poly (ethylene oxide) nanogels from their interpolymer complexes by radiation-induced intramolecular crosslinking. Colloid Polym. Sci. 2018, 296, 1599–1608. [Google Scholar] [CrossRef]
- Achilli, E.; Casajus, G.; Siri, M.; Flores, C.; Kadłubowski, S.; Alonso, S.d.V.; Grasselli, M. Preparation of protein nanoparticle by dynamic aggregation and ionizing-induced crosslinking. Colloids Surf. A Physicochem. Eng. Asp. 2015, 486, 161–171. [Google Scholar] [CrossRef]
- Achilli, E.; Siri, M.; Flores, C.; Kikot, P.; Flor, S.; Martinefski, M.; Lucangioli, S.; Alonso, S.d.V.; Grasselli, M. Radiolysis effect of the high proportion of ethanol in the preparation of albumin nanoparticle. Radiat. Phys. Chem. 2020, 169, 108775. [Google Scholar] [CrossRef]
- Siri, M.; Achilli, E.; Grasselli, M.; del V Alonso, S. Albumin nanocarriers, γ-irradiated crosslinked, combined with therapeutic drugs for cancer therapy. Curr. Pharm. Des. 2017, 23, 5272–5282. [Google Scholar] [CrossRef]
- Siri, M.; Ruocco, M.J.F.; Achilli, E.; Pizzuto, M.; Delgado, J.F.; Ruysschaert, J.-M.; Grasselli, M.; Alonso, S.d.V. Effect of structure in ionised albumin based nanoparticle: Characterisation, Emodin interaction, and in vitro cytotoxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109813. [Google Scholar] [CrossRef]
- Espinoza, S.L.S.; Sánchez, M.L.; Risso, V.; Smolko, E.E.; Grasselli, M. Radiation synthesis of seroalbumin nanoparticles. Radiat. Phys. Chem. 2012, 81, 1417–1421. [Google Scholar] [CrossRef]
- Grimaldi, N.; Sabatino, M.; Przybytniak, G.; Kaluska, I.; Bondì, M.; Bulone, D.; Alessi, S.; Spadaro, G.; Dispenza, C. High-energy radiation processing, a smart approach to obtain PVP-graft-AA nanogels. Radiat. Phys. Chem. 2014, 94, 76–79. [Google Scholar] [CrossRef]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef]
- Adamoa, G.; Grimaldib, N.; Camporaa, S.; Antonietta, M.; Sabatinob, C.D.; Ghersia, G. Glutathione-sensitive nanogels for drug release. Chem. Eng. Trans. 2014, 38, 457–462. [Google Scholar]
- Pasanphan, W.; Rimdusit, P.; Choofong, S.; Piroonpan, T.; Nilsuwankosit, S. Systematic fabrication of chitosan nanoparticle by gamma irradiation. Radiat. Phys. Chem. 2010, 79, 1095–1102. [Google Scholar] [CrossRef]
- Pasanphan, W.; Choofong, S.; Rimdusit, P. Deoxycholate-chitosan nanospheres fabricated by γ-irradiation and chemical modification: Nanoscale synthesis and controlled studies. J. Appl. Polym. Sci. 2012, 123, 3309–3320. [Google Scholar] [CrossRef]
- Pasanphan, W.; Rattanawongwiboon, T.; Rimdusit, P.; Piroonpan, T. Radiation-induced graft copolymerization of poly (ethylene glycol) monomethacrylate onto deoxycholate-chitosan nanoparticles as a drug carrier. Radiat. Phys. Chem. 2014, 94, 199–204. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Pasanphan, W. Light stabilizer–conjugated–stearylate chitosan nanoparticles: A bio-based additive for free radical stabilization of healthcare plastics under irradiation. Polym. Degrad. Stab. 2014, 109, 405–415. [Google Scholar] [CrossRef]
- Pasanphan, W.; Rattanawongwiboon, T.; Choofong, S.; Güven, O.; Katti, K.K. Irradiated chitosan nanoparticle as a water-based antioxidant and reducing agent for a green synthesis of gold nanoplatforms. Radiat. Phys. Chem. 2015, 106, 360–370. [Google Scholar] [CrossRef]
- Piroonpan, T.; Katemake, P.; Pasanphan, W. Comparative study of different chitosan solutions to assist the green synthesis of gold nanoparticles under irradiation. Radiat. Phys. Chem. 2020, 169, 108250. [Google Scholar] [CrossRef]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K.V.; Pasanphan, W. Water-Soluble Chitosan Conjugated DOTA-Bombesin Peptide Capped Gold Nanoparticles as a Targeted Therapeutic Agent for Prostate Cancer. Nanotechnol. Sci. Appl. 2021, 14, 69. [Google Scholar] [CrossRef]
- Flores, C.Y.; Achilli, E.; Grasselli, M. Radiosynthesis of gold/albumin core/shell nanoparticles for biomedical applications. MRS Adv. 2017, 2, 2675–2681. [Google Scholar] [CrossRef]
- Flores, C.Y.; Achilli, E.; Grasselli, M. Radiation-induced preparation of core/shell gold/albumin nanoparticles. Radiat. Phys. Chem. 2018, 142, 60–64. [Google Scholar] [CrossRef]
- Flores, C.Y.; Achilli, E.; Schinca, D.; Grasselli, M. Plasmon properties of multilayer albumin/gold hybrid nanoparticles. Mater. Res. Express. 2019, 6, 055005. [Google Scholar] [CrossRef] [Green Version]
- Achilli, E.; Flores, C.Y.; Temprana, C.F.; Alonso, S.d.V.; Radrizzani, M.; Grasselli, M. Enhanced gold nanoparticle-tumor cell recognition by albumin multilayer coating. OpenNano 2021, 6, 100033. [Google Scholar] [CrossRef]
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; San Biagio, P.L.; Di Giacinto, M.L.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondì, M.L.; Casaletto, M.P.; Rigogliuso, S.; Adamo, G.; Ghersi, G. Minimalism in radiation synthesis of biomedical functional nanogels. Biomacromolecules 2012, 13, 1805–1817. [Google Scholar] [CrossRef] [Green Version]
- Adamo, G.; Grimaldi, N.; Sabatino, M.A.; Walo, M.; Dispenza, C.; Ghersi, G. E-beam crosslinked nanogels conjugated with monoclonal antibodies in targeting strategies. Biol. Chem. 2017, 398, 277–287. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.; Ajovalasit, A.; Ditta, L.; Ragusa, M.; Purrello, M.; Costa, V.; Conigliaro, A.; Alessandro, R. Nanogel-antimiR-31 conjugates affect colon cancer cells behaviour. RSC Adv. 2017, 7, 52039–52047. [Google Scholar] [CrossRef] [Green Version]
- Wongkrongsak, S.; Tangthong, T.; Pasanphan, W. Electron beam induced water-soluble silk fibroin nanoparticles as a natural antioxidant and reducing agent for a green synthesis of gold nanocolloid. Radiat. Phys. Chem. 2016, 118, 27–34. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Haema, K.; Pasanphan, W. Stearyl methacrylate-grafted-chitosan nanoparticle as a nanofiller for PLA: Radiation-induced grafting and characterization. Radiat. Phys. Chem. 2014, 94, 205–210. [Google Scholar] [CrossRef]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K.V.; Pasanphan, W. Bombesin Peptide Conjugated Water-Soluble Chitosan Gallate—A New Nanopharmaceutical Architecture for the Rapid One-Pot Synthesis of Prostate Tumor Targeted Gold Nanoparticles. Int. J. Nanomed. 2021, 16, 6957. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Mohandoss, D.K.D.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine–pre-clinical and pilot human clinical investigations. Int. J. Nanomed. 2020, 15, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoobchandani, M.; Khan, A.; Katti, K.K.; Thipe, V.C.; Al-Yasiri, A.Y.; MohanDoss, D.K.; Nicholl, M.B.; Lugão, A.B.; Hans, C.P.; Katti, K.V. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci. Rep. 2021, 11, 16797. [Google Scholar] [CrossRef] [PubMed]
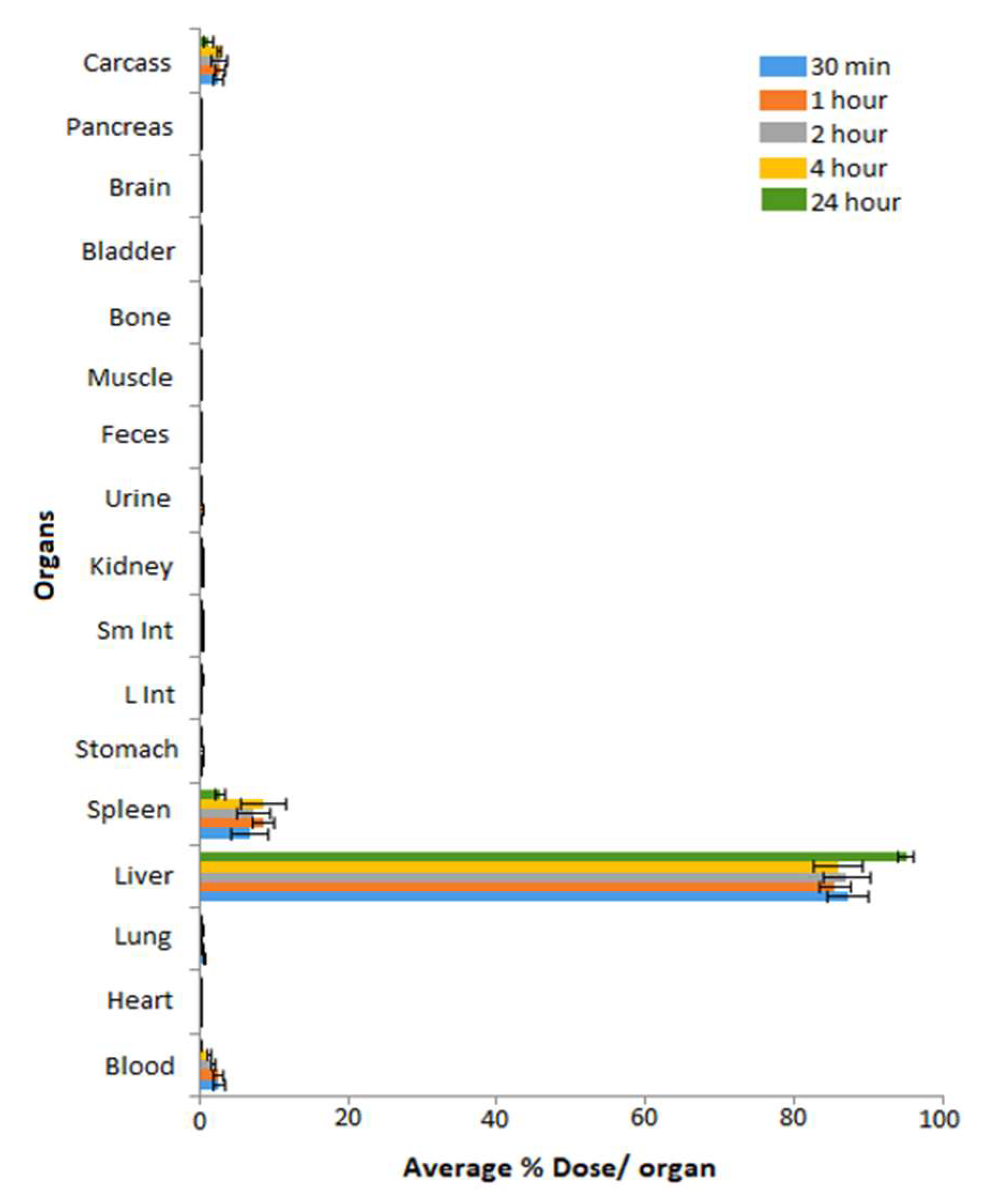
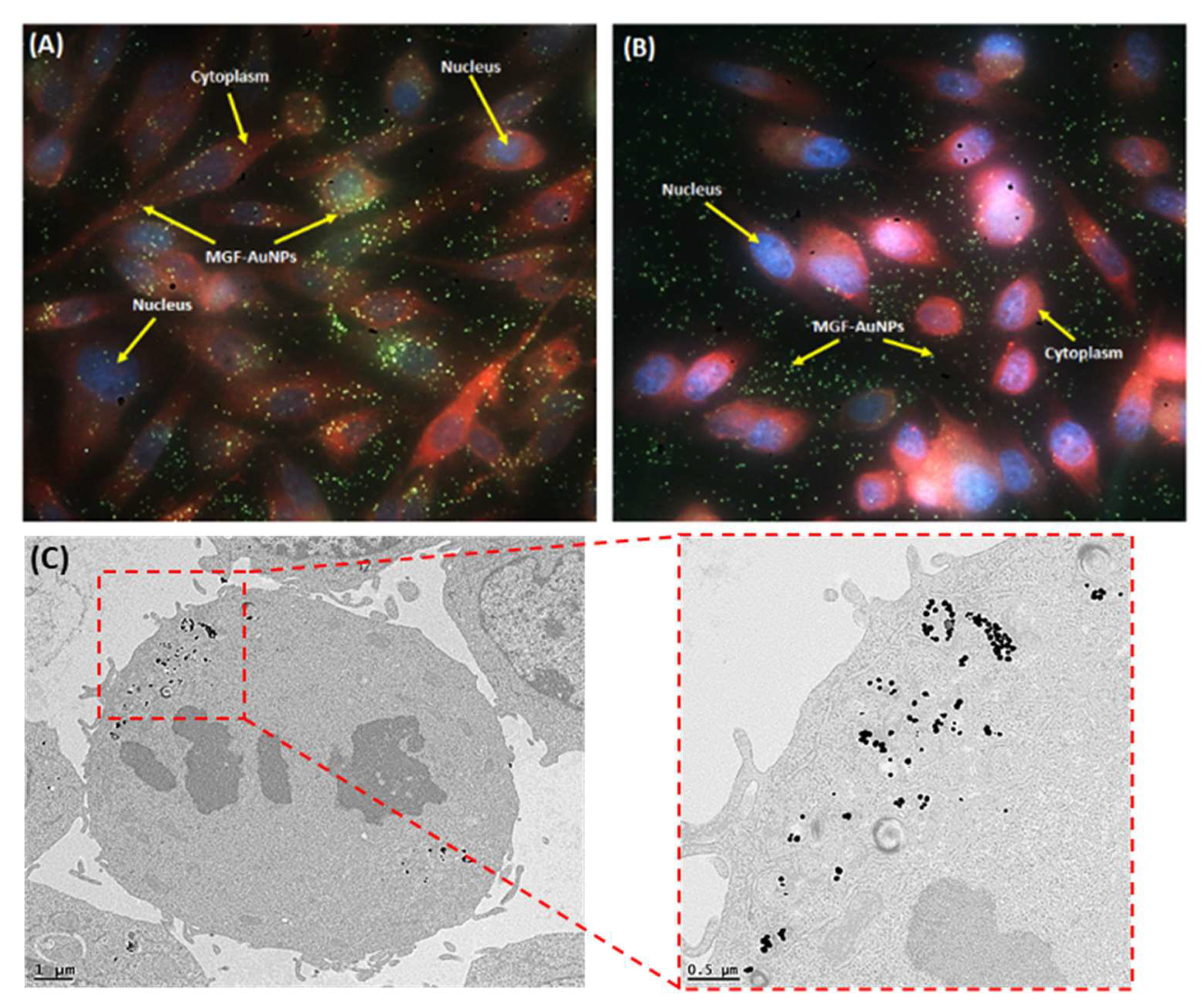
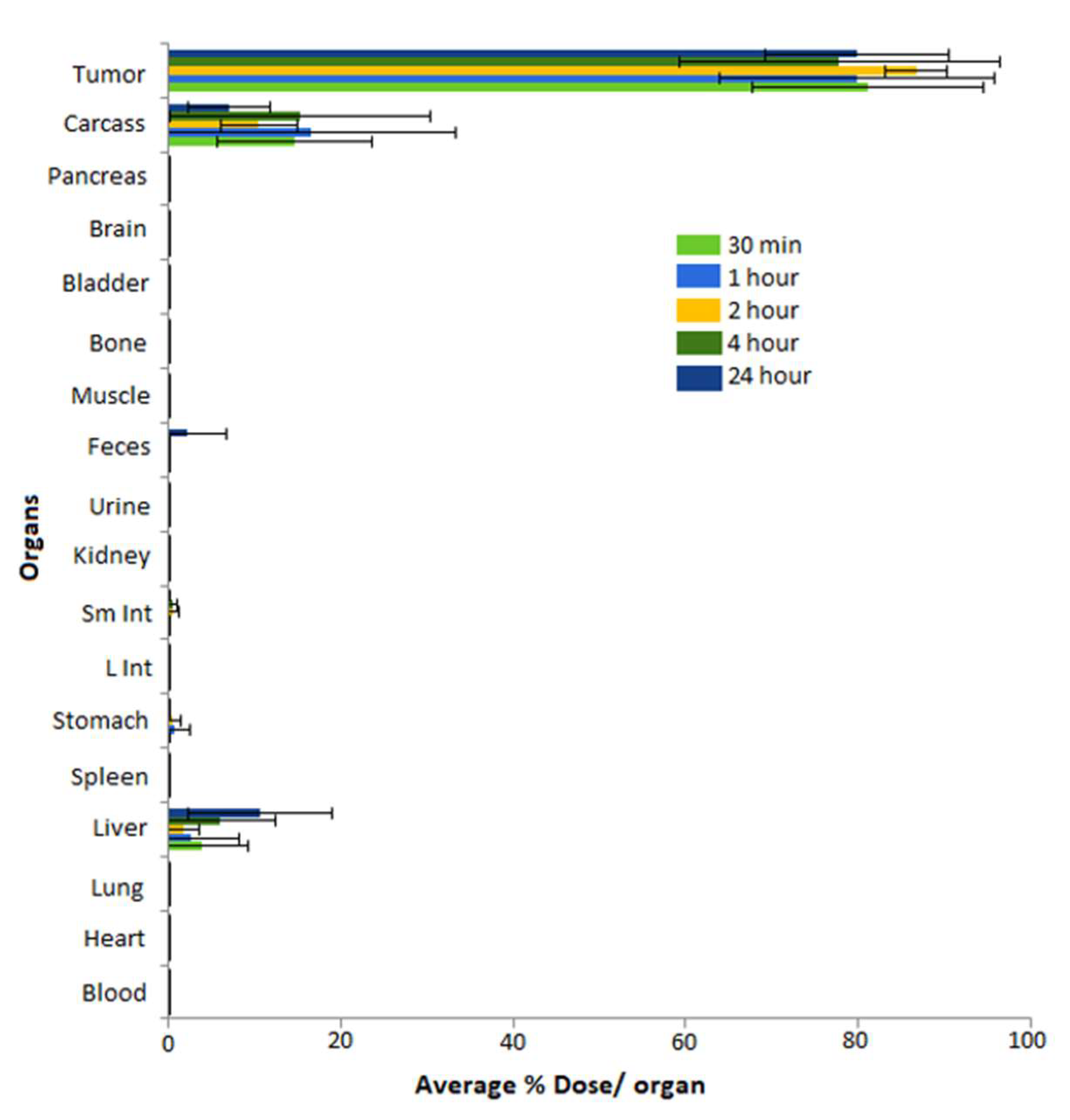
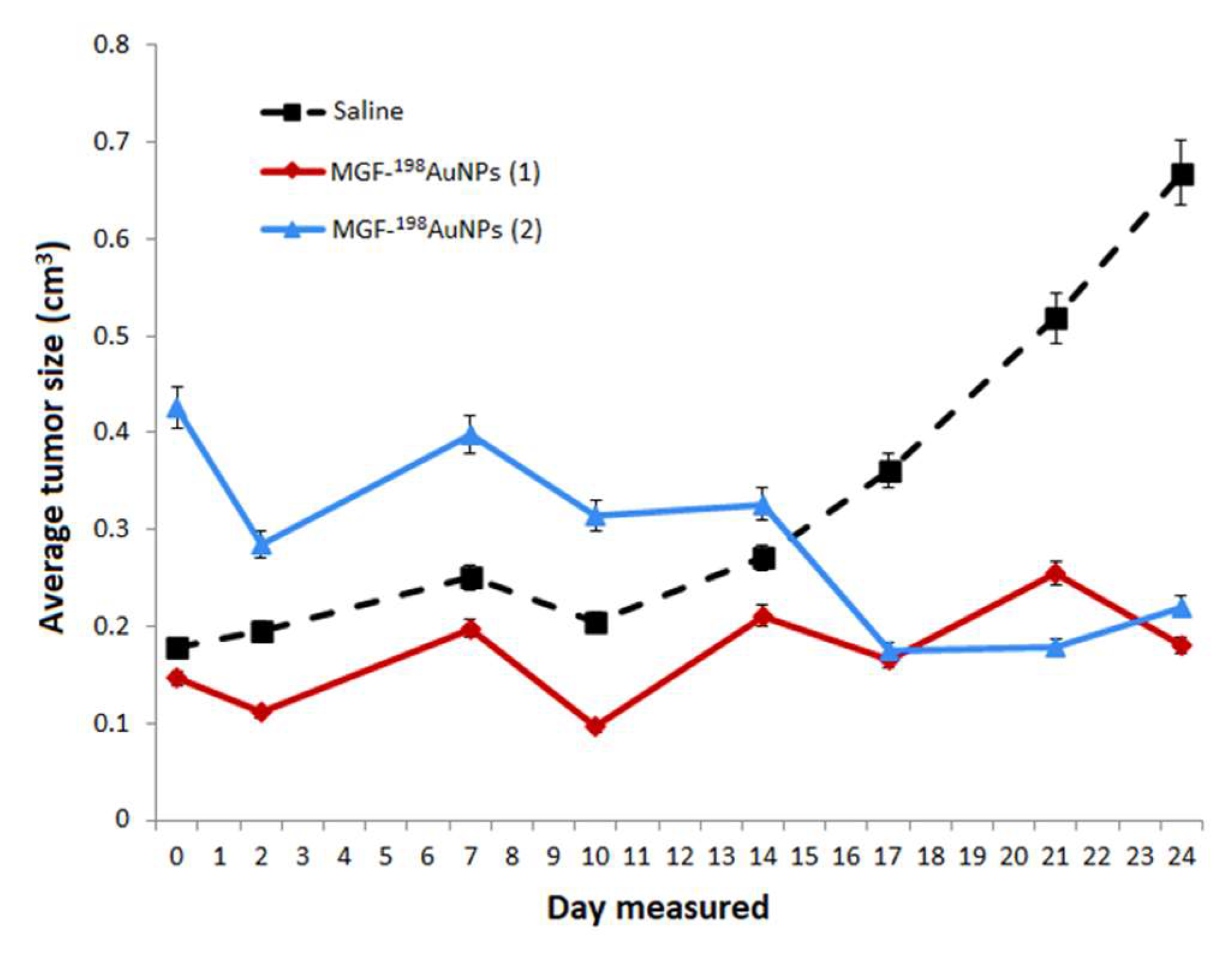
| Categories of NPs | Name of NPs | Size (nm) | Information of NPs | Ref. |
|---|---|---|---|---|
| Inorganic | AgNP | 21 | AgNP prepared using ascorbic acid reducing agent and polyethylene glycol stabilizing agent for delivering 131I radioisotope. | [52] |
| SeNP | 43 | SeNP prepared using sodium dithionate reducing agent and glutathione stabilizing agent for 99mTc. | [49] | |
| 23 | SeNP prepared using sodium dithionate reducing agent and ascorbic acid as stabilizing agent for delivering 99mTc radioisotope. | [50] | ||
| AuNP | 30–85 | AuNP prepared using mangiferin as reducing and stabilizing agents for delivering 198Au radioisotope. | [42] | |
| 20.3 | AuNP prepared using Trisodium citrate as reducing and stabilizing agents for delivering 99mTc radioisotope. | [44,47] | ||
| 50 | AuNP prepared using gallic acid as reducing and stabilizing agent for delivering 99mTc radioisotope. | [22,45,60] | ||
| Fe oxide NP | 24 | Fe oxide NPs prepared by co-precipitation method and using polyethylene glycol as stabilizing agent for delivering 99mTc radioisotope. | [51] | |
| Cu oxide NP | 32.4 | Cu oxide NPs prepared by biological synthesis using Aspergillus flavus for delivering 99mTc. | [48] | |
| Polymers | 99mTc-PAMAM-Tyr3-Octreotide 99mTc -AuNP-Tyr3-Octreotide. | 20 b | Dendrimer-based or gold-based nanoradiopharmaceuticals for somatostatin receptors imaging on neuroendocrine tumors. | [17,46] |
| 177Lu-DenAuNP-folate-bombesin | 18.60 ± 8.00 b | 177Lu-dendrimer (PAMAM-G4)-folate-bombesin with AuNPs in the dendritic cavity for targeted radiotherapy and the simultaneous detection of folate receptors (FRs) and gastrin-releasing peptide receptors (GRPRs) overexpressed in breast cancer cells. | [46,55,56] | |
| DOX-PLGA/γ-PGA-FA | 597 ± 45.0 a | Poly(L-γ-glutamic acid) (γ-PGA) conjugated to modified folic acid (FA) as a targeting ligand for specific doxorubicin delivery. | [61] | |
| PMAA(PTX)-RGD | 17.5 ± 7.4 b | Multimeric system of RGD-grafted PMMA- nanoparticles as a targeted drug-delivery system for paclitaxel. | [62] | |
| 177Lu-PLGA(PTX)-BN | 163.54 ± 33.25 a | A targeted paclitaxel delivery system with concomitant radiotherapeutic effect for the treatment of GRPr-positive breast cancer. | [30] | |
| 177Lu-DOTA-HA-PLGA(MTX) | 167.6 ± 57.4 a | Multifunctional chemo/radiotherapy agent based on PLGA, modified with hyaluronic acid and DOTA as a chelating agent for radiosynovectomy and specific targeted anti-rheumatic therapy. | [54] | |
| 177Lu-DOTA-DN(PTX)-BN | 16.37 a | Bombesin targeted polymeric NPs designed to produce radiotherapy and chemotherapy towards GRP receptors. | [29,55] | |
| 177Lu-DN(C19)-CXCR4 | 67.0 ± 23.17 a | Nanoradiopharmaceutical dendrimer-based for combinatorial therapy (targeted chemotherapy and radiotherapy) in pancreatic cancer cell lines overexpressing the CXCR4 receptor. | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalilian, A.R.; Ocampo-García, B.; Pasanphan, W.; Sakr, T.M.; Melendez-Alafort, L.; Grasselli, M.; Lugao, A.B.; Yousefnia, H.; Dispenza, C.; Janib, S.M.; et al. IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery. Pharmaceutics 2022, 14, 1060. https://doi.org/10.3390/pharmaceutics14051060
Jalilian AR, Ocampo-García B, Pasanphan W, Sakr TM, Melendez-Alafort L, Grasselli M, Lugao AB, Yousefnia H, Dispenza C, Janib SM, et al. IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery. Pharmaceutics. 2022; 14(5):1060. https://doi.org/10.3390/pharmaceutics14051060
Chicago/Turabian StyleJalilian, Amir R., Blanca Ocampo-García, Wanvimol Pasanphan, Tamer M. Sakr, Laura Melendez-Alafort, Mariano Grasselli, Ademar B. Lugao, Hassan Yousefnia, Clelia Dispenza, Siti Mohd Janib, and et al. 2022. "IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery" Pharmaceutics 14, no. 5: 1060. https://doi.org/10.3390/pharmaceutics14051060
APA StyleJalilian, A. R., Ocampo-García, B., Pasanphan, W., Sakr, T. M., Melendez-Alafort, L., Grasselli, M., Lugao, A. B., Yousefnia, H., Dispenza, C., Janib, S. M., Khan, I. U., Maurin, M., Ulański, P., Loo, S. C. J., Safrany, A., Osso, J. A., Jr., Duatti, A., & Katti, K. V. (2022). IAEA Contribution to Nanosized Targeted Radiopharmaceuticals for Drug Delivery. Pharmaceutics, 14(5), 1060. https://doi.org/10.3390/pharmaceutics14051060











