Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence
Abstract
1. Introduction
2. Physicochemical Characterization
2.1. Particle Size and Polydispersity
2.1.1. Dynamic Light Scattering Spectroscopy
2.1.2. Static Light Scattering
2.1.3. Atomic Force Microscopy
2.1.4. Centrifugal Liquid Sedimentation
2.2. Surface Charge and Hydrophobicity
2.3. Morphology of Nanocarriers
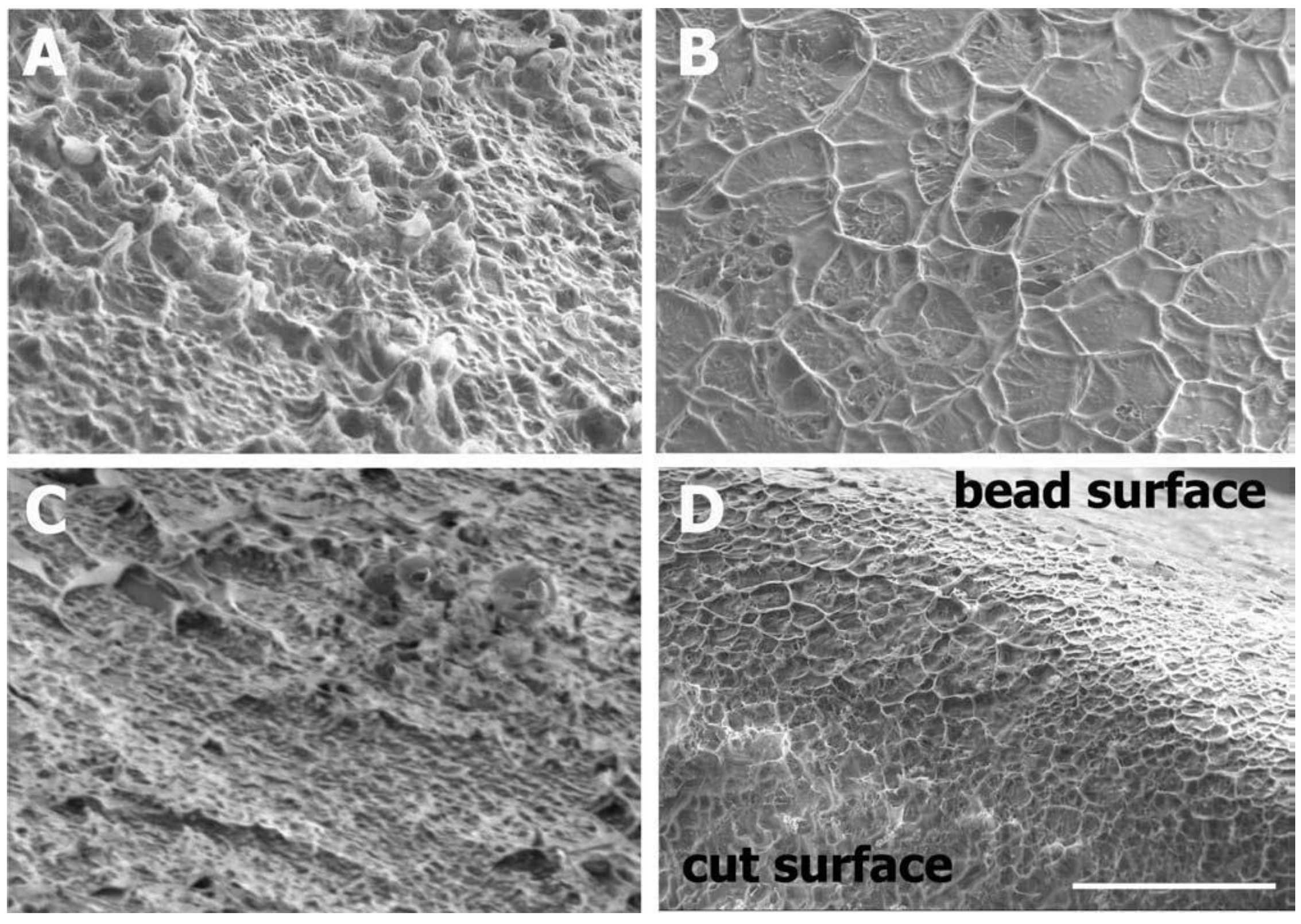
2.3.1. Scanning Electron Microscopy
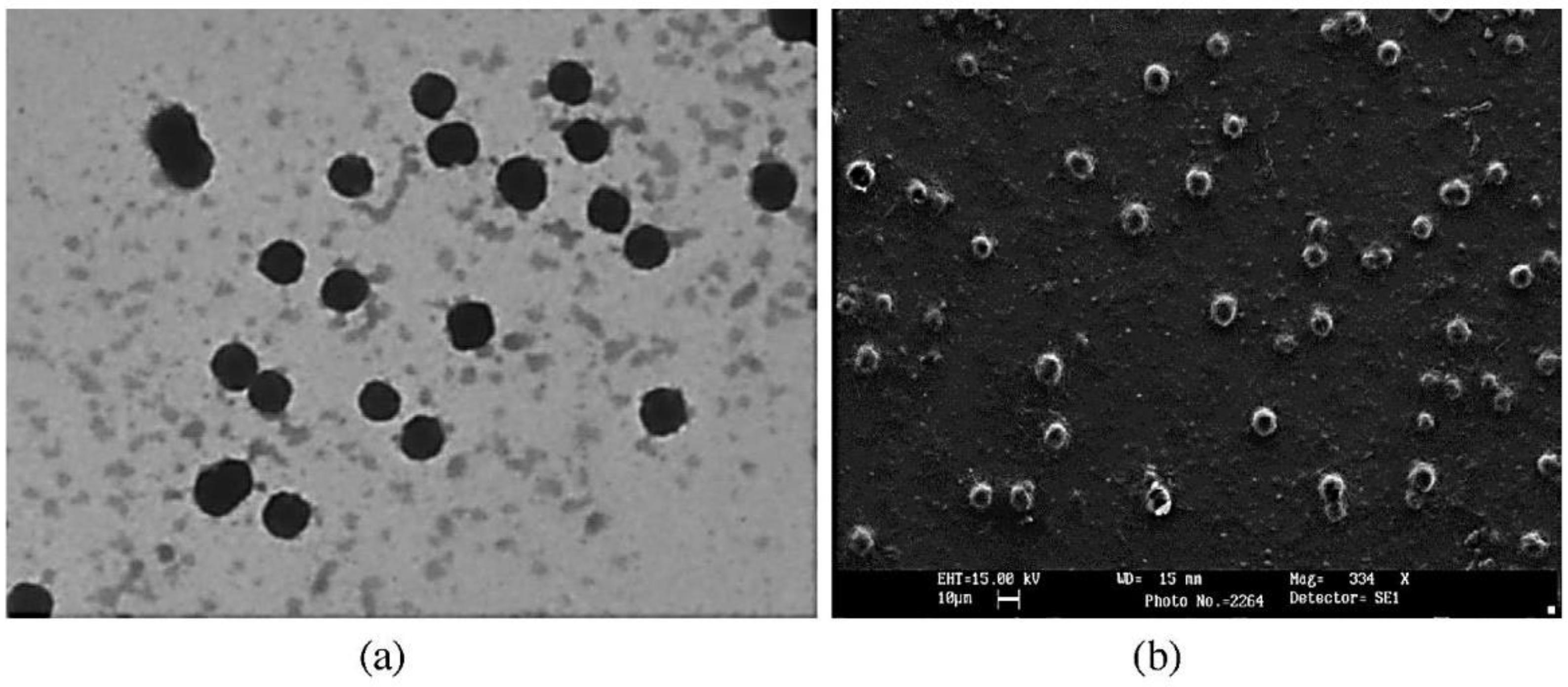
2.3.2. Transmission Electron Microscopy
3. Composition, Loading Efficiency and Mechanical Properties
4. In Vitro Drug Release
4.1. Dialysis Method
4.2. Sample and Separation Method
4.3. Continuous Flow Method
4.4. Dynamic Dissolution Method
4.5. Microdialysis Method
5. Stability Studies
5.1. Stability Studies for Vesicular Nanocarriers
5.2. Physical Stability of Self-Assembled Nanocarrier Systems
5.3. Thermal Stability of Polymeric Nanocarriers
5.4. Stability of Nanocarrier Suspensions and Nanoemulsions
5.5. Stability of Nanocarriers in Biological Matrices
6. Permeability Assessment
6.1. Ex Vivo Models
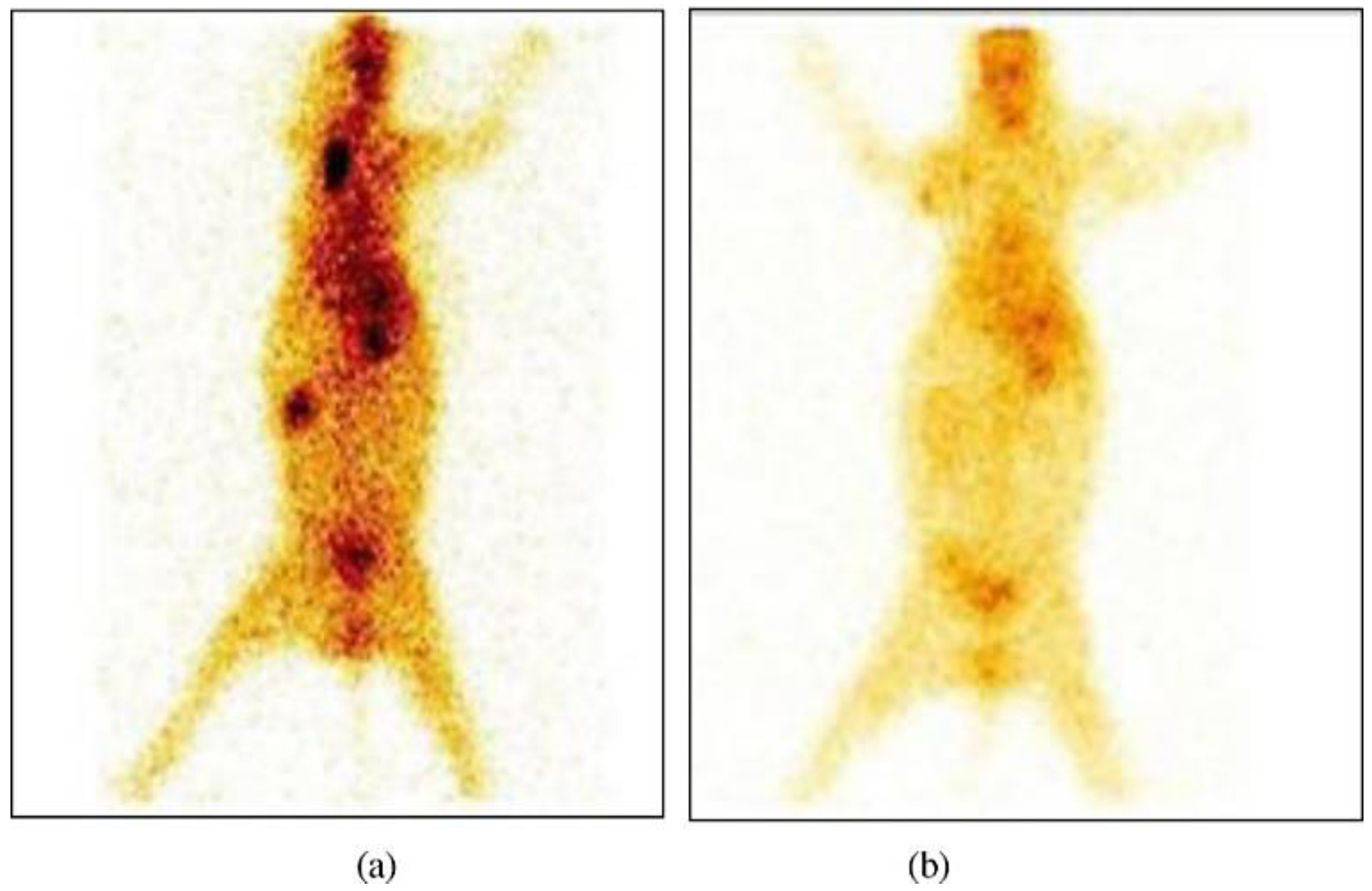
6.2. In Vivo Methods
6.3. In Situ Organ Perfusion Models
6.4. Cell Culture-Based Models
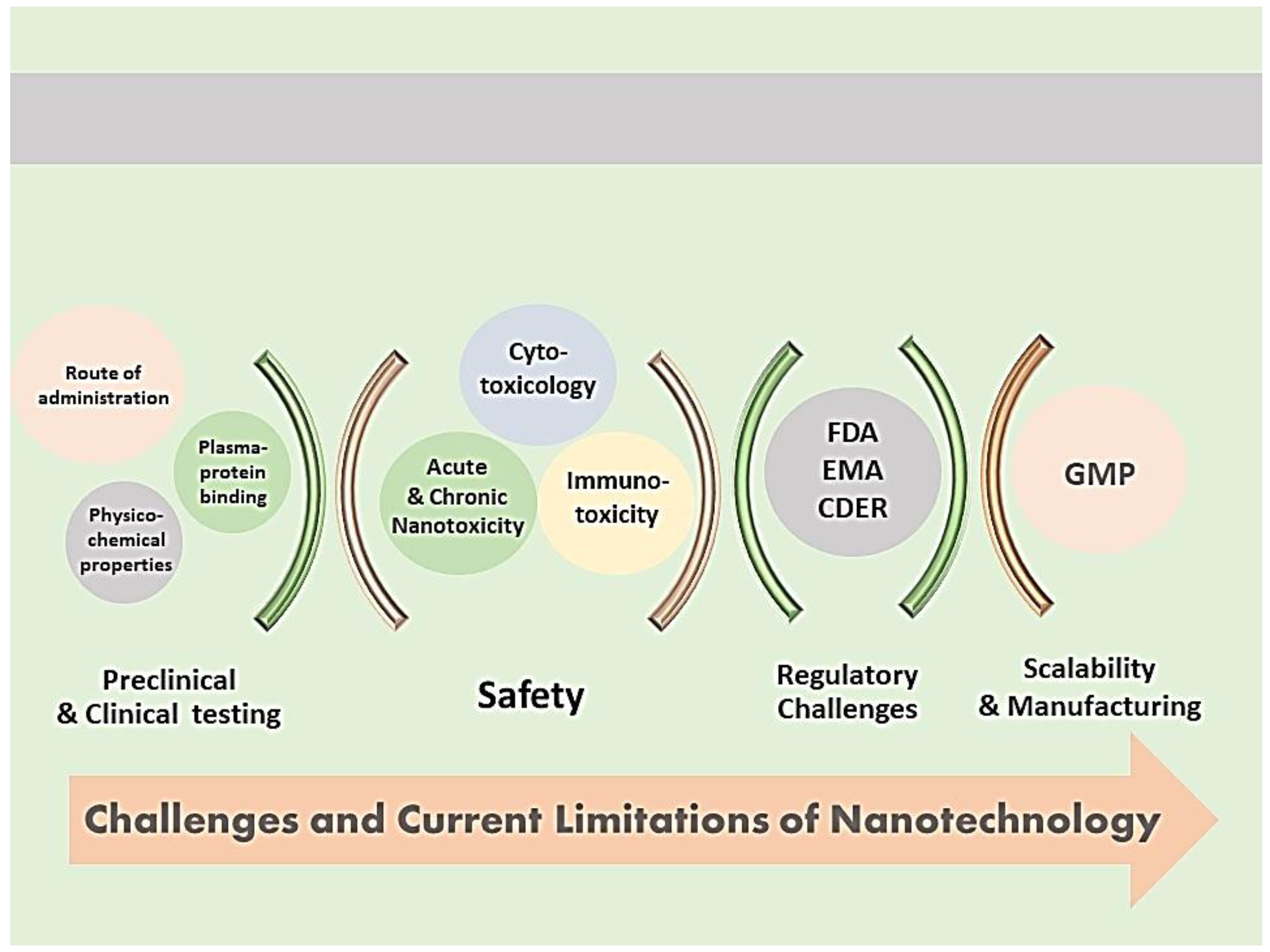
7. Challenges and Limitations of Nanocarrier Characterization
7.1. Correlation of Preclinical Characterization to Clinical Testing
7.2. Safety Considerations
7.3. Regulatory Challenges in Nanomedicine Development
7.4. Manufacturing Considerations
8. Integration of Artificial Intelligence (AI) with Nanotechnology
8.1. AI in Pharmaceutics and Drug Delivery
8.2. Applications of AI in the Development and Optimization of Nanocarriers
8.3. AI Problems in the Development and Optimization of Nanocarriers and Pharmaceuticals
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pontes, J.F.; Grenha, A. Multifunctional Nanocarriers for Lung Drug Delivery. Nanomaterials 2020, 10, 183. [Google Scholar] [CrossRef]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.U.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Sliman, Y.; Ercan, I.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Kaewsaneha, C.; Elaissari, A. Magnetic and ph-responsive magnetic nanocarriers. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–85. [Google Scholar]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, L.; Atre, P.; Rizvi, S. Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations. Pharmaceuticals 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Vachhani, S.; Kleinstreuer, C. Comparison of micron- and nano-particle transport in the human nasal cavity with a focus on the olfactory region. Comput. Biol. Med. 2021, 128, 104103. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Reboredo, C.; González-Navarro, C.; Martínez-Oharriz, C.; Martínez-López, A.; Irache, J. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120287. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, Z.; Sun, J.; Luo, J.; Wei, Y.; Zou, J. Deoxycholic acid-functionalised nanoparticles for oral delivery of rhein. Eur. J. Pharm. Sci. 2021, 159, 105713. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Duan, X.P.; Li, Y.P. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.W.; Palazuelos, M.; Moudgil, B.M.; Roberts, S.M. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 2007, 1, 42–51. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Gupta, A.S. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zellnitz, S.; Müller, M.; Meindl, C.; Schröttner, H.; Fröhlich, E. Impact of drug particle shape on permeability and cellular uptake in the lung. Eur. J. Pharm. Sci. 2019, 139, 105065. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Fischer, K.; Schmidt, M. Pitfalls and novel applications of particle sizing by dynamic light scattering. Biomaterials 2016, 98, 79–91. [Google Scholar] [CrossRef]
- Ramirez, L.M.F.; Rihouey, C.; Chaubet, F.; Le Cerf, D.; Picton, L. Characterization of dextran particle size: How frit-inlet asymmetrical flow field-flow fractionation (FI-AF4) coupled online with dynamic light scattering (DLS) leads to enhanced size distribution. J. Chromatogr. A 2021, 1653, 462404. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Le Cerf, D.; Picton, L.; Muller, G. Aggregation of amphiphilic pullulan derivatives evidenced by on-line flow field flow fractionation/multi-angle laser light scattering. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 115–122. [Google Scholar] [CrossRef]
- Picton, L.; Bataille, I.; Muller, G. Analysis of a complex polysaccharide (gum arabic) by multi-angle laser light scattering coupled on-line to size exclusion chromatography and flow field flow fractionation. Carbohydr. Polym. 2000, 42, 23–31. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, L.M.F.; Gobin, E.; Aid-Launais, R.; Journe, C.; Moraes, F.C.; Picton, L.; Le Cerf, D.; Letourneur, D.; Chauvierre, C.; Chaubet, F. Gd(DOTA)-grafted submicronic polysaccharide-based particles functionalized with fucoidan as potential MR contrast agent able to target human activated platelets. Carbohydr. Polym. 2020, 245, 116457. [Google Scholar] [CrossRef]
- Agrahari, V.; Burnouf, P.-A.; Burnouf, T. Nanoformulation properties, characterization, and behavior in complex biological matrices: Challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv. Drug Deliv. Rev. 2019, 148, 146–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Light scattering: A review of particle characterization applications. Particuology 2015, 18, 11–21. [Google Scholar] [CrossRef]
- Sitterberg, J.; Özcetin, A.; Ehrhardt, C.; Bakowsky, U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 74, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hinterdorfer, P.; Dufrêne, Y. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 2006, 3, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Coleman, V.A.; Gilliland, D. Analytical centrifugation. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–247. [Google Scholar]
- Kestens, V.; Coleman, V.; De Temmerman, P.-J.; Minelli, C.; Woehlecke, H.; Roebben, G. Improved Metrological Traceability of Particle Size Values Measured with Line-Start Incremental Centrifugal Liquid Sedimentation. Langmuir 2017, 33, 8213–8224. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Couteau, O.; Franks, K.; Kestens, V.; Roebben, G.; Lamberty, A.; Linsinger, T. Validation of dynamic light scattering and centrifugal liquid sedimentation methods for nanoparticle characterisation. Adv. Powder Technol. 2011, 22, 766–770. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 25. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: New York, NY, USA, 2011; pp. 63–70. [Google Scholar]
- Scholes, P.; Coombes, A.; Illum, L.; Davis, S.; Watts, J.; Ustariz, C.; Vert, M.; Davies, M. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J. Control. Release 1999, 59, 261–278. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning electron microscopy: Principle and applications in nanomaterials characterization. In Handbook of Materials Characterization; Sharma, S., Ed.; Springer: New York, NY, USA, 2018; pp. 113–145. [Google Scholar] [CrossRef]
- Bogner, A.; Thollet, G.; Basset, D.; Jouneau, P.-H.; Gauthier, C. Wet STEM: A new development in environmental SEM for imaging nano-objects included in a liquid phase. Ultramicroscopy 2005, 104, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, Y.; Zou, W.; Duan, J.; Chen, Y. Preparation and Characterization of Magnetic Chitosan Microcapsules. J. Chem. 2013, 2013, 585613. [Google Scholar] [CrossRef]
- Hoesli, C.A.; Kiang, R.L.J.; Mocinecová, D.; Speck, M.; Mošková, D.J.; Donald-Hague, C.; Lacík, I.; Kieffer, T.J.; Piret, J.M. Reversal of diabetes by βTC3 cells encapsulated in alginate beads generated by emulsion and internal gelation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1017–1028. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. The transmission electron microscope. In Transmission Electron Microscopy; Springer: Boston, MA, USA, 1996; pp. 3–17. [Google Scholar]
- Pascucci, L.; Scattini, G. Imaging extracelluar vesicles by transmission electron microscopy: Coping with technical hurdles and morphological interpretation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129648. [Google Scholar] [CrossRef]
- Chen, L.; Subirade, M. Chitosan/β-lactoglobulin core–shell nanoparticles as nutraceutical carriers. Biomaterials 2005, 26, 6041–6053. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Korin, E.; Froumin, N.; Cohen, S. Surface Analysis of Nanocomplexes by X-ray Photoelectron Spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Saupe, A.; Gordon, K.C.; Rades, T. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int. J. Pharm. 2006, 314, 56–62. [Google Scholar] [CrossRef]
- Pratiwi, D.; Fawcett, J.; Gordon, K.C.; Rades, T. Quantitative analysis of polymorphic mixtures of ranitidine hydrochloride by Raman spectroscopy and principal components analysis. Eur. J. Pharm. Biopharm. 2002, 54, 337–341. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Pispas, S.; Demetzos, C. Differential Scanning Calorimetry (DSC): An Invaluable Tool for the Thermal Evaluation of Advanced Chimeric Liposomal Drug Delivery Nanosystems. In Thermodynamics and Biophysics of Biomedical Nanosystems. Applications and Practical Considerations. Series: Series in BioEngineering; Demetzos, C., Pippa, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 297–337. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Theophile, T. Infrared Spectroscopy: Materials Science, Engineering and Technology; BoD–Books on Demand: Rijeka, Croatia, 2012. [Google Scholar]
- Sawyer, L.; Grubb, D.T.; Meyers, G.F. Polymer Microscopy; Springer Science & Business Media: New York, NY, USA, 2008. [Google Scholar]
- Fresta, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, K.; Cilurzo, F.; Cosco, D.; Iannone, M.; Paolino, D. Targeting of the Pilosebaceous Follicle by Liquid Crystal Nanocarriers: In Vitro and In Vivo Effects of the Entrapped Minoxidil. Pharmaceutics 2020, 12, 1127. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Pandey, M.M.; Singhvi, G. UV Spectrophotometric method for characterization of curcumin loaded nanostructured lipid nanocarriers in simulated conditions: Method development, in-vitro and ex-vivo applications in topical delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117392. [Google Scholar] [CrossRef]
- Zhang, Z.; Stenson, J.D.; Thomas, C.R. Micromanipulation in Mechanical Characterization of Single Particles in Characterization of Flow, Particles and Interfaces; Academic Press: San Diego, CA USA, 2009; pp. 29–85. [Google Scholar]
- Fischer-Cripps, A.C. Applications of Nanoindentation; Springer: New York, NY, USA, 2011; pp. 213–233. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Sheshala, R.; Anuar, N.K.; Abu Samah, N.H.; Wong, T.W. In Vitro Drug Dissolution/Permeation Testing of Nanocarriers for Skin Application: A Comprehensive Review. AAPS PharmSciTech 2019, 20, 164. [Google Scholar] [CrossRef]
- Nothnagel, L.; Wacker, M.G. How to measure release from nanosized carriers? Eur. J. Pharm. Sci. 2018, 120, 199–211. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouni, N.S.; Farid, R.M.; Elkamel, A.; El-Gamal, S.S. Nanostructured lipid carriers for intraocular brimonidine localisation: Development, in-vitro and in-vivo evaluation. J. Microencapsul. 2018, 35, 102–113. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouni, N.S.; Gowayed, M.A.; Seiffein, N.L.; Moneim, R.A.A.; Kamel, M.A.; Labib, G.S. Valsartan solid lipid nanoparticles integrated hydrogel: A challenging repurposed use in the treatment of diabetic foot ulcer, in-vitro/in-vivo experimental study. Int. J. Pharm. 2021, 592, 120091. [Google Scholar] [CrossRef] [PubMed]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic potential of L-carnosine from stimuli-responsive magnetic nanoparticles against breast cancer model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.; Labib, G.; Elkamel, A. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015, 10, 893–902. [Google Scholar] [CrossRef]
- Morais, J.M.; Burgess, D.J. In vitro release testing methods for vitamin E nanoemulsions. Int. J. Pharm. 2014, 475, 393–400. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Y.; Gao, Y.; Ma, H.; Xu, H.; Zhang, X.; Pan, W. Preparation and characterization of 5-fluorouracil pH-sensitive niosome and its tumor-targeted evaluation: In vitro and in vivo. Drug Dev. Ind. Pharm. 2012, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgess, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef]
- Kilfoyle, B.E.; Sheihet, L.; Zhang, Z.; Laohooa, M.; Kohn, J.; Michniak-Kohna, B.B. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Control. Release 2012, 163, 18–24. [Google Scholar] [CrossRef]
- Uprit, S.; Sahu, R.K.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.; Neumann, D.; Lamprecht, A. In vitro drug release mechanism from lipid nanocapsules (LNC). Int. J. Pharm. 2010, 390, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Shono, Y.; Jantratid, E.; Kesisoglou, F.; Reppas, C.; Dressman, J.B. Forecasting in vivo oral absorption and food effect of micronized and nanosized aprepitant formulations in humans. Eur. J. Pharm. Biopharm. 2010, 76, 95–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, C.; Sun, B.; Wang, Y.; Wang, S.; Gao, C. A Novel Three-Dimensional Large-Pore Mesoporous Carbon Matrix as a Potential Nanovehicle for the Fast Release of the Poorly Water-soluble Drug, Celecoxib. Pharm. Res. 2014, 31, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Farid, R.M.; El Gamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef]
- Wallace, S.J.; Li, J.; Nation, R.; Boyd, B.J. Drug release from nanomedicines: Selection of appropriate encapsulation and release methodology. Drug Deliv. Transl. Res. 2012, 2, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Pedreschi, E.; Di Colo, G. Is dialysis a reliable method for studying drug release from nanoparticulate systems? A case study. Int. J. Pharm. 2012, 434, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sievens-Figueroa, L.; Pandya, N.; Bhakay, A.; Keyvan, G.; Michniak-Kohn, B.; Bilgili, E.; Davé, R.N. Using USP I and USP IV for Discriminating Dissolution Rates of Nano- and Microparticle-Loaded Pharmaceutical Strip-Films. AAPS Pharmscitech 2012, 13, 1473–1482. [Google Scholar] [CrossRef]
- Rudd, N.D.; Reibarkh, M.; Fang, R.; Mittal, S.; Walsh, P.L.; Brunskill, A.P.J.; Forrest, W.P. Interpreting In Vitro Release Performance from Long-Acting Parenteral Nanosuspensions Using USP-4 Dissolution and Spectroscopic Techniques. Mol. Pharm. 2020, 17, 1734–1747. [Google Scholar] [CrossRef]
- Paaver, U.; Heinämäki, J.; Kassamakov, I.; Ylitalo, T.; Hæggström, E.; Laidmäe, I.; Kogermann, K. Quasi-Dynamic Dissolution of Electrospun Polymeric Nanofibers Loaded with Piroxicam. Pharmaceutics 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Hitzman, C.J.; Elmquist, W.F.; Wattenberg, L.W.; Wiedmann, T.S. Development of a respirable, sustained release microcarrier for 5-Fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J. Pharm. Sci. 2006, 95, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-P.; Zong, R.-F.; Li, L.; Fu, T.; Liu, F.; Yu, X.-H. Anticancer Drug-Loaded Nanospheres Based on Biodegradable Amphiphilic ε-Caprolactone and Carbonate Copolymers. Pharm. Res. 2010, 27, 2743–2752. [Google Scholar] [CrossRef]
- Kumar, R.; Nagarwal, R.C.; Dhanawat, M.; Pandit, J.K. In-vitro and in-vivo study of indomethacin loaded gelatin nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 325–333. [Google Scholar] [CrossRef]
- Modi, S.; Anderson, B.D. Determination of Drug Release Kinetics from Nanoparticles: Overcoming Pitfalls of the Dynamic Dialysis Method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bautista, G.; Tam, K.C. Evaluation of dialysis membrane process for quantifying the in vitro drug-release from colloidal drug carriers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 299–303. [Google Scholar] [CrossRef]
- Juenemann, D.; Jantratid, E.; Wagner, C.; Reppas, C.; Vertzoni, M.; Dressman, J.B. Biorelevant in vitro dissolution testing of products containing micronized or nanosized fenofibrate with a view to predicting plasma profiles. Eur. J. Pharm. Biopharm. 2010, 77, 257–264. [Google Scholar] [CrossRef]
- Yue, P.-F.; Lu, X.-Y.; Zhang, Z.-Z.; Yuan, H.-L.; Zhu, W.-F.; Zheng, Q.; Yang, M. The Study on the Entrapment Efficiency and In Vitro Release of Puerarin Submicron Emulsion. AAPS PharmSciTech 2009, 10, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Sebak, S.; Mirzaei, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525. [Google Scholar] [CrossRef]
- Heng, D.; Cutler, D.J.; Chan, H.; Yun, J.; Judy, A. Raper What is a suitable dissolution method for drug nanoparticles? Pharm. Res. 2008, 25, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; De Wulf, O.; Laru, J.; Heikkilä, T.; Van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Peltonen, L.; Laaksonen, T. Dissolution Studies of Poorly Soluble Drug Nanosuspensions in Non-sink Conditions. AAPS PharmSciTech 2013, 14, 748–756. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Burgess, D.J. A novel USP apparatus 4 based release testing method for dispersed systems. Int. J. Pharm. 2010, 388, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.P.; Goh, C.H.; Tam, K.C. Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur. J. Pharm. Sci. 2007, 32, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Chumbimuni-Torres, K.Y.; Clawson, C.; Hernandez, L.; Zhang, L.; Wang, J. Real-time electrochemical monitoring of drug release from therapeutic nanoparticles. J. Control. Release 2009, 140, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kayaert, P.; Li, B.; Jimidar, I.; Rombaut, P.; Ahssini, F.; Mooter, G.V.D. Solution calorimetry as an alternative approach for dissolution testing of nanosuspensions. Eur. J. Pharm. Biopharm. 2010, 76, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.T.; Tucker, C.J.; Rogers, T.L.; Williams, R.O.; Johnston, K.P. Turbidimetric measurement and prediction of dissolution rates of poorly soluble drug nanocrystals. J. Control. Release 2007, 117, 351–359. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Michalowski, C.; Guterres, S.; Costa, T.D. Microdialysis for evaluating the entrapment and release of a lipophilic drug from nanoparticles. J. Pharm. Biomed. Anal. 2004, 35, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Beyer, S.; Vogel, V.; Wacker, M.G.; Mäntele, W. Assessing the drug release from nanoparticles: Overcoming the shortcomings of dialysis by using novel optical techniques and a mathematical model. Int. J. Pharm. 2015, 488, 108–119. [Google Scholar] [CrossRef]
- Janas, C.; Mast, M.-P.; Kirsamer, L.; Angioni, C.; Gao, F.; Mäntele, W.; Dressman, J.; Wacker, M.G. The dispersion releaser technology is an effective method for testing drug release from nanosized drug carriers. Eur. J. Pharm. Biopharm. 2017, 115, 73–83. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Ignoring the modeling approaches: Towards the shadowy paths in nanomedicine. J. Control. Release 2018, 280, 58–75. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling Drug-Carrier Interaction in the Drug Release from Nanocarriers. J. Drug Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pandya, K.; Aggarwal, D. Establishing Prospective IVIVC for Generic Pharmaceuticals: Methodologies Assessment. Open Drug Deliv. J. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Baconnier, S.; Bastogne, T.; Bazile, D.; Boisseau, P.; Borchard, G.; Borgos, S.E.; Calzolai, L.; Cederbrant, K.; Di Felice, G.; et al. Bridging communities in the field of nanomedicine. Regul. Toxicol. Pharmacol. 2019, 106, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Deng, W.; Fu, M.; Wang, L.; Tong, S.; Wei, Y.; Xu, Y.; Su, W.; Xu, X.; Yu, J. In vitro release and in vitro–in vivo correlation for silybin meglumine incorporated into hollow-type mesoporous silica nanoparticles. Int. J. Nanomed. 2012, 7, 753. [Google Scholar] [CrossRef]
- Singh, G.; Pai, R.S. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J. Pharm. Pharmacol. 2014, 66, 1062–1076. [Google Scholar] [CrossRef]
- Hernández-Caselles, T.; Villalaín, J.; Gómez-Fernández, J.C. Stability of Liposomes on Long Term Storage. J. Pharm. Pharmacol. 1990, 42, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Shimizu, T.; Ishima, Y.; Ishida, T. Long-term storage of PEGylated liposomal oxaliplatin with improved stability and long circulation times in vivo. Int. J. Pharm. 2019, 564, 237–243. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Bummer, P.M. Simple Chromatographic Method for Simultaneous Analyses of Phosphatidylcholine, Lysophosphatidylcholine, and Free Fatty Acids. AAPS PharmSciTech 2010, 11, 1084–1091. [Google Scholar] [CrossRef][Green Version]
- Shibata, H.; Yomota, C.; Okuda, H. Simultaneous Determination of Polyethylene Glycol-Conjugated Liposome Components by Using Reversed-Phase High-Performance Liquid Chromatography with UV and Evaporative Light Scattering Detection. AAPS PharmSciTech 2013, 14, 811–817. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef]
- Filippov, A.; Tarabukina, E.; Simonova, M.; Kirila, T.; Fundueanu, G.; Harabagiu, V.; Constantin, M.; Popescu, I. Synthesis and Investigation of Double Stimuli-Responsive Behavior ofN-Isopropylacrylamide and Maleic Acid Copolymer in Solutions. J. Macromol. Sci. Part B 2015, 54, 1105–1121. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kwon, I.C.; Bae, Y.H.; Kim, S.W. Saccharide Effect on the Lower Critical Solution Temperature of Thermosensitive Polymers. Macromolecules 1995, 28, 939–944. [Google Scholar] [CrossRef]
- Na, K.; Bae, Y.H. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: Characterization, aggregation, and adriamycin release in vitro. Pharm. Res. 2002, 19, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; McClements, D.J. Overview of Nanoemulsion Properties: Stability, Rheology, and Appearance. Nanoemulsions 2018, 21–49. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2009, 7, 49–62. [Google Scholar] [CrossRef]
- Miller, T.; Rachel, R.; Besheer, A.; Uezguen, S.; Weigandt, M.; Goepferich, A. Comparative Investigations on In Vitro Serum Stability of Polymeric Micelle Formulations. Pharm. Res. 2012, 29, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Hühmer, A.F.; Biringer, R.G.; Amato, H.; Fonteh, A.N.; Harrington, M. Protein Analysis in Human Cerebrospinal Fluid: Physiological Aspects, Current Progress and Future Challenges. Dis. Markers 2006, 22, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Hadjistilianou, T.; Giglioni, S.; Micheli, L.; Vannoni, D.; Brogi, E.; Cevenini, G.; Cortelazzo, A.; De Francesco, S.; Menicacci, F.; Leoncini, R. Analysis of aqueous humour proteins in patients with retinoblastoma. Clin. Exp. Ophthalmol. 2012, 40, e8–e15. [Google Scholar] [CrossRef]
- Angi, M.; Kalirai, H.; Coupland, S.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, 148039. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yonezawa, T.; Matsuki, N. Synovial fluid total protein concentration as a possible marker for canine idiopathic polyarthritis. J. Vet. Med. Sci. 2015, 77, 1715–1717. [Google Scholar] [CrossRef]
- Gobezie, R.; Kho, A.; Krastins, B.; Sarracino, D.A.; Thornhill, T.S.; Chase, M.; Millett, P.J.; Lee, D.M. High abundance synovial fluid proteome: Distinct profiles in health and osteoarthritis. Arthritis Res. Ther. 2007, 9, R36. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Gaucher, G.; Marchessault, R.H.; Leroux, J.-C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release 2010, 143, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrion, C.; Carril, M.; Parak, W.J. Techniques for the experimental investigation of the protein corona. Curr. Opin. Biotechnol. 2017, 46, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kawano, K.; Yokoyama, M.; Opanasopit, P.; Okano, T.; Maitani, Y. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int. J. Pharm. 2006, 308, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, B.; Tao, J.; Hec, Y.; Dengb, H.; Wangb, X.; Zhengc, Y. Application of Förster Resonance Energy Transfer (FRET) technique to elucidate intracellular and In Vivo biofate of nanomedicines. Adv. Drug Deliv. Rev. 2019, 143, 177–205. [Google Scholar] [CrossRef]
- Lu, J.; Owen, S.C.; Shoichet, M.S. Stability of Self-Assembled Polymeric Micelles in Serum. Macromolecules 2011, 44, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Basalious, E.B.; Shamma, R.N. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: In vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int. J. Pharm. 2015, 493, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Saifi, Z.; Rizwanullah; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Chavda, K.; Vyas, B.; Patel, S. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: Role of P-gp inhibition. Drug Deliv. Transl. Res. 2020, 11, 1166–1185. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.E.-S.; Sokar, M.S.; Abdelmonsif, D.A.; El-Kamel, A.H. Mucopenetrating nanoparticles for enhancement of oral bioavailability of furosemide: In vitro and in vivo evaluation/sub-acute toxicity study. Int. J. Pharm. 2017, 526, 366–379. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Yang, W.; Niu, P.; Li, X.; Chen, Y.; Li, Z.; Liu, Y.; An, Y.; Liu, Y.; et al. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35, 100970. [Google Scholar] [CrossRef]
- Trousil, J.; Pavliš, O.; Kubíčková, P.; Škorič, M.; Marešová, V.; Pavlova, E.; Knudsen, K.D.; Dai, Y.-S.; Zimmerman, M.; Dartois, V.; et al. Antitubercular nanocarrier monotherapy: Study of In Vivo efficacy and pharmacokinetics for rifampicin. J. Control. Release 2020, 321, 312–323. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M. Comparative evaluation of intranasally delivered quetiapine loaded mucoadhesive microemulsion and polymeric nanoparticles for brain targeting: Pharmacokinetic and gamma scintigraphy studies. Futur. J. Pharm. Sci. 2021, 7, 6. [Google Scholar] [CrossRef]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and In Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Hou, R.; Zhang, J.; Wang, P.; Liu, X.; Zhang, Z. Bubble-generating nano-lipid carriers for ultrasound/CT imaging-guided efficient tumor therapy. Int. J. Pharm. 2017, 534, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Inchaurraga, L.; Martínez-López, A.L.; Abdulkarim, M.; Gumbleton, M.; Quincoces, G.; Peñuelas, I.; Martin-Arbella, N.; Irache, J.M. Modulation of the fate of zein nanoparticles by their coating with a Gantrez® AN-thiamine polymer conjugate. Int. J. Pharm. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Zhao, D.; Fang, C.; He, D.; Yang, Q.; Yang, L.; Chen, R.; Tan, Q.; Zhang, J. Oral administration of natural polyphenol-loaded natural polysaccharide-cloaked lipidic nanocarriers to improve efficacy against small-cell lung cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102261. [Google Scholar] [CrossRef]
- Hu, X.; Fan, W.; Yu, Z.; Lu, Y.; Qi, J.; Zhang, J.; Dong, X.; Zhao, W.; Wu, W. Evidence does not support absorption of intact solid lipid nanoparticles via oral delivery. Nanoscale 2015, 8, 7024–7035. [Google Scholar] [CrossRef]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Salman, A.; Alahmad, A. In-vitro anticancer activity against Caco-2 cell line of colloidal nano silver synthesized using aqueous extract of Eucalyptus Camaldulensis leaves. Heliyon 2020, 6, e04594. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; He, L.; You, Y.; Mo, J.; Chen, T. Controlled synthesis and size effects of multifunctional mesoporous silica nanosystem for precise cancer therapy. Drug Deliv. 2018, 25, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Vajedi, F.S.; Dehghani, H.; Zarrabi, A. Design and characterization of a novel pH-sensitive biocompatible and multifunctional nanocarrier for in vitro paclitaxel release. Mater. Sci. Eng. C 2021, 119, 111627. [Google Scholar] [CrossRef] [PubMed]
- Minuesa, G.; Huber-Ruano, I.; Pastor-Anglada, M.; Koepsell, H.; Clotet, B.; Martinez-Picado, J. Drug uptake transporters in antiretroviral therapy. Pharmacol. Ther. 2011, 132, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Volpe, D.A. Application of Method Suitability for Drug Permeability Classification. AAPS J. 2010, 12, 670–678. [Google Scholar] [CrossRef]
- Dixit, P.; Jain, D.K.; Dumbwani, J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J. Pharmacol. Toxicol. Methods 2012, 65, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.-P.; Chen, S.; Yu, B.-Y.; Zhu, D.-N.; Cordell, G.; Qiu, S. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur. J. Med. Chem. 2006, 41, 605–610. [Google Scholar] [CrossRef]
- Musther, H.; Olivares-Morales, A.; Hatley, O.J.D.; Liua, B.; Hodjegana, A.R. Animal versus human oral drug bioavailability: Do they correlate? Eur. J. Pharm. Sci. 2014, 57, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Yoon, I.-S.; Cho, H.-J.; Kim, D.-H.; Choi, Y.-H.; Kim, D.-D. Emulsion-based colloidal nanosystems for oral delivery of doxorubicin: Improved intestinal paracellular absorption and alleviated cardiotoxicity. Int. J. Pharm. 2014, 464, 117–126. [Google Scholar] [CrossRef] [PubMed]
- El-Ghareb, W.I.; Swidana, M.M.; Ibrahim, I.T.; El-Bary, A.A.; Tadros, M.I.; Sakrde, T.M. 99mTc-Doxorubicin-loaded gallic acid-gold nanoparticles (99mTc-DOX-loaded GA-Au NPs) as a multifunctional theranostic agent. Int. J. Pharm. 2020, 586, 119514. [Google Scholar] [CrossRef] [PubMed]
- Javed, I.; Hussaina, S.Z.; Shahzad, A.; Khan, J.M.; ur-Rehmana, H.; Rehman, M.; Usman, F.; Razi, M.T.; Shah, M.R.; Hussain, I. Lecithin-gold hybrid nanocarriers as efficient and pH selective vehicles for oral delivery of diacerein—In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Duarte, Í.; Corvo, M.L.; Serôdio, P.; Vicente, J.; Pinto, J.; Temtem, M. Production of nano-solid dispersions using a novel solvent-controlled precipitation process–Benchmarking their in vivo performance with an amorphous micro-sized solid dispersion produced by spray drying. Eur. J. Pharm. Sci. 2016, 93, 203–214. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Newman, S.; Wilding, I. Gamma scintigraphy: An in vivo technique for assessing the equivalence of inhaled products. Int. J. Pharm. 1998, 170, 1–9. [Google Scholar] [CrossRef]
- Stappaerts, J.; Brouwers, J.; Annaert, P.; Augustijns, P. In situ perfusion in rodents to explore intestinal drug absorption: Challenges and opportunities. Int. J. Pharm. 2014, 478, 665–681. [Google Scholar] [CrossRef]
- Singh, B.; Singh, R.; Bandopadhyay, S.; Kapil, R.; Garg, B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf. B Biointerfaces 2013, 101, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Caldeira, T.G.; Ruiz-Picazo, A.; Lozoya-Agullo, I.; Saúde-Guimarães, D.A.; González-Álvarez, M.; de Souza, J.; González-Álvarez, I.; Bermejo, M. Determination of intestinal permeability using in situ perfusion model in rats: Challenges and advantages to BCS classification applied to digoxin. Int. J. Pharm. 2018, 551, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Fischer, S.M.; Fricker, G.; Brandl, M. In vitro models to evaluate the permeability of poorly soluble drug entities: Challenges and perspectives. Eur. J. Pharm. Sci. 2012, 45, 235–250. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S. Cell culture-based models for intestinal permeability: A critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- A Volpe, D. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Futur. Med. Chem. 2011, 3, 2063–2077. [Google Scholar] [CrossRef]
- Sevin, E.; Dehouck, L.; Fabulas-da Costa, A.; Cecchelli, R.; Dehouck, M.P.; Lundquist, S.; Culot, M. Accelerated Caco-2 cell permeability model for drug discovery. J. Pharmacol. Toxicol. Methods 2013, 68, 334–339. [Google Scholar] [CrossRef]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Walker., J.M. Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: New York, NY, USA, 2011. [Google Scholar]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and Pharmacodynamic Features of Nanoemulsion Following Oral, Intravenous, Topical and Nasal Route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Zhang, Y.; Yi, S.; Du, F.; Song, K.-H.; Scott, E.A.; Sun, C.; Zhang, H.F. Super-Resolution Imaging of Self-Assembled Nanocarriers Using Quantitative Spectroscopic Analysis for Cluster Extraction. Langmuir 2020, 36, 2291–2299. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2018, 17, 849–865. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Landesman-Milo, D.; Peer, D. Altering the immune response with lipid-based nanoparticles. J. Control. Release 2012, 161, 600–608. [Google Scholar] [CrossRef]
- Roursgaard, M.; Knudsen, K.B.; Northeved, H.; Persson, M.; Christensen, T.; Kumar, P.E.; Permin, A.; Andresen, T.L.; Gjetting, T.; Lykkesfeldt, J.; et al. In vitro toxicity of cationic micelles and liposomes in cultured human hepatocyte (HepG2) and lung epithelial (A549) cell lines. Toxicol. Vitr. 2016, 36, 164–171. [Google Scholar] [CrossRef]
- Gupta, R.; Shea, J.; Scaife, C.; Shurlygina, A.; Rapoport, N. Polymeric micelles and nanoemulsions as drug carriers: Therapeutic efficacy, toxicity, and drug resistance. J. Control. Release 2015, 212, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, M.; Dudek, M.; Knutelska, J.; Nowiński, L.; Sapa, J.; Zygmunt, M.; Nowak, G.; Luty-Błocho, M.; Wojnicki, M.; Fitzner, K.; et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: In vivo studies. Pharmacol. Rep. 2014, 67, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ciappellano, S.G.; Tedesco, E.; Venturini, M.; Benetti, F. In vitro toxicity assessment of oral nanocarriers. Adv. Drug Deliv. Rev. 2016, 106, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of nanomaterials: Exposure, pathways, assessment, and recent advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of Nanomaterial Dispersion in Solution Prior to In Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci. 2007, 101, 239–253. [Google Scholar] [CrossRef]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Caster, J.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed. Nanobiotechnology 2016, 9, e1416. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: Recent developments and challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Jansson, M.; Boholm, Å. Expert stakeholders’ perception of nanotechnology: Risk, benefit, knowledge, and regulation. J. Nanoparticle Res. 2019, 21, 1–17. [Google Scholar] [CrossRef]
- Wang, W.; Ye, Z.; Gao, H.; Ouyang, D. Computational pharmaceutics—A new paradigm of drug delivery. J. Control. Release 2021, 338, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xiong, H.; Ye, Z.; Yang, Y.; Huang, T.; Jing, Q.; Lu, J.; Pan, H.; Ren, F.; Ouyang, D. Predicting physical stability of solid dispersions by machine learning techniques. J. Control. Release 2019, 311–312, 16–25. [Google Scholar] [CrossRef]
- Thota, N.; Jiang, J. Computational Amphiphilic Materials for Drug Delivery. Front. Mater. 2015, 2, 64. [Google Scholar] [CrossRef]
- Huynh, L.; Neale, C.; Pomès, R.; Allen, C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 20–36. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, Z.; Liu, X.; Wei, Z.; Qiu, F.; Li, H.-F.; Zheng, Y.; Ouyang, D. Can machine learning predict drug nanocrystals? J. Control. Release 2020, 322, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.; Pieters, C.; Korach-Rechtman, H.; Shklover, J.; Schroeder, A. Robotics, microfluidics, nanotechnology and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Duarte, Y.; Márquez-Miranda, V.; Miossec, M.J.; González-Nilo, F. Integration of target discovery, drug discovery and drug delivery: A review on computational strategies. WIREs Nanomed. Nanobiotechnology 2019, 11, e1554. [Google Scholar] [CrossRef]
- Maas, J.; Kamm, W.; Hauck, G. An integrated early formulation strategy–from hit evaluation to preclinical candidate profiling. Eur. J. Pharm. Biopharm. 2007, 66, 1–10. [Google Scholar] [CrossRef]
- Gonzalez-Ibanez, A.M.; Nilo, F.D.G.; Cachau, R. The Collaboratory for Structural Nanobiology. Biophys. J. 2009, 96, 49a. [Google Scholar] [CrossRef]
- Mills, K.C.; Ostraat, M.L.; A Guzan, K.; Murry, D. The Nanomaterial Registry: Facilitating the sharing and analysis of data in the diverse nanomaterial community. Int. J. Nanomed. 2013, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C.; Hersam, M.C.; Mirkin, C.A. The Long View of Nanotechnology Development: The National Nanotechnology Initiative at 10 Years; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–28. [Google Scholar] [CrossRef]
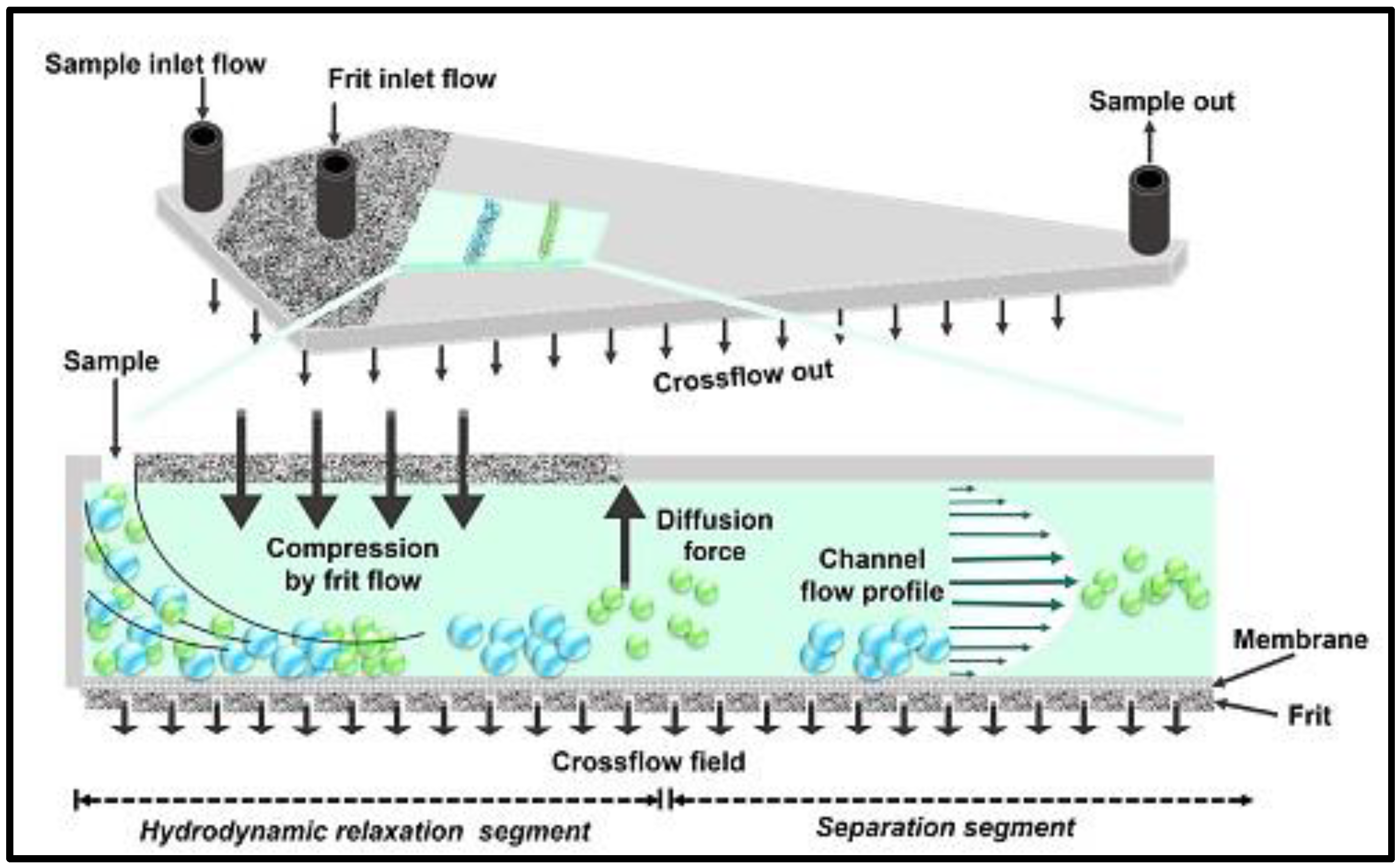
| In Vitro Release Model | Subtype Model | Nanocarriers System | Reference |
|---|---|---|---|
| Dialysis | Regular Dialysis | Solid Lipid Nanoparticles | [62,63] |
| Proniosomes | [64] | ||
| Magnetic Nanoparticles | [65] | ||
| Nanosponges | [66] | ||
| Reverse Dialysis | Nanoemulsion | [67] | |
| Niosomes | [68] | ||
| Liposomes | [69] | ||
| Side-by-Side Dialysis | Nanospheres | [70] | |
| Nanostructured Lipid Nanoparticles | [71] | ||
| Lipid Nanocapsules | [72] | ||
| Sample and Separation | Membrane Filters | Nanocrystals | [73] |
| Mesoporous Nanoparticles | [74] | ||
| Centrifugation | Chitosan Nanoparticles | [75] | |
| Ultracentrifugation | Liposomes | [76] | |
| Ultrafiltration | Chitosan Nanoparticles | [77] | |
| Liposomes | [76] | ||
| Continuous Flow | Nanoparticles Incorporated in Strip-Films | [78] | |
| Dynamic Dissolution Microdialysis | Nanosuspension | [79] | |
| Nanofibers | [80] | ||
| Nanoparticles | [81] |
| Permeability Assessment | Main Information | Nanocarrier Systems/Drugs (Technique or Part Used) | References |
|---|---|---|---|
| 1. Ex vivo models | Examples of organs used:
|
| [133] |
| [134] | ||
| [135] | ||
| [136] | ||
| [137] | ||
| 2. In vivo methods | Experimental animal models:
|
| [138] |
| [139] | ||
| [140] | ||
| [141] | ||
| [142] | ||
| 3. In situ organ perfusion models | Advantages:
|
| [135] |
| [143] | ||
| [144] | ||
| 4. Cell culture-based models | Examples: Cell line/origin:
|
| [145] |
| [146] | ||
| [65] | ||
| [147] | ||
| [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. https://doi.org/10.3390/pharmaceutics14040883
Alshawwa SZ, Kassem AA, Farid RM, Mostafa SK, Labib GS. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics. 2022; 14(4):883. https://doi.org/10.3390/pharmaceutics14040883
Chicago/Turabian StyleAlshawwa, Samar Zuhair, Abeer Ahmed Kassem, Ragwa Mohamed Farid, Shaimaa Khamis Mostafa, and Gihan Salah Labib. 2022. "Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence" Pharmaceutics 14, no. 4: 883. https://doi.org/10.3390/pharmaceutics14040883
APA StyleAlshawwa, S. Z., Kassem, A. A., Farid, R. M., Mostafa, S. K., & Labib, G. S. (2022). Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics, 14(4), 883. https://doi.org/10.3390/pharmaceutics14040883






