Neuroprotective Effect of Ramipril Is Mediated by AT2 in a Mouse MODEL of Paclitaxel-Induced Peripheral Neuropathy
Abstract
1. Introduction
2. Materials and Methods
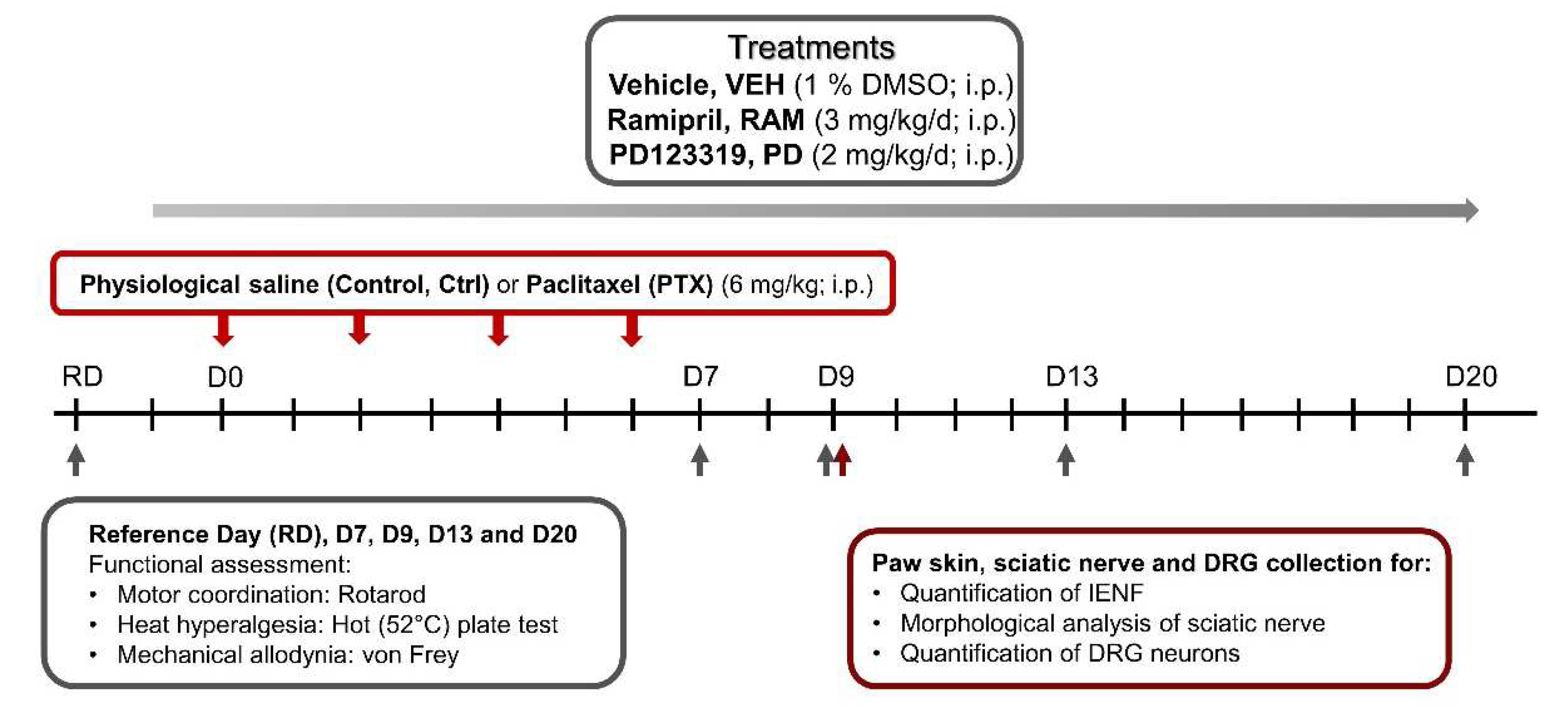
2.1. Treatments
2.2. Animals
- Ctrl-VEH
- Ctrl-RAM
- Ctrl-PD
- Ctrl-RAM+PD
- PTX-VEH
- PTX-RAM
- PTX-PD
- PTX-RAM+PD
2.3. Behavioral Tests
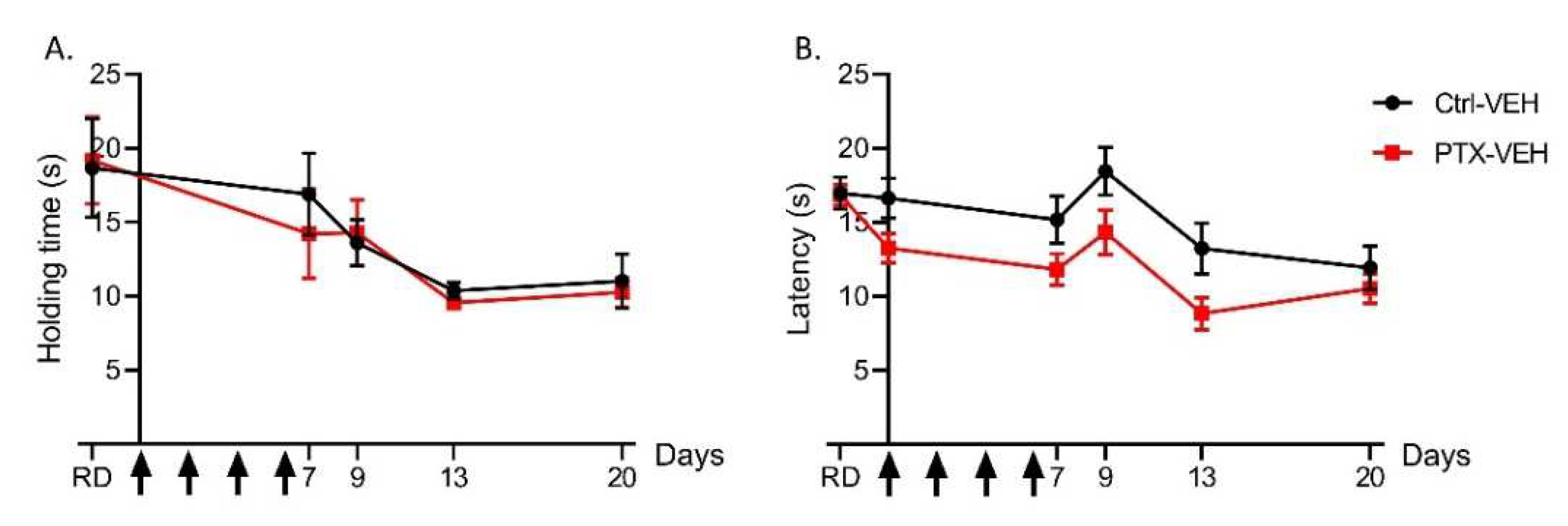
2.3.1. Motor Coordination
2.3.2. Heat Hyperalgesia
2.3.3. Tactile Allodynia
2.4. Immunohistochemistry of Footpad Skin and Dorsal Root Ganglia Neurons
2.5. Morphological Analysis of Sciatic Nerves
2.6. Data Analysis
3. Results
3.1. Characterization of Paclitaxel-Induced Peripheral Neuropathy
3.1.1. Paclitaxel Does Not Affect Motor Coordination and Heat Nociception
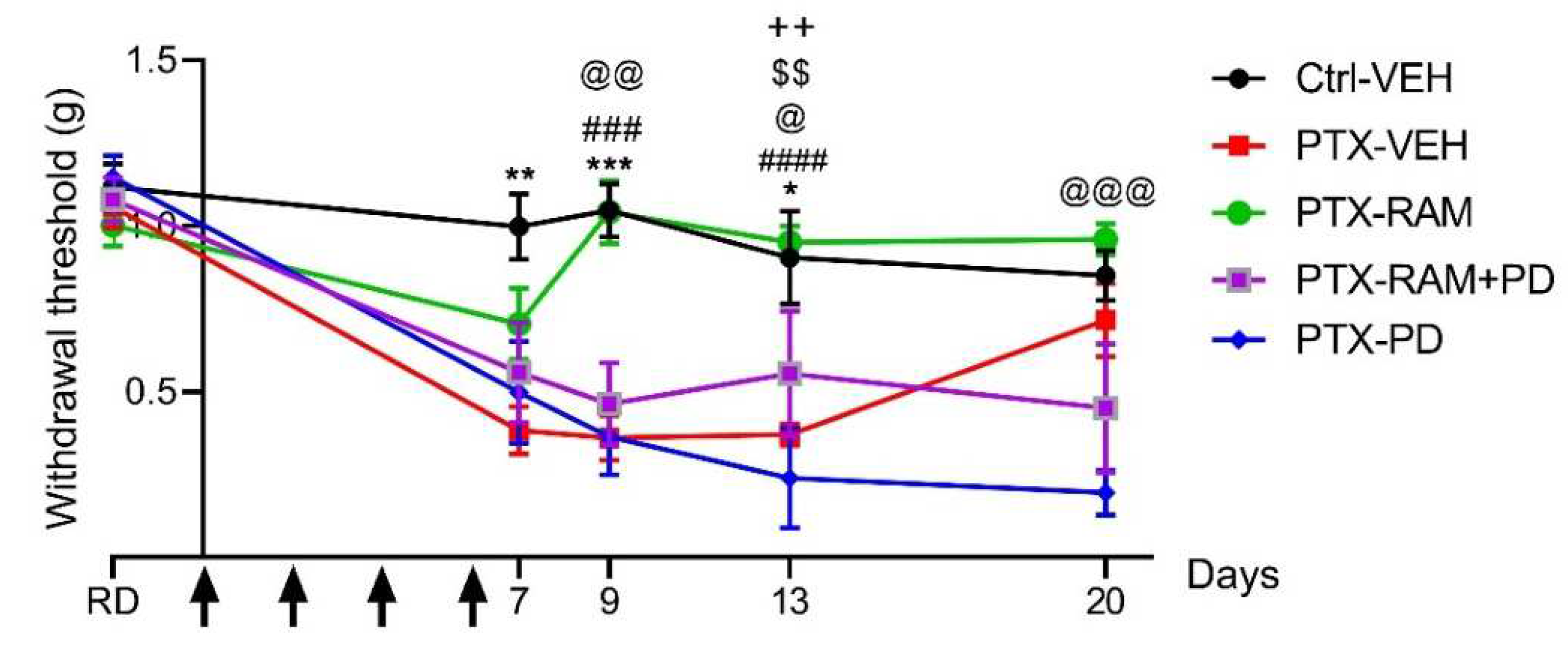
3.1.2. Paclitaxel Induces Tactile Allodynia
3.1.3. Paclitaxel Induces a Decrease in Sensory Neuron Terminals in the Epidermis, and a Decrease of Myelinated and Unmyelinated Nerve Fiber Densities in the Sciatic Nerve
3.2. Effect of Ramipril and Involvement of AT2 on PIPN
3.2.1. Ramipril Accelerates Recovery of Normal Mechanical Sensitivity in Wild Type Mice and this Effect Was Counteracted by Blockade of AT2
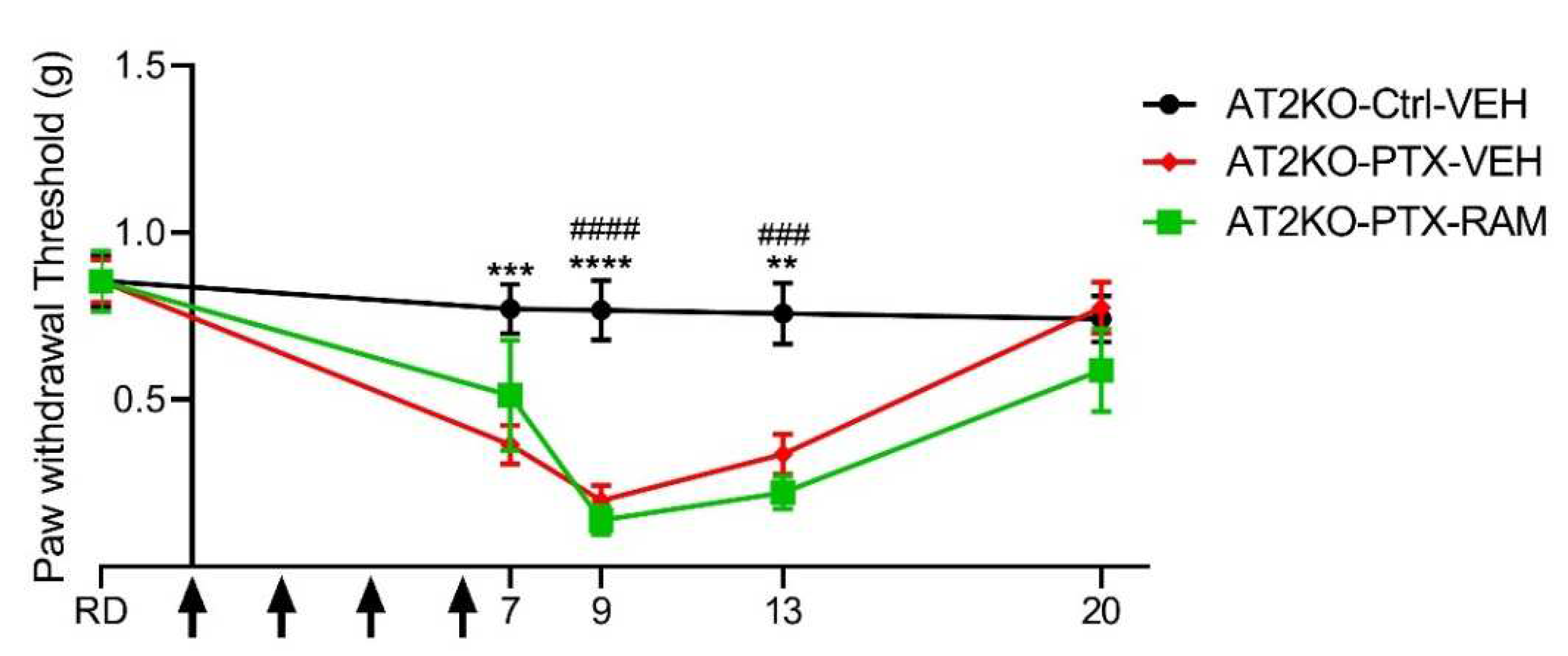
3.2.2. Ramipril Accelerates Recovery of Normal Mechanical Sensitivity in Wild Type but Not in AT2KO Mice
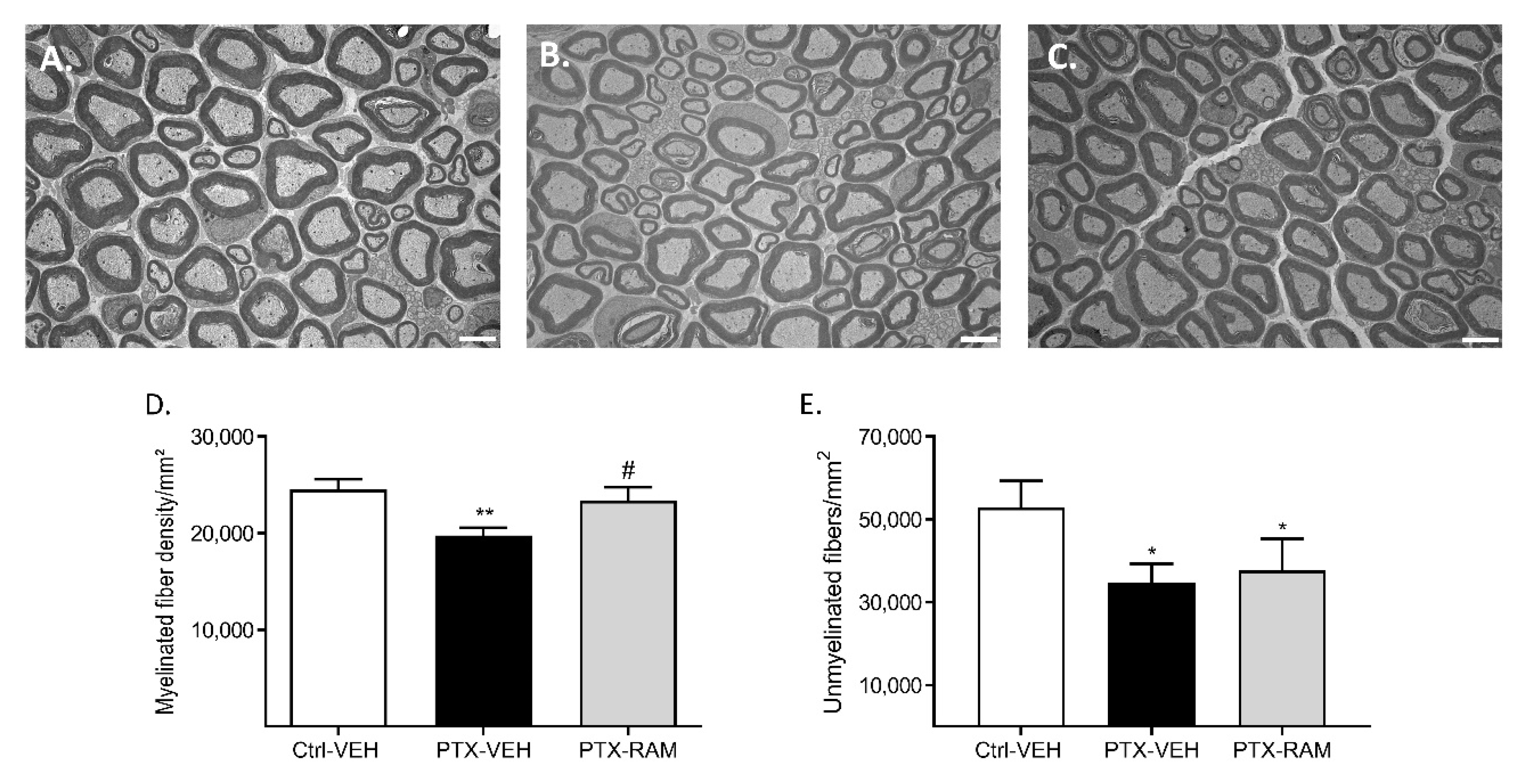
3.2.3. Ramipril Ameliorates Morphological Alterations Induced by PTX
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Scripture, C.D.; Figg, W.D.; Sparreboom, A. Peripheral neuropathy induced by paclitaxel: Recent insights and future perspectives. Curr. Neuropharmacol. 2006, 4, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Danigo, A.; Rovini, A.; Bessaguet, F.; Bouchenaki, H.; Bernard, A.; Sturtz, F.; Bourthoumieu, S.; Desmoulière, A.; Magy, L.; Demiot, C. The Angiotensin II Type 2 Receptor, a Target for Protection and Regeneration of the Peripheral Nervous System? Pharmaceuticals 2021, 14, 175. [Google Scholar] [CrossRef]
- Bessaguet, F.; Magy, L.; Desmoulière, A.; Demiot, C. The Therapeutic Potential of Renin Angiotensin Aldosterone System (RAAS) in Chronic Pain: From Preclinical Studies to Clinical Trials. Expert Rev. Neurother. 2016, 16, 331–339. [Google Scholar] [CrossRef]
- Kaur, P.; Muthuraman, A.; Kaur, J. Ameliorative potential of angiotensin-converting enzyme inhibitor (ramipril) on chronic constriction injury of sciatic nerve induced neuropathic pain in mice. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2015, 16, 103–112. [Google Scholar] [CrossRef]
- Yuksel, T.N.; Halici, Z.; Demir, R.; Cakir, M.; Calikoglu, C.; Ozdemir, G.; Unal, D. Investigation of the effect of telmisartan on experimentally induced peripheral nerve injury in rats. Int. J. Neurosci. 2015, 125, 464–473. [Google Scholar] [CrossRef]
- Hegazy, N.; Rezq, S.; Fahmy, A. Renin-angiotensin system blockade modulates both the peripheral and central components of neuropathic pain in rats: Role of calcitonin gene-related peptide, substance P and nitric oxide. Basic Clin. Pharm. Toxicol. 2020, 127, 451–460. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Holmes, A.; Yorek, M.A. Effect of inhibition of angiotensin converting enzyme and/or neutral endopeptidase on vascular and neural complications in high fat fed/low dose streptozotocin-diabetic rats. Eur. J. Pharmacol. 2012, 677, 180–187. [Google Scholar] [CrossRef][Green Version]
- Hashikawa-Hobara, N.; Hashikawa, N.; Inoue, Y.; Sanda, H.; Zamami, Y.; Takatori, S.; Kawasaki, H. Candesartan cilexetil improves angiotensin II type 2 receptor-mediated neurite outgrowth via the PI3K-Akt pathway in fructose-induced insulin-resistant rats. Diabetes 2012, 61, 925–932. [Google Scholar] [CrossRef]
- Alkhudhayri, S.; Sajini, R.; Alharbi, B.; Qabbani, J.; Al-Hindi, Y.; Fairaq, A.; Yousef, A. Investigating the beneficial effect of aliskiren in attenuating neuropathic pain in diabetic Sprague-Dawley rats. Endocrinol. Diabetes Metab. 2021, 4, e00209. [Google Scholar] [CrossRef] [PubMed]
- Bessaguet, F.; Danigo, A.; Magy, L.; Sturtz, F.; Desmoulière, A.; Demiot, C. Candesartan prevents resiniferatoxin-induced sensory small-fiber neuropathy in mice by promoting angiotensin II-mediated AT2 receptor stimulation. Neuropharmacology 2017, 126, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Bouchenaki, H.; Danigo, A.; Bernard, A.; Bessaguet, F.; Richard, L.; Sturtz, F.; Balayssac, D.; Magy, L.; Demiot, C. Ramipril Alleviates Oxaliplatin-Induced Acute Pain Syndrome in Mice. Front. Pharmacol. 2021, 12, 712442. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, S.-H.; Kim, H.-K.; Abdi, S.; Kim, H.K. Losartan, an Angiotensin II Type 1 Receptor Antagonist, Alleviates Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain by Inhibiting Inflammatory Cytokines in the Dorsal Root Ganglia. Mol. Neurobiol. 2019, 56, 7408–7419. [Google Scholar] [CrossRef]
- Bessaguet, F.; Danigo, A.; Bouchenaki, H.; Duchesne, M.; Magy, L.; Richard, L.; Sturtz, F.; Desmoulière, A.; Demiot, C. Neuroprotective effect of angiotensin II type 2 receptor stimulation in vincristine-induced mechanical allodynia. Pain 2018, 159, 2538–2546. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. Boutron I, editor. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Rosenberg, N.; Sommer, C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2005, 12, 747–758. [Google Scholar] [CrossRef]
- Smith, S.B.; Crager, S.E.; Mogil, J.S. Paclitaxel-induced neuropathic hypersensitivity in mice: Responses in 10 inbred mouse strains. Life Sci. 2004, 74, 2593–2604. [Google Scholar] [CrossRef]
- De Iuliis, F.; Taglieri, L.; Salerno, G.; Lanza, R.; Scarpa, S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit. Rev. Oncol. Hematol. 2015, 96, 34–45. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Bennett, G.J. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Polomano, R.C.; Mannes, A.J.; Clark, U.S.; Bennett, G.J. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 2001, 94, 293–304. [Google Scholar] [CrossRef]
- Ruiz-Medina, J.; Baulies, A.; Bura, S.A.; Valverde, O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur. J. Pain 2013, 17, 75–85. [Google Scholar] [CrossRef]
- Authier, N.; Fialip, J.; Eschalier, A.; Coudoré, F. Assessment of allodynia and hyperalgesia after cisplatin administration to rats. Neurosci. Lett. 2000, 291, 73–76. [Google Scholar] [CrossRef]
- Cavaletti, G.; Cavalletti, E.; Montaguti, P.; Oggioni, N.; De Negri, O.; Tredici, G. Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology 1997, 18, 137–145. [Google Scholar]
- Apfel, S.C.; Lipton, R.B.; Arezzo, J.C.; Kessler, J.A. Nerve growth factor prevents toxic neuropathy in mice. Ann. Neurol. 1991, 29, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Wiesen, M.H.J.; Albert, V.; Bobylev, I.; Joshi, A.R.; Müller, C.; Lehmann, H.C. Impact of drug formulations on kinetics and toxicity in a preclinical model of paclitaxel-induced neuropathy. J. Peripher. Nerv. Syst. 2021, 26, 216–226. [Google Scholar] [CrossRef]
- Bouchenaki, H.; Danigo, A.; Sturtz, F.; Hajj, R.; Magy, L.; Demiot, C. An overview of ongoing clinical trials assessing pharmacological therapeutic strategies to manage chemotherapy-induced peripheral neuropathy, based on preclinical studies in rodent models. Fundam. Clin. Pharmacol. 2021, 35, 506–523. [Google Scholar] [CrossRef]
- Dina, O.A.; Chen, X.; Reichling, D.; Levine, J.D. Role of protein kinase Cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience 2001, 108, 507–515. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Ikeda, K.; Fujita, M.; Sasaki, A.; Kato, A.; Kuraishi, Y. Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: Different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biol. Pharm. Bull. 2009, 32, 732–734. [Google Scholar] [CrossRef]
- Cavaletti, G.; Cavalletti, E.; Oggioni, N.; Sottani, C.; Minoia, C.; D’Incalci, M.; Zucchetti, M.; Marmiroli, P.; Tredici, G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 2000, 21, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, A.; Rammelkamp, Z.; Slusher, A.B.; Wozniak, K.; Slusher, B.S.; Farah, M.H. Paclitaxel causes degeneration of both central and peripheral axon branches of dorsal root ganglia in mice. BMC Neurosci. 2016, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Liljencrantz, J.; Björnsdotter, M.; Bergstrand, S.; Ceko, M.; Seminowicz, D.A.; Cole, J.; Bushnell, M.C.; Olausson, H. Altered C-tactile processing in human dynamic tactile allodynia. Pain 2013, 154, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Kim, Y.H.; Bang, S.; Zhang, Y.; Berta, T.; Wang, F.; Oh, S.B.; Ji, R.R. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 2015, 21, 1326–1331. [Google Scholar] [CrossRef]
- Hughes, D.I.; Todd, A.J. Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurotherapeutics 2020, 17, 874–885. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.; Smith, E.M.L.; Kaggal, S.; Novotny, P.; Ruddy, K.J.; Lafky, J.M.; Ta, L.E.; Beutler, A.S.; et al. Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 2016, 24, 5059–5068. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Reeves, B.N.; Dakhil, S.R.; Sloan, J.A.; Wolf, S.L.; Burger, K.N.; Kamal, A.; Le-Lindqwister, N.A.; Soori, G.S.; Jaslowski, A.J.; et al. Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. J. Clin. Oncol. 2011, 29, 1472–1478. [Google Scholar] [CrossRef]
- Klein, I.; Lehmann, H.C. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Siau, C.; Xiao, W.; Bennett, G.J. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: Loss of epidermal innervation and activation of Langerhans cells. Exp. Neurol. 2006, 201, 507–514. [Google Scholar] [CrossRef]
- Muthuraman, A.; Kaur, P. Renin-Angiotensin-Aldosterone System: A Current Drug Target for the Management of Neuropathic Pain. Curr. Drug Targets 2016, 17, 178–195. [Google Scholar] [CrossRef]
- Ismael, M.A.; Talbot, S.; Carbonneau, C.L.; Beauséjour, C.M.; Couture, R. Blockade of sensory abnormalities and kinin B1 receptor expression by N-Acetyl-l-Cysteine and ramipril in a rat model of insulin resistance. Eur. J. Pharmacol. 2008, 589, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Silva, C.R.; Trevisan, G.; de Campos Velho Gewehr, C.; Rigo, F.K.; La Rocca Tamiozzo, L.; Rossato, M.F.; Tonello, R.; Dalmolin, G.D.; de Almeida Cabrini, D.; et al. Potentiation of Paclitaxel-Induced Pain Syndrome in Mice by Angiotensin I Converting Enzyme Inhibition and Involvement of Kinins. Mol. Neurobiol. 2017, 54, 7824–7837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, S.; Wu, I.; Mata, M.; Fink, D.J. Activation of TLR-4 to produce tumour necrosis factor-α in neuropathic pain caused by paclitaxel. Eur. J. Pain 2015, 19, 889–898. [Google Scholar] [CrossRef]
- Sumners, C.; Fleegal, M.A.; Zhu, M. Angiotensin AT1 receptor signalling pathways in neurons. Clin. Exp. Pharmacol. Physiol. 2002, 29, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F.; Jankowski, J.; Jankowski, V.; Sousa, F.; Alzamora, A.; et al. Discovery and Characterization of Alamandine: A Novel Component of the Renin–Angiotensin System. Circ Res. 2013, 112, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kittana, N. Angiotensin-converting enzyme 2-Angiotensin 1-7/1-9 system: Novel promising targets for heart failure treatment. Fundam Clin. Pharmacol. 2018, 32, 14–25. [Google Scholar] [CrossRef]
- Ranjbar, R.; Shafiee, M.; Hesari, A.; Ferns, G.A.; Ghasemi, F.; Avan, A. The potential therapeutic use of renin–angiotensin system inhibitors in the treatment of inflammatory diseases. J. Cell Physiol. 2019, 234, 2277–2295. [Google Scholar] [CrossRef]
- Isaksson, R.; Casselbrant, A.; Elebring, E.; Hallberg, M.; Larhed, M.; Fändriks, L. Direct stimulation of angiotensin II type 2 receptor reduces nitric oxide production in lipopolysaccharide treated mouse macrophages. Eur. J. Pharmacol. 2020, 868, 172855. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Domoto, R.; Nakashima, K.; Yamasoba, D.; Yamanishi, H.; Tsubota, M.; Wake, H.; Nishibori, M.; Kawabata, A. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: Evidence for a neuroimmune crosstalk. Neuropharmacology 2018, 141, 201–213. [Google Scholar] [CrossRef]
- Smith, M.T.; Anand, P.; Rice, A.S.C. Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: Preclinical and clinical studies. Pain 2016, 157, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Mohapatra, D.P. Attenuation of Unevoked Mechanical and Cold Pain Hypersensitivities Associated with Experimental Neuropathy in Mice by Angiotensin II Type-2 Receptor Antagonism. Anesth. Analg. 2019, 128, e84. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Facer, P.; Yiangou, Y.; Sinisi, M.; Fox, M.; McCarthy, T.; Bountra, C.; Korchev, Y.; Anand, P. Angiotensin II type 2 receptor (AT2R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur. J. Pain 2013, 17, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Zanata, G.C.; Pinto, L.G.; da Silva, N.R.; Lopes, A.H.; de Oliveira, F.F.; Schivo, I.R.; Cunha, F.Q.; McNaughton, P.; Cunha, T.M.; Silva, R.L. Blockade of Bradykinin Receptors or Angiotensin II Type 2 Receptor Prevents Paclitaxel-Associated Acute Pain Syndrome in Mice. Eur. J. Pain 2020, 23, 1660. [Google Scholar] [CrossRef]
- Uchida, M.; Kawazoe, H.; Takatori, S.; Namba, H.; Uozumi, R.; Tanaka, A.; Kawasaki, H.; Araki, H. Preventive Effects of Renin-angiotensin System Inhibitors on Oxaliplatin-induced Peripheral Neuropathy: A Retrospective Observational Study. Clin. Ther. 2018, 40, 1214–1222.e1. [Google Scholar] [CrossRef]
- Roldan, C.J.; Song, J.; Engle, M.P.; Dougherty, P.M. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Modulate the Function of Myelinated Fibers after Chemotherapy: A Quantitative Sensory Testing Study. Pain Physician 2017, 20, 281–292. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchenaki, H.; Bernard, A.; Bessaguet, F.; Frachet, S.; Richard, L.; Sturtz, F.; Magy, L.; Bourthoumieu, S.; Demiot, C.; Danigo, A. Neuroprotective Effect of Ramipril Is Mediated by AT2 in a Mouse MODEL of Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics 2022, 14, 848. https://doi.org/10.3390/pharmaceutics14040848
Bouchenaki H, Bernard A, Bessaguet F, Frachet S, Richard L, Sturtz F, Magy L, Bourthoumieu S, Demiot C, Danigo A. Neuroprotective Effect of Ramipril Is Mediated by AT2 in a Mouse MODEL of Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics. 2022; 14(4):848. https://doi.org/10.3390/pharmaceutics14040848
Chicago/Turabian StyleBouchenaki, Hichem, Amandine Bernard, Flavien Bessaguet, Simon Frachet, Laurence Richard, Franck Sturtz, Laurent Magy, Sylvie Bourthoumieu, Claire Demiot, and Aurore Danigo. 2022. "Neuroprotective Effect of Ramipril Is Mediated by AT2 in a Mouse MODEL of Paclitaxel-Induced Peripheral Neuropathy" Pharmaceutics 14, no. 4: 848. https://doi.org/10.3390/pharmaceutics14040848
APA StyleBouchenaki, H., Bernard, A., Bessaguet, F., Frachet, S., Richard, L., Sturtz, F., Magy, L., Bourthoumieu, S., Demiot, C., & Danigo, A. (2022). Neuroprotective Effect of Ramipril Is Mediated by AT2 in a Mouse MODEL of Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics, 14(4), 848. https://doi.org/10.3390/pharmaceutics14040848







