Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Formulation
- Evaluation of PEGDA/PEG/water ratio: different amounts of PEGDA MW 575, PEG 400, and water with 0.1% addition of riboflavin were tested in PVC molds to optimize photosensitive resin curable with visible light. The compositions of the tested formulations were selected based on the full factorial design of the experiment.
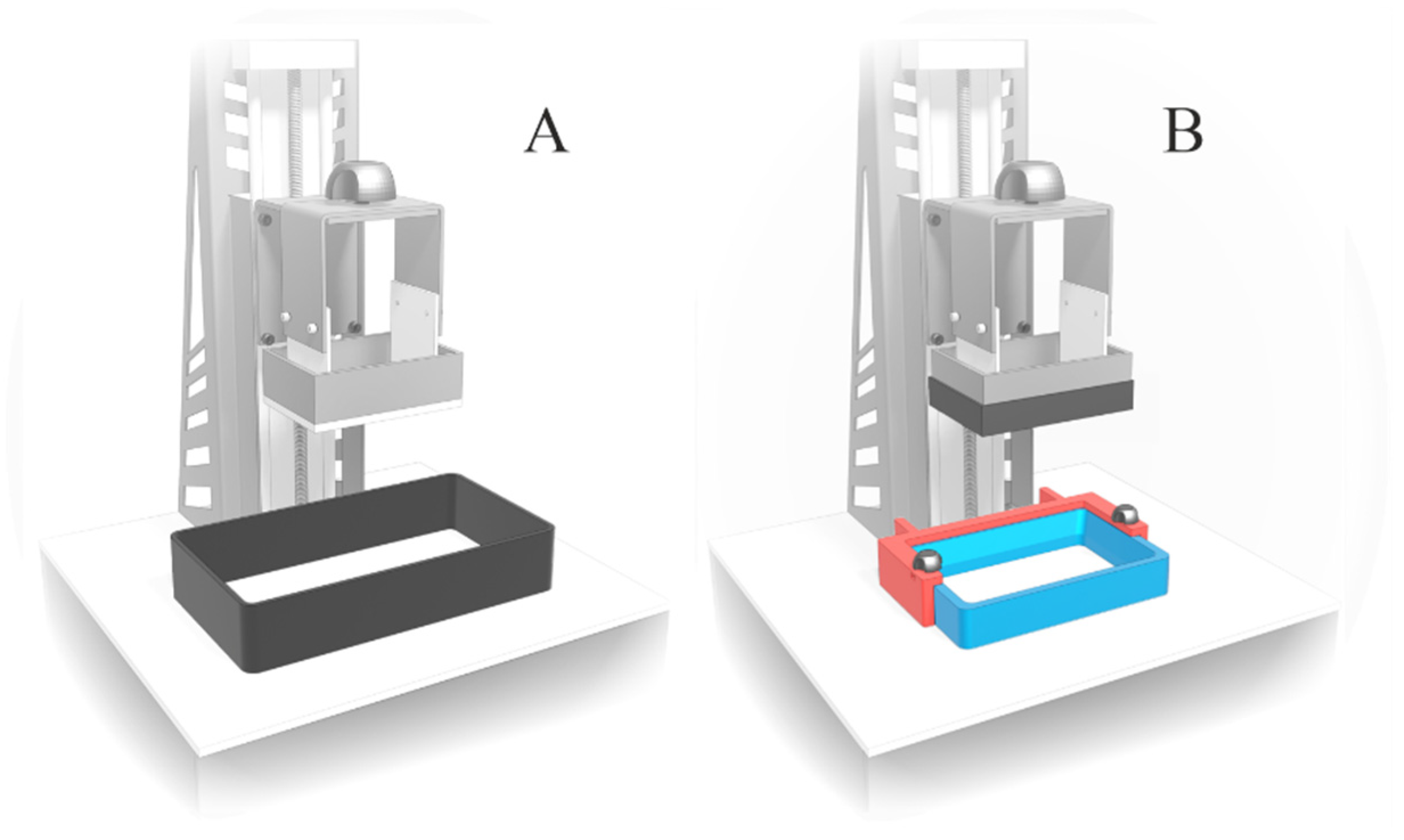
- 3D printing of placebo tablets and printer customization: optimized resin was used to print placebo tablets using a Daylight Polymer Printing technology. To improve the quality of the printed tablets and reduce the amount of resin needed for printing, an overlay on the printer’s platform and a smaller resin tank with an adapted holder were used.
- Assessment of the impact of co-initiators: the best resin composition was used to evaluate the effectiveness of ascorbic acid and triethanolamine to improve the mechanical properties of the tablets and reduce the printing time.
- Evaluation of the possibility of reusing the uncured resin left over from printing.
- 3D printing of drug-loaded tablets: process optimization and analysis.
2.3. Evaluation of PEGDA/PEG/Water Ratio
2.4. Mechanical Properties
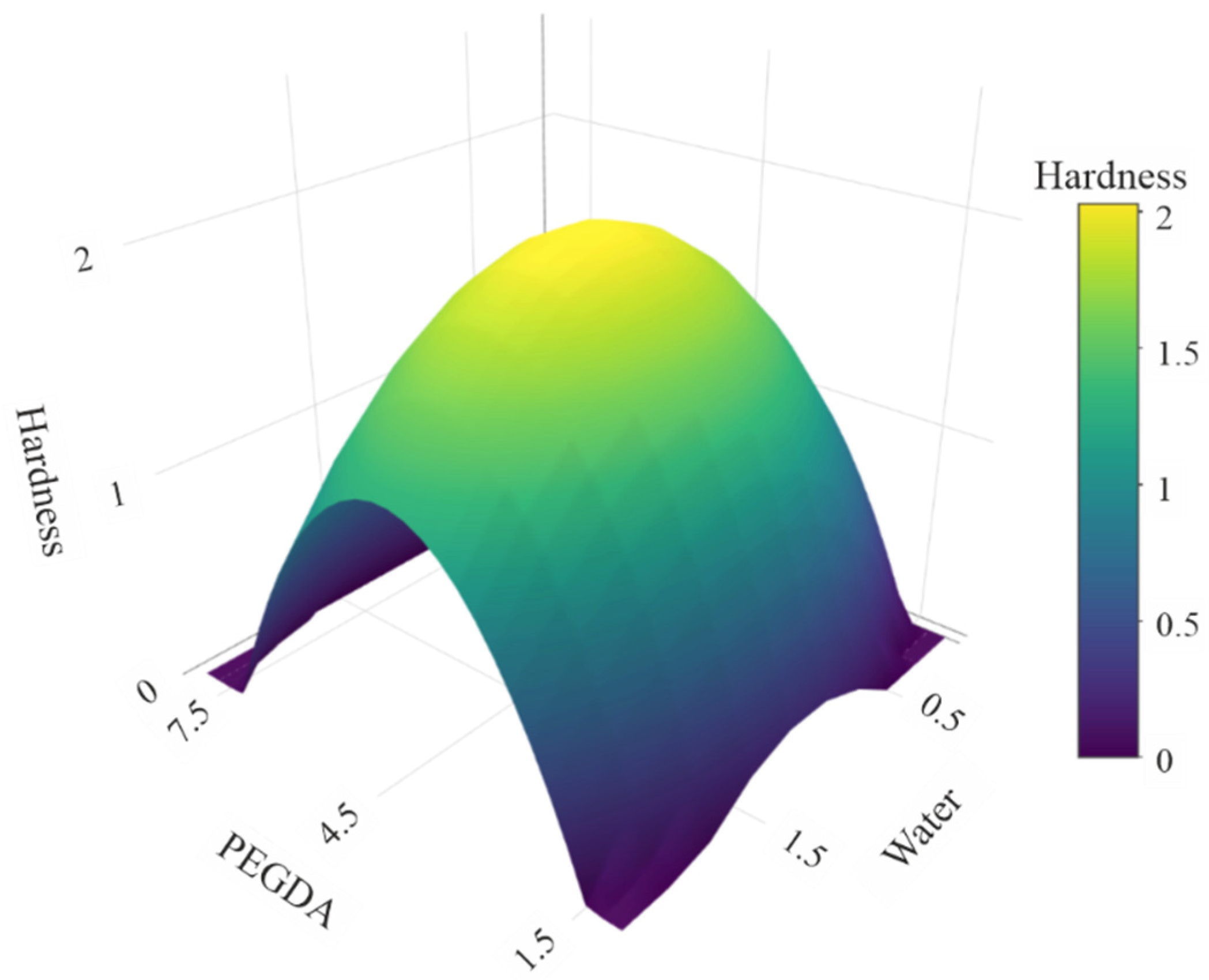
2.5. Model-Driven Optimization of Resin Composition
2.6. 3D Printing of Placebo Tablets
2.7. Assessment of the Impact of Co-Initiators
2.8. Evaluation of Resin Reusability
2.9. Preparation of Drug-Loaded Tablets
2.10. Drug Content in Tablets
2.11. Dissolution Study
2.12. Project Volume–Dosage Correlation
3. Results and Discussion
3.1. Optimization of Formulation
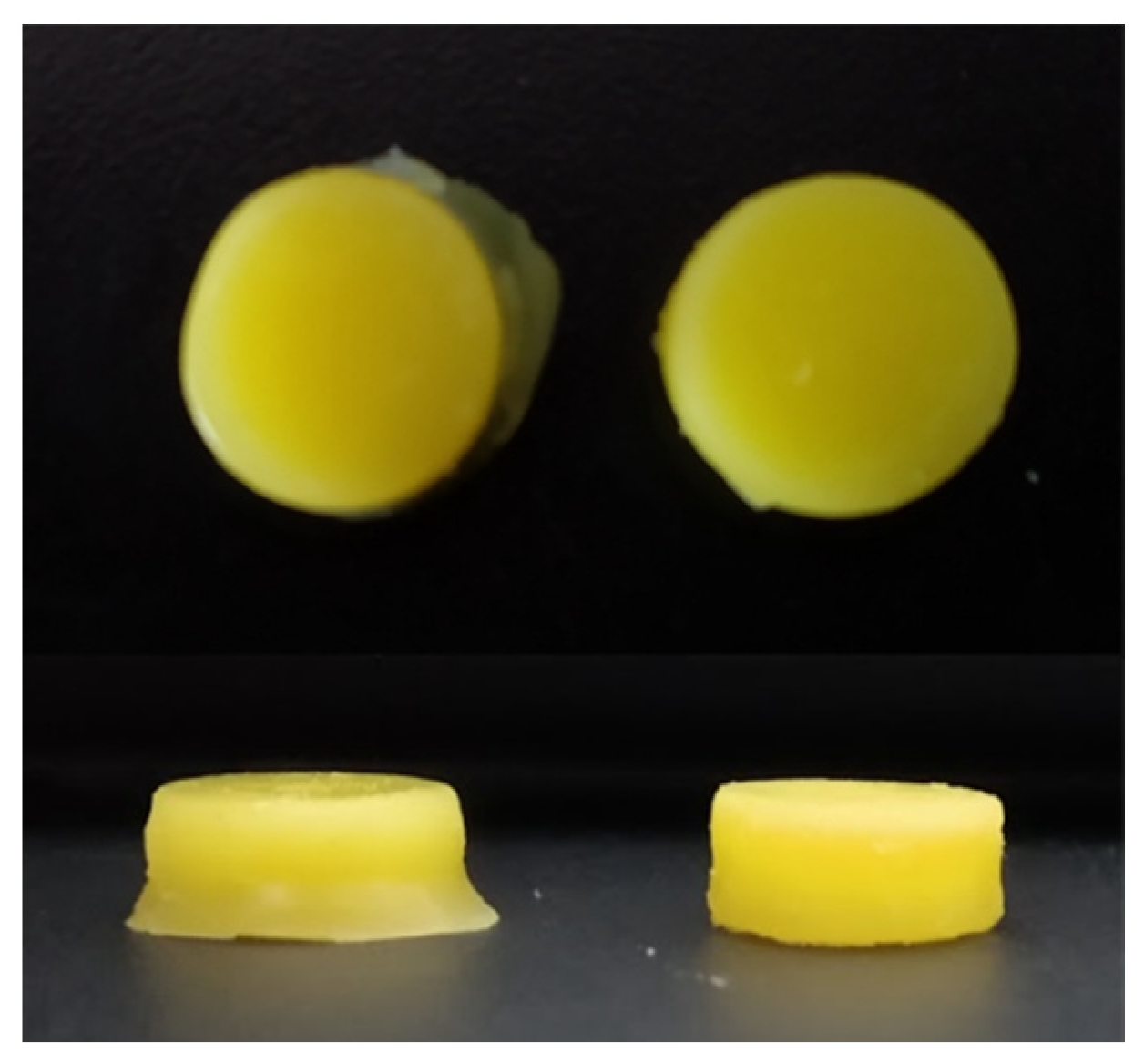
3.2. 3D Printing of Placebo Tablets and Customization of the 3D Printer
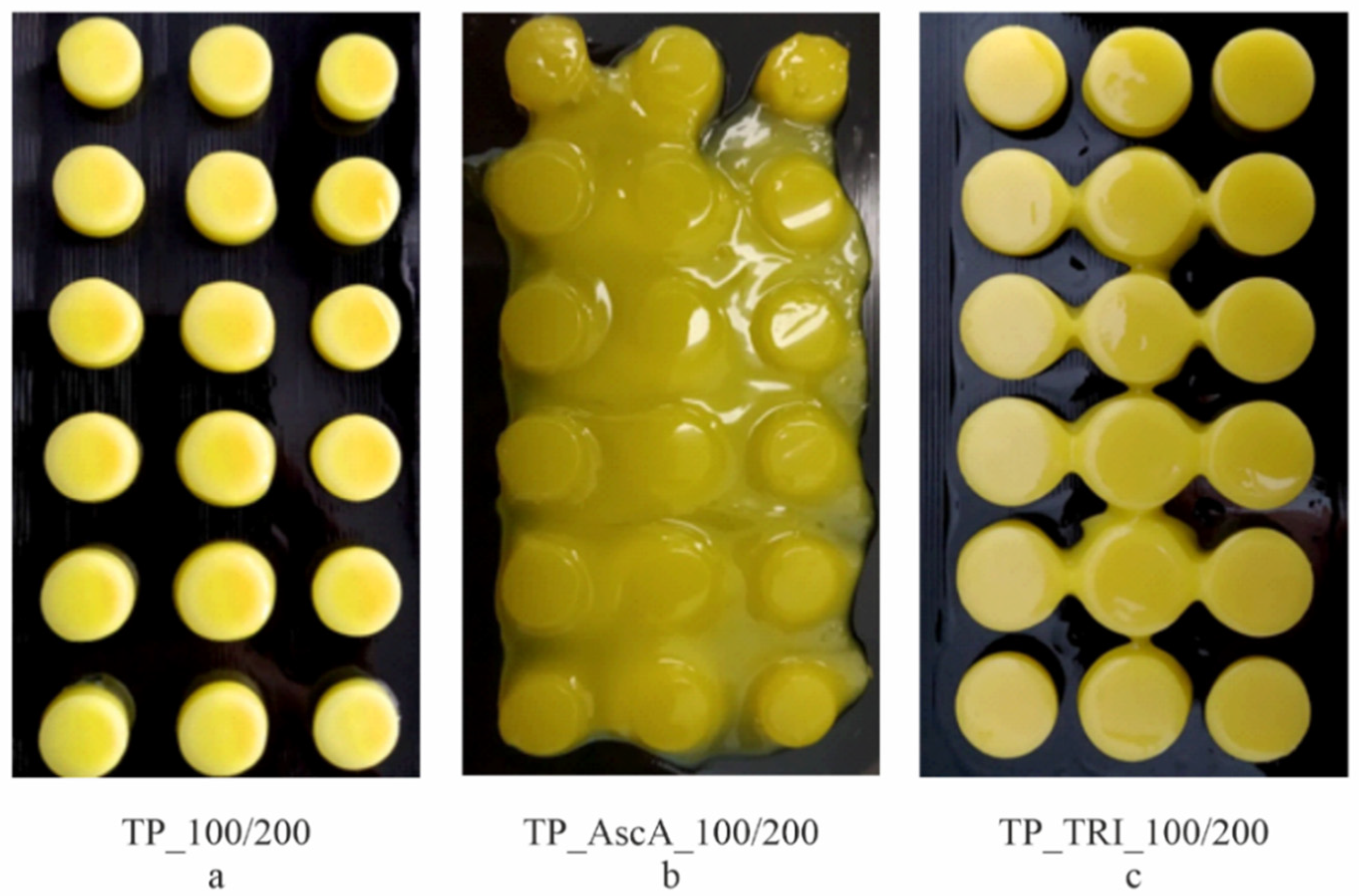
3.3. Co-Initiator Impact Assessment and Optimization of Printing Settings
3.4. Drug-Loaded Tablets
3.5. Project Volume–Dosage Correlation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D Printing for Enhanced Drug Delivery: Current State-of-the-Art and Challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D Printing: An Appealing Route for Customized Drug Delivery Systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A Graphical Review on the Escalation of Fused Deposition Modeling (FDM) 3D Printing in the Pharmaceutical Field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D Printing Promotes the Development of Drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef]
- Govender, R.; Abrahmsén-Alami, S.; Larsson, A.; Folestad, S. Therapy for the Individual: Towards Patient Integration into the Manufacturing and Provision of Pharmaceuticals. Eur. J. Pharm. Biopharm. 2020, 149, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Triastek, Inc. MEDTM 3D Printed Pharmaceutical Product Receives IND Clearance from the US FDA. Available online: https://www.triastek.com/detail/6.html (accessed on 9 November 2021).
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications–Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D Printing of Oral Modified-Release Dosage Forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Khatami, M.; Sarani, M.; Mosazadeh, F.; Rajabalipour, M.; Izadi, A.; Abdollahpour-Alitappeh, M.; Lima Nobre, M.A.; Borhani, F. Nickel-Doped Cerium Oxide Nanoparticles: Green Synthesis Using Stevia and Protective Effect against Harmful Ultraviolet Rays. Molecules 2019, 24, 4424. [Google Scholar] [CrossRef] [Green Version]
- Berwick, M.; Lachiewicz, A.; Pestak, C.; Thomas, N. Solar UV Exposure and Mortality from Skin Tumors. In Sunlight, Vitamin D and Skin Cancer; Reichrath, J., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 624, pp. 117–124. ISBN 978-0-387-77573-9. [Google Scholar]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital Light Processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2020, 26, 111. [Google Scholar] [CrossRef]
- Madzarevic, M.; Medarevic, D.; Vulovic, A.; Sustersic, T.; Djuris, J.; Filipovic, N.; Ibric, S. Optimization and Prediction of Ibuprofen Release from 3D DLP Printlets Using Artificial Neural Networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef] [Green Version]
- Madžarević, M. Evaluation of Exposure Time and Visible Light Irradiation in LCD 3D Printing of Ibuprofen Extended Release Tablets. Eur. J. Pharm. Sci. 2021, 158, 10568. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D Printing of Ascorbic Acid Loaded Hydrogels: A Controlled Release Study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Curti, C.; Kirby, D.J.; Russell, C.A. Stereolithography Apparatus Evolution: Enhancing Throughput and Efficiency of Pharmaceutical Formulation Development. Pharmaceutics 2021, 13, 616. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J. Control Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of Drug-Loaded Hydrogels with Stereolithographic 3D Printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konasch, J.; Riess, A.; Mau, R.; Teske, M.; Rekowska, N.; Eickner, T.; Grabow, N.; Seitz, H. A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots. Pharmaceutics 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Introduction to 3D Printing-Additive Processes. Available online: https://make.3dexperience.3ds.com/processes/photopolymerization (accessed on 6 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-Project.org/ (accessed on 7 October 2021).
- Lenth, R.V. Response-Surface Methods in R, Using Rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Rsm Package for R v. 2.10.3. Available online: https://cran.r-project.org/web/packages/rsm/index.html (accessed on 1 April 2022).
- Council of Europe. EDQM-European Directorate for the Quality of Medicines; European Pharmacopoeia 10th Edition; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Mendyk, A.; Jachowicz, R.; Fijorek, K.; Dorożyński, P.; Kulinowski, P.; Polak, S. KinetDS: An Open Source Software for Dissolution Test Data Analysis. Dissol. Technol. 2012, 19, 6–11. [Google Scholar] [CrossRef]
- Beztsinna, N.; Solé, M.; Taib, N.; Bestel, I. Bioengineered Riboflavin in Nanotechnology. Biomaterials 2016, 80, 121–133. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/ (accessed on 9 November 2021).
- Kim, S.; Chu, C.-C. Visible Light Induced Dextran-Methacrylate Hydrogel Formation Using (−)-Riboflavin Vitamin B2 as a Photoinitiator and L-Arginine as a Co-Initiator. Fibers Polym. 2009, 10, 14–20. [Google Scholar] [CrossRef]
- Zhang, T.; Yeow, J.; Boyer, C. A Cocktail of Vitamins for Aqueous RAFT Polymerization in an Open-to-Air Microtiter Plate. Polym. Chem. 2019, 10, 4643–4654. [Google Scholar] [CrossRef]
- Ahmad, I.; Iqbal, K.; Sheraz, M.A.; Ahmed, S.; Ali, S.A.; Kazi, S.H.; Mirza, T.; Bano, R.; Aminuddin, M. Solvent Effect on Photoinitiator Reactivity in the Polymerization of 2-Hydroxyethyl Methacrylate. Adv. Phys. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Orellana, B.; Rufs, A.M.; Encinas, M.V.; Previtali, C.M.; Bertolotti, S. The Photoinitiation Mechanism of Vinyl Polymerization by Riboflavin/Triethanolamine in Aqueous Medium. Macromolecules 1999, 32, 6570–6573. [Google Scholar] [CrossRef]
- Encinas, M.V.; Rufs, A.M.; Bertolotti, S.; Previtali, C.M. Free Radical Polymerization Photoinitiated by Riboflavin/Amines. Effect of the Amine Structure. Macromolecules 2001, 34, 2845–2847. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Riboflavin-Mediated Radical Polymerization–Outlook for Eco-Friendly Synthesis of Functional Materials. Eur. Polym. J. 2021, 142, 110152. [Google Scholar] [CrossRef]
- Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. ISBN 978-0-08-100092-2.







| Name | Tested Formulation Composition (% w/w) | |||
|---|---|---|---|---|
| PEGDA 575 | PEG 400 | Water | Riboflavin | |
| P1 | 15.00 (−1) | 79.90 | 5.00 (−1) | 0.10 |
| P2 | 45.00 (0) | 49.90 | 5.00 (−1) | |
| P3 | 75.00 (+1) | 29.90 | 5.00 (−1) | |
| P4 | 15.00 (−1) | 69.90 | 15.00 (0) | |
| P5 | 45.00 (0) | 39.90 | 15.00 (0) | |
| P5′ | 45.00 (0) | 39.90 | 15.00 (0) | |
| P5″ | 45.00 (0) | 39.90 | 15.00 (0) | |
| P6 | 75.00 (+1) | 9.90 | 15.00 (0) | |
| P7 | 15.00 (−1) | 59.90 | 25.00 (+1) | |
| P8 | 45.00 (0) | 29.90 | 25.00 (+1) | |
| P9 | 75.00 (+1) | - | 24.90 (+1) | |
| Name | Resin Composition (% w/w) | Printing Parameters | |||||
|---|---|---|---|---|---|---|---|
| PEGDA 575 | PEG 400 | Water | Riboflavin | Additional Compound | Layer Height (µm) | Exposure Time (s) | |
| TP_100/200 | 45.00 | 39.90 | 15.00 | 0.10 | - | 100 | 200 |
| TP_50/100 | 50 | 100 | |||||
| TP_50/100′ | 50 | 100 | |||||
| TP_AscA1_100/200 | 45.00 | 38.90 | 15.00 | 0.10 | AscA 1.00 | 100 | 200 |
| TP_AscA2_100/200 | 45.00 | 39.40 | 15.00 | 0.10 | AscA 0.50 | 100 | 200 |
| TP_AscA3_100/200 | 45.00 | 39.80 | 15.00 | 0.10 | AscA 0.10 | 100 | 200 |
| TP_TRI_100/200 | 45.00 | 39.75 | 15.00 | 0.10 | TRI 0.15 | 100 | 200 |
| TP_TRI_50/50 | 50 | 50 | |||||
| TP_TRI_50/25 | 50 | 25 | |||||
| TP_TRI_50/25′ | 50 | 25 | |||||
| TP_TRI_50/12.5 | 50 | 12.5 | |||||
| Name | Layer Height (µm) | Exposure Time (s) |
|---|---|---|
| TMEB_50/25 | 50 | 25 |
| TMEB_50/25′ | ||
| TMEB_50/50 | 50 | 50 |
| TMEB_50/50′ |
| Name | Force (N) ± SD |
|---|---|
| P1 | 0.11 ± 0.02 |
| P2 | 1.66 ± 0.23 |
| P3 | resin uncured |
| P4 | 0.11 ± 0.01 |
| P5 | 2.05 ± 0.19 |
| P5′ | 2.07 ± 0.44 |
| P5″ | 2.13 ± 0.19 |
| P6 | 0.98 ± 0.13 |
| P7 | 0.09 ± 0.01 |
| P8 | 1.00 ± 0.11 |
| P9 | resin uncured |
| First-Order Model | Second-Order Model | |
|---|---|---|
| Formula | Y = C1 × X1 + C2 × X2 + Intercept | Y = C1 × X1 + C2 × X2 + C3 × X1 × X2 + C4 × X12 + C5 × X22 + Intercept |
| p-value | 0.9277 | 0.0046 |
| Coefficients | Intercept = 0.9276 (p = 0.0159) C1 = 0.112 (p = 0.792) C2 = −0.115 (p = 0.788) | Intercept = 2.0294 (p = 5.94 × 10−5) C1 = 0.112 (p = 0.427) C2 = −0.115 (p = 0.417) C3 = 0.0057 (p = 0.973) C4 = −1.402 (p = 0.00091) C5 = −0.618 (p = 0.027) |
| Residual standard error | 1.01 | 0.3179 |
| Multiple R-squared | 0.0186 | 0.9393 |
| Adjusted R-squared | −0.2268 | 0.8786 |
| Shapiro–Wilk normality test of residuals | 0.1229 | 0.4518 |
| Name | Mass 1 (mg) ± SD | Diameter 1 (mm) ± SD | Force 2 (N) ± SD |
|---|---|---|---|
| TP_100/200 | 442.61 ± 41.60 | 10.81 ± 5.47 | 8.40 ± 0.79 |
| TP_50/100 | 369.77 ± 49.98 | 9.90 ± 0.70 | 9.29 ± 0.73 |
| TP_50/100′ | Printing failed | ||
| TP_AscA1_100/200 | Printing failed | ||
| TP_AscA2_100/200 | |||
| TP_AscA3_100/200 | |||
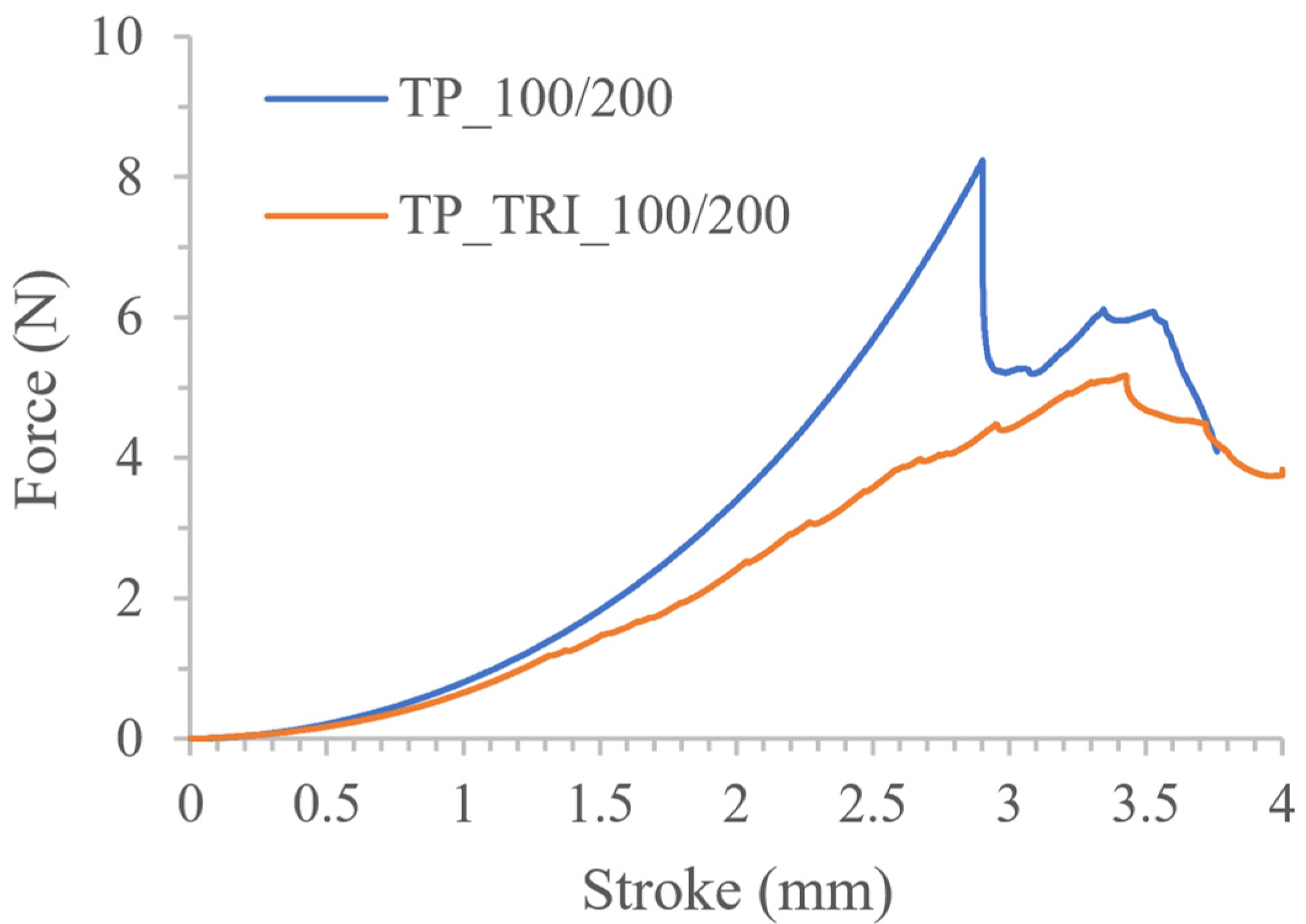
| TP_TRI_100/200 | 760.06 ± 103.19 | 12.08 ± 0.51 | 4.93 ± 3.43 |
| TP_TRI_50/50 | 563.53 ± 55.58 537.62 ± 19.30 * | 11.55 ± 0.64 11.26 ± 0.37 * | 5.93 ± 0.57 * |
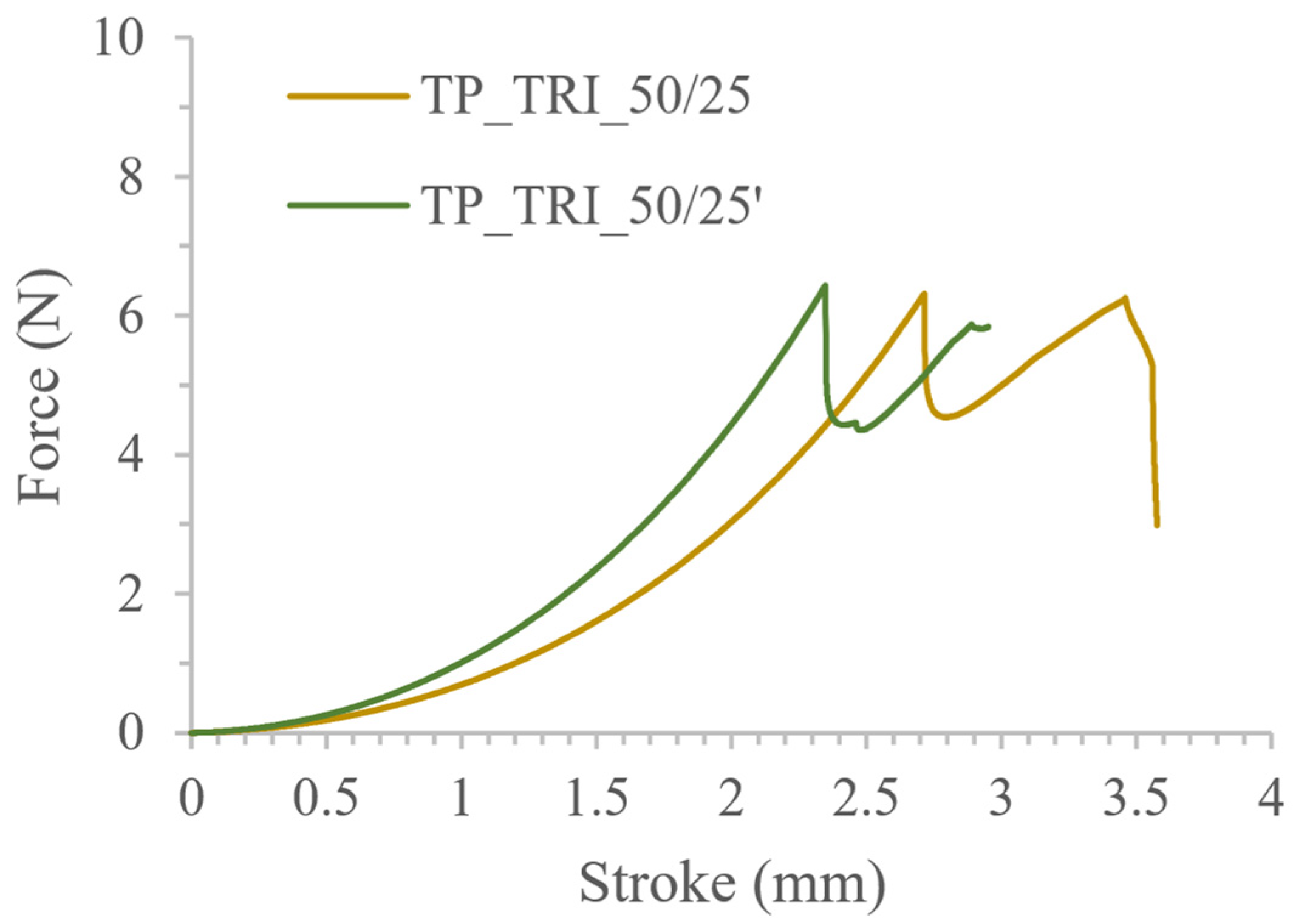
| TP_TRI_50/25 | 490.49 ± 28.50 477.15 ± 18.78 * | 10.45 ± 0.23 10.38 ± 0.13 * | 6.52 ± 0.26 * |
| TP_TRI_50/25′ | 487.67 ± 27.56 476.09 ± 17.88 * | 10.32 ± 0.20 10.23 ± 0.08 * | 6.28 ± 0.75 * |
| TP_TRI_50/12.5 | Printing failed | ||
| Name of Tablets | Mass 1 (mg) ± SD | Diameter 1 (mm) ± SD | Force 2 (N) ± SD | Drug-Loading 3 (%) ± SD | Dosage (mg) |
|---|---|---|---|---|---|
| TMEB_50/25 | 388.39 ± 13.71 | 9.78 ± 0.17 | 6.44 ± 0.66 | 10.83 ± 0.32 | 41.94 |
| TMEB_50/25′ | 384.06 ± 17.41 * | 9.41 ± 0.30 * | - | - | - |
| TMEB_50/50 | 423.66 ± 9.96 | 10.12 ± 0.19 | 7.73 ± 0.53 | 11.21 ± 0.02 | 47.73 |
| TMEB_50/50′ | 426.66 ± 5.79 | 10.02 ± 0.11 | 7.60 ± 0.26 | 11.00 ± 0.17 | 47.23 |
| Project | 3D Printed Tablets | ||||
|---|---|---|---|---|---|
| Diameter (mm) | Height (mm) | Volume (mm3) | Diameter (mm) ± RSD (%) | Height (mm) ± RSD (%) | Mass (mg) ± RSD (%) |
| 14 | 7 | 1070.66 | 14.01 ± 0.51 | 6.72 ± 0.43 | 1194.70 ± 1.59 |
| 12 | 6 | 674.23 | 12.01 ± 0.34 | 5.72 ± 0.49 | 766.16 ± 2.00 |
| 10 | 5 | 390.18 | 10.13 ± 0.05 | 4.80 ± 0.77 | 464.35 ± 3.59 |
| 8 | 4 | 199.77 | 8.03 ± 0.20 | 3.77 ± 0.87 | 234.54 ± 5.31 |
| 6 | 3 | 84.28 | 5.94 ± 0.95 | 2.77 ± 1.68 | 94.66 ± 7.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyteraf, J.; Pacławski, A.; Jamróz, W.; Mendyk, A.; Paluch, M.; Jachowicz, R. Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products. Pharmaceutics 2022, 14, 843. https://doi.org/10.3390/pharmaceutics14040843
Pyteraf J, Pacławski A, Jamróz W, Mendyk A, Paluch M, Jachowicz R. Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products. Pharmaceutics. 2022; 14(4):843. https://doi.org/10.3390/pharmaceutics14040843
Chicago/Turabian StylePyteraf, Jolanta, Adam Pacławski, Witold Jamróz, Aleksander Mendyk, Marian Paluch, and Renata Jachowicz. 2022. "Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products" Pharmaceutics 14, no. 4: 843. https://doi.org/10.3390/pharmaceutics14040843
APA StylePyteraf, J., Pacławski, A., Jamróz, W., Mendyk, A., Paluch, M., & Jachowicz, R. (2022). Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products. Pharmaceutics, 14(4), 843. https://doi.org/10.3390/pharmaceutics14040843











