Abstract
Palbociclib is a good candidate for therapeutic drug monitoring (TDM) due to its narrow therapeutic range and frequency of toxicities, particularly high-grade neutropenia. In this prospective, bicentric clinical trial, we evaluated the palbociclib exposure–toxicity relationship and determined the relevant sources of palbociclib pharmacokinetic variability, including drug–drug interactions (DDI). We followed 58 patients (mean age: 62.9 years) for 1 year. The geometric median of palbociclib plasma trough concentration (Ctrough) was 74.1 ng/mL. Neutropenia occurred in 70.7% of patients (high grade in 67.2% of patients). High-grade neutropenia occurrence during the first two palbociclib cycles was higher in patients with lower neutrophil count at initiation (p = 0.002). Palbociclib plasma Ctrough was correlated with high-grade neutropenia occurrence during the first two cycles (p = 0.024, OR 5.51). Co-treatment with agents that may interfere with palbociclib PK significantly influenced palbociclib Ctrough (p < 0.05). CYP3A4/P-glycoprotein inhibitors increased by 25% palbociclib Ctrough (p = 0.035), while antacids reduced it by 20% (p = 0.036). However, DDI did not have any significant effect on high-grade neutropenia occurrence (p > 0.05). This study confirms the major role of TDM to manage palbociclib safe use from the first week of treatment, particularly the significant incidence of hematological toxicity. Moreover, this first dedicated prospective study confirmed the importance of characterizing co-treatments to limit the DDI risk with oral-targeted therapies.
1. Introduction
Neutropenia is one of the most frequently reported toxicities when using oncologic drugs. Neutropenia may even be considered a biomarker of exposure for drugs targeting the cell cycle, and may be used as a surrogate marker of efficacy [1,2,3]. CKD4/6 inhibitors, such as palbociclib, are the gold standard for the treatment of metastatic breast cancer. Although these drugs cause high-grade neutropenia in almost 50% of patients, no predictive biomarker of their toxicity has been identified yet, and there is no consensus on the correlation between palbociclib exposure and neutropenia occurrence (i.e., exposure–toxicity relationship), or between the target plasma concentration and treatment efficacy (palbociclib pharmacokinetics, PK/pharmacodynamics, PD) [4,5]. PK models suggest a link between the occurrence of neutropenia and palbociclib exposure or dose [1,6,7]. Moreover, data from clinical trials indicate that a dose reduction or a prolonged pause (>7 days) decreases the toxicity grade, or even normalizes neutrophil count [8,9]. As these findings suggest a link between dose, plasma concentration, and toxicity, therapeutic drug monitoring (TDM) could be used to monitor palbociclib plasma concentration.
Practical recommendations on TDM use for targeted therapies are based on PK data, availability of analytical techniques, and clinical trials that used TDM for dose adjustments [10]. However, TDM guidelines for palbociclib are not available yet. Therefore, it is important to characterize the PK–PD–toxicity relationships of palbociclib, especially because this drug presents PK variabilities, for instance caused by drug–drug interactions (DDI) or pharmacogenetic variants [11]. Palbociclib bioavailability is moderate and pH dependent (46%) [12]. Moreover, it is largely bound to plasma protein (85.3%), leading to a significant risk of intra- and inter-individual PK variabilities.
In addition, as a substrate and inhibitor of CYP3A4, palbociclib plasmatic concentration may be modulated by co-treatments (i.e., DDI victim), but it may also lead to DDI (i.e., perpetrator). To date, only clinical cases highlighted the potential clinical relevance of these DDI (palbociclib associated with ciclosporin or verapamil), without a specific analysis of palbociclib PK [13,14]. A recent review suggested that empirical dose adjustments should be performed in function of the other drugs taken by the patient [15].
In this context, in a prospective cohort of patients with breast cancer receiving first-line palbociclib treatment, we determined palbociclib plasma concentration and evaluated its correlation with neutropenia occurrence. We also investigated the causes of PK variability, including DDI that may influence plasma palbociclib concentration.
2. Materials and Methods
2.1. Trial Design and Patients
This study used the clinical data collected in the framework of a dedicated, prospective, bicentric clinical trial to determine palbociclib exposure–toxicity correlations carried out at the Institut du Cancer de Montpellier (ICM, France) and Nîmes University Hospital (France). The trial was performed in accordance with Good Clinical Practice standards (NCT04025541). Patients with metastatic, hormone-sensitive, HER2-negative breast cancer were enrolled between June 2018 and July 2020. They all received first-line treatment with palbociclib (125 mg per day for 3–4 weeks) associated with an aromatase inhibitor. Patients were included after signature of the informed consent. After the oncology consultation and inclusion in the clinical trial, patients were interviewed by a hospital pharmacist to identify co-treatments and DDI risk, particularly CYP450 inducers or inhibitors. Treatment compliance was assessed at each visit.
2.2. Endpoint Analysis (Palbociclib Exposure–Toxicity Relationship)
The primary endpoint was the percentage of patients with grade 3–4 neutropenia, according to the NCI-CTCAE v4.03 criteria, during the first two palbociclib cycles, in function of its steady-state concentration (day 15 of the first cycle, D15C1). Exploratory analyses were carried out to evaluate the impact of concomitant treatments and DDI occurrence on palbociclib steady-state concentration (D15C1).
2.3. Pharmacokinetics
The steady-state concentration of palbociclib (plasma trough concentration; Ctrough) was quantified in all patients. For the PK analysis, blood samples were collected at D15C1 before the next dose to determine plasma concentration (Ctrough) using our previously published HPLC-MS/MS method, validated according to the Food and Drug Administration and European Medicines Agency recommendations [16]. Non-compliant patients or those whose samples were not at the residual concentration were excluded from the analysis.
2.4. Exposure–Toxicity Analysis
Clinical and biological toxicities were recorded at each visit, i.e., every 15 days during the first two treatment cycles. Patients were divided into two groups in function of the occurrence or not of palbociclib-induced high-grade (3–4) neutropenia during the first two treatment cycles. For each patient, the geometric median of all available palbociclib Ctrough levels was calculated. This was compared to the geometric median value of palbociclib Ctrough in the whole population.
2.5. Exposure-DDI Relationship Analysis
After the oncology consultation and inclusion in the clinical trial, patients were interviewed by a hospital pharmacist to identify co-treatments and DDI risk (part of the medication reconciliation process). Patients were classified in function of their risk of DDI that might lead to CYP3A4 and/or P-glycoprotein inhibition (P-gp) and to gastric pH increase by gastric acid-suppressive agents (e.g., proton pump inhibitors, histamine type 2-receptor blockers) using databases (e.g., DDI predictor®, Drugs.com®, PubMed®).
2.6. Statistical Analysis
Quantitative variables were described as the number of observations (N), median, interquartile range, mean and standard deviation. The Kruskal–Wallis test was used to compare the distribution of quantitative variables. Qualitative variables were described as number of observations (N) and frequency (%) of each modality. Missing values for each variable were counted. Percentages were calculated relative to the total population after exclusion of missing data. The Chi-2 test was used to compare frequencies (or the Fisher’s exact test if the expected frequencies were <5). Adjusted odds ratios (OR) and their 95% confidence interval (CI) were estimated using a logistic regression model for the occurrence of grade 3–4 neutropenia during the first two palbociclib cycles. Palbociclib Ctrough was log-transformed and modeled using a multivariate linear regression. Multivariate model for occurrence of grade neutropenia was constructed using a backward variable selection procedure. All variables that showed a significant or moderately significant correlation (i.e., p < 0.20) with the primary endpoint were included as candidate variables in the initial model. Potential confounding factors were assessed at each step of the selection procedure. Functional forms of continuous variables were checked in order to assess any potential deviation from linearity in the multivariate model. All statistical tests were two-sided, and the significance level was set at 5% (i.e., p < 0.05). Statistical analyses were performed with STATA v16.0 and R v4.0.3.
3. Results
3.1. Patients
In total, 62 patients were included in the study between 18 June 2018 and 16 July 2020 (intention-to-treat population). However, four patients withdrew from the study before palbociclib treatment initiation (Figure S1). Among the 58 patients (n = 57 women; median age: 66 years), the ECOG performance status at inclusion was 0 in more than 60%. Patients were mostly menopausal (80.7%), and 67.2% of them had received at least one previous treatment at the localized stage of the disease (Table S1). Most patients (98.3%) were treated for metastatic breast cancer (except one patient with locally advanced, unresectable breast cancer), and half of them had de novo metastatic disease, with a median of one metastatic site, mainly in bone (78.9%), lymph nodes (35.1%), lung (15.8%), or liver (14%) (Table S1).
At palbociclib treatment initiation, blood count was normal in more than 80% of patients. High-grade (3–4) neutropenia was the most frequent side effect during the first two cycles of palbociclib (67.2% of patients; all grades combined: 70.7% of patients) (Table 1). Palbociclib dose was reduced by at least one dose level in 32.8% (19/58) of patients, mostly due to hematological toxicities (89.5%, 17/19 patients), and treatment was interrupted for hematological toxicity in 5.2% of patients (3/58) (Table 1). All adverse events were prospectively recorded during the follow-up, although only neutropenia was evaluated in the endpoint analysis.

Table 1.
Treatment interruption/dose modification and toxicity occurrence (safety population, n = 58).
3.2. Clinical–Biological Data and Palbociclib-Induced Toxicity
Biological data at inclusion (blood count, kidney and liver function) were in the normal range in >80% of patients. Plasma palbociclib concentration could be quantified in 54 patients (Figure S1), and the steady-state plasma Ctrough at D15C1 was used as an indicator for TDM. The mean ± standard deviation (range) palbociclib Ctrough was 80.3 ± 26.7 ng/mL (21.2–130 ng/mL), and the median was 74.1 ng/mL. Median palbociclib Ctrough (IQR) was 66.7 ng/mL (52.0–82.7) and 76.7 ng/mL (61.3–101.5) in patients without and with high grade neutropenia, respectively (Figure 1).
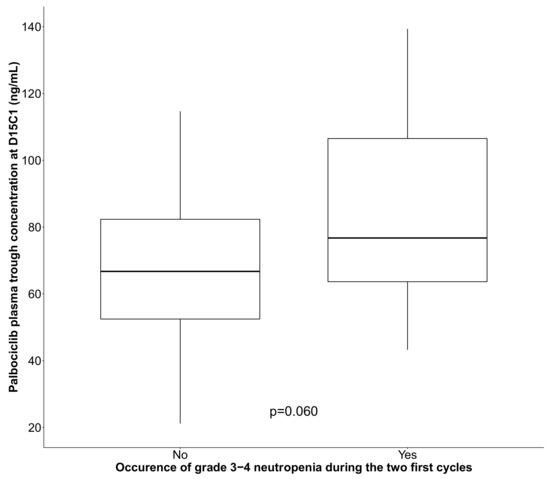
Figure 1.
Box plot showing palbociclib plasma trough concentration at D15C1 in function of the occurrence or not of grade 3–4 neutropenia during the first two treatment cycles (black line: median). p value is derived from Kruskal–Wallis test.
In univariate analysis, higher BMI (ORincrease 1.14, 95% CI (1.00; 1.31) p = 0.038), lower leukocytes (ORincrease = 0.61, 95% CI (0.44; 0.84)) and neutrophils (ORincrease = 0.62, 95% IC (0.42; 0.92)) at inclusion were significantly associated with high-grade neutropenia (Table 2). Higher palbociclib Ctrough was also correlated with increased risk of high-grade neutropenia (OR = 1.28, 95% CI (1.01; 1.64), p = 0.031) (Table 2).

Table 2.
Univariable and multivariable analysis for occurrence of grade 3–4 neutropenia during the first two palbociclib cycle. Patients evaluable for safety (n = 58).
The final multivariate model included neutrophils count at inclusion and palbociclib Ctrough. As observed in the univariate analysis, lower neutrophils (ORincrease = 0.56, 95% IC (0.36; 0.86), p = 0.002) and higher palbociclib Ctrough (ORincrease = 1.42, 95% IC (1.06; 1.90), p = 0.008) were significantly associated with increased risk of high-grade neutropenia (Table 2).
From the multivariate model, a probability model of neutropenia risk in function of palbociclib Ctrough at D15C1 was generated (Figure 2). According to this model, the probability of developing high grade neutropenia for patient with a palbociclib Ctrough of 61, 74 and 101 ng/mL and a neutrophil count of 4.3 109/L was 52% (95%CI (34%, 70%)) 63% (95% CI (47%, 76%)) and 82% (95% CI (62%, 92%)), respectively. This indicates the presence of a concentration–toxicity relationship.
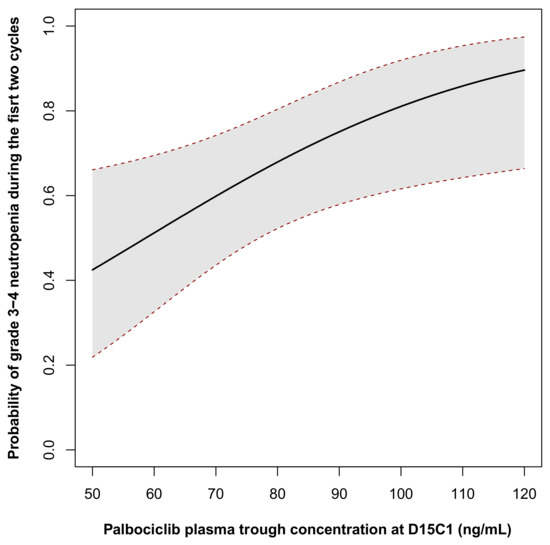
Figure 2.
Probability of grade 3–4 neutropenia occurrence during the first two cycles in function of palbociclib trough concentration at D15C1. The probability was calculated for a patient neutrophil count at inclusion corresponded to the mean value of the cohort (62 years of age, neutrophils count = 4.3 × 109/L).
3.3. Palbociclib Pharmacokinetics and Clinicopathological Features
In univariate analysis, plasma palbociclib Ctrough was correlated with clinical and biological features, such as age and kidney function and albuminemia, but not with BMI.
Specifically, palbociclib Ctrough was higher than the median concentration (74 ng/mL) in older patients (71- vs. 57-year-old, p = 0.002) (Table 3) and in patients with reduced kidney function (glomerular filtration rate of 80.3 vs. 93.6 mL/min, p = 0.017).

Table 3.
Univariate analysis of the correlation between plasma trough concentration of palbociclib at D15C1 and selected variables. Patients evaluable for safety with usable Ctrough data (n = 54).
3.4. Palbociclib Exposure and Co-Medication
Among the causes of PK variability that may modulate palbociclib Ctrough, the impact of DDI (i.e., drugs taken at D15C1) was also evaluated. To this aim, the number of patients who were still taking CYP3A4 and P-glycoprotein inhibitors at D15C1 (despite the medication reconciliation at inclusion) was recorded. One third of patients (33.3%) were taking at least one CYP3A4 or P-glycoprotein inhibitor (e.g., amlodipine, nifedipine, atorvastatin, simvastatin). As palbociclib absorption is pH dependent, the influence of antacid intake was also evaluated. In our cohort, 25% of patients used antacids (proton pump inhibitors, such as pantoprazole or omeprazole, and histamine type 2-receptor blockers, such as ranitidine) at D15C1, despite the initial medication reconciliation.
Palbociclib Ctrough was higher in patients that had taken at least one CYP3A4 or P-glycoprotein inhibitor (106.1 ng/mL vs. 71.3 ng/mL, p = 0.007; univariate analysis, Figure 3). Median palbociclib Ctrough was 80 ng/mL and 72.2 ng/mL for patients who took at least one antacid and those who did not, respectively (p = 0.390, univariate analysis, Figure 3).
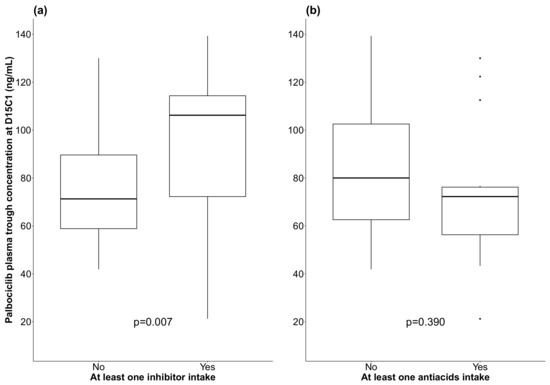
Figure 3.
Box plots showing palbociclib plasma trough concentration at D15C1 in function of the co-intake or not of CYP3A4/P-glycoprotein inhibitors (a) and of antacids (b); (black line: median).
To assess the impact of co-medication on palbociclib Ctrough, a multivariate analysis was carried out using a linear regression including intake of CYP3A4 or P-glycoprotein inhibitor and antacids, adjusted for age and body surface area at D15C1 (Table 4). After adjustment, the mean palbociclib Ctrough in patients taking at least one CYP3A4 or P-glycoprotein inhibitor was significantly increased by 25% (95% CI (0.4%; 56%), p = 0.035) compared with patients not taking inhibitors. The mean palbociclib Ctrough was significantly decreased by 20% in patients taking at least one antacid (95% CI (−36%; −0.3%), p = 0.036) compared with patients not taking them. The risk of interaction between CYP3A4 inhibitors and antacids was also tested, but it was not significant (p = 0.788). However, DDI was not associated with high-grade neutropenia occurrence (p = 0.372 for CYP3A4 inhibitors and p = 0.206 for antacids) (Table 3, univariate analysis).

Table 4.
Adjusted association between comedications at D15C1 and log-concentration at D15C. Multivariate linear regression (n = 52).
4. Discussion
This prospective study investigated palbociclib exposure–toxicity relationship and PK variability in real life in 62 patients with metastatic hormone-sensitive breast cancer. The clinical–biological data were consistent with those of the PALOMA 1–3 trials: similar mean age (62.9 years vs. 60 years), but better general condition (63.8% of patients with ECOG performance status of 0 vs. 58.2% in the combined PALOMA trials) [17]. During the first two palbociclib cycles, 70.7% of patients reported neutropenia (vs. 80.6% in the combined PALOMA trials) and 67.2% high-grade neutropenia (vs. 67.1% in the PALOMA 2 and 57% in the PALOMA 1 trial) [18]. The use of dose reduction was similar (more than 30% in our study and the PALOMA trials). Conversely, treatment interruption was required for 74% of patients in the PALOMA trials but only for 20% of our patients, probably due to less stringent rules for neutrophil count thresholds in clinical settings, following the integration of the PALOMA trial data in clinical practice. In the PALOMA 2 trial, dose reduction for toxicity, mainly following high-grade neutropenia occurrence, did not result in a reduction of treatment effectiveness [19]. Palbociclib plasma Ctrough could be estimated in 54 patients (mean: 80.3 ng/mL; median: 74.1 ng/mL). The mean Ctrough was similar to the estimated concentration reported in the PALOMA 1 trial (88.5 ng/mL), but was higher than in the PALOMA 2 cohort (61.7 ng/mL in the Caucasian subgroup, relative to Japanese (95.4 ng/mL) and other Asians (90.1 ng/mL)) [20]. As our cohort consisted exclusively of Caucasian patients, it seems important to consider performing subgroup analyses according to the patient ethnicity.
We then tried to identify factors that may influence neutropenia occurrence. We found that the risk of developing neutropenia during the first two cycles of palbociclib was higher for patients with lower baseline neutrophil count (p = 0.007). This suggests that the patient’s bone marrow reserve (i.e., standard blood count) should be routinely analyzed before palbociclib initiation to characterize the risk of toxicity, because the occurrence of high-grade toxicity leads to therapeutic pauses and dose reduction. These data are comparable to the pooled analysis of the PALOMA 1 and 2 trials [21]. Although not confirmed in the multivariate analysis, higher BMI was also related to the occurrence of neutropenia, as reported in a recent study (n = 78) [22]. Importantly, in our patients, palbociclib was the first-line treatment for metastatic disease. Therefore, in patients receiving palbociclib as second (or more) line treatment, the risk of neutropenia could be higher because of their treatment history.
In our cohort, after adjustment, the risk of high-grade neutropenia was significantly increased with higher values of palbociclib Ctrough (p = 0.008, ORincrease = 1.42, 95% CI (1.06; 1.90)). We also estimated the probability of high-grade neutropenia at 63% (95% CI (47%, 76%)) in patients with palbociclib Ctrough at the median value (74 ng/mL). This prospective trial demonstrated the palbociclib pharmacokinetic–toxicity relationship and also investigated possible causes of palbociclib PK variability, thus completing a previously reported PK/PD model for palbociclib [23]. Our model estimated at 51% the risk of developing high grade neutropenia (95% CI (32%; 69%)) in patients with a palbociclib Ctrough of ~60 ng/mL (approximately the mean value of the PALOMA clinical trials). Univariate analysis shows a higher palbociclib concentration in patients with lower renal clearance, despite the low proportion of palbociclib elimination by the renal route (17%). A clinical study showed that in patients with impaired renal function, palbociclib plasma concentration is higher, but can be used safely in this population [24]. DDI impact on palbociclib-induced neutropenia was also assessed, based on concomitant treatments at D15C1. The use of CYP3A4 or P-glycoprotein inhibitors and antacids influences palbociclib plasma concentration significantly. Palbociclib concentration was increased (+26%) when combined with at least one CYP3A4 or P-glycoprotein inhibitor (p < 0.05; multivariate analysis). The influence of such inhibitors on palbociclib PK has been increasingly characterized, for instance for erythromycin, a moderate CYP3A4 inhibitor (n = 11) [25]. Although the drugs involved in our analysis are not described as major inhibitors (simvastatin, atorvastatin, amlodipine, losartan or nifedipine), their influence was found to be statistically significant. Conversely, we observed a significant reduction in palbociclib Ctrough concentration (20%, p < 0.05) in patients taking antacids at D15C1. However, we did not find any correlation between these DDI and the occurrence of neutropenia (p = 0.239). This can certainly be explained by the small size of our cohort (n = 62). The impact of these co-treatments in terms of survival will be evaluated later, especially because the correlation between palbociclib plasma concentration and hematologic toxicity can lead to dose reductions. Recent studies suggest a link between co-medication (statin use) and neutropenia occurrence (n = 78), and a negative influence of antacids on the survival of patients treated with palbociclib (p < 0.0001) [22,26]. It would be relevant to analyze the various PK/PD correlations and specifically the modulation of palbociclib concentration on treatment efficacy in our cohort. However, we could not investigate this point because the survival data of our cohort are not available yet. The clinical impact of DDI is becoming better characterized, for instance the negative influence of antacid use on survival in patients with sarcoma treated with pazopanib [27]. Although target concentrations are not yet clearly defined for palbociclib, TDM appears to be a relevant tool for improving patient management, especially in view of the frequent occurrence of hematological toxicity. TDM is also a way to characterize and estimate the relevance of the causes of PK variability.
5. Conclusions
The pharmacokinetic–toxicity relationship and PK variability of palbociclib were characterized in real-life metastatic breast cancer patients (n = 62). The risk of high-grade neutropenia was significantly associated with higher values of palbociclib Ctrough (p = 0.008, ORincrease = 1.42, 95% CI (1.06; 1.90)). Cotreatment, as CYP3A4 or P-glycoprotein inhibitors or antacids, were significantly modulated palbociclib Ctrough (+/−20%). Clinical pharmacy activity and TDM allows characterization of DDI risk and ensures safety and efficacy of the CDK4/6 inhibitor as palbociclib.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14040841/s1, Figure S1: Study flow chart; Table S1: Socio-demographic and tumor anatomopathological characteristics at inclusion. Population evaluable for toxicity (n = 58).
Author Contributions
F.L.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Roles/Writing—original draft. F.F.: Investigation, Validation, Writing—review and editing. L.G.: Methodology, Data curation, Formal analysis, Roles/Writing—original draft. M.A.: Investigation, Validation. S.G.: Investigation, Validation. N.F.: Investigation, Validation. S.P.: Investigation, Validation. M.V.: Investigation, Validation. G.L.: Project administration. C.G (Chloé Gauthier): Investigation. C.M.: Conceptualization, Data curation. M.G.: Formal analysis, Resources. C.G. (Celine Gongora): Supervision. L.M.: Resources. A.E.: Conceptualization, Methodology, Supervision, Validation, Roles/Writing—original draft, Writing—review and editing. W.J.: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Roles/Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by INCa-Cancéropôle, grant number No. 2018-E13.
Institutional Review Board Statement
This study used the clinical data collected in the framework of a prospective and bicentric pharmacokinetic–toxicity correlation study (Clinicaltrials.gov identifier NCT04025541) carried out at the Institut du Cancer de Montpellier (ICM), France, in accordance with Good Clinical Practice (GCP) and in accordance with the Declaration of Helsinki. The ethics committee (Comité de protection des personnes; Committee for the protection of persons) authorized this trial on 7 March 2018; IdRCP N. 2018-A00064-51.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on request due to restrictions, e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author.
Acknowledgments
Biological Resource Center (BIOBANQUES—BB-0033-00032), Nîmes University Hospital, Carémeau Hospital Group, 30029 Nîmes cedex 09, France. Biological Resource Center (BIOBANQUES—BB-0033-00059), ICM, 34000 Montpellier, France). Medical writer: Elisabetta Andermarcher.
Conflicts of Interest
All conflicts of interest have been declared in conflicts of interest statement. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sun, W.; O’Dwyer, P.J.; Finn, R.S.; Ruiz-Garcia, A.; Shapiro, G.I.; Schwartz, G.K.; DeMichele, A.; Wang, D. Characterization of Neutropenia in Advanced Cancer Patients Following Palbociclib Treatment Using a Population Pharmacokinetic-Pharmacodynamic Modeling and Simulation Approach. J. Clin. Pharmacol. 2017, 57, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Matsuo, K.; Takahari, D.; Yokota, T.; Shibata, T.; Ura, T.; Ito, S.; Tajika, M.; Kawai, H.; Muro, K. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second-line chemotherapy with weekly paclitaxel. Ann. Oncol. 2010, 21, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhong, X.; Ma, J.; Sun, W.; Han, H.S.; Soliman, H.H.; Loftus, L.S.; Costa, R.L.B.; Armaghani, A.J.; Soyano-Muller, A.E.; et al. Real-world benefit of combination palbociclib and endocrine therapy for metastatic breast cancer and correlation with neutropenia. Cancer Med. 2021, 10, 7665–7672. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 175883591879332. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, D.D. A Population Pharmacokinetic (Pk) Analysis of Palbociclib (Pd-0332991) in Patients (Pts) with Advanced Solid Tumors. Ann. Oncol. 2014, 25, iv154. [Google Scholar] [CrossRef]
- Yu, Y.; Loi, C.-M.; Hoffman, J.; Wang, D. Physiologically Based Pharmacokinetic Modeling of Palbociclib: PBPK Modeling of Palbociclib. J. Clin. Pharmacol. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Hu, W.; Sung, T.; Jessen, B.A.; Thibault, S.; Finkelstein, M.B.; Khan, N.K.; Sacaan, A.I. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin. Cancer Res. 2016, 22, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- DeMichele, A.; Clark, A.S.; Tan, K.S.; Heitjan, D.F.; Gramlich, K.; Gallagher, M.; Lal, P.; Feldman, M.; Zhang, P.; Colameco, C.; et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, M.E.; Danesi, R.; Girmenia, C.; Invernizzi, P.; Elvevi, A.; Uguccioni, M.; on behalf of NetworkER+. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: A multidisciplinary approach is the key to success. Breast Cancer Res. Treat. 2019, 176, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs part two—Targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef]
- Bellet, M.; Ahmad, F.; Villanueva, R.; Valdivia, C.; Palomino-Doza, J.; Ruiz, A.; Gonzalez, X.; Adrover, E.; Azaro, A.; Valls-Margarit, M.; et al. Palbociclib and ribociclib in breast cancer: Consensus workshop on the management of concomitant medication. Ther. Adv. Med. Oncol. 2019, 11, 175883591983386. [Google Scholar] [CrossRef]
- Sun, W.; Klamerus, K.J.; Yuhas, L.M.; Pawlak, S.; Plotka, A.; O’Gorman, M.; Kirkovsky, L.; Kosa, M.; Wang, D. Impact of Acid-Reducing Agents on the Pharmacokinetics of Palbociclib, a Weak Base with pH-Dependent Solubility, with Different Food Intake Conditions. Clin. Pharmacol. Drug Dev. 2017, 6, 614–626. [Google Scholar] [CrossRef]
- Momper, J.D.; Yang, J.; Kerr, J.; Saunders, I.; Smith, J.; Shah, M.M. Interaction Between Cyclosporine and Palbociclib in a Renal Transplant Patient: Case Report and Pharmacokinetic Perspective. J. Pharm. Pract. 2020, 33, 912–914. [Google Scholar] [CrossRef]
- Gowarty, J.L.; Herrington, J.D. Verapamil as a culprit of palbociclib toxicity. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2019, 25, 743–746. [Google Scholar] [CrossRef]
- Roncato, R.; Angelini, J.; Pani, A.; Cecchin, E.; Sartore-Bianchi, A.; Siena, S.; De Mattia, E.; Scaglione, F.; Toffoli, G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int. J. Mol. Sci. 2020, 21, 76350. [Google Scholar] [CrossRef]
- Leenhardt, F.; Gracia, M.; Perrin, C.; Muracciole-Bich, C.; Marion, B.; Roques, C.; Alexandre, M.; Firmin, N.; Pouderoux, S.; Mbatchi, L.; et al. Liquid chromatography–tandem mass spectrometric assay for the quantification of CDK4/6 inhibitors in human plasma in a clinical context of drug-drug interaction. J. Pharm. Biomed. Anal. 2020, 188, 113438. [Google Scholar] [CrossRef]
- Diéras, V.; Rugo, H.S.; Schnell, P.; Gelmon, K.; Cristofanilli, M.; Loi, S.; Colleoni, M.; Lu, D.R.; Mori, A.; Gauthier, E.; et al. Long-term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for HR+/HER2- Advanced Breast Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Diéras, V.; Harbeck, N.; Joy, A.A.; Gelmon, K.; Ettl, J.; Verma, S.; Lu, D.R.; Gauthier, E.; Schnell, P.; Mori, A.; et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2− Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019, 24, 1514–1525. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yu, Y.; Durairaj, C.; Diéras, V.; Finn, R.S.; Wang, D.D. Impact of Dose Reduction on Efficacy: Implications of Exposure-Response Analysis of Palbociclib. Target Oncol. 2020, 16, 69–76. [Google Scholar] [CrossRef]
- Mukai, H.; Shimizu, C.; Masuda, N.; Ohtani, S.; Ohno, S.; Takahashi, M.; Yamamoto, Y.; Nishimura, R.; Sato, N.; Ohsumi, S.; et al. Palbociclib in combination with letrozole in patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int. J. Clin. Oncol. 2019, 24, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Rugo, H.S.; Gelmon, K.A.; Cristofanilli, M.; Colleoni, M.; Loi, S.; Colleoni, M.; Lu, D.R.; Mori, A.; Gauthier, E.; et al. Long-Term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Updated Analysis with up to 5 Years of Follow-Up. Oncologist 2021, 26, e749–e755. [Google Scholar] [CrossRef] [PubMed]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Takayama, K.; Taguchi, T. Predictors for development of palbociclib-induced neutropenia in breast cancer patients as determined by ordered logistic regression analysis. Sci. Rep. 2021, 11, 20055. [Google Scholar] [CrossRef] [PubMed]
- Marouille, A.L.; Petit, E.; Kaderbhaï, C.; Desmoulins, I.; Hennequin, A.; Mayeur, D.; Fumet, J.-D.; Ladoire, S.; Tharin, Z.; Ayati, S.; et al. Pharmacokinetic/Pharmacodynamic Model of Neutropenia in Real-Life Palbociclib-Treated Patients. Pharmaceutics 2021, 13, 1708. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hoffman, J.; Plotka, A.; O’Gorman, M.; Shi, H.; Wang, D. Palbociclib (PD-0332991) pharmacokinetics in subjects with impaired renal function. Cancer Chemother. Pharmacol. 2020, 86, 701–710. [Google Scholar] [CrossRef]
- Molenaar-Kuijsten, L.; Braal, C.L.; Groenland, S.L.; Vries, N.; Rosing, H.; Beijnen, J.H.; Koolen, S.L.W.; Vulink, A.J.E.; van Dongen, M.G.J.; Mathijssen, R.H.J.; et al. Effects of the Moderate CYP3A4 Inhibitor Erythromycin on the Pharmacokinetics of Palbociclib: A Randomized Crossover Trial in Patients with Breast Cancer. Clin. Pharmacol. Ther. 2021, 111, 477–484. [Google Scholar] [CrossRef]
- Del Re, M.; Omarini, C.; Diodati, L.; Palleschi, M.; Meattini, I.; Crucitta, S.; Lorenzini, G.; Isca, C.; Fontana, A.; Livi, L.; et al. Drug–drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open 2021, 6, 100231. [Google Scholar] [CrossRef]
- Mir, O.; Touati, N.; Lia, M.; Litière, S.; Le Cesne, A.; Sleijfer, S.; Blay, J.-Y.; Leahy, M.; Young, R.; Mathijssen, R.H.J.; et al. Impact of Concomitant Administration of Gastric Acid-Suppressive Agents and Pazopanib on Outcomes in Soft-Tissue Sarcoma Patients Treated within the EORTC 62043/62072 Trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).