Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date
Abstract
1. Introduction
2. Anatomy and Physiology of the CNS
2.1. Barriers to CNS Drug Delivery
2.1.1. The Blood-Brain Barrier (BBB)
2.1.2. The Blood-Cerebrospinal Fluid Barrier (BCSFB)
2.1.3. The Blood-Brain–Tumor Barrier (BBTB)
2.1.4. Efflux Transporters
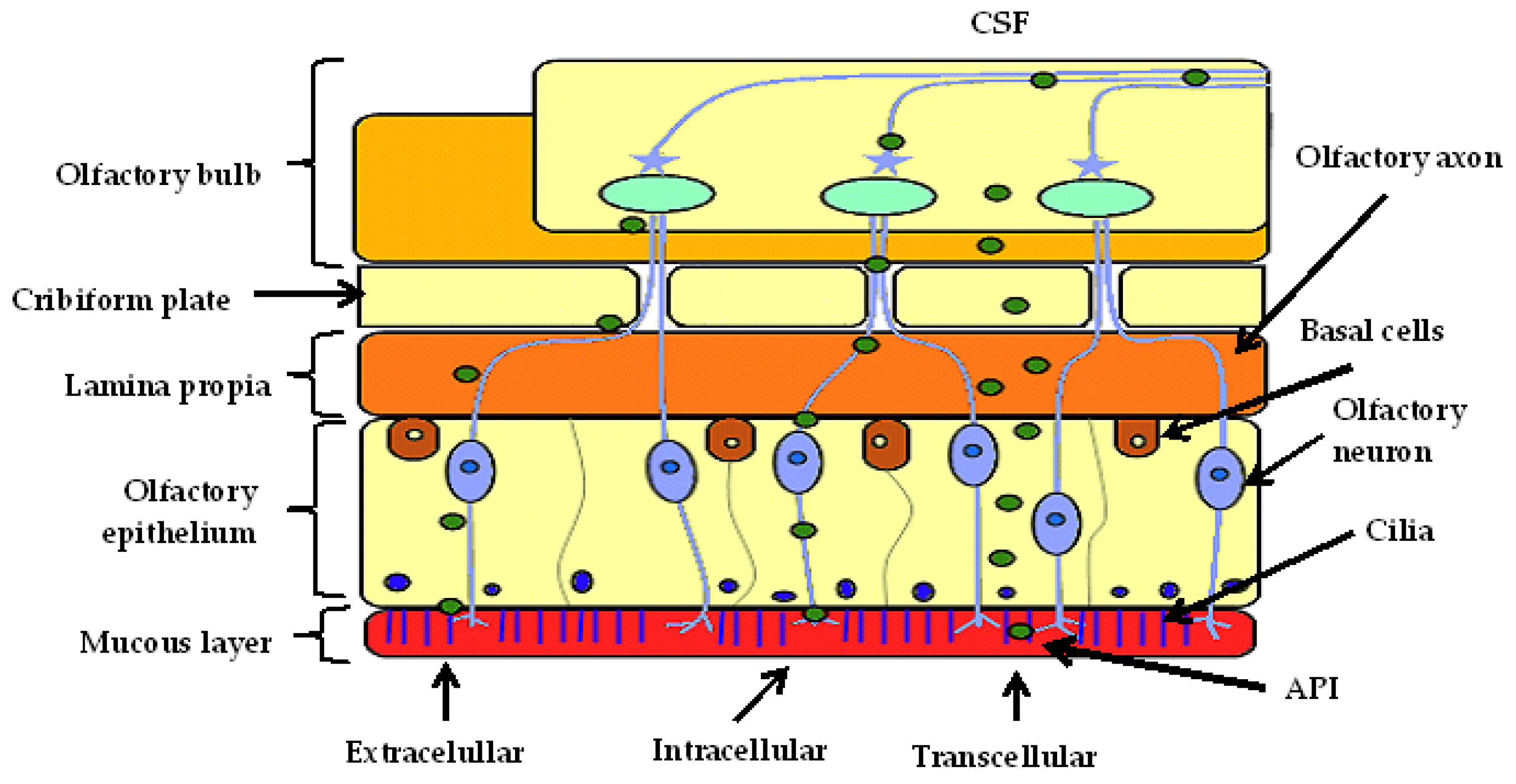
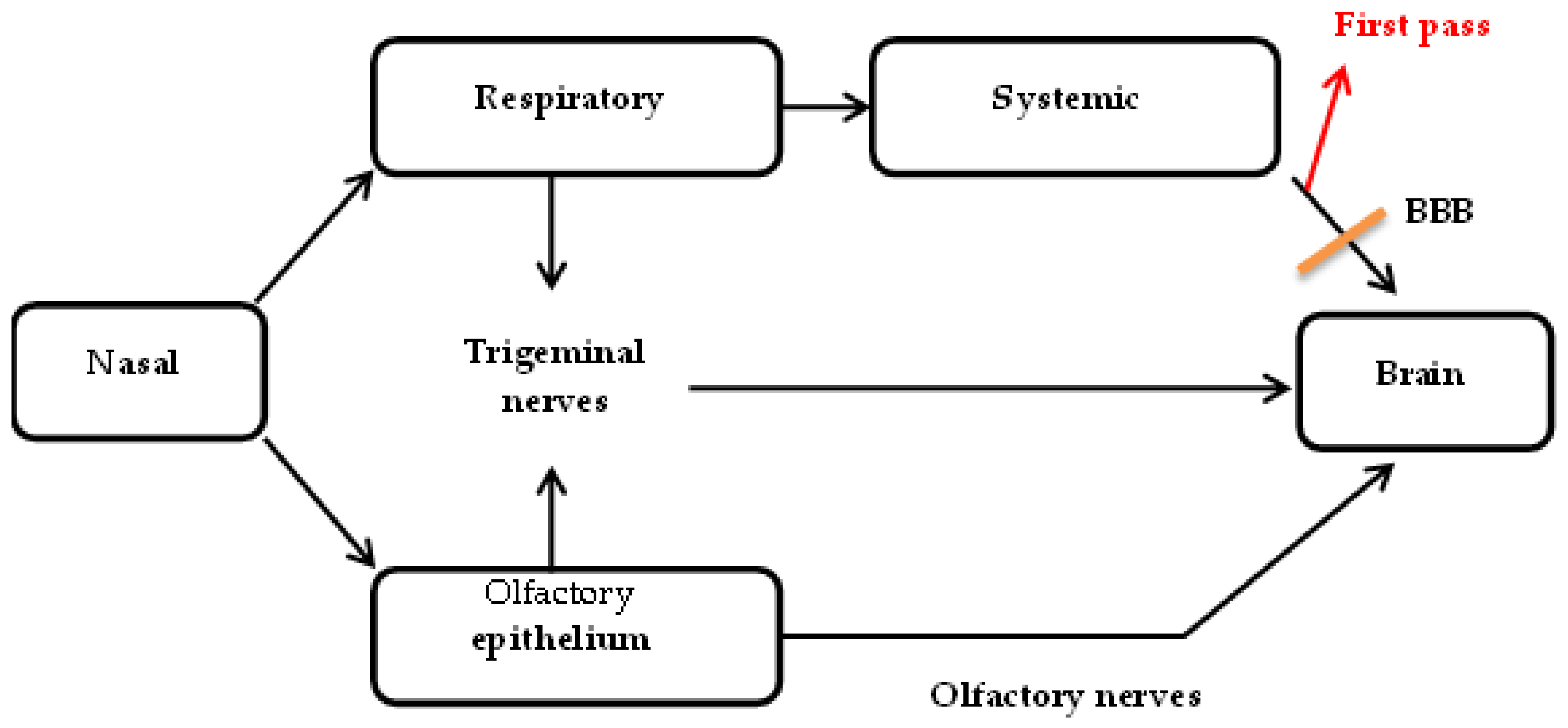
2.2. Nose-to-Brain Drug Delivery
2.2.1. Intracellular Transport
2.2.2. Extracellular Transport
2.2.3. Transcellular Transport
2.2.4. Enzymatic Activity
2.2.5. Elimination
2.2.6. Factors Affecting API Transport
Nasal pH
Isotonicity
Deposition and Access Volume
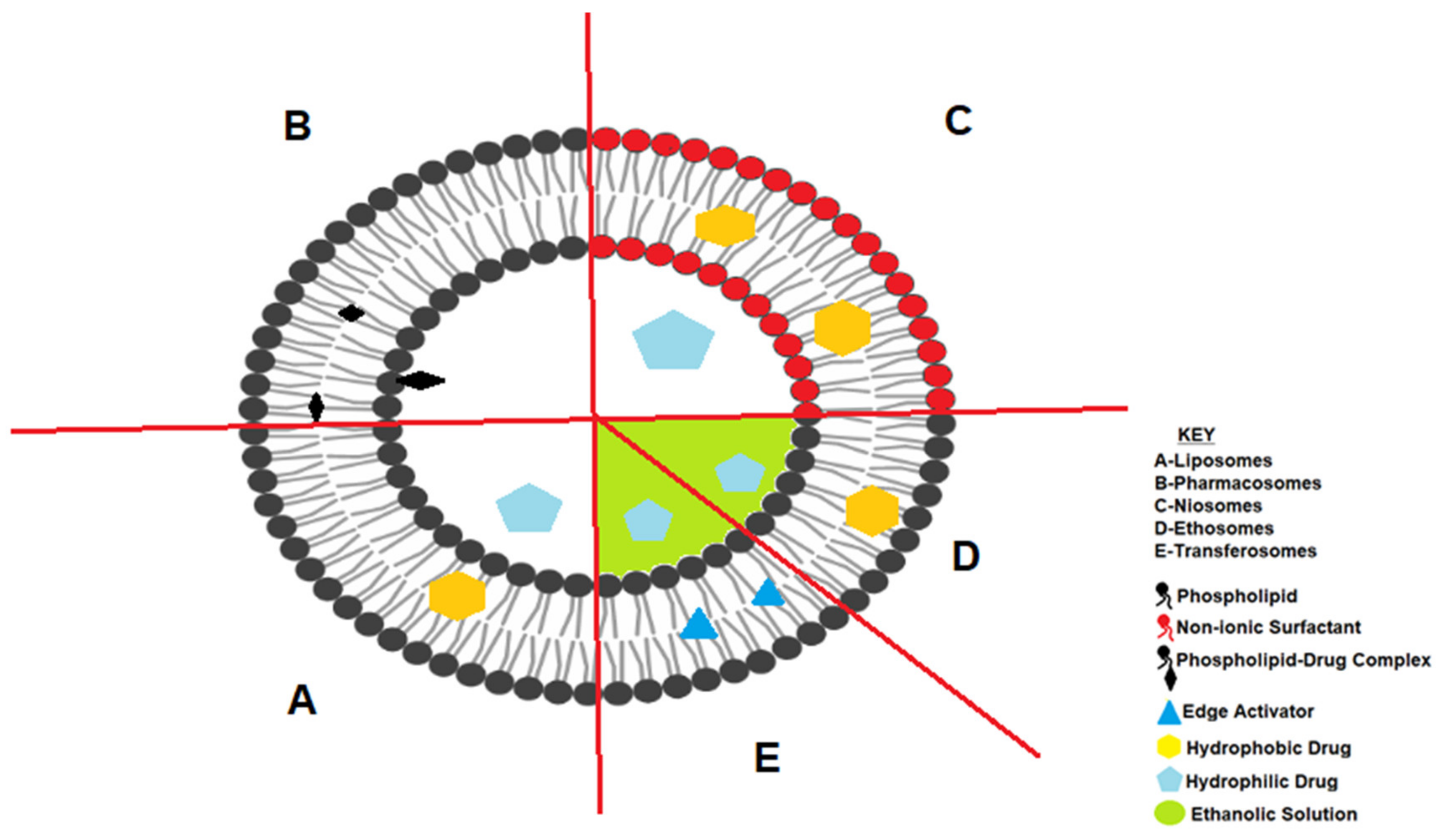
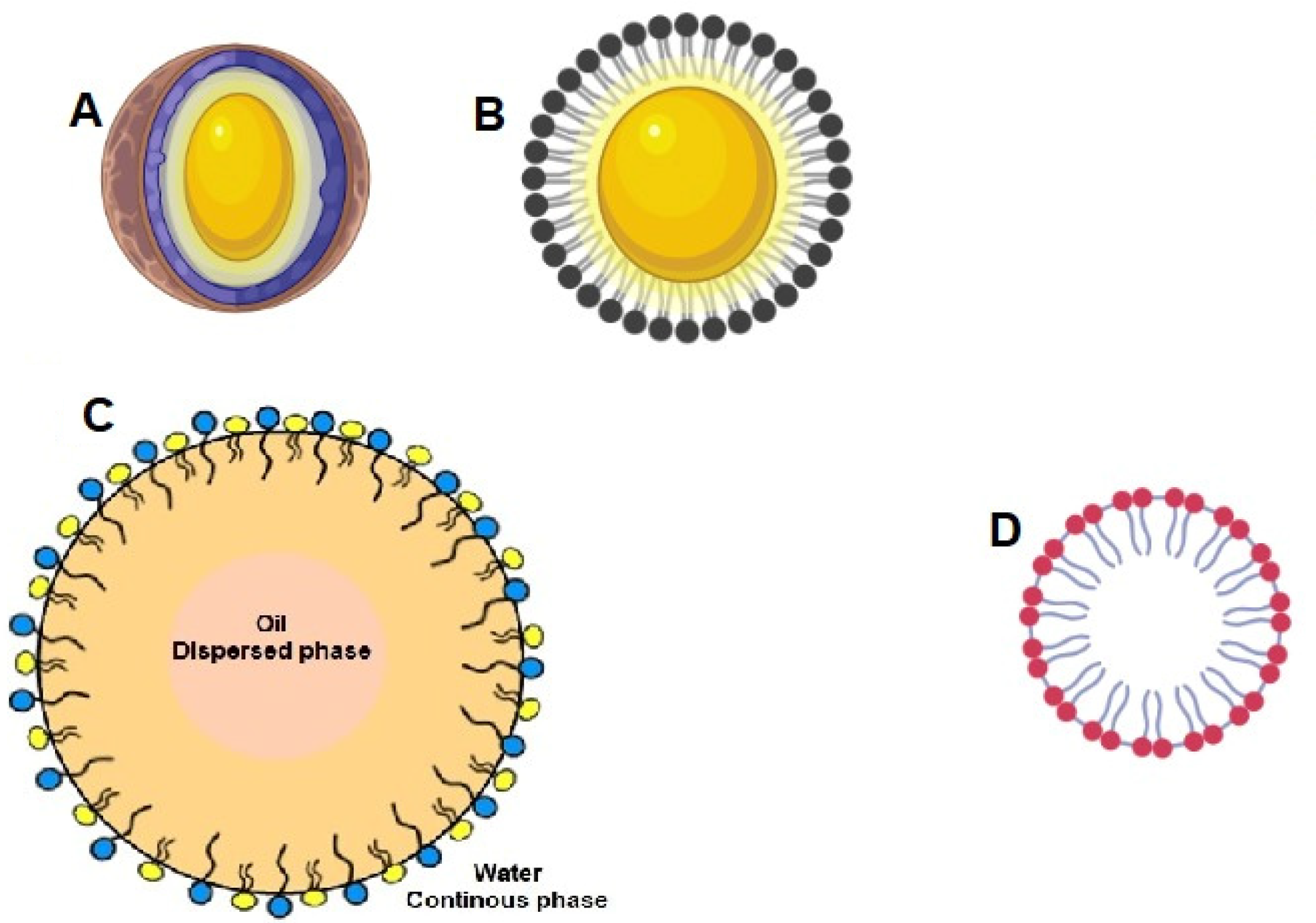
3. Bilayered Lipid Nanocarriers
3.1. Liposomes
3.2. Pharmacosomes
3.3. Ethosomes and Transferosomes
3.4. Niosomes
3.5. Unilayered Lipid Drug Delivery Systems
3.5.1. Solid Lipid Nanoparticles
3.5.2. Nanostructured Lipid Carriers
3.5.3. Lipid-Drug Conjugates (LDC)
3.5.4. Nanocapsules
3.5.5. Micelles
3.5.6. Self-Nanoemulsifying Drug Delivery Systems
3.6. Submicron Emulsions
Microemulsions and Nanoemulsions
4. The Application of Lipid Based Nanocarriers in the Treatment of Neurological Disorders
4.1. Neurodegenerative Disorders
4.1.1. Alzheimer’s Disease
4.1.2. Parkinson’s Disease
4.1.3. Dementia
4.2. Epilepsy
4.3. Ischaemic Stroke
4.4. CNS Neoplastic Disease
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pottoo, F.H.; Sharma, S.; Javed, M.N.; Barkat, M.A.; Harshita; Alam, M.S.; Naim, M.J.; Alam, O.; Ansari, M.A.; Barreto, G.E.; et al. Lipid-based nanoformulations in the treatment of neurological disorders. Drug Metab. Rev. 2020, 52, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Koul, V.; Singh, Y.; Anand, A. Targeted Drug Delivery to Central Nervous System (CNS) for the Treatment of Neurodegenerative Disorders: Trends and Advances. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Allhenn, D.; Shetab Boushehri, M.A.; Lamprecht, A. Drug delivery strategies for the treatment of malignant gliomas. Int. J. Pharm. 2012, 436, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Micheli, M.R.; Bova, R.; Magini, A.; Polidoro, M.; Emiliani, C. Lipid-Based Nanocarriers for CNS-Targeted Drug Delivery. Recent Pat. CNS Drug Discov. 2012, 7, 71–86. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csóka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Odeh, F.; Al-Adaileh, F.; Al-Taher, S.; Jaber, A.M.; Alshaer, W.; Al Bawa, A.; Mubarak, M.S. Lipid nanostructures for targeting brain cancer. Heliyon 2021, 7, e07994. [Google Scholar] [CrossRef]
- Ali, O.A.M.A.; Shaikh, M.F.; Hasnain, M.S.; Sami, F.; Khan, A.; Ansari, M.T. Nanotechnological Advances in the Treatment of Epilepsy. CNS Neurol. Disord. Drug Targets 2021, 21. E-pub Ahead of Print. [Google Scholar] [CrossRef]
- Shilpi, S.; Jain, A.; Dixit, S.; Saraogi, G.; Yadav, A.K.; Jain, S.K. Lipid nanocarrier-based drug delivery for the treatment of brain-related disorders. In Nanomedical Drug Delivery for Neurodegenerative Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 55–65. [Google Scholar]
- Nguyen, T.T.; Nguyen, T.T.D.; Tran, N.M.A.; Van Vo, G. Lipid-Based Nanocarriers via Nose-to-Brain Pathway for Central Nervous System Disorders. Neurochem. Res. 2022, 47, 552–573. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug targeting to the brain. Pharm. Res. 2007, 24, 1733–1744. [Google Scholar] [CrossRef]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for enhancing the permeation of cns-active drugs through the blood-brain barrier: A review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Medicin. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Sorokin, L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin. Immunopathol. 2009, 31, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Martin, D.; Byrne, M. Recent advances in delivery through the blood-brain barrier. Curr. Top. Med. Chem. 2014, 14, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Ha, S.; Hochman, J.; Sheridan, R. Mini review on molecular modeling of P-glycoprotein (Pgp). Curr. Top. Med. Chem. 2007, 7, 1525–1529. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2, a002907. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudin interactions in and out of the tight junction. Tissue Barriers 2013, 1, e25247. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Segal, M.B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 2004, 56, 1695–1716. [Google Scholar] [CrossRef] [PubMed]
- Quintela, T.; Albuquerque, T.; Lundkvist, G.; Carmine Belin, A.; Talhada, D.; Gonçalves, I.; Carro, E.; Santos, C.R.A. The choroid plexus harbors a circadian oscillator modulated by estrogens. Chronobiol. Int. 2017, 35, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Noe, C.R.; Noe-Letschnig, M.; Handschuh, P.; Noe, C.A.; Lanzenberger, R. Dysfunction of the Blood-Brain Barrier—A Key Step in Neurodegeneration and Dementia. Front. Aging Neurosci. 2020, 12, 185. [Google Scholar] [CrossRef]
- Groblewska, M.; Mroczko, B. Pro-and antiangiogenic factors in gliomas: Implications for novel therapeutic possibilities. Int. J. Mol. Sci. 2021, 22, 6126. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balca-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight junction proteins and signaling pathways in cancer and inflammation: A functional crosstalk. Front. Physiol. 2019, 10, 1942. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. Biomed Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta Biomembr. 2009, 1788, 872–891. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-W.O.; Kim, C.-H.; Cheong, J.-H.; Bak, K.-H.; Kim, J.-M.; Oh, S.-J. Occludin Expression in Brain Tumors and its Relevance to Peritumoral Edema and Survival. Cancer Res. Treat. 2006, 38, 139–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bendayan, R.; Ronaldson, P.T.; Gingras, D.; Bendayan, M. In Situ Localization of P-glycoprotein (ABCB1) in Human and Rat Brain. J. Histochem. Cytochem. 2006, 54, 1159. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef]
- Meyer, J.; Rauh, J.; Galla, H.-J. The susceptibility of cerebral endothelial cells to astroglial induction of blood-brain barrier enzymes depends on their proliferative state. J. Neurochem. 1991, 57, 1971–1977. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Perrin, R.; Leininger-Muller, B.; Grassiot, M.C.; Jeandel, C.; Floquet, J.; Cuny, G.; Siest, G.; Minn, A. Subcellular localization of cytochrome P450, and activities of several enzymes responsible for drug metabolism in the human brain. Biochem. Pharmacol. 1993, 45, 647–658. [Google Scholar] [CrossRef]
- Eyal, S.; Hsiao, P.; Unadkat, J.D. Drug interactions at the blood-brain barrier: Fact or fantasy? Pharmacol. Ther. 2009, 123, 80. [Google Scholar] [CrossRef]
- Nicolazzo, J.; Katneni, K. Drug transport across the blood-brain barrier and the impact of breast cancer resistance protein (ABCG2). Curr. Top. Med. Chem. 2009, 9, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, A.; Niemi, M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 2009, 158, 693. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.A.; Foreman, K.B.; Albertine, K.H. Nasal Cavity. In The Big Picture: Gross Anatomy; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; David, T.P. Perivascular and perineural pathways involved in brain delivery and distribution of drugs after intranasal administration. Pharmaceutics 2019, 11, 598. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Vyas, T.K.; Shahiwala, A.; Marathe, S.; Misra, A. Intranasal drug delivery for brain targeting. Curr. Drug Deliv. 2005, 2, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.T. Transport of molecules from nose to brain: Transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res. Bull. 1985, 15, 129–142. [Google Scholar] [CrossRef]
- Broadwell, R.D.; Balin, B.J. Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J. Comp. Neurol. 1985, 242, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef]
- Haberly, L.B.; Price, J.L. The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res. 1977, 129, 152–157. [Google Scholar] [CrossRef]
- Robertson, B.; Grant, G. A comparison between wheat germ agglutinin- and choleragenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurones in the rat with some observations in the cat. Neuroscience 1985, 14, 895–905. [Google Scholar] [CrossRef]
- Bojsen-Moller, F. Demonstration of terminalis, olfactory, trigeminal and perivascular nerves in the rat nasal septum. J. Comp. Neurol. 1975, 159, 245–256. [Google Scholar] [CrossRef]
- Wauthoz, N.; van Woensel, M.; Rosiere, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; van Gool, S.W.; de Vleeschouwer, S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers 2013, 5, 1020–1048. [Google Scholar]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef]
- Hoekman, J.D.; Ho, R.J.Y. Effects of localized hydrophilic mannitol and hydrophobic nelfinavir administration targeted to olfactory epithelium on brain distribution. AAPS Pharm. Sci. Tech. 2011, 12, 534–543. [Google Scholar] [CrossRef]
- Balin, B.J.; Broadwell, R.D.; Salcman, M.; El-Kalliny, M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol. 1986, 251, 260–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, Y.; Chen, J.; Gao, X.; Feng, C.; Wang, L.; Zhang, Q.; Jiang, X. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm. Res. 2012, 29, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Broadwell, R.D. The morphological approach to the study of normal and abnormal brain permeability. Adv. Exp. Med. Biol. 1976, 69, 41–54. [Google Scholar] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcao, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–331. [Google Scholar] [CrossRef]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef]
- Alagusundaram, M.; Chengaiah, B.; Gnanaprakash, K.; Ramkanth, S.; Madhusudhana, C.C.; Dhachinamoorthi, D. Nasal drug delivery system—An overview. Int. J. Res. Pharm. Sci. 2010, 1, 454–465. [Google Scholar]
- Oliveira, P.; Fortuna, A.; Alves, G.; Falcao, A. Drug-metabo-lizing enzymes and efflux transporters in nasal epithelium: Influence on the bioavailability of intranasally adminis-tered drugs. Curr. Drug Metab. 2016, 17, 628–647. [Google Scholar] [CrossRef]
- Heydel, J.M.; Faure, P.; Neiers, F. Nasal odorant metabolism: Enzymes, activity and function in olfaction. Drug Metab. Rev. 2019, 51, 224–245. [Google Scholar] [CrossRef]
- Heydel, J.M.; Coelho, A.; Thiebaud, N.; Legendre, A.; Le Bon, A.M.; Faure, P.; Neiers, F.; Artur, Y.; Golebiowski, J.; Briand, L. Odorant-binding proteins and xenobiotic metabolizing enzymes: Implications in olfactory perireceptor events. Anat. Rec. 2013, 296, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Mundlia, J.; Mukesh, K. Nasal drug delivery: An overview. Int. J. Pharm. Sci. Res. 2015, 6, 19. [Google Scholar]
- Prajapati, S.T.; Pathak, S.P.; Thakkar, J.H.; Patel, C.N. Nanoemulsion based intranasal delivery of risperidone for nose to brain targeting. Bull. Pharm. Res. 2015, 5, 6–13. [Google Scholar]
- Perucca, E. The Clinical Pharmacokinetics of the New Antiepileptic Drugs. Epilepsia 1999, 40, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Sakane, T.; Yamashita, S.; Yata, N.; Sezaki, H. Transnasal delivery of 5-fluorouracil to the brain in the rat. J. Drug Target. 1999, 7, 233–240. [Google Scholar] [CrossRef]
- Wu, I.Y.; Nikolaisen, T.E.; Skalko-Basnet, N.; di Cagno, M.P. The Hypotonic Environmental Changes Affect Liposomal Formulations for Nose-to-Brain Targeted Drug Delivery. J. Pharm. Sci. 2019, 108, 2570–2579. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Hounam, R.F.; Black, A.; Walsh, M. The deposition of aerosol particles in the nasopharyngeal region of the human respiratory tract. Inhaled Part 1970, 1, 47–61. [Google Scholar]
- Proctor, D.F.; Anderson, I.; Lundqvist, G. Clearance of inhaled particle from human nose. Arch. Intern. Med. 1973, 131, 132–139. [Google Scholar] [CrossRef]
- Bangham, A.D. Surrogate Cells or Trojan Horses: The Discovery of Liposomes. BioEssays 1995, 17, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, IN26–IN27. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Mishra, P.; Khar, R.; Biju, S. Vesicular systems: An overview. Indian J. Pharm. Sci. 2006, 68, 141. [Google Scholar] [CrossRef]
- Allen, T.M. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs 1998, 56, 747–756. [Google Scholar] [CrossRef]
- Isalomboto Nkanga, C.; Murhimalika Bapolisi, A.; Ikemefuna Okafor, N.; Werner Maçedo Krause, R. General Perception of Liposomes: Formation, Manufacturing and sApplications. In Liposomes—Advances and Perspectives; Catala, A., Ed.; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar]
- Witika, B.A. The Development, Manufacture and Characterisation of Niosomes Intended to Deliver Nevirapine to the Brain. Master’s Thesis, Rhodes University, Makhanda, South Africa, 2017. [Google Scholar]
- Abdelbary, G.; El-gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef]
- Désormeaux, A.; Bergeron, M.G. Lymphoid tissue targeting of anti-HIV drugs using liposomes. Methods Enzymol. 2005, 391, 330–351. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Q. Development of liposomal formulations: From concept to clinical investigations. Asian J. Pharm. Sci. 2013, 8, 79–90. [Google Scholar] [CrossRef]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 3–53. ISBN 978-0-8247-5056-5. [Google Scholar]
- Nkanga, C.I.; Krause, R.W.M. Conjugation of isoniazid to a zinc phthalocyanine via hydrazone linkage for pH-dependent liposomal controlled release. Appl. Nanosci. 2018, 8, 1313–1323. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Roth, M.; Walker, R.B.; Noundou, X.S.; Krause, R.W.M. Co-loading of isoniazid-grafted phthalocyanine-in-cyclodextrin and rifampicin in crude soybean lecithin liposomes: Formulation, spectroscopic and biological characterization. J. Biomed. Nanotechnol. 2020, 16, 14–28. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Vorauer-Uhl, K. Liposome Technology for Industrial Purposes, 3rd ed.; Informa Healthcare Inc.: New York, NY, USA, 2011; Volume 2011, ISBN 9780849388217. [Google Scholar]
- Gregoriadis, G. Immunological adjuvants: A role for liposomes. Immunol. Today 1990, 11, 89–97. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Rawat, B.S.; Singh, D.; Rawat, M. Pharmacosomes: The lipid-based new drug delivery system. Expert Opin. Drug Deliv. 2009, 6, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, M.; Singh, R.; Goel, R.K.; Nath, G. Recent advances in various emerging vescicular systems: An overview. Asian Pac. J. Trop. Biomed. 2012, 2, S1176–S1188. [Google Scholar] [CrossRef]
- Venkatesh, D.N.; Kalyani, K.; Tulasi, K.; Priyanka, V.S.; Ali, S.A.; Kumar, S.S. Pharmacosomes: A Potential Vesicular Drug Delivery System. Int. J. Pharm. Sci. Drug Res. 2014, 6, 90–94. [Google Scholar]
- Pandita, A.; Sharma, P.; Pandita, A.; Sharma, P. Pharmacosomes: An Emerging Novel Vesicular Drug Delivery System for Poorly Soluble Synthetic and Herbal Drugs. ISRN Pharm. 2013, 2013, 348186. [Google Scholar] [CrossRef]
- Goyal, T.; Rawat, A.S.; Chauhan, M. Pharmacosomes: Opening new doors for drug delivery. Int. J. Pharm. Pharm. Sci. 2012, 4, 25–29. [Google Scholar]
- Kavitha, D.; Naga Sowjanya, J.; Panaganti, S. Pharmacosomes: An emerging vesicular system. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 168–171. [Google Scholar]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M.S.M. Development and physicochemical evaluation of pharmacosomes of diclofenac. Acta Pharm. 2009, 59, 335–344. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M.S.M. Development and Evaluation of Pharmacosomes of Aceclofenac. Indian J. Pharm. Sci. 2010, 72, 576–581. [Google Scholar] [CrossRef]
- Singh, D.; Pradhan, M.; Nag, M.; Singh, M.R. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artif. Cells Nanomed. Biotechnol. 2015, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Cevc, G.; Schätzlein, A.; Blume, G. Transdermal drug carriers: Basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J. Control. Release 1995, 36, 3–16. [Google Scholar] [CrossRef]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schätzlein, A.; Blume, G. Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta Biomembr. 1998, 1368, 201–215. [Google Scholar] [CrossRef]
- Lai, F.; Caddeo, C.; Manca, M.L.; Manconi, M.; Sinico, C.; Fadda, A.M. What’s new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 2020, 583, 119398. [Google Scholar] [CrossRef]
- Solanki, D.; Kushwah, L.; Motiwale, M.; Chouhan, V. Transferosomes—A Review. World J. Pharm. Pharm. Sci. 2016, 5, 435–449. [Google Scholar] [CrossRef]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Oestradiol skin delivery from ultradeformable liposomes: Refinement of surfactant concentration. Int. J. Pharm. 2000, 196, 63–74. [Google Scholar] [CrossRef]
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Arami, S. Formulation and evaluation of piroxicam transferosomal gel: An approach for penetration enhancement. J. Drug Deliv. Sci. Technol. 2013, 23, 587–590. [Google Scholar] [CrossRef]
- Rai, K.; Gupta, Y.; Jain, A.; Jain, S.K. Transfersomes: Self-optimizing carriers for bioactives. PDA J. Pharm. Sci. Technol. 2008, 62, 362–379. [Google Scholar]
- Ramsden, J.J. Applied Nanotechnology: The Conversion of Research Results to Products (Micro and Nano Technologies); Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9781455731893. [Google Scholar]
- Cevc, G.; Blume, G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim. Biophys. Acta Biomembr. 2001, 1514, 191–205. [Google Scholar] [CrossRef]
- Kumar, K.K.; Sasikanth, K.; Sabareesh, M.; Dorababu, N. Formulation and evaluation of diacerein cream. Asian J. Pharm. Clin. Res. 2011, 4, 93–98. [Google Scholar] [CrossRef]
- Agarwal, R.; Katare, O.P.; Vyas, S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Srinivas, S.; Anand Kumar, Y.; Hemanth, A.; Anitha, M. Preparation and evaluation of niosomes containing aceclofenac. Dig. J. Nanomater. Biostructures 2010, 5, 249–254. [Google Scholar]
- Shakya, V.; Bansal, B.K. Niosomes: A Novel Trend in Drug Delivery. Int. J. Res. Dev. Pharm. Life Sci. 2014, 3, 1036–1041. [Google Scholar]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Double, J.A.; Turton, J.A.; Florence, A.T. Distribution, Metabolism and Tumoricidal Activity of Doxorubicin Administered in Sorbitan Monostearate (Span 60) Niosomes in the Mouse. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 1019–1024. [Google Scholar]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int. J. Pharm. 2006, 314, 83–89. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2002, 47, 165–196. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers-Lipid Nanoparticles for Medicals and Pharmaceuticals. Encycl. Nanosci. Nanotechnol. 2011, 23, 313–328. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fang, C.L.; Liu, C.H.; Su, Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Shah, J.C.; Sadhale, Y.; Chilukuri, D.M. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Muller, R.H. Comparative study between the viscoelastic behaviors of different lipid nanoparticle formulations. Int. J. Cosmet. Sci. 2004, 55, 36. [Google Scholar] [CrossRef]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F. Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Gautam, L.; Jain, A.; Vishwakarma, N.; Vyas, S.; Vyas, S.P. Lipid Drug Conjugates for Improved Therapeutic Benefits. Curr. Pharm. Des. 2020, 26, 3187–3202. [Google Scholar] [CrossRef]
- Mouzouvi, C.R.A.; Umerska, A.; Bigot, A.K.; Saulnier, P. Surface active properties of lipid nanocapsules. PLoS ONE 2017, 12, e0179211. [Google Scholar] [CrossRef]
- Matougui, N.; Boge, L.; Groo, A.C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. A Novel Phase Inversion-Based Process for the Preparation of Lipid Nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Bryant, A.A.; Vanden Berg-Foels, W.S.; Wen, X. Bioengineering Strategies for Designing Targeted Cancer Therapies. Adv. Cancer Res. 2013, 118, 1–59. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Okano, T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J. Control. Release 2000, 65, 93–103. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Effect of molecular architecture of hydrophobically modified poly(N-isopropylacrylamide) on the formation of thermoresponsive core-shell micellar drug carriers. J. Control. Release 1998, 53, 119–130. [Google Scholar] [CrossRef]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, properties, and applications of solid self-emulsifying delivery systems (S-SEDS) in the food and pharmaceutical industries. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Komaiko, J.S.; Mcclements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Karunaratne, N.; Pamunuwa, G.; Ranatunga, U. Introductory Chapter: Microemulsions. In Properties and Uses of Microemulsions; Intech: Gonawila, Sri Lanka, 2017. [Google Scholar]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, M.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS PharmSciTech 2011, 13, 184–192. [Google Scholar] [CrossRef]
- Kumara, P.; Mohanb, C.; Uma Shankara, M.K.S.; Gulatia, M. Physiochemical characterization and release rate studies of solid dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran. J. Pharm. Res. 2011, 10, 685–694. [Google Scholar] [CrossRef]
- Neubauer, R.; Höhn, S.; Dulle, M.; Lapp, A.; Schulreich, C.; Hellweg, T. Protein diffusion in a bicontinuous microemulsion: Inducing sub-diffusion by tuning the water domain size. Soft Matter 2017, 13, 1998–2003. [Google Scholar] [CrossRef]
- Kronberg, B. The hydrophobic effect. Curr. Opin. Colloid Interface Sci. 2016, 22, 14–22. [Google Scholar] [CrossRef]
- Bardhan, S.; Kundu, K.; Chakraborty, G.; Saha, S.K.; Swapan, B.; Bidyut, B.; Paul, B.K. The Schulman Method of Cosurfactant Titration of the Oil/Water Interface (Dilution Method): A Review on a Well-Known Powerful Technique in Interfacial Science for Characterization of Water-in-Oil Microemulsions. J. Surfactants Deterg. 2015, 18, 547–567. [Google Scholar] [CrossRef]
- Paul, K.B.; Moulik, S.P. The viscosity behaviour of microemulsions: An overview. Proc. Indian Natl. Sci. Acad. 2000, 66, 499. [Google Scholar]
- Sarhan, O.; Ibrahim, M.M.; Mahdy, M. Microemulsions as antidiabetic drug delivery systems. Int. J. Pharm. Sci. Res. 2012, 3, 4442–4456. [Google Scholar] [CrossRef]
- Moulik, S.P.; Paul, B.K. Structure, dynamics and transport properties of microemulsions. Adv. Colloid Interface Sci. 1998, 78, 99–195. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Improved oral bioavailability of BCS class 2 compounds by self nano-emulsifying drug delivery systems (SNEDDS): The underlying mechanisms for amiodarone and talinolol. Pharm. Res. 2013, 30, 3029–3044. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Katare, O.P.; Singh, B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf. B. Biointerfaces 2012, 100, 50–61. [Google Scholar] [CrossRef]
- Kogan, A.; Shalev, D.E.; Raviv, U.; Aserin, A.; Garti, N. Formation and Characterization of Ordered Bicontinuous Microemulsions. J. Phys. Chem. 2009, 113, 10669–10678. [Google Scholar] [CrossRef]
- Solanki, S.S.; Sarkar, B.; Dhanwani, R.K. Microemulsion Drug Delivery System: For Bioavailability Enhancement of Ampelopsin. ISRN Pharm. 2012, 2012, 108164. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Formulation consideration and characterization of microemulsion drug delivery system for transnasal administration of carbamazepine. Bull. Fac. Pharmacy, Cairo Univ. 2013, 51, 243–253. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Kunstman, P.; Roszak, R.; Białas, W. Rheological and textural properties of microemulsion-based polymer gels with indomethacin. Drug Dev. Ind. Pharm. 2016, 42, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Balakrishnan, P.; Song, C.-K.; Choi, J.-H.; Noh, G.-Y.; Park, C.-G.; Choi, A.-J.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Microemulsion-based Hydrogel Formulation of Itraconazole for Topical Delivery. J. Pharm. Investig. 2010, 40, 305–311. [Google Scholar] [CrossRef]
- Mojeiko, G.; Brito, M.; Salata, G.C.; Lopes, L.B. Combination of microneedles and microemulsions to increase celecoxib topical delivery for potential application in chemoprevention of breast cancer. Int. J. Pharm. 2019, 560, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, X.-H.; Yao, G.-I.; Zhang, W.-T.; Zhao, Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Florence, K.; Manisha, L.; Kumar, B.A.; Ankur, K.; Kumar, M.A.; Ambikanandan, M. Intranasal clobazam delivery in the treatment of status epilepticus. J. Pharm. Sci. 2011, 100, 692–703. [Google Scholar] [CrossRef]
- Krstic, M.; Medarevic, Ð.; Ðuriš, J.; Ibric, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and selfmicroemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 473–508. [Google Scholar]
- Karasulu, H.Y. Microemulsions as novel drug carriers: The formation, stability, applications and toxicity. Expert Opin. Drug Deliv. 2008, 5, 119–135. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. Fabrication of Nanoemulsions by Ultrasonication. In Nanoemulsions: Formulation, Applications, and Characterization; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Gorgan, Iran, 2018. [Google Scholar]
- Calligaris, S.; Plazzotta, S.; Bot, F.; Grasselli, S.; Malchiodi, A.; Anese, M. Nanoemulsion preparation by combining high pressure homogenization and high power ultrasound at low energy densities. Food Res. Int. 2016, 83, 25–30. [Google Scholar] [CrossRef]
- Borrin, T.R.; Georges, E.L.; Moraes, I.C.F.; Pinho, S.C. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: An evaluation of process parameters and physico-chemical stability. J. Food Eng. 2016, 169, 1–9. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Feng, J.; Roché, M.; Vigolo, D.; Arnaudov, L.N.; Stoyanov, S.D.; Gurkov, T.D.; Tsutsumanova, G.G.; Stone, H.A. Nanoemulsions obtained via bubble-bursting at a compound interface. Nat. Phys. 2014, 10, 606–612. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Beg, S.; Kaur, R.; Shukla, G.; Bandopadhyay, S.; Singh, B. Systematic development of optimized SNEDDS of artemether with improved biopharmaceutical and antimalarial potential. Drug Deliv. 2016, 23, 3209–3223. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Sandhu, P.S.; Batra, R.S.; Khurana, R.K.; Singh, B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv. 2015, 22, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Hiranphinyophat, S.; Otaka, A.; Asaumi, Y.; Fujii, S.; Iwasaki, Y. Particle-stabilized oil-in-water emulsions as a platform for topical lipophilic drug delivery. Colloids Surfaces B Biointerfaces 2021, 197, 111423. [Google Scholar] [CrossRef] [PubMed]
- Bhushani, A.J.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Sureshkumar, R.; Gowthamarajan, K.; Bhavani, P. Nanoemulsion for lymphatic absorption: Investigation of fenofibrate nanoemulsion system for lymphatic uptake. Int. J. ChemTech Res. 2015, 7, 832–841. [Google Scholar]
- Ye, J.Y.; Chen, Z.Y.; Huang, C.L.; Huang, B.; Zheng, Y.R.; Zhang, Y.F.; Lu, B.Y.; He, L.; Liu, C.S.; Long, X.Y. A non-lipolysis nanoemulsion improved oral bioavailability by reducing the first-pass metabolism of raloxifene, and related absorption mechanisms being studied. Int. J. Nanomed. 2020, 15, 6503–6518. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Duong, V.A.; Maeng, H.J. Pharmaceutical formulations with p-glycoprotein inhibitory effect as promising approaches for enhancing oral drug absorption and bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Figueiredo, K.A.; Neves, J.K.O.; Da Silva, J.; De Freitas, R.M.; Carvalho, A.L.M. Phenobarbital loaded microemulsion: Development, kinetic release and quality control. Brazillian J. Pharm. Sci. 2016, 52. [Google Scholar] [CrossRef]
- Alzheimer Association Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [CrossRef]
- Thakur, K.A.; Kamboj, P.; Goswami, K.; Ahuja, K. Pathophysiology and management of alzheimer’s disease: An overview. J. Anal. Pharm. Res. 2018, 7, 226–235. [Google Scholar] [CrossRef]
- Fan, L. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2020, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.M.; Keating, G.M. Memantine A Review of its Use in Alzheimer’s Disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- Patnaik, N. Cure for Alzheimer’s Disease. World J. Neurosci. 2015, 5, 328–330. [Google Scholar] [CrossRef][Green Version]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Banerjee, D. A Primer on the Evolution of Aducanumab: The First Antibody Approved for Treatment of Alzheimer’s Disease. J. Alzheimers. Dis. 2021, 83, 1537–1552. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- ALZFORUM. Therapeutics. Available online: https://www.alzforum.org/therapeutics (accessed on 12 January 2022).
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Fischer, H.; Gottschlich, R.; Seelig, A. Blood-brain barrier permeation: Molecular parameters governing passive diffusion. J. Membr. Biol. 1998, 165, 201–211. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of alzheimer’s disease and blood-brain barrier drug delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Ganesan, P.; Park, S.Y.; Kim, J.S.; Choi, D.K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Kristensson, K.; Olsson, Y. Uptake of exogenous proteins in mouse olfactory cells. Acta Neuropathol. 1971, 19, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Layek, B.; Singh, J. Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood-Brain Barrier for Effective Treatment of Alzheimer’s Disease. Mol. Pharm. 2021, 18, 714–725. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S.; Chauhan, I.; Gaur, P.K.; Singh, A.P.; Puri, D.; Ameeduzzafar, A. Solid lipid nanoparticles for nose to brain delivery of donepezil: Formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1838–1851. [Google Scholar] [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Curcumin-donepezil–loaded nanostructured lipid carriers for intranasal delivery in an Alzheimer’s disease model. Alzheimer’s Dement. 2013, 9, P299. [Google Scholar] [CrossRef]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016, 23, 1434–1443. [Google Scholar] [CrossRef]
- Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 2015, CD001191. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Fouad Ismail, M.; Nabil ElMeshad, A.; Abdel-Hameed Salem, N. Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer’s disease. Int. J. Nanomed. 2013, 8, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.B.; Deǧim, Z.; Yilmaz, Ş.; Eiz, D.; Nacar, A. New perspective for the treatment of Alzheimer diseases: Liposomal rivastigmine formulations. Drug Dev. Ind. Pharm. 2011, 37, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Tanner, C.M. Chapter 10. Epidemiology of Parkinson’s Disease. In Movement Disorders; Watts, R.L., Standaert, D.G., Obeso, J.A., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2012. [Google Scholar]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. The Non-motor symptoms of Parkinson’ s disease: Diagnosis and management Related papers management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- De Lau, L.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Rodriguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef]
- Caiazza, M.C.; Lang, C.; Wade-Martins, R. What we can learn from iPSC-derived cellular models of Parkinson’s disease. Prog. Brain Res. 2020, 252, 3–25. [Google Scholar] [CrossRef]
- Mao, Q.; Qin, W.Z.; Zhang, A.; Ye, N. Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol. Sin. 2020, 41, 471–482. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Rajesh, R. Current development of nanocarrier delivery systems for Parkinson’s disease pharmacotherapy. J. Taiwan Inst. Chem. Eng. 2018, 87, 15–25. [Google Scholar] [CrossRef]
- Stoker, T.B.; Barker, R.A. Recent developments in the treatment of Parkinson’s Disease. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, C.T.; Ravi, P.R.; Dalvi, A.V. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int. J. Pharm. 2021, 606, 120881. [Google Scholar] [CrossRef]
- Esposito, E.; Fantin, M.; Marti, M.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Sivieri, E.; Lain, F.; Menegatti, E.; Morari, M.; et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm. Res. 2008, 25, 1521–1530. [Google Scholar] [CrossRef]
- Demirel, M.; Yazan, Y.; Müller, R.H.; Kilic, F.; Bozan, B. Formulation and in vitro-in vivo evaluation of piribedil solid lipid micro- and nanoparticles. J. Microencapsul. 2001, 18, 359–371. [Google Scholar] [CrossRef]
- Tsai, M.J.; Huang, Y.B.; Wu, P.C.; Fu, Y.S.; Kao, Y.R.; Fang, J.Y.; Tsai, Y.H. Oral apomorphine delivery from solid lipid nanoparticleswith different monostearate emulsifiers: Pharmacokinetic and behavioral evaluations. J. Pharm. Sci. 2011, 100, 547–557. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Rajput, P.V.; Belgamwar, V.S.; Tekade, A.R.; Surana, S.J. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: Application of factorial design approach. Drug Deliv. 2013, 20, 47–56. [Google Scholar] [CrossRef]
- Axelsen, T.M.; Woldbye, D.P.D. Gene therapy for Parkinson’s disease, an update. J. Parkinsons. Dis. 2018, 8, 195–215. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.E.; Kordower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010, 184, 237–264. [Google Scholar] [CrossRef]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhães, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Li, X.; Lu, C.T.; Lin, M.; Chen, L.J.; Xiang, Q.; Zhang, M.; Jin, R.R.; Jiang, X.; Shen, X.T.; et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tyson, J.; Patel, S.; Patel, M.; Katakam, S.; Mao, X.; He, W. Emerging Nanotechnology for Treatment of Alzheimer’s and Parkinson’s Disease. Front. Bioeng. Biotechnol. 2021, 9, 25. [Google Scholar] [CrossRef]
- Piersimoni, M.E.; Teng, X.; Cass, A.E.G.; Ying, L. Antioxidant lipoic acid ligand-shell gold nanoconjugates against oxidative stress caused by α-synuclein aggregates. Nanoscale Adv. 2020, 2, 5666–5681. [Google Scholar] [CrossRef]
- Hayley, S.; Kasahara, J.; Ganguly, A.; Liu, Z.; Yan, A.; Liu, Z.; Song, L.; Wang, X.; Zhang, Y.; Wu, N.; et al. Idebenone Alleviates Neuroinflammation and Modulates Microglial Polarization in LPS-Stimulated BV2 Cells and MPTP-Induced Parkinson’s Disease Mice. Front. Cell. Neurosci. 2019, 12, 529. [Google Scholar] [CrossRef]
- Gupta, B.K.; Kumar, S.; Kaur, H.; Ali, J.; Baboota, S. Attenuation of oxidative damage by coenzyme Q10 loaded nanoemulsion through oral route for the management of Parkinson’s disease. Rejuvenation Res. 2018, 21, 232–248. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Wang, Y.; Guo, Y.; Xu, X.; Huang, P.; Lian, B.; Zhang, R.; Chen, Y.; Ha, Y. Protective effects of resveratrol liposomes on mitochondria in substantia nigra cells of parkinsonized rats. Ann. Palliat. Med. 2021, 10, 2458–2468. [Google Scholar] [CrossRef]
- Kumar, S.; Dang, S.; Nigam, K.; Ali, J.; Baboota, S. Selegiline Nanoformulation in Attenuation of Oxidative Stress and Upregulation of Dopamine in the Brain for the Treatment of Parkinson’s Disease. Rejuvenation Res. 2018, 21, 464–476. [Google Scholar] [CrossRef]
- Qamar, Z.; Ashhar, M.U.; Annu; Qizilibash, F.F.; Sahoo, P.K.; Ali, A.; Ali, J.; Baboota, S. Lipid nanocarrier of selegiline augmented anti-Parkinson’s effect via P-gp modulation using quercetin. Int. J. Pharm. 2021, 609, 121131. [Google Scholar] [CrossRef] [PubMed]
- Mursaleen, L.; Noble, B.; Somavarapu, S.; Zariwala, M.G. Micellar nanocarriers of hydroxytyrosol are protective against parkinson’s related oxidative stress in an in vitro hcmec/d3-sh-sy5y co-culture system. Antioxidants 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.-d.; Wei, Y.-h. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Rahman, M.; Kumar, V. Fabrication of solid lipid nanoparticles containing vitexin protects dopaminergic neurons against 6-hydroxydopamine induced Parkinson’s disease model via altered the genetic backgrounds. J. Neurol. Sci. 2019, 405, 248. [Google Scholar] [CrossRef]
- Pardridge, W.M. Brain Delivery of Nanomedicines: Trojan Horse Liposomes for Plasmid DNA Gene Therapy of the Brain. Front. Med. Technol. 2020, 2, 8. [Google Scholar] [CrossRef]
- Shariare, M.H.; Rahman, M.; Lubna, S.R.; Roy, R.S.; Abedin, J.; Marzan, A.L.; Altamimi, M.A.; Ahamad, S.R.; Ahmad, A.; Alanazi, F.K.; et al. Liposomal drug delivery of Aphanamixis polystachya leaf extracts and its neurobehavioral activity in mice model. Sci. Rep. 2020, 10, 6938. [Google Scholar] [CrossRef]
- Spuch, C.; Navarro, C. Liposomes for Targeted Delivery of Active Agents against Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). J. Drug Deliv. 2011, 2011, 469679. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Yadav, A.; Sunkaria, A.; Singhal, N.; Sandhir, R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 239–254. [Google Scholar] [CrossRef]
- Prathipati, B.; Rohini, P.; Kola, P.K.; Reddy Danduga, R.C.S. Neuroprotective effects of curcumin loaded solid lipid nanoparticles on homocysteine induced oxidative stress in vascular dementia. Curr. Res. Behav. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Wa Kasongo, K.; Shegokar, R.; Müller, R.H.; Walker, R.B. Formulation development and in vitro evaluation of didanosine-loaded nanostructured lipid carriers for the potential treatment of AIDS dementia complex. Drug Dev. Ind. Pharm. 2011, 37, 396–407. [Google Scholar] [CrossRef]
- Kasongo, K.W.; Jansch, M.; Rainer, H.; Müller, R.B.W. Evaluation of the in vitro differential protein adsorption patterns of didanosine-loaded nanostructured lipid carriers (NLCs) for potential targeting to the brain. J. Liposome Res. 2011, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.K.; Sharma, P.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef] [PubMed]
- Takalani, F.; Kumar, P.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Lipid–drug conjugates and associated carrier strategies for enhanced antiretroviral drug delivery. Pharm. Dev. Technol. 2019, 25, 267–280. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Contado, C.; Mari, L.; Perrone, D.; Pavan, B.; Paganetto, G.; Hanuskovà, M.; Vighi, E.; Leo, E. Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid. Drug Deliv. 2014, 21, 221–232. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, K.; Xie, X.; Zhu, X.; Feng, J.; Jin, Z.; Zhang, H.; Tian, M.; Chen, H. Self-assembly hollow manganese Prussian white nanocapsules attenuate Tau-related neuropathology and cognitive decline. Biomaterials 2020, 231, 119678. [Google Scholar] [CrossRef]
- Wang, X.J.; Gao, Y.P.; Lu, N.N.; Li, W.S.; Xu, J.F.; Ying, X.Y.; Wu, G.; Liao, M.H.; Tan, C.; Shao, L.X.; et al. Endogenous Polysialic Acid Based Micelles for Calmodulin Antagonist Delivery against Vascular Dementia. ACS Appl. Mater. Interfaces 2016, 8, 35045–35058. [Google Scholar] [CrossRef]
- Choi, S.H.; Aid, S.; Caracciolo, L.; Sakura Minami, S.; Niikura, T.; Matsuoka, Y.; Turner, R.S.; Mattson, M.P.; Bosetti, F. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2013, 124, 59–68. [Google Scholar] [CrossRef]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by design based formulation study of meloxicam-loaded polymeric micelles for intranasal administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef]
- Institute for Quality and Efficiency in Health Care (IQWiG). Epilepsy: Overview. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343313/ (accessed on 22 February 2022).
- Bromfield, E.B.; Cavazos, J.E.; Sirven, J.I. Basic Mechanisms Underlying Seizures and Epilepsy. In An Introduction to Epilepsy; American Epilepsy Society: West Hartford, CN, USA, 2006. [Google Scholar]
- Ahmad, N.; Ahmad, R.; Qatifi, A.; Alessa, M.; Hajji, H.; Sarafroz, M. A bioanalytical UHPLC based method used for the quantification of Thymoquinone-loaded-PLGA-nanoparticles in the treatment of epilepsy. BMC Chem. 2020, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Chiang, B.H.; Huang, D.W.; Li, P.H. Skin permeation of d-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason. Sonochem. 2014, 21, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Porecha, S.; Pundarikakshudu, A.K.; Panchal, A.; Lalwani, A. Development of carbamazepine transnasal microemulsion for treament of epilepsy. Drug Deliv. Transl. Res. 2013, 3, 252–259. [Google Scholar]
- Acharya, S.P.; Pundarikakshudu, K.; Panchal, A.; Lalwani, A. Preparation and evaluation of transnasal microemulsionof carbamazepine. Asian J. Pharm. Sci. 2013, 8, 64–70. [Google Scholar] [CrossRef]
- Zhang, C.; Kwan, P.; Zuo, Z.; Baum, L. The transport of antiepileptic drugs by P-glycoprotein. Adv. Drug Deliv. Rev. 2012, 64, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.S. Selecting surfactants for the maximum inhibition of the activity of the multi drug resistance efflux pump transporter, P-glycoprotein: Conceptual development. J. Excipients Food Chem. 2010, 1, 51–59. [Google Scholar]
- Patel, R.J.; Parikh, R.H. Intranasal delivery of topiramate nanoemulsion: Pharmacodynamic, pharmacokinetic and brain uptake studies. Int. J. Pharm. 2020, 585, 119486. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohta, R.; Machida, Y.; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996, 134, 173–181. [Google Scholar] [CrossRef]
- Finger, T.E.; St Jeor, V.L.; Kinnamon, J.C.; Silver, W.L. Ultrastructure substance P- and CGRP-immunoreactive nerve fibres in the nasal epithelium of rodents. J. Comp. Neurol. 1990, 294, 293–305. [Google Scholar] [CrossRef]
- Bali, V.; Bhavna, M.; Baboota, A.; Ali, J. Potential of microemulsions in drug delivery and therapeutics: A patient review. Recent Patents Drug Deliv. Formul. 2008, 2, 136–144. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N. Superparamagnetic Iron Oxide–Loaded Lipid Nanocarriers Incorporated in Thermosensitive In Situ Gel for Magnetic Brain Targeting of Clonazepam. J. Pharm. Sci. 2018, 107, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhandari, S.; Deshmukh, R.; Yadav, A.K.; Mishra, N. Development and characterization of embelin-loaded nanolipid carriers for brain targeting. Artif. Cells Nanomed. Biotechnol. 2017, 45, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.; Pandit, J.; Vohora, D.; Aqil, M.; Ali, A.; Sultana, Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: In vitro characterization and in vivo efficacy in epilepsy. Expert Opin. Drug Deliv. 2015, 12, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Varshosaz, J.; Minaiyan, M.; Tabbakhian, M. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: In vivo pharmacodynamic studies using rat electroshock model. Int. J. Nanomed. 2011, 6, 363–371. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Tsai, M.J.; Wu, P.C.; Huang, Y.B.; Chang, J.S.; Lin, C.L.; Tsai, Y.H.; Fang, J.Y. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; He, Q.; Liu, X.; Chukwunweike Ikechukwu, O.; Tong, L.; Guo, L.; Yang, H.; Zhang, Q.; Zhao, H.; et al. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia-reperfusion in rats. Int. J. Pharm. 2015, 489, 131–138. [Google Scholar] [CrossRef]
- Lu, Y.-m.; Huang, J.-y.; Wang, H.; Lou, X.-f.; Liao, M.-h.; Hong, L.-j.; Tao, R.-r.; Ahmed, M.M.; Shan, C.-l.; Wang, X.-l.; et al. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials 2014, 35, 530–537. [Google Scholar] [CrossRef]
- Bönöczk, P.; Gulyás, B.; Adam-Vizi, V.; Nemes, A.; Kárpáti, E.; Kiss, B.; Kapás, M.; Szántay, C.; Koncz, I.; Zelles, T.; et al. Role of sodium channel inhibition in neuroprotection: Effect of vinpocetine. Brain Res. Bull. 2000, 53, 245–254. [Google Scholar] [CrossRef]
- Szakácz, T.; Veres, Z.; Vereczkey, L. In vitro-in vivo correlation of the pharmacokinetics of vinpocetine. Pol. J. Pharmacol. 2001, 53, 623–628. [Google Scholar]
- Lin, C.; Chen, F.; Ye, T.; Zhang, L.; Zhang, W.; Liu, D.; Xiong, W.; Yang, X.; Pan, W. A novel oral delivery system consisting in “drug-in cyclodextrin-in nanostructured lipid carriers” for poorly water-soluble drug: Vinpocetine. Int. J. Pharm. 2014, 465, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.M.; Ghorab, D.M.; Badie, H.A. Brain targeted solid lipid nanoparticles for brain ischemia: Preparation and in vitro characterization. Pharm. Dev. Technol. 2013, 18, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Frozza, R.L.; Horn, A.P.; Campos, M.M.; Calixto, J.B.; Salbego, C.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M.O. Protective effects of indomethacin-loaded nanocapsules against oxygen-glucose deprivation in organotypic hippocampal slice cultures: Involvement of neuroinflammation. Neurochem. Int. 2010, 57, 629–636. [Google Scholar] [CrossRef]
- Liu, C.; Wen, J.; Li, D.; Qi, H.; Nih, L.; Zhu, J.; Xu, D.; Ren, Y.; Zhang, S.; Han, D.; et al. Systemic delivery of microRNA for treatment of brain ischemia. Nano Res. 2021, 14, 3319–3328. [Google Scholar] [CrossRef]
- Jin, W.; Wu, Y.; Chen, N.; Wang, Q.; Wang, Y.; Li, Y.; Li, S.; Han, X.; Yang, E.; Tong, F.; et al. Early administration of MPC-n(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics 2021, 11, 8197–8217. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cai, Y.; Li, S.; Liu, H.; Zhou, X.; Lu, C.; Gao, X.; Qian, J.; Zhang, J.; Ju, S.; et al. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics 2017, 7, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, C.; Chen, Q.; Liu, P.; Guo, Q.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhou, W.; Liang, D.; et al. Microthrombus-Targeting Micelles for Neurovascular Remodeling and Enhanced Microcirculatory Perfusion in Acute Ischemic Stroke. Adv. Mater. 2019, 31, 1808361. [Google Scholar] [CrossRef]
- Fukushima, Y.; Uchida, S.; Imai, H.; Nakatomi, H.; Kataoka, K.; Saito, N.; Itaka, K. Treatment of ischemic neuronal death by introducing brain-derived neurotrophic factor mRNA using polyplex nanomicelle. Biomaterials 2021, 270, 120681. [Google Scholar] [CrossRef]
- Singh, V.; Krishan, P.; Shri, R. Amelioration of ischaemia reperfusion-induced cerebral injury in mice by liposomes containing Allium cepa fraction administered intranasally. Artif. Cells Nanomed. Biotechnol. 2018, 46, S982–S992. [Google Scholar] [CrossRef]
- Bruch, G.E.; Fernandes, L.F.; Bassi, B.L.T.; Alves, M.T.R.; Pereira, I.O.; Frézard, F.; Massensini, A.R. Liposomes for drug delivery in stroke. Brain Res. Bull. 2019, 152, 246–256. [Google Scholar] [CrossRef]
- Srikanth, M.; Kessler, J.A. Nanotechnology—Novel therapeutics for CNS disorders. Nat. Rev. Neurol. 2012, 8, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Serwer, L.P.; James, C.D. Challenges in drug delivery to tumors of the central nervous system: An overview of pharmacological and surgical considerations. Adv. Drug Deliv. Rev. 2012, 64, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, N.; Bi, X.; Dou, M. Solid lipid nanoparticles of temozolomide: Potential reduction of cardial and nephric toxicity. Int. J. Pharm. 2008, 355, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Benoit, J.P. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J. Control. Release 2006, 112, 208–213. [Google Scholar] [CrossRef]
- Garcion, E.; Lamprecht, A.; Heurtault, B.; Paillard, A.; Aubert-Pouessel, A.; Denizot, B.; Menei, P.; Benoît, J.P. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol. Cancer Ther. 2006, 5, 1710–1722. [Google Scholar] [CrossRef]
- Martins, S.; Tho, I.; Reimold, I.; Fricker, G.; Souto, E.; Ferreira, D.; Brandl, M. Brain delivery of camptothecin by means of solid lipid nanoparticles: Formulation design, in vitro and in vivo studies. Int. J. Pharm. 2012, 439, 49–62. [Google Scholar] [CrossRef]
- Martins, S.M.; Sarmento, B.; Nunes, C.; Lúcio, M.; Reis, S.; Ferreira, D.C. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur. J. Pharm. Biopharm. 2013, 85, 488–502. [Google Scholar] [CrossRef]
- Koziara, J.M.; Lockman, P.R.; Allen, D.D.; Mumper, R.J. Paclitaxel nanoparticles for the potential treatment of brain tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef]
- Fundarò, A.; Cavalli, R.; Bargoni, A.; Vighetto, D.; Zara, G.P.; Gasco, M.R. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: Pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res. 2000, 42, 337–343. [Google Scholar] [CrossRef]
- Zara, G.P.; Cavalli, R.; Fundarò, A.; Bargoni, A.; Caputo, O.; Gasco, M.R. Pharmacokinetics of doxorubicin incorporated in solid lipid nanospheres (SLN). Pharmacol. Res. 1999, 40, 281–286. [Google Scholar] [CrossRef]
- Allard, E.; Passirani, C.; Garcion, E.; Pigeon, P.; Vessières, A.; Jaouen, G.; Benoit, J.P. Lipid nanocapsules loaded with an organometallic tamoxifen derivative as a novel drug-carrier system for experimental malignant gliomas. J. Control. Release 2008, 130, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Yakati, V.; Shankar, G.; Jaggarapu, M.M.C.S.; Moku, G.; Madhusudana, K.; Banerjee, R.; Ramkrishna, S.; Srinivas, R.; Chaudhuri, A. Amphetamine decorated cationic lipid nanoparticles cross the blood-brain barrier: Therapeutic promise for combating glioblastoma. J. Mater. Chem. B 2020, 8, 4318–4330. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Sadeghi, H.; Khadivar, K. Development and optimization of transferrin-conjugated nanostructured lipid carriers for brain delivery of paclitaxel using Box–Behnken design. Pharm. Dev. Technol. 2017, 22, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Brain Targeting of Temozolomide via the Intranasal Route Using Lipid-Based Nanoparticles: Brain Pharmacokinetic and Scintigraphic Analyses. Mol. Pharm. 2016, 13, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dube, B.; Sawant, K. Development and evaluation of nanostructured lipid carriers of cytarabine for treatment of meningeal leukemia. J. Nanosci. Nanotechnol. 2011, 11, 6676–6682. [Google Scholar] [CrossRef]
- Cros, E.; Jordheim, L.; Dumontet, C.; Galmarini, C.M. Problems related to resistance to cytarabine in acute myeloid leukemia. Leuk. Lymphoma 2004, 45, 1123–1132. [Google Scholar] [CrossRef]
- Capizzi, R.L.; White, J.C.; Powell, B.L.; Perrino, F. Effect of dose on the pharmacokinetic and pharmacodynamic effects of cytarabine. Semin. Hematol. 1991, 28, 54–69. [Google Scholar]
- Wu, M.; Fan, Y.; Lv, S.; Xiao, B.; Ye, M.; Zhu, X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: A comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016, 23, 2720–2725. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, X.; Song, S.; Zhu, X.; Zhu, J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016, 23, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Husna Ibrahim, N.; Yahaya, M.F.; Mohamed, W.; Teoh, S.L.; Hui, C.K.; Kumar, J. Pharmacotherapy of Alzheimer’s Disease: Seeking Clarity in a Time of Uncertainty. Front. Pharmacol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Ashwood, T.; Odergren, T.; Jackson, D.M. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharmacol. Ther. 2003, 100, 195–214. [Google Scholar] [CrossRef]

| Disease | API | Nanocarrier Type | Lipid System | Technique | RoA | In Vivo Model | In Vivo Results | Refs |
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s | Donepezil | SLN | Glyceryl monostearate | Solvent Emulsification–Diffusion Technique | IN | Wistar Rats | Improved bio distribution and pharmacokinetics | [207] |
| Donepezil + Curcumin | NLC | Precirol (Solid lipid) and Capmul MCM (liquid lipid) | Hot High-Pressure Homogenization | IN | Wistar Rats | Higher drug concentration in the brain | [208] | |
| Galantamine | SLN | Glyceryl behnate (Compritol®) | Micro-Emulsification | IN | Wistar Rats | Significant memory restoration capability and two-fold increase in bioavailability | [209] | |
| Rivastigmine | Liposomes | Egg phosphatidylcholine and Cholesterol | Ammonium Sulphate Gradient Loading Method. | IN SC PO and IP | Male Sprague Dawley rats | Enhanced BBB penetration and improved brain delivery | [211,212,213] | |
| Rivastigmine | Liposomes | Phosphocholine, dihexadecyl phosphate and cholesterol | Lipid Layer Hydration and The Heating Methods | SC | Wistar Rats | Preventing amyloid plaque formation | [211,212,213] | |
| Rivastigmine | Liposomes | Dipalmitoylphosfotidyl choline (DPPC) and cholesterol | Film Formation Method | IP and PO | Balb-C type mice | Inhibition of AChE | [211,212,213] | |
| Parkinson’s | Ropinirole HCL | SLN | Dynasan 114 and stearylamine | Emulsification-Solvent Diffusion | IN | Mice | Reduction in signs of Parkinsonism-like symptoms and comparable with marketed oral formulation | [230] |
| Piribedil | SLN | palmitic acid (PA) and polyvinyl alcohol (PVA) | Hot Homogenization Followed by Ultrasonication | IN | Wistar rats | Four-fold increase in AUC, increased nose-to-brain uptake | [226] | |
| Bromocriptine | NLC | Tristearin, stearic triglyceride (tristearin), Miglyol 812, caprylic/capric triglycerides (tricaprin) Mivaplex 600, stearic monoglyceride (monostearin) | Homogenization Or Ultrasonication | IV | Sprague–Dawley rats | Rapid onset of action, long lasting activity (5 h) | [227] | |
| Apomorphine hydrochloride | SLNs | tripalmitin, Hydrogenated soybean phosphatidylcholine | Emulsification | PO | Wistar rats | A 12- to 13- fold higher bioavailability | [229] | |
| Basic fibroblast growth factor | Phospholipid-based gelatin nanoparticles | N/A | Water-In-Water Emulsion and Freeze-Drying Technique | IN | hemiparkinsonian Sprague–Dawley rats | Stimulated dopaminergic function | [234] | |
| Glial cell-derived neurotrophic factor | TAT- LNC | (Precirol ATO and Mygliol® | Melt Emulsification Technique | IN | male C57BL/6J mice | Reduction in microgliosis and motor recovery | [235] | |
| Selegiline | NE | grape seed oil and Sefsol 218 | High Energy Emulsification Method | IN | Wistar rats | Decreased dopamine loss, Brain: blood ratio of 2.207 indicating the ability to deliver the drug into brain | [242] | |
| Selegiline and quercetin | LNC | Labrafil M 2130 CS and Capryol 90 | Modified Emulsiosonication Method | IP | Wistar rats | Increased behavioral response and effect of quercetin on increasing the bioavailability by modulating the P-gp inhibitor | [243] | |
| Vitexin | SLN | N/A | Hot Melt Emulsification And Ultrasonication Method | N/A | Mice | Neuroprotective effect observed via improved total reactive antioxidant in striatum | [247] | |
| Dementia | Resveratrol | SLN | stearic acid, lecithin and taurocholate | Refer To Method | PO | Sprague Dawley (SD) rats | Reduced mitochondrial oxidative stress | [252] |
| Aphanamixis Polystachya leaf extract (ethanol) | Liposomes | Phospholipid extracted from Egg yolk | Solvent Injection Method | PO | Swiss albino mice | Significant improvement in memory function, locomotor activity, and ambulatory performance | [249] | |
| Curcumin | SLN | D-L-Homocysteine, Glyceryl monostearate, and Stearic acid | Modified Solvent Evaporation Method | caudal vein | Sprague Dawley rats | Reduced oxidative stress | [253] | |
| Rivastigmine | NLC | Combination of solid and liquid lipids | High Pressure Homogenization Technique | TD | Wistar rats | Increased Cmax and AUC, resulting in increased bioavailability | [256] | |
| Epilepsy | Carbamazepine | ME | oleic acid | Oil- In- Water Emulsion | IN | Sprague Dawley rats. | Significantly higher CBZ ME via intranasal route and protection from seizures | [267] |
| Topiramate | NE | Capmul MCM C8 | Oil- In- Water Emulsion | IN and PO | Wistar rats | Improved bioavailability | [271] | |
| Clonazepam | SLN and NLC | Glycerol oleate and oleic acid | High Pressure Homogenization Technique | IN | Swiss Albino mice | NLCs showed prolonged the onset times for convulsion | [275] | |
| Embelin | NLC | solid lipid (cetyl palmitate) and liquid lipid (octyldodecanol) | Emulsification And Ultrasonication Method | IN | Wistar rats | Higher concentration of drug into brain for NLCs as compared to plain embelin and marketed formulation | [276] | |
| Lamotrigine | NLC | Glyceryl monostearate as solid lipid and oleic acid as liquid lipid a | Solvent Evaporation Method | PO and IN | Wistar rats | Accumulation and longer retention of the drug in the brain Significant improvement in latency and duration of tonic hindlimb extension, up to 24 h post seizure induction. | [277] | |
| Valproic acid | NLC | Cetyl palmitate soy lecithin S100, 0.1 mL of octyldodecanol, and 400 mg of VPA, | Emulsion–Solvent Diffusion and Evaporation Method | IN and IP | Wistar rats | NLCs administered via IN showed higher brain:plasma concentration in comparison with NLCs via IP NLCs administered via IN and IP showed similar protective effects to seizure induction | [278] | |
| Ischaemic Stroke | Baicalein | NLC | Tripalmitin, Gelucire® and Hydrogenated soybean phosphatidylcholine 80% | Sonication | IV | Wistar rats | Significantly higher accumulation of the drug from NLCs in all parts of the brain | [280] |
| Baicalein | SLN | N/A | N/A | IV | Sprague Dawley rats | A 5.69-fold higher AUC, 6.84-fold higher Cmax than that of the Baicalein solution. Improved bioavailability of baicalin in cerebral spinal fluid | [281] | |
| Fas ligand Antibody | PEG-lipid nanoparticles | Monostearin, medium chain triglyceride, Polyethylene glycol monostearate and ODARITC | Solvent Diffusion Method | IV | C57BL/6J wild-type mice | Significant improvements in brain injury and in neurological deficit after ischaemia at significantly lower dose in comparison with regular dl-NB | [282] | |
| Vinpocetine | Cyclodextrin- NLC | Compritol® 888 ATO and Miglyol® 812N | Water In Oil Emulsification | PO | New Zealand white male rabbits | The relative bioavailability of VP in cyclodextrin-loaded NLC was 592% compared with VP suspension and 92% higher than VP–NLC. | [285] | |
| Edaravone | Micelle | methoxypoly (ethylene glycol)-b-poly (D,L-lactic acid) (PEG-PLA) | Solvent Evaporation Strategy | IV | ICR mice | The agonistic micelle (EDV-AM) delivered more EDV into brain Ischemia, more rapidly salvaged ischemic tissue EDV-AM showed highest efficiency of in accelerating axonal remodeling and improved functional behaviors | [290] | |
| mRNA—brain derived neurotrophic factor (BDNF) | Nano -Micelle | N/A | Synthesis Of Block-Copolymers | intraventricular injection | Wistar rats | Prevention of ischemic neuronal death | [292] | |
| Allium cepa fraction (Ethyl Acetate Fraction) | liposomes | phosphatidylcholine and cholesterol | N/A | IN | Swiss Albino mice | Significant neuroprotection observed at 1/10th the oral dose | [293] | |
| CNS Neoplastic Disease | Temozolomide | SLN | lecithin and Poloxamer 188 | Sonication | IV | Rabbits –Pharmacokinetics Kunming mice-Tissue distribution | Higher AUC/dose and the mean residence times in brain and reticuloendothelial cells-containing organs | [297] |
| Paclitaxel | LNC | Labrafac® WL 1349, Lipoiïd S75-3 and Solutol® HS 15 | Emulsion Inversion Phase Process | N/A | Syngeneic Fischer F344 male rats | Inhibitory effects on efflux pump activity and reduced tumor expansion | [299] | |
| Camptothecin | SLN | cetyl palmitate, Dynasan® 114 and Witepsol® E85 | Oil-In-Water Nanoemulsion Method | IV | Wistar rats | A 6-fold increase in the drug in the brain over the free drug | [300] | |
| Camptothecin | SLN | cetyl palmitate | High Shear Homogenization and Ultra-Sonication | IV | Wistar rats | A 4.3-fold increase in brain concentration of the drug and detected until 24 h in brain | [301] | |
| Doxorubicin | SLN | stearic acid, Epikuron 200 and taurocholate sodium salt | Oil-In-Water (O/W) Micro Emulsion | IV | Wistar rats | Significant amount of doxorubicin was detectable in the brain and CSF | [303,304] | |
| Ferrocenyl diphenol tamoxifen derivative | LNC | Solutol® HS15, Lipoid® and Labrafac® | Multi-inversion Phase Processes | SC | Syngeneic Fischer F344 female rats | Significant reduction in both tumor mass and glioma volume | [305] | |
| Amphetamine | LNC | DOPC, cholesterol and amphetaminylated lipid | Conventional Thin Film Hydration Method | IV | female C57BL/6 mice | LNCs with hexadecyl saturated hydrocarbon chain (16-BACL) showed highest accumulation into the brain, co-solubilized paclitaxel and PD-L1siRNA significantly enhanced the overall survivability of the mice | [306] | |
| Temozolomide (TMZ) | LNC | Gelucire® 44/14 (solid lipid) and Vit. E (liquid lipid) | High Pressure Homogenization (HPH) Technique | IN | Wistar rats | Higher bioavailability and increased residence time of drug in brain | [308] | |
| Temozolomide | LNC | Compritol® 888 ATO, Cremophor ELP and soybean phosphatidylcholine | Solvent Diffusion Method | IV | BALB/c nude mice | Significant inhibition (3 times) of tumor growth | [309,310] | |
| Vincristine (VT) and temozolomide (T) | SLN & LNC | SLN—stearic acid (1 g) and injectable soya lecithin LNC—Compritol® 888 ATO, Cremophor® ELP and soybean phosphatidylcholine | Solvent Displacement Technique | IV | BALB/c nude mice | VT-NLCs inhibited tumor growth over 80%, followed by VT-SLNs (56%) and T-NLCs (70%) | [314] | |
| Temozolomide (gene-loaded) | (TMZ/DNA-LNCs) | Compritol® 888 ATO, Cremophor® ELP and soybean phosphatidylcholine | Solvent Diffusion Method | IV | BALB/c nude mice | TMZ/DNA-NLCs inhibited tumor growth 3.3 times higher than that of free TMZ | [315] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witika, B.A.; Poka, M.S.; Demana, P.H.; Matafwali, S.K.; Melamane, S.; Malungelo Khamanga, S.M.; Makoni, P.A. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics 2022, 14, 836. https://doi.org/10.3390/pharmaceutics14040836
Witika BA, Poka MS, Demana PH, Matafwali SK, Melamane S, Malungelo Khamanga SM, Makoni PA. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics. 2022; 14(4):836. https://doi.org/10.3390/pharmaceutics14040836
Chicago/Turabian StyleWitika, Bwalya Angel, Madan Sai Poka, Patrick Hulisani Demana, Scott Kaba Matafwali, Siyabonga Melamane, Sandile Maswazi Malungelo Khamanga, and Pedzisai Anotida Makoni. 2022. "Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date" Pharmaceutics 14, no. 4: 836. https://doi.org/10.3390/pharmaceutics14040836
APA StyleWitika, B. A., Poka, M. S., Demana, P. H., Matafwali, S. K., Melamane, S., Malungelo Khamanga, S. M., & Makoni, P. A. (2022). Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics, 14(4), 836. https://doi.org/10.3390/pharmaceutics14040836








