Add Sugar to Chitosan: Mucoadhesion and In Vitro Intestinal Permeability of Mannosylated Chitosan Nanocarriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Caco-2 Cells
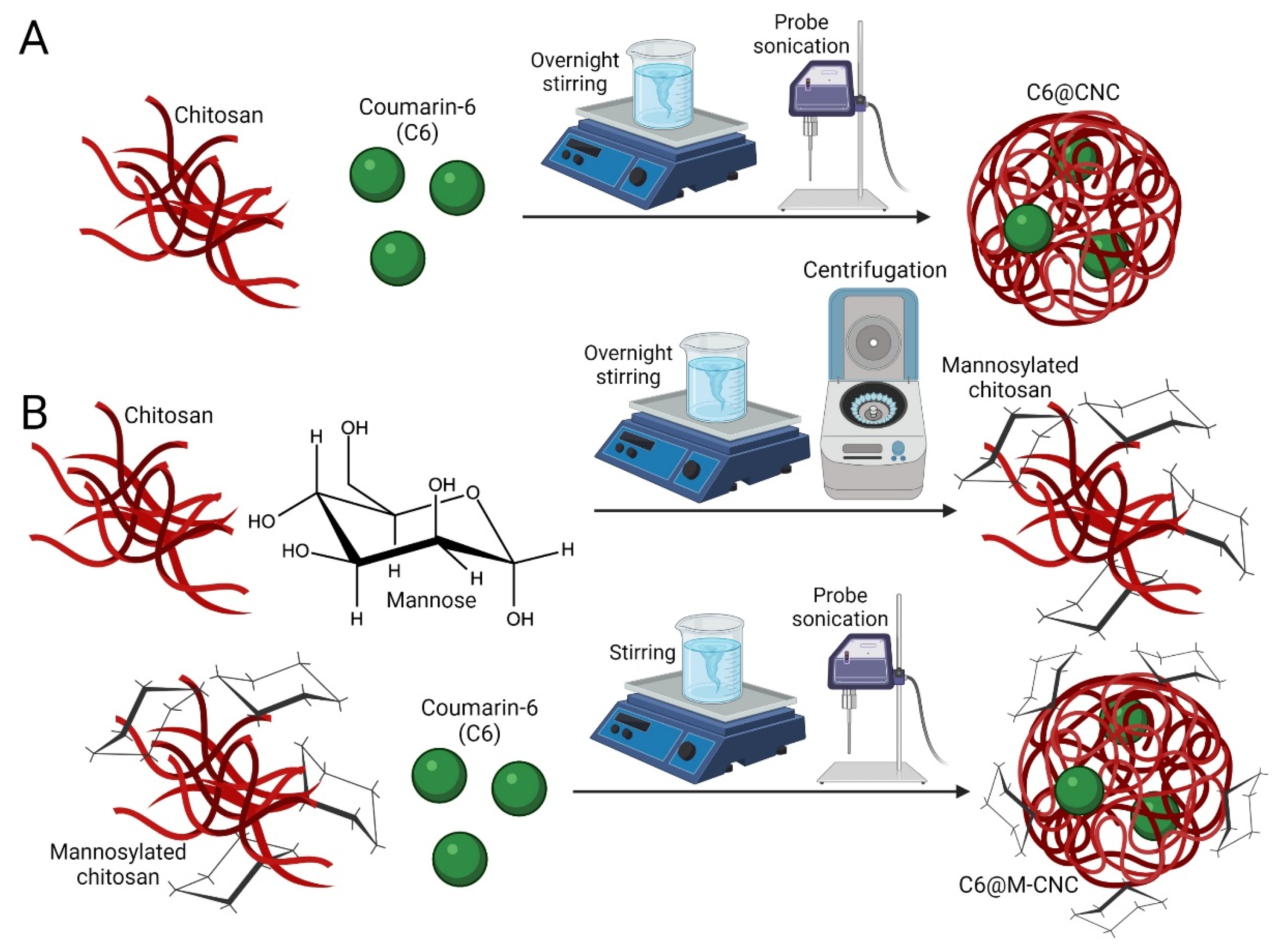
2.3. Synthesis of C6@CNCs and C6@M-CNCs
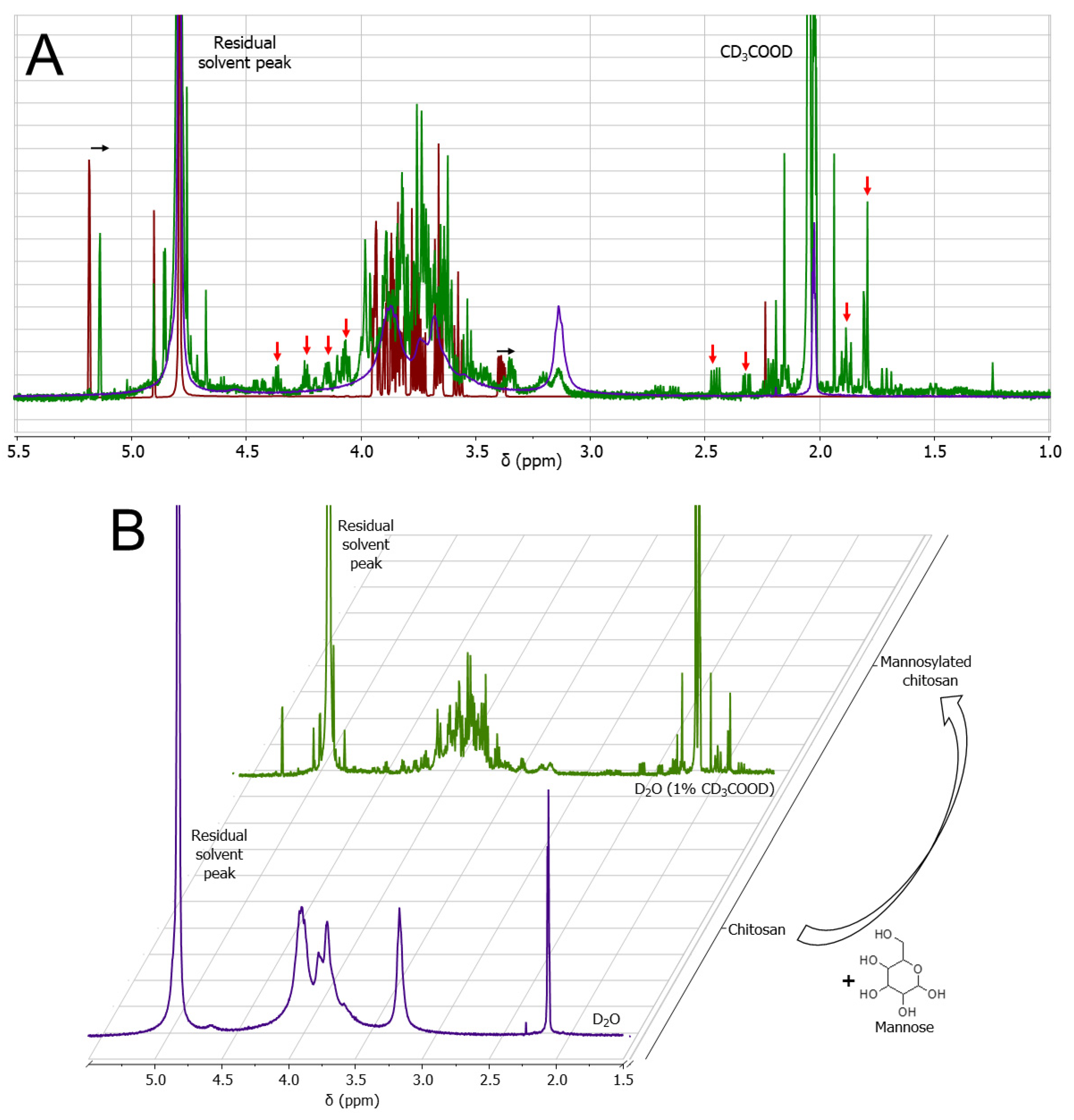
2.4. Nuclear Magnetic Resonance (NMR) Analyses
2.5. Scanning Electron Microscopy of C6@CNCs and C6@M-CNCs
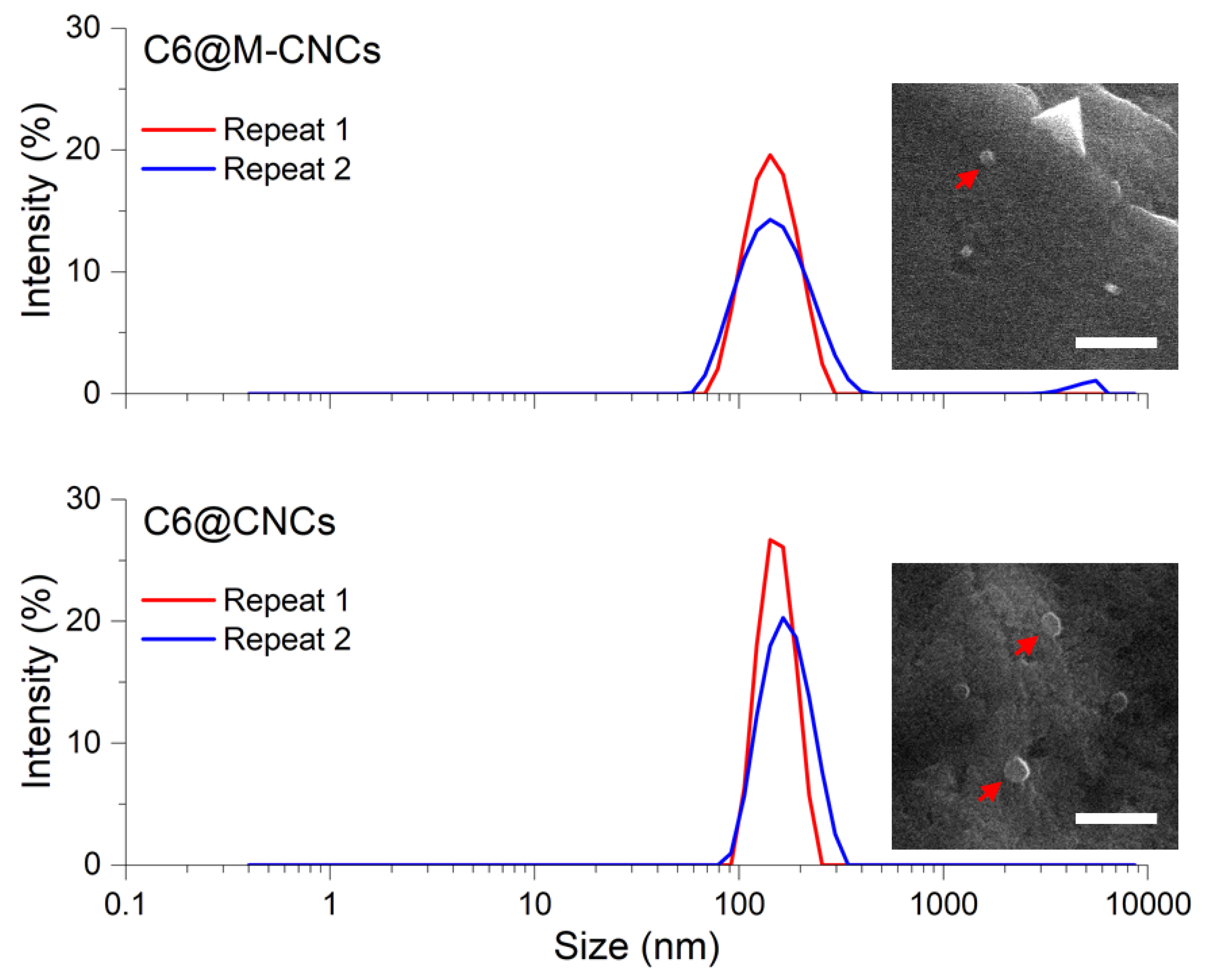
2.6. Hydrodynamic Size Determination of C6@CNCs and C6@M-CNCs
2.7. C6 Quantification
2.8. Release of C6 from C6@CNCs and C6@M-CNCs
2.9. MTS Assay
2.10. Caco-2 Adherence and Uptake of CNCs
2.11. Transepithelial Flux of C6 in NCs across Caco-2 Monolayers
2.12. Purification of Porcine Gut Mucin
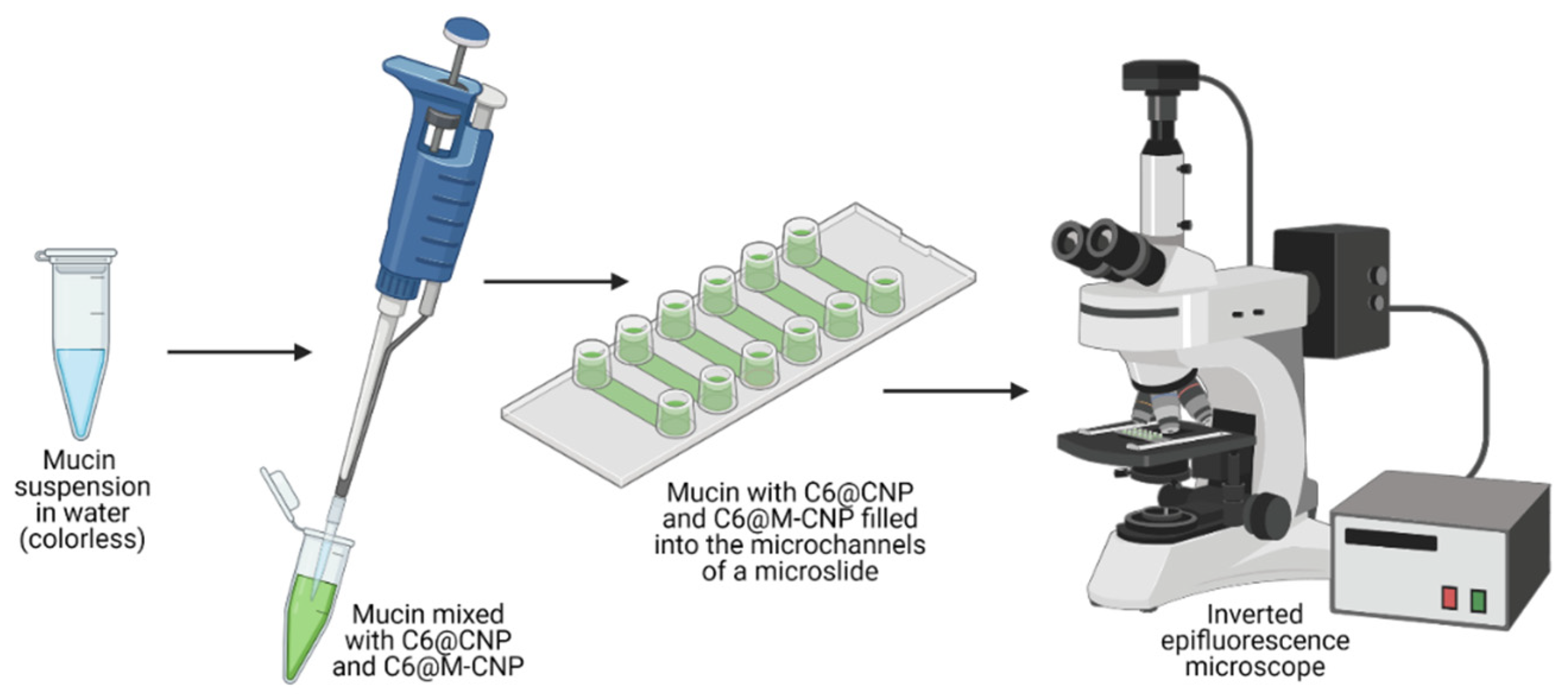
2.13. Mucoadhesion Studies
2.14. Statistical Analyses
3. Results and Discussion
3.1. Mannosylation of Chitosan
3.2. Size of C6@CNCs and C6@M-CNCs
3.3. Loading Efficiency (LE) of C6 in C6@CNCs and C6@M-CNCs
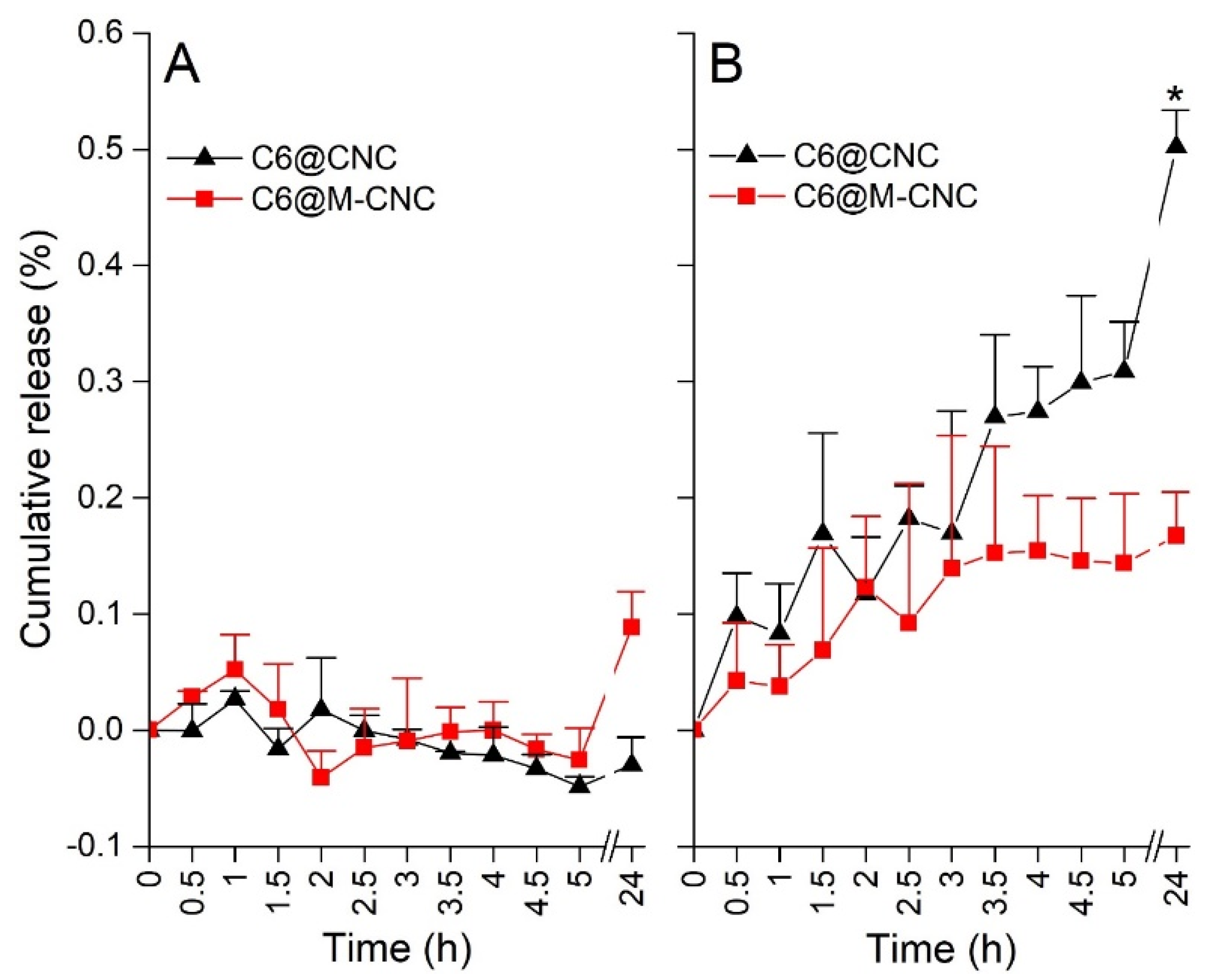
3.4. Release of C6 from C6@CNCs and C6@M-CNCs
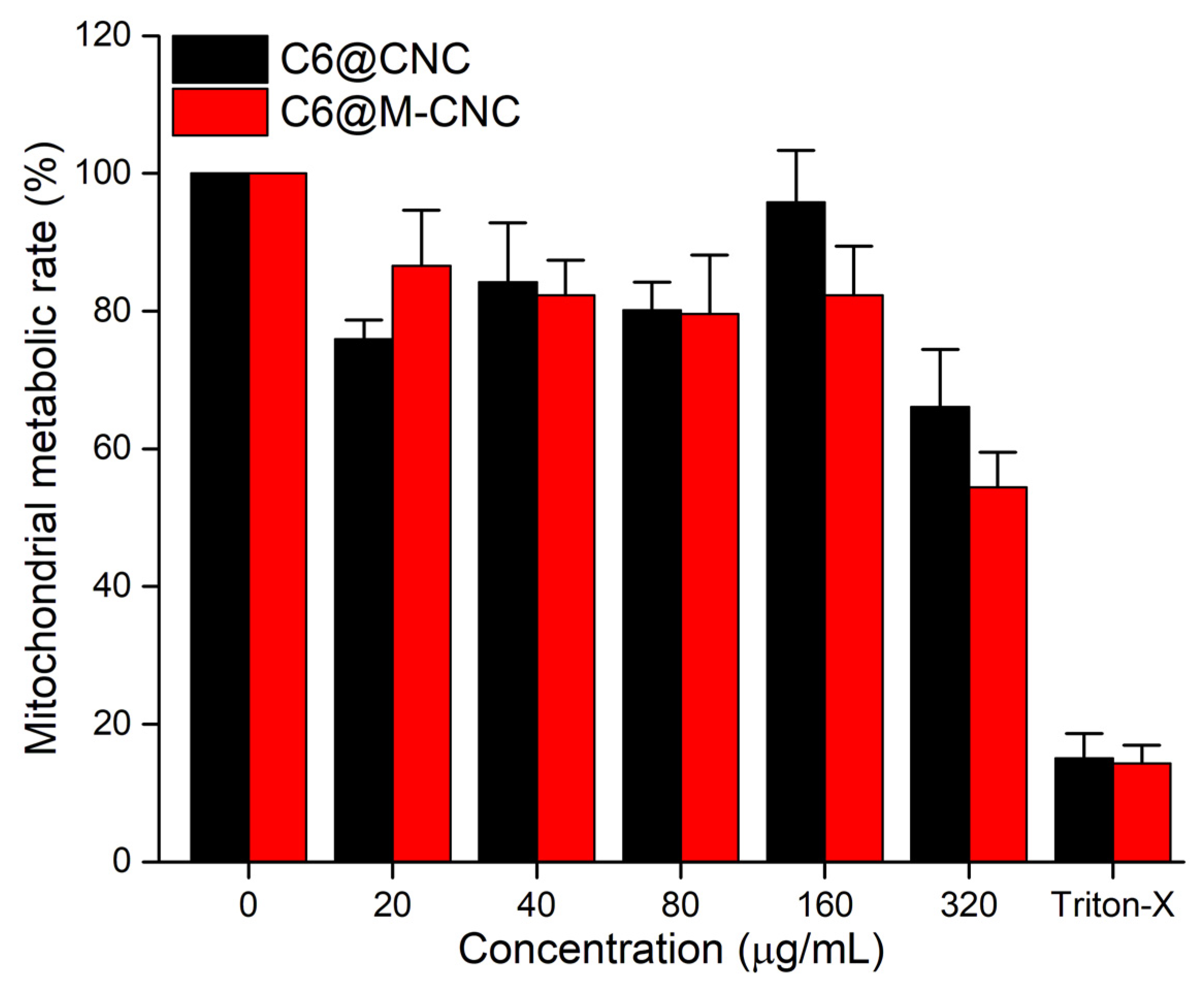
3.5. MTS Assay of CNCs
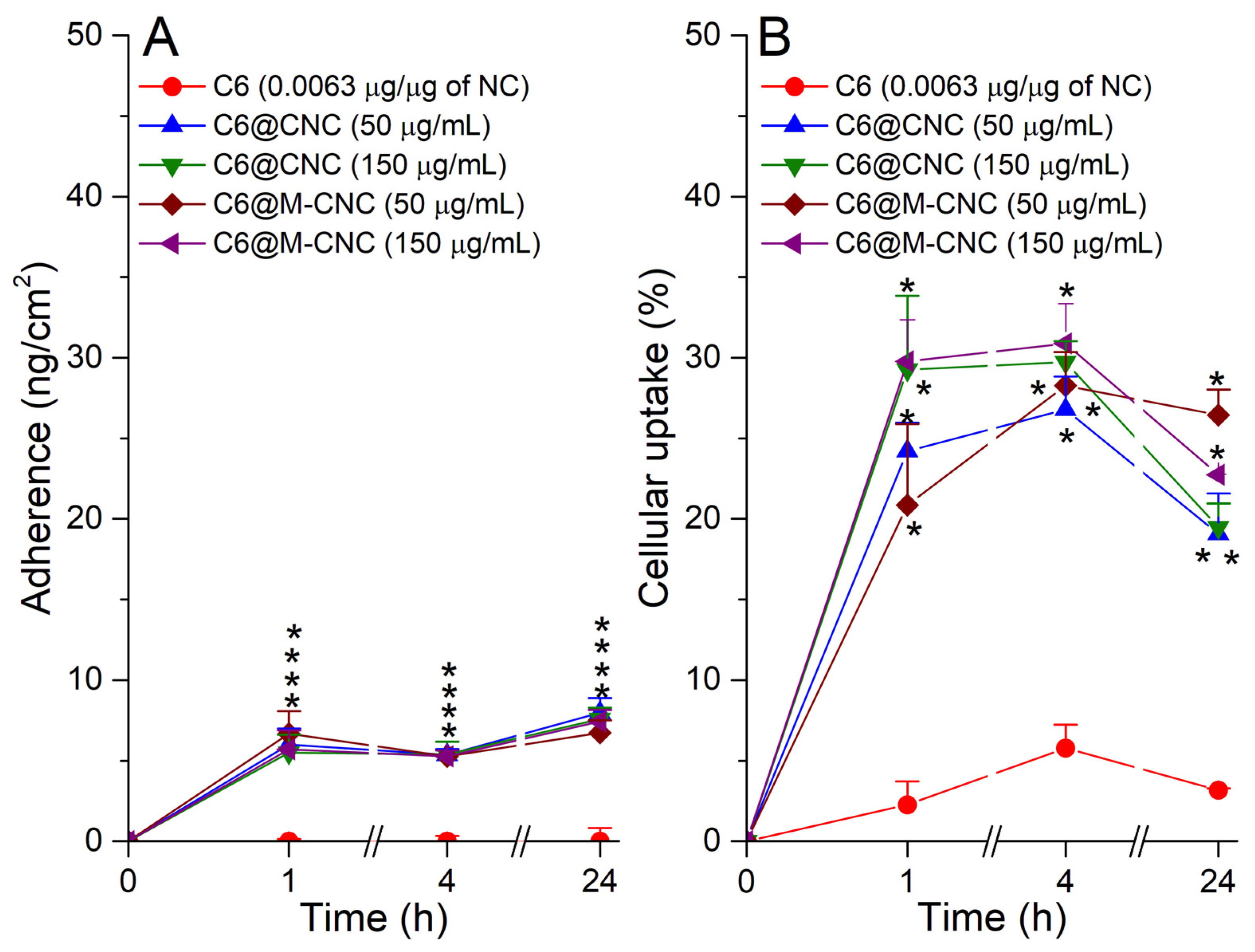
3.6. Adherence and Uptake Analyses of C6@CNCs and C6@M-CNCs in Caco-2 Cells
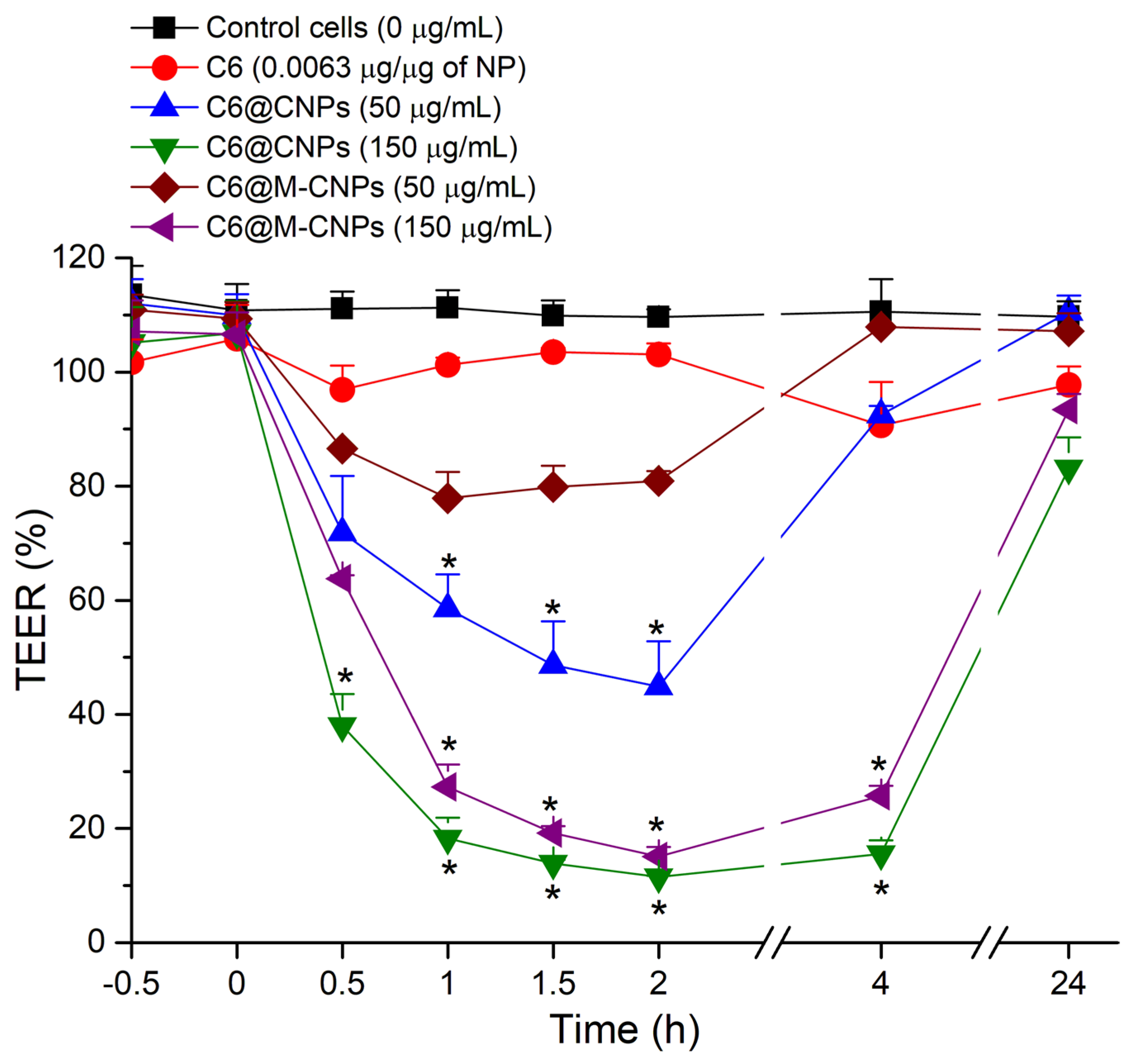
3.7. TEER Studies on Caco-2 Monolayers
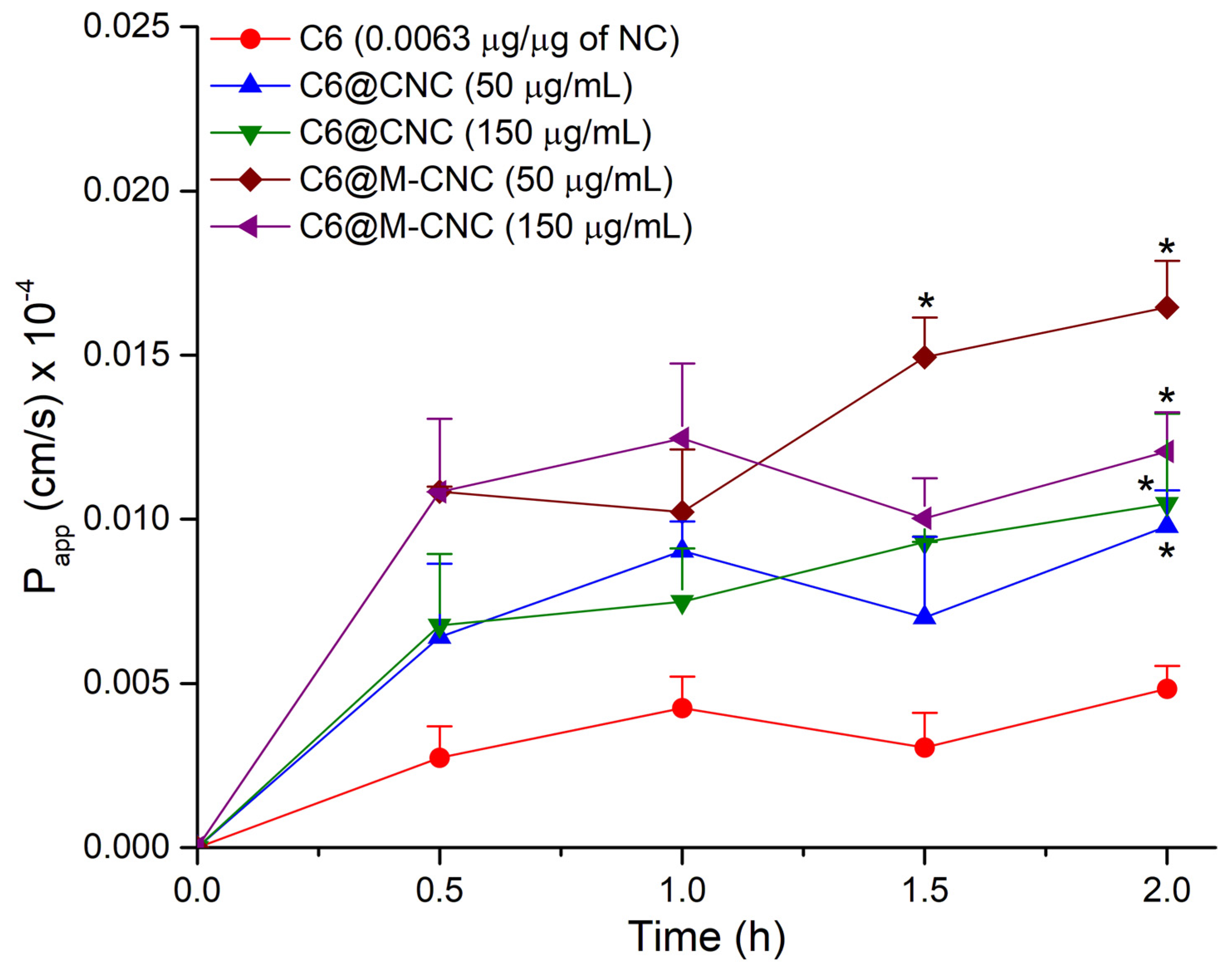
3.8. Transport of the C6@CNCs and C6@M-CNCs across the Caco-2 Monolayers
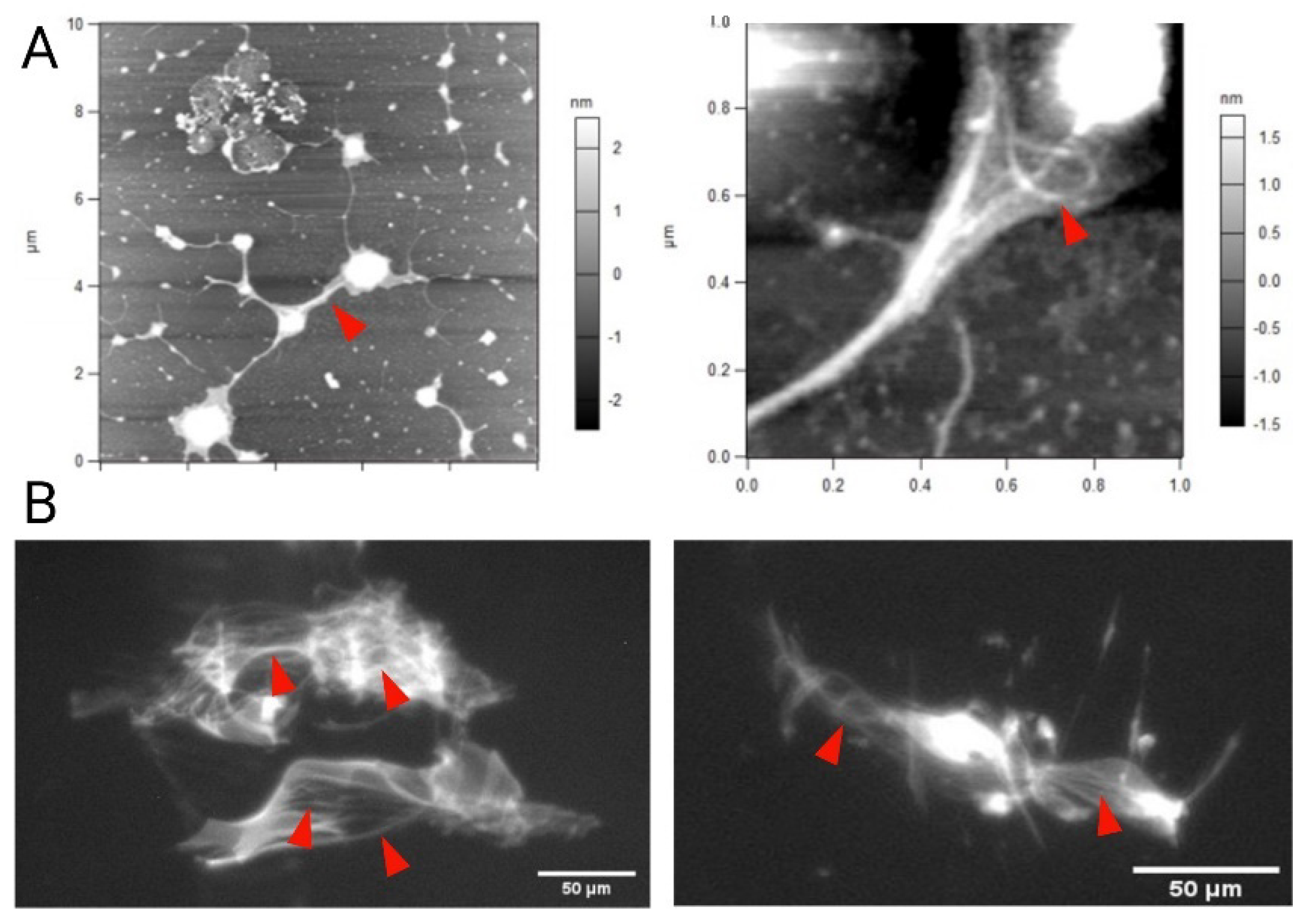
3.9. Mucoadhesion Studies on Porcine Gut Mucin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle technologies for oral drug delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddhanta, S.; Bhattacharjee, S.; Harrison, S.M.; Scholz, D.; Barman, I. Shedding light on the trehalose-enabled mucopermeation of nanoparticles with label-free Raman spectroscopy. Small 2019, 15, 1901679. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Mahon, E.; Harrison, S.M.; McGetrick, J.; Muniyappa, M.; Carrington, S.D.; Brayden, D.J. Nanoparticle passage through porcine jejunal mucus: Microfluidics and rheology. Nanomed. NBM 2017, 13, 863–873. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Witten, J.; Samad, T.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef]
- Hristov, D.; McCartney, F.; Beirne, J.; Mahon, E.; Reid, S.; Bhattacharjee, S.; Penarier, G.; Werner, U.; Bazile, D.; Brayden, D.J. Silica-coated nanoparticles with a core of zinc, L-arginine, and a peptide designed for oral delivery. ACS Appl. Mater. Interfaces 2020, 12, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; van Opstal, E.J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M. Surface charge-specific interactions between polymer nanoparticles and ABC transporters in Caco-2 cells. J. Nanopart. Res. 2013, 15, 1695. [Google Scholar] [CrossRef]
- Weiss, M.; Fan, J.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021, 19, 5. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Pengpong, T.; Sangvanich, P.; Sirilertmukul, K.; Muangsin, N. Design, synthesis and in vitro evaluation of mucoadhesive p-coumarate-thiolated-chitosan as a hydrophobic drug carriers. Eur. J. Pharm. Biopharm. 2014, 86, 487–497. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, J.; Liu, K.; Wu, C.; Liang, F. Stealth PEGylated chitosan polyelectrolyte complex nanoparticles as drug delivery carrier. J. Biomater. Sci. 2021, 32, 1387–1405. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Venter, J.P.; Kotzé, A.F.; Auzély-Velty, R.; Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006, 313, 36–42. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ichikawa, M.; Freeze, H.H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 2014, 453, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagimori, M.; Chinda, Y.; Suga, T.; Yamanami, K.; Kato, N.; Inamine, T.; Fuchigami, Y.; Kawakami, S. Synthesis of high functionality and quality mannose-grafted lipids to produce macrophage-targeted liposomes. Eur. J. Pharm. Sci. 2018, 123, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man-Kupisinska, A.; Swierzko, A.S.; Maciejewska, A.; Hoc, M.; Rozalski, A.; Siwinska, M.; Lugowski, C.; Cedzynski, M.; Lukasiewicz, J. Interaction of mannose-binding lectin with lipopolysaccharide outer core region and its biological consequences. Front. Immunol. 2018, 9, 1498. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, characterization, and insecticidal activity of avermectin-grafted-carboxymethyl chitosan. BioMed Res. Int. 2016, 2016, 9805675. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, J.; Li, B. Mannose-modificated polyethylenimine: A specific and effective antibacterial agent against Escherichia coli. Langmuir 2018, 34, 1574–1580. [Google Scholar] [CrossRef]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef]
- Hristov, D.R.; Lopez, H.; Ortin, Y.; O'Sullivan, K.; Dawson, K.A.; Brougham, D.F. Impact of dynamic sub-populations within grafted chains on the protein binding and colloidal stability of PEGylated nanoparticles. Nanoscale 2021, 13, 5344–5355. [Google Scholar] [CrossRef]
- Kim, E.-M.; Jeong, H.-J.; Park, I.-K.; Cho, C.-S.; Kim, C.-G.; Bom, H.-S. Hepatocyte-targeted nuclear imaging using 99mTc-galactosylated chitosan: Conjugation, targeting, and biodistribution. J. Nucl. Med. 2005, 46, 141–145. [Google Scholar]
- Bhattacharjee, S.; Ershov, D.; Fytianos, K.; van der Gucht, J.; Alink, G.M.; Rietjens, I.M.C.M.; Marcelis, A.T.M.; Zuilhof, H. Cytotoxicity and cellular uptake of tri-block copolymer nanoparticles with different size and surface characteristics. Part. Fibre Toxicol. 2012, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Abosabaa, S.A.; ElMeshad, A.N.; Arafa, M.G. Chitosan nanocarrier entrapping hydrophilic drugs as advanced polymeric system for dual pharmaceutical and cosmeceutical application: A comprehensive analysis using Box-Behnken design. Polymers 2021, 13, 677. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS ONE 2018, 13, e0190976. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Je, H.J.; Kim, E.S.; Lee, J.-S.; Lee, H.G. Release properties and cellular uptake in Caco-2 Cells of size-controlled chitosan nanoparticles. J. Agric. Food Chem. 2017, 65, 10899–10906. [Google Scholar] [CrossRef] [PubMed]
- JanssenDuijghuijsen, L.M.; Grefte, S.; de Boer, V.C.J.; Zeper, L.; van Dartel, D.A.M.; van der Stelt, I.; Bekkenkamp-Grovenstein, M.; van Norren, K.; Wichers, H.J.; Keijer, J. Mitochondrial ATP depletion disrupts Caco-2 monolayer integrity and internalizes claudin 7. Front. Physiol. 2017, 8, 794. [Google Scholar] [CrossRef] [Green Version]
- Twarog, C.; Liu, K.; O'Brien, P.J.; Dawson, K.A.; Fattal, E.; Illel, B.; Brayden, D.J. A head-to-head Caco-2 assay comparison of the mechanisms of action of the intestinal permeation enhancers: SNAC and sodium caprate (C10). Eur. J. Pharm. Biopharm. 2020, 152, 95–107. [Google Scholar] [CrossRef]
- Ranaldi, G.; Marigliano, I.; Vespignani, I.; Perozzi, G.; Sambuy, Y. The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line. J. Nutr. Biochem. 2002, 13, 157–167. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Hsu, L.-W.; Tseng, M.T.; Lee, P.-L.; Sonjae, K.; Ho, Y.-C.; Sung, H.-W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The mucus barrier to inhaled gene therapy. Mol. Ther. 2016, 24, 2043–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, P.C.; Cattoz, B.; Ibrahim, M.S.; Anuonye, J.C. Probing the interaction of nanoparticles with mucin for drug delivery applications using dynamic light scattering. Eur. J. Pharm. Biopharm. 2015, 97, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Quan, X.; Peng, C.; Dong, J.; Zhou, J. Structural properties of polymer-brush-grafted gold nanoparticles at the oil–water interface: Insights from coarse-grained simulations. Soft Matter 2016, 12, 3352–3359. [Google Scholar] [CrossRef]
- Malhaire, H.; Gimel, J.-C.; Roger, E.; Benoît, J.-P.; Lagarce, F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv. Drug Deliv. Rev. 2016, 106, 320–336. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejaz, S.; Hogg, B.; Hristov, D.R.; Brayden, D.J.; Imran, M.; Bhattacharjee, S. Add Sugar to Chitosan: Mucoadhesion and In Vitro Intestinal Permeability of Mannosylated Chitosan Nanocarriers. Pharmaceutics 2022, 14, 830. https://doi.org/10.3390/pharmaceutics14040830
Ejaz S, Hogg B, Hristov DR, Brayden DJ, Imran M, Bhattacharjee S. Add Sugar to Chitosan: Mucoadhesion and In Vitro Intestinal Permeability of Mannosylated Chitosan Nanocarriers. Pharmaceutics. 2022; 14(4):830. https://doi.org/10.3390/pharmaceutics14040830
Chicago/Turabian StyleEjaz, Sadaf, Bridget Hogg, Delyan R. Hristov, David J. Brayden, Muhammad Imran, and Sourav Bhattacharjee. 2022. "Add Sugar to Chitosan: Mucoadhesion and In Vitro Intestinal Permeability of Mannosylated Chitosan Nanocarriers" Pharmaceutics 14, no. 4: 830. https://doi.org/10.3390/pharmaceutics14040830
APA StyleEjaz, S., Hogg, B., Hristov, D. R., Brayden, D. J., Imran, M., & Bhattacharjee, S. (2022). Add Sugar to Chitosan: Mucoadhesion and In Vitro Intestinal Permeability of Mannosylated Chitosan Nanocarriers. Pharmaceutics, 14(4), 830. https://doi.org/10.3390/pharmaceutics14040830







