Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification and Identification of Exosomes
2.3. Preparation of siRNA-Loaded Exosomes
2.4. Cell Culture
2.5. Measuring GPC3 Levels in B16/BL6 Cells Treated with siRNA-Loaded Exosomes Derived from Serum
2.6. Cell Viability Assay
2.7. Mice
2.8. The B16/BL6 Spontaneous Lung Metastasis Mouse Model
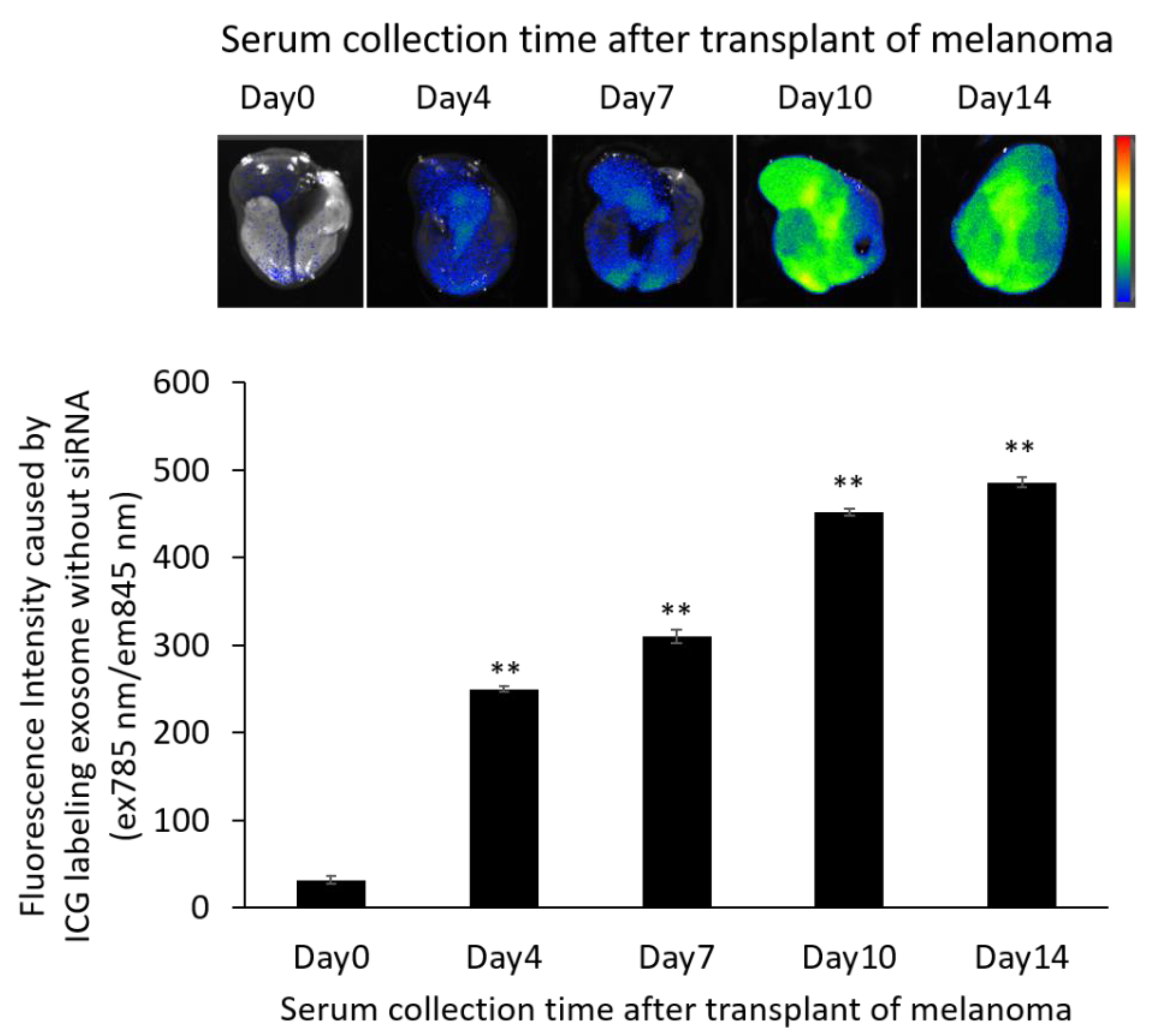
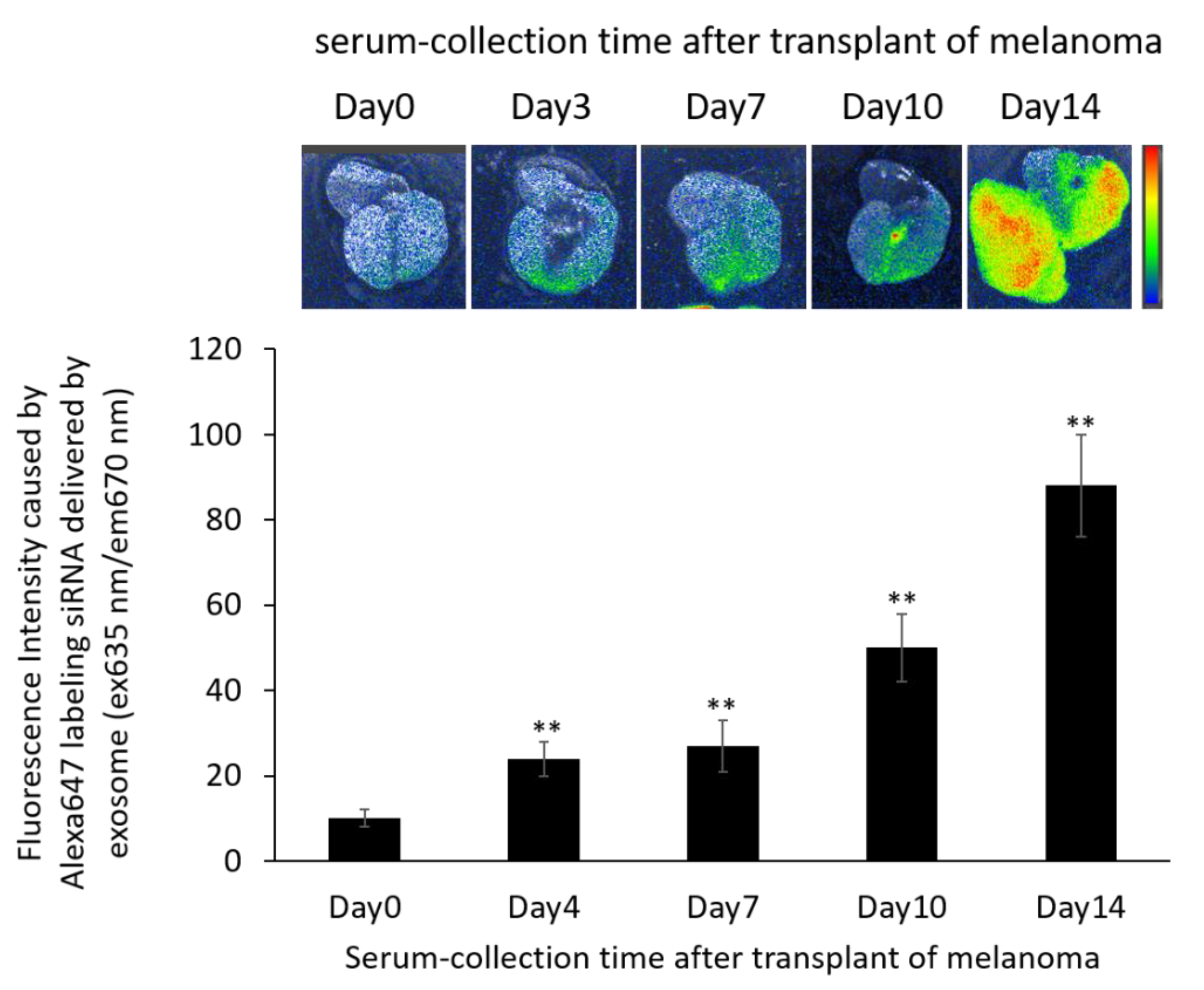
2.9. Distribution of Exosomes and siRNA Delivered by Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model
2.10. Measuring the Anti-Metastatic Effects of siRNA-Loaded Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model
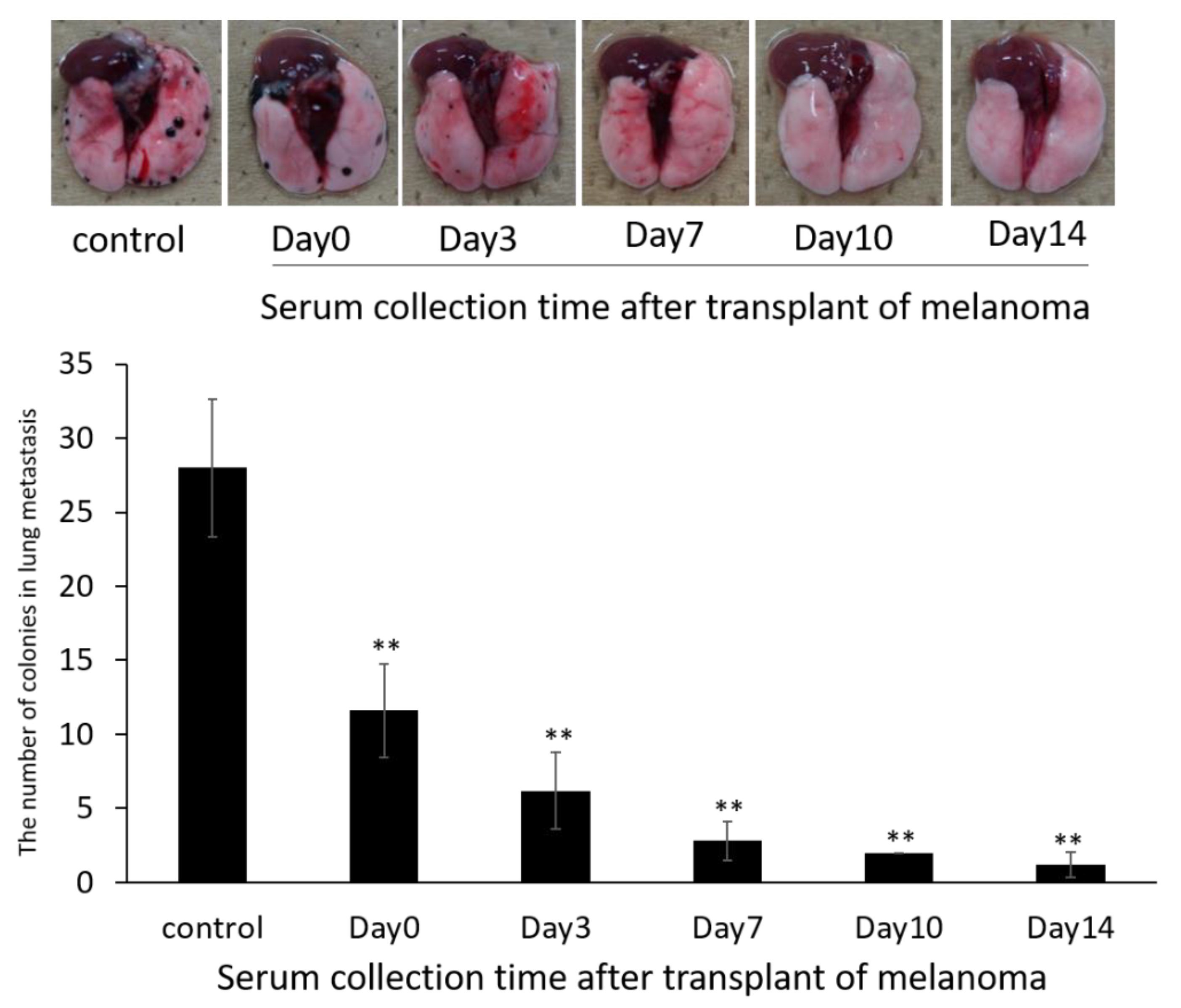
2.10.1. Evaluation of Exosome Collection Time to Use for siRNA Carrier
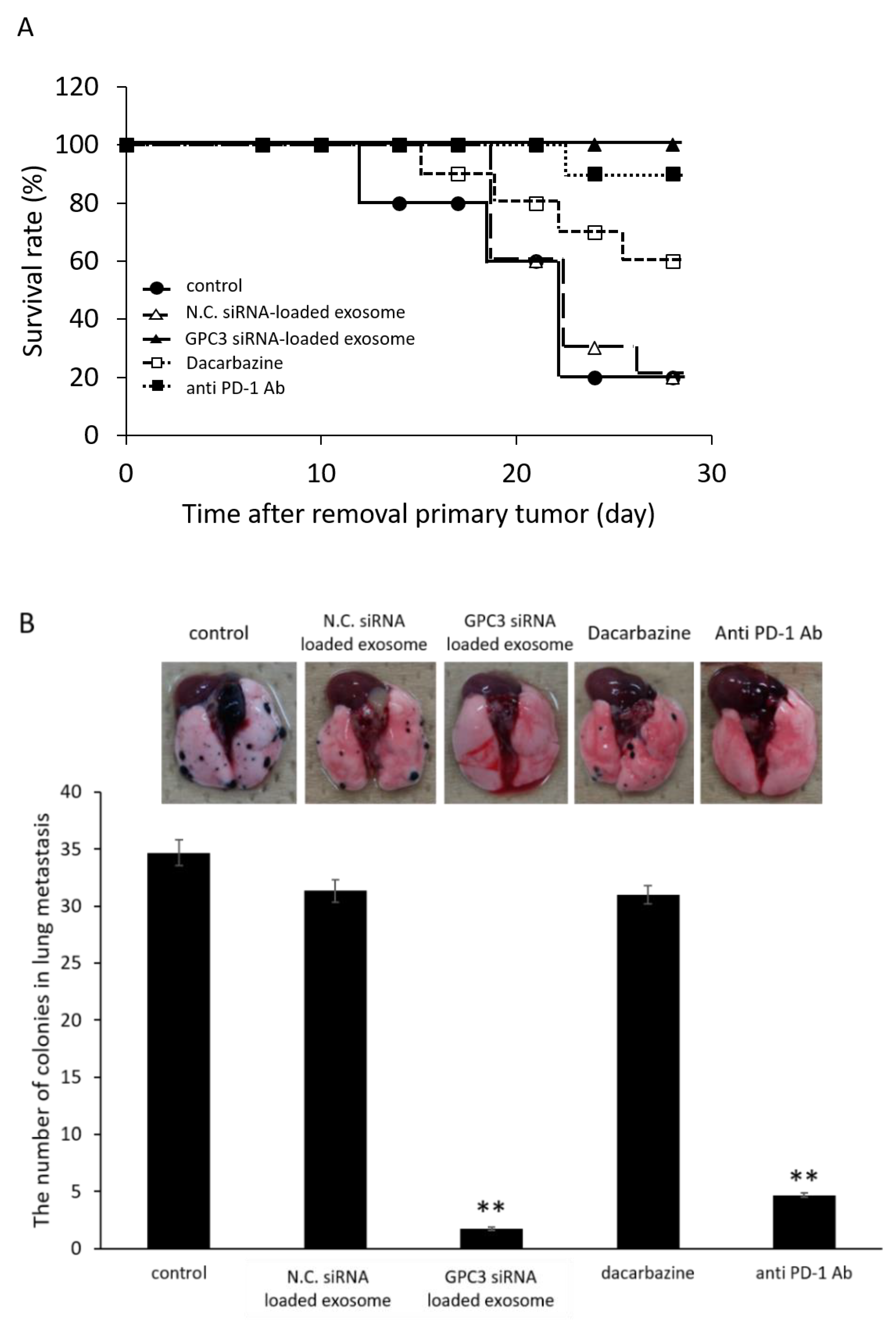
2.10.2. Effect of Survival Rate and Anti-Metastasis Effects of siRNA Loaded Exosome Compared with Dacarbazine or Anti PD-1 Antibody
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Size Distribution and Detection of Exosome Markers in Exosomes Isolated from Serum
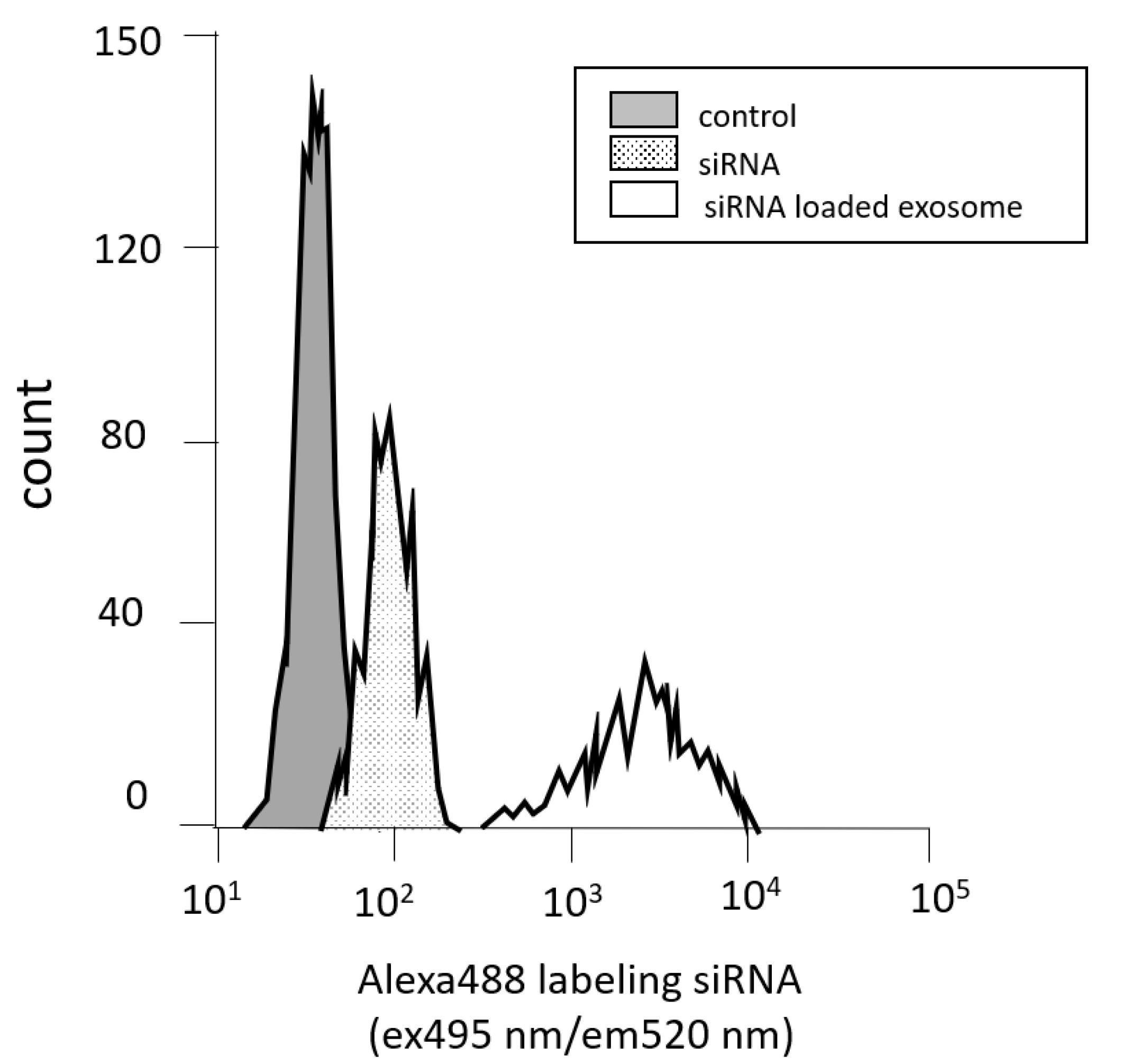
3.2. Measuring Cell Permeability by Flow Cytometry
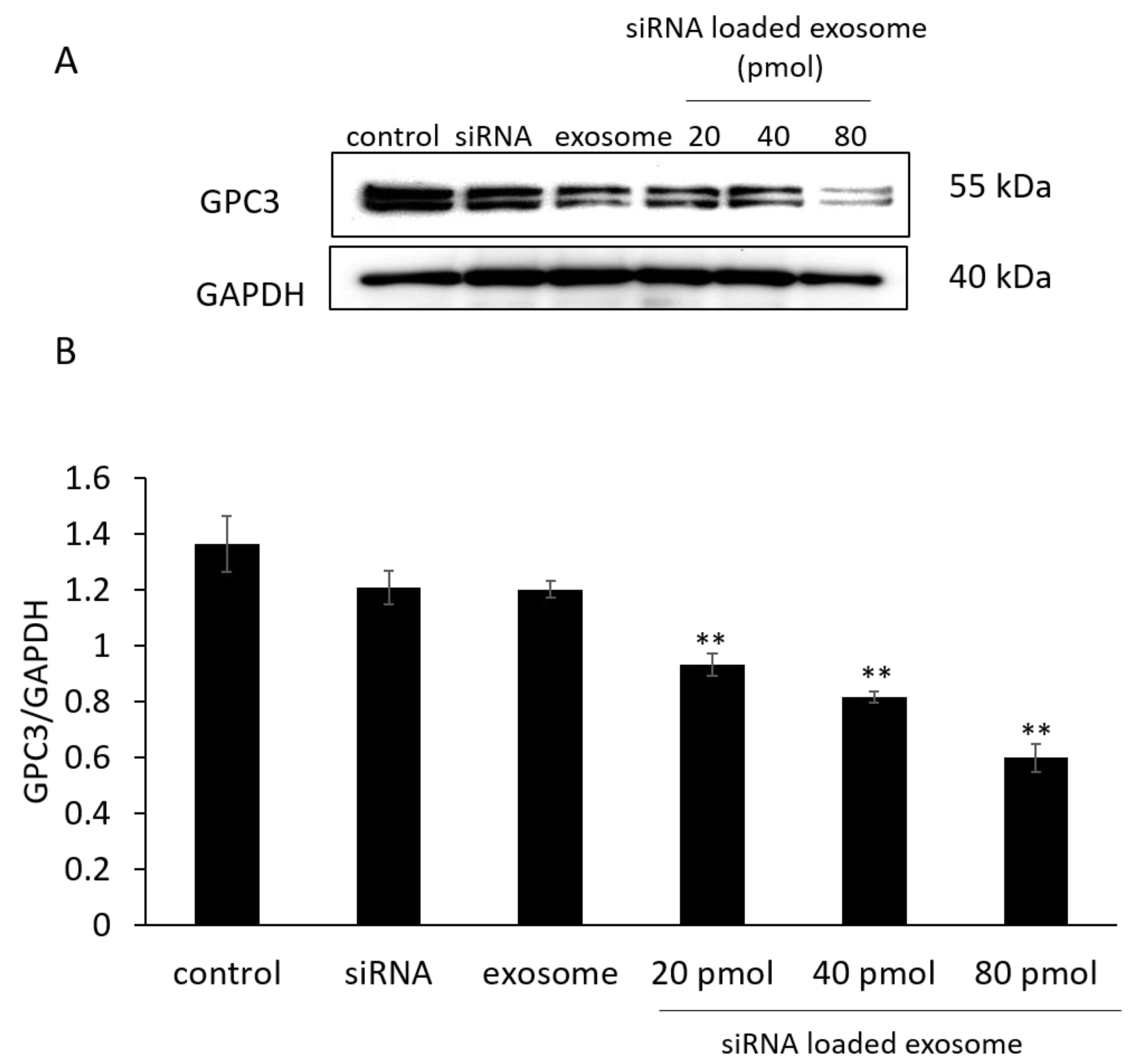
3.3. GPC3 Expression in B16/BL6 Cells Treated with siRNA-Loaded Exosomes
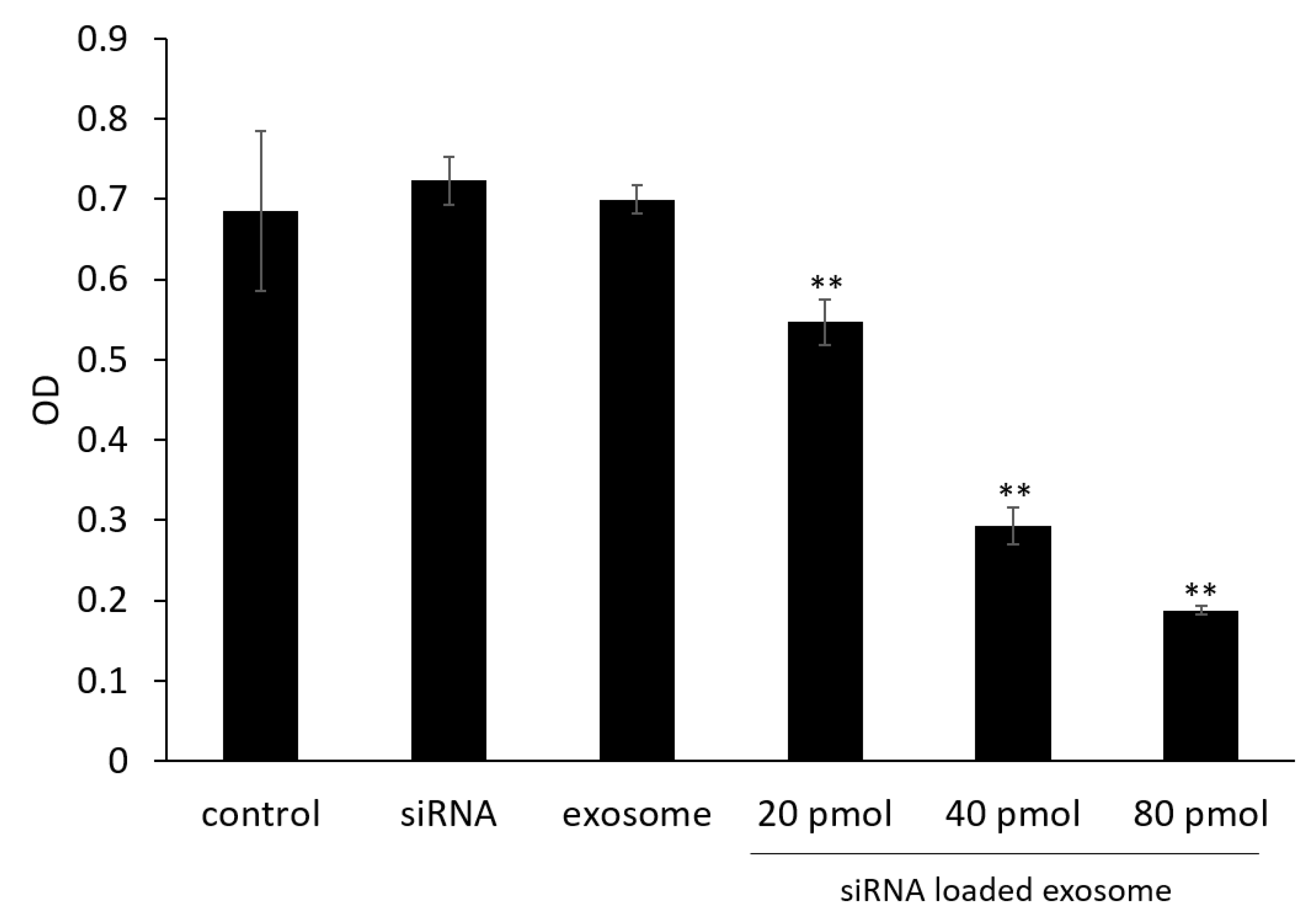
3.4. Cell Viability Assay
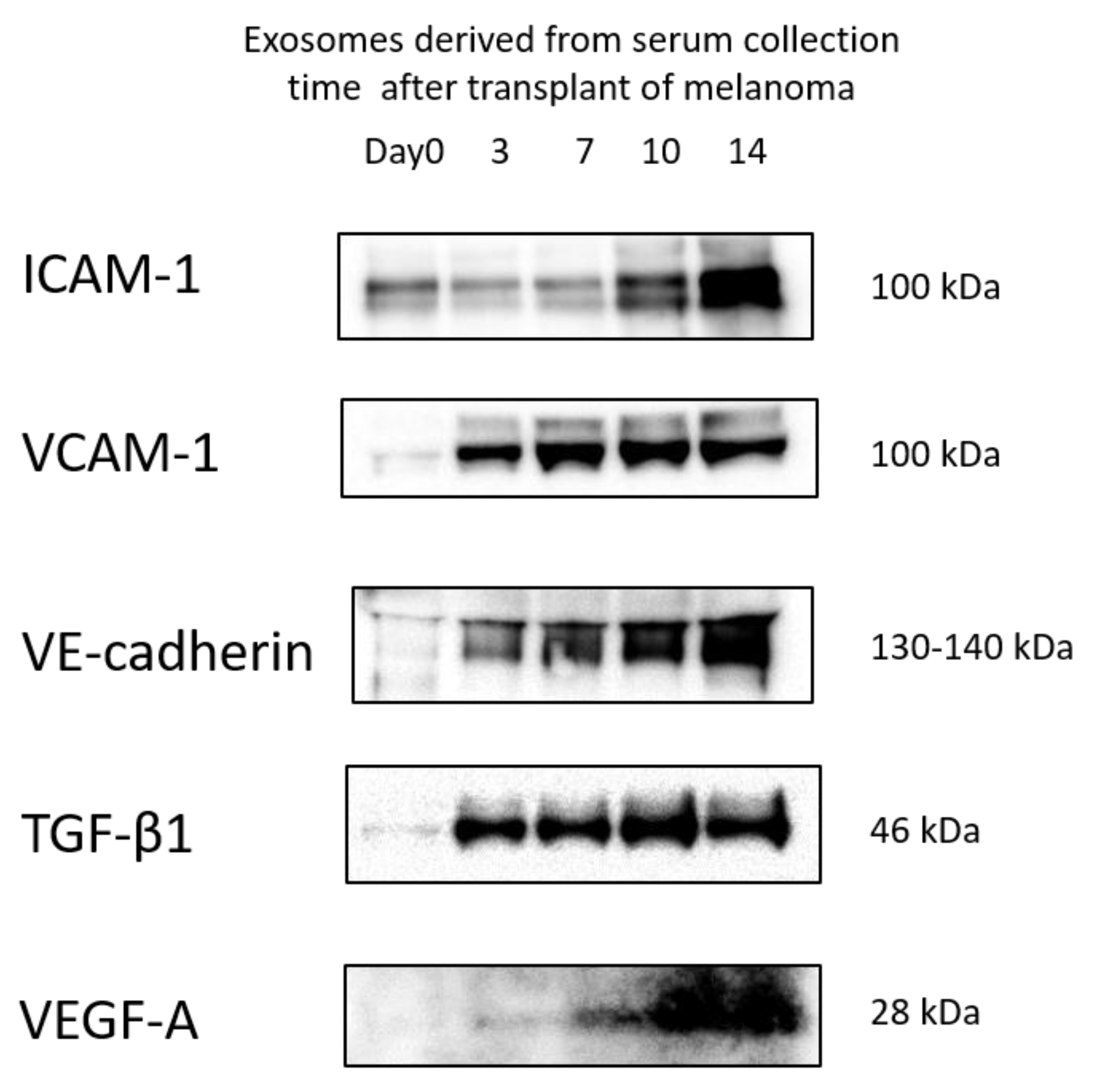
3.5. Protein Levels in Exosomes
3.6. Anti-Metastatic Effects of siRNA-Loaded Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model
3.7. Localization of ICG-Labeled Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model
3.8. Localization of Alexa 647-Labeled siRNA-Loaded Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model
3.9. Survival Rate and Anti-Metastatic Effects of siRNA-Loaded Exosomes in the B16/BL6 Spontaneous Lung Metastasis Mouse Model Compared with Dacarbazine and Anti-PD-1 Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Gumireddy, K.; Schrier, M.; Ie Sage, C.; Nagel, R.; Nair, S.; Egan, D.A.; Li, A.; Huang, G.; Klein-Szanto, A.J.; et al. The microRNAs miR-373 and miR-520c promote tumor invasion and metastasis. Nat. Cell Biol. 2008, 10, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hayashi, Y.; Nishinakagawa, T.; Hazekawa, M.; Kawano, S.; Nakamura, S.; Nakashima, M. The SQUU-B cell line spreads its metastatic properties to nonmetastatic clone SQUU-A from the same patient through exosomes. J. Oral Biosci. 2016, 58, 33–38. [Google Scholar] [CrossRef]
- Morioka, M.; Kawakubo-Yasukochi, T.; Hayashi, Y.; Hazekawa, M.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M.J. Exosomes from oral squamous carcinoma cell lines, SQUU-A and SQUU-B, define the tropism of lymphatic dissemination. Oral Biosci. 2016, 58, 180–184. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumor exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Tuschl, T.; Zamore, P.D.; Lehmann, R.; Bartel, D.P.; Sharp, P.A. Targeted mRNA degradation by double-stranded RNA in vitro. Gene Dev. 1999, 13, 3191–3197. [Google Scholar] [CrossRef] [Green Version]
- Hazekawa, M.; Nishinakagawa, T.; Kawakubo-Yasukochi, T.; Nakashima, M. Glypican-3 gene silencing for ovarian cancer using siRNA-PLGA hybrid micelles in a murine peritoneal dissemination model. J. Pharmacol. Sci. 2019, 139, 231–239. [Google Scholar] [CrossRef]
- Hazekawa, M.; Nishinakagawa, T.; Mori, T.; Yoshida, M.; Uchida, T.; Ishibashi, D. Preparation of siRNA-PLGA/Fab’-PLGA mixed micellar system with target cell-specific recognition. Sci. Rep. 2021, 11, 16789. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Hazekawa, M.; Yoshida, M.; Nishinakagawa, T.; Uchida, T.; Ishibashi, D. Enhancing the anticancer efficacy of a LL-37 peptide fragment analog using peptide-linked PLGA conjugate micelles in tumor cells. Int. J. Pharm. 2021, 606, 120891. [Google Scholar] [CrossRef] [PubMed]
- Hyoudou, K.; Nishikawa, M.; Ikemura, M.; Kobayashi, Y.; Mendelsohn, A.; Miyazaki, N.; Tabata, Y.; Yamashita, F.; Hashida, M. Prevention of pulmonary metastasis from subcutaneous tumors by binary system-based sustained delivery of catalase. J. Control. Release 2009, 137, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hyoudou, K.; Nishikawa, M.; Kobayashi, Y.; Ikemura, M.; Yamashita, F.; Hashida, M. SOD derives prevent metastatic tumor growth aggravated by tumor removal. Clin. Exp. Metastasis 2008, 25, 531–536. [Google Scholar] [CrossRef]
- Oh, E.; Hong, J.; Yun, C.O. Regulatory T cells induce metastasis by increasing Tgf-beta and enhancing the epithelial-mesenchymal transition. Cells 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, J.; Jia, X.J.; Yan, J.; Wang, H.C.; Feng, B.; Xing, H.Y.; Jia, Y.T. BRAF(V600E) mutant colorectal cancer cells mediate local immunosuppressive microenvironment through exosomal long noncoding RNAs. World J. Gastorointest. Oncol. 2021, 13, 2129–2148. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lim, J.W.; Moritz, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Okahara, H.; Yagita, H.; Miyake, K.; Okumura, K. Involvement of very late activation antigen 4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) in tumor necrosis factor alpha enhancement of experimental metastasis. Cancer Res. 1994, 54, 3233–3236. [Google Scholar] [PubMed]
- Chen, L.; Wang, L.; Zhu, L.; Xu, Z.; Liu, Y.; Li, Z.; Zhou, J.; Luo, F. Exosomes as drug carrier in anti-cancer therapy. Front. Cell Dev. Biol. 2022, 10, 728616. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, S.; Xiao, Z.; Chen, S.; Yang, L.; Peng, Q.; Li, H.; Fu, J.; Yu, X.; Zhang, L. Epigenetic inhibition assisted chemotherapeutic treatment of lung cancer based on artificial exosomes. Pharmacol. Res. 2021, 171, 105787. [Google Scholar] [CrossRef]
- Nakatsura, T.; Kageshita, T.; Ito, S.; Wakamatsu, K.; Monji, M.; Ikuta, Y.; Senju, S.; Ono, T.; Nishimura, Y. Identification of glypican-3 as a novel tumor marker for melanoma. Clin. Cancer Res. 2004, 10, 6612–6621. [Google Scholar] [CrossRef] [Green Version]
- Stadlmann, S.; Gueth, U.; Baumhoer, D.; Moch, H.; Terracciano, L.; Singer, G. Glypican-3 expression in primary and recurrent ovarian carcinomas. Int. J. Gynecol. Pathol. 2007, 26, 341–344. [Google Scholar] [CrossRef]
- Lin, Q.; Xiong, L.W.; Pan, X.F.; Gen, J.F.; Bao, G.L.; Sha, H.F.; Feng, J.X.; Ji, C.Y.; Chen, M. Expression of GPC3 protein and its significance in lung squamous cell carcinoma. Med. Oncol. 2012, 29, 663–669. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazekawa, M.; Nishinakagawa, T.; Hosokawa, M.; Ishibashi, D. Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model. Pharmaceutics 2022, 14, 815. https://doi.org/10.3390/pharmaceutics14040815
Hazekawa M, Nishinakagawa T, Hosokawa M, Ishibashi D. Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model. Pharmaceutics. 2022; 14(4):815. https://doi.org/10.3390/pharmaceutics14040815
Chicago/Turabian StyleHazekawa, Mai, Takuya Nishinakagawa, Masato Hosokawa, and Daisuke Ishibashi. 2022. "Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model" Pharmaceutics 14, no. 4: 815. https://doi.org/10.3390/pharmaceutics14040815
APA StyleHazekawa, M., Nishinakagawa, T., Hosokawa, M., & Ishibashi, D. (2022). Development of an Organ-Directed Exosome-Based siRNA-Carrier Derived from Autologous Serum for Lung Metastases and Testing in the B16/BL6 Spontaneous Lung Metastasis Model. Pharmaceutics, 14(4), 815. https://doi.org/10.3390/pharmaceutics14040815





