Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology
Abstract
:1. Introduction
2. Nanoparticles for Immunotherapy
3. Bacteria for Antitumor Therapy
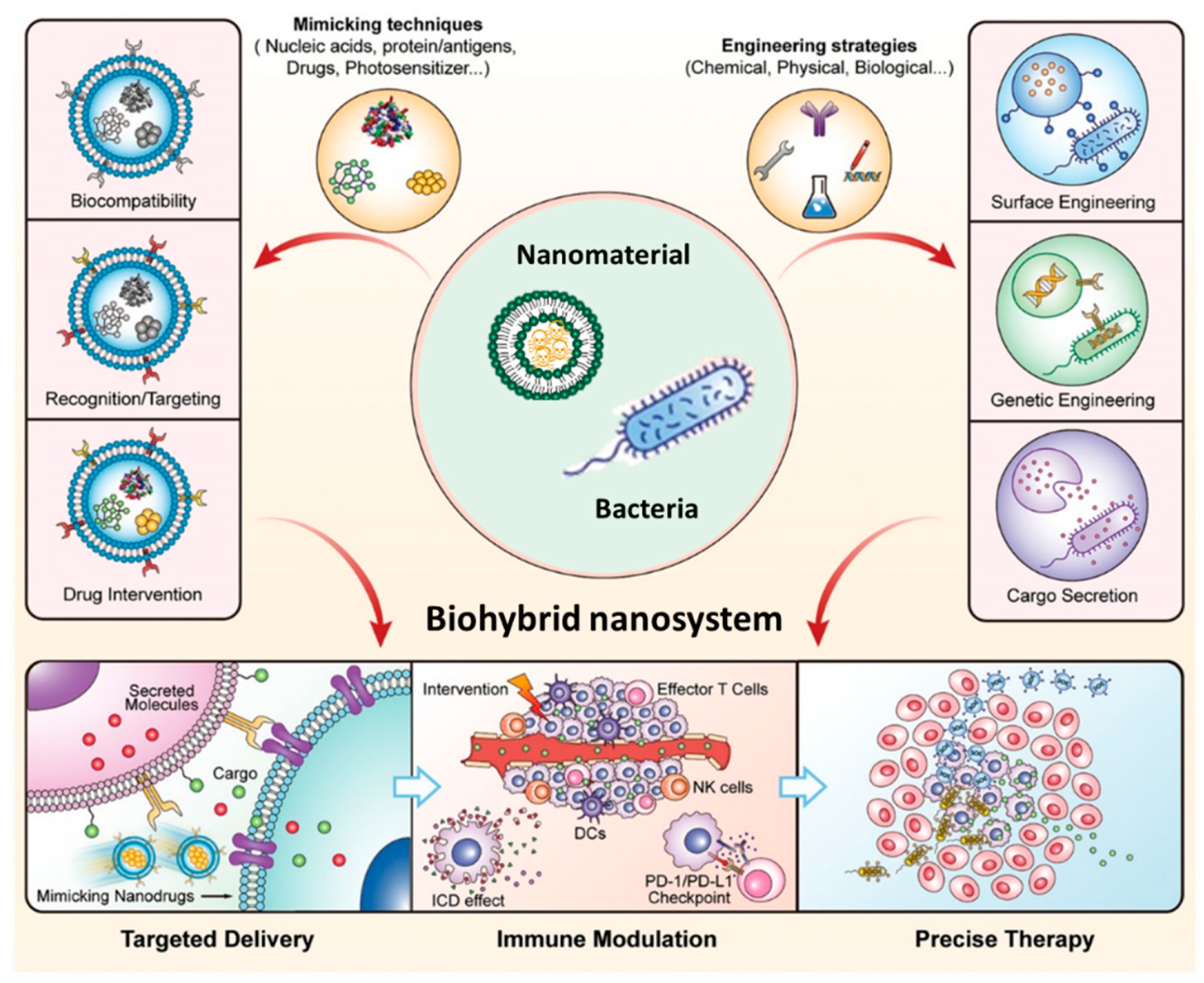
4. Bacterial Nanocarriers for Cancer Immunotherapy
4.1. Vectors of Oral DNA Vaccines
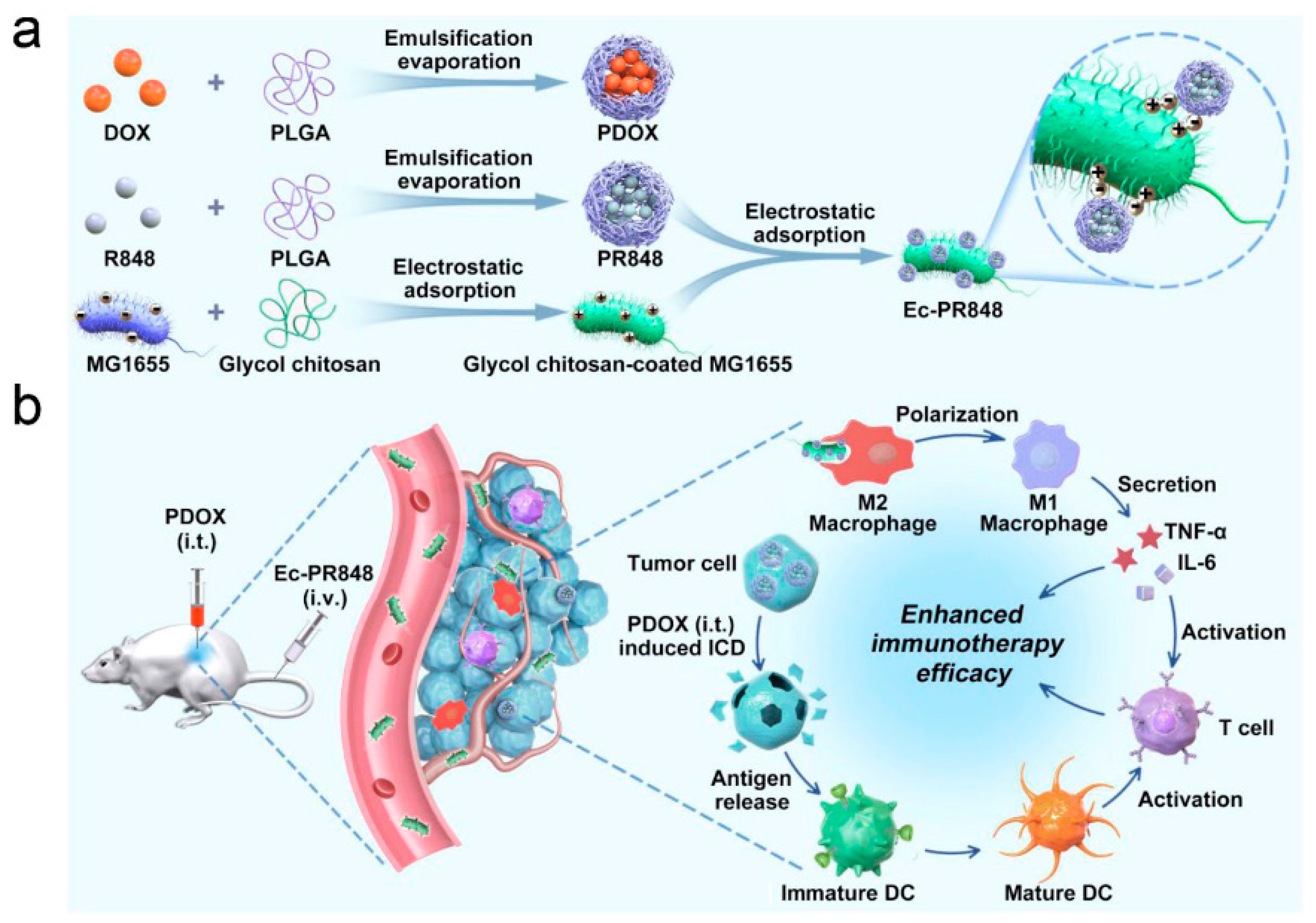
4.2. Agents for Macrophage Polarization
4.3. Providers of Bacterial Metabolites for Immunostimulation
4.4. Carriers for Immunogenic PDT/PTT
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Yang, P.; Peng, S.; Cao, Y.; Yao, X.; Guo, S.; Yang, W. A phosphorylcholine-based zwitterionic copolymer coated ZIF-8 nanodrug with a long circulation time and charged conversion for enhanced chemotherapy. J. Mater. Chem. B 2020, 8, 6128–6138. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, M.; Zheng, D.; Hong, S.; Feng, J.; Zhang, X.-Z. A Universal Approach to Render Nanomedicine with Biological Identity Derived from Cell Membranes. Biomacromolecules 2018, 19, 2043–2052. [Google Scholar] [CrossRef]
- Park, K. The beginning of the end of the nanomedicine hype. J. Control. Release 2019, 305, 221–222. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Villegas, M.R.; Baeza, A.; Noureddine, A.; Durfee, P.N.; Butler, K.S.; Agola, J.O.; Brinker, C.J.; Vallet-Regí, M. Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices. Chem. Mater. 2018, 30, 112–120. [Google Scholar] [CrossRef]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; von Roemeling, C.A.; Chen, Y.; Qie, Y.; Liu, X.; Chen, J.; Kim, B.Y.S. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng. 2017, 1, 0029. [Google Scholar] [CrossRef]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging Bio–Nano Science and Cancer Nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef]
- Chen, Y.; Du, M.; Yu, J.; Rao, L.; Chen, X.; Chen, Z. Nanobiohybrids: A Synergistic Integration of Bacteria and Nanomaterials in Cancer Therapy. BIO Integr. 2020, 1, 25–36. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.-W.; Singh, A.V.; Sitti, M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef]
- Carlsen, R.W.; Sitti, M. Bio-Hybrid Cell-Based Actuators for Microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, K.R.; Loeb, L.A. Genetic Instability and the Mutator Phenotype. Am. J. Pathol. 1999, 154, 1621–1626. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeza, A. Tumor Targeted Nanocarriers for Immunotherapy. Molecules 2020, 25, 1508. [Google Scholar] [CrossRef] [Green Version]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Ochyl, L.J.; Bazzill, J.D.; Park, C.; Xu, Y.; Kuai, R.; Moon, J.J. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials 2018, 182, 157–166. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.T.; Asthana, S.; Gao, S.P.; Lee, B.H.; Chapman, J.S.; Kandoth, C.; Gao, J.; Socci, N.D.; Solit, D.B.; Olshen, A.B.; et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat. Biotechnol. 2016, 34, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Laga, R.; Jewell, C.M. Induction of anti-cancer T cell immunity by in situ vaccination using systemically administered nanomedicines. Cancer Lett. 2019, 459, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Dudek, A.M.; Garg, A.D.; Krysko, D.V.; De Ruysscher, D.; Agostinis, P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013, 24, 319–333. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.P.; Salazar, F.; Sun, B.; Jiang, W.; Chang, C.H.; Jiang, J.; Wang, X.; Wu, A.M.; et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Löb, S.; Königsrainer, A.; Rammensee, H.-G.; Opelz, G.; Terness, P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat. Rev. Cancer 2009, 9, 445–452. [Google Scholar] [CrossRef]
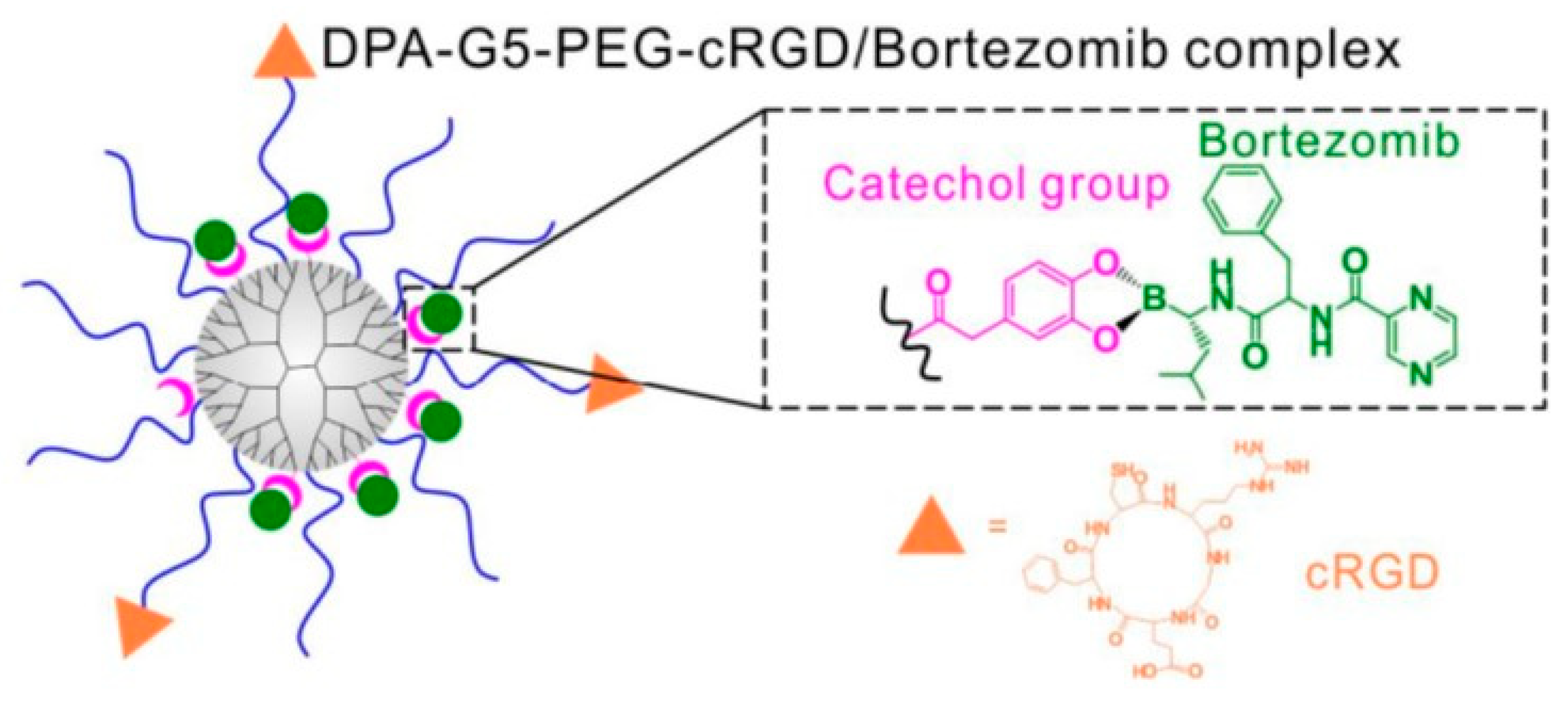
- Wang, M.; Cai, X.; Yang, J.; Wang, C.; Tong, L.; Xiao, J.; Li, L. A Targeted and pH-Responsive Bortezomib Nanomedicine in the Treatment of Metastatic Bone Tumors. ACS Appl. Mater. Interfaces 2018, 10, 41003–41011. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, 2004788. [Google Scholar] [CrossRef]
- Beola, L.; Grazú, V.; Fernández-Afonso, Y.; Fratila, R.M.; De Las Heras, M.; De La Fuente, J.M.; Gutiérrez, L.; Asín, L. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Appl. Mater. Interfaces 2021, 13, 12982–12996. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shim, M.K.; Yang, S.; Hwang, H.S.; Cho, H.; Kim, J.; Yun, W.S.; Moon, Y.; Kim, J.; Yoon, H.Y.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 12086–12098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Yang, Z.; Xu, J.; Xu, L.; Liang, C.; Han, X.; Zhuang, L. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019, 31, 1802228. [Google Scholar] [CrossRef] [PubMed]
- Alhallak, K.; Sun, J.; Wasden, K.; Guenthner, N.; O’Neal, J.; Muz, B.; King, J.; Kohnen, D.; Vij, R.; Achilefu, S.; et al. Nanoparticle T-cell engagers as a modular platform for cancer immunotherapy. Leukemia 2021, 35, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ortega, L.; Rojas, J.M.; Marcos, A.; Portilla, Y.; Stein, J.V.; Barber, D.F. T cells loaded with magnetic nanoparticles are retained in peripheral lymph nodes by the application of a magnetic field. J. Nanobiotechno. 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.A.; Drake, V.; Huang, H.S.; Chiu, S.C.; Zheng, L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Chen, H.; Luan, X.; Paholak, H.J.; Burnett, J.P.; Stevers, N.O.; Sansanaphongpricha, K.; He, M.; Chang, A.E.; Li, Q.; Sun, D. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine 2020, 15, 77–92. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.-H.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Poh, A.R.; Ernst, M. Targeting macrophages in cancer: From bench to bedside. Front. Oncol. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Liu, L.; Li, X.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J. Control. Release 2019, 313, 42–53. [Google Scholar] [CrossRef]
- Chen, X.-J.; Zhang, X.-Q.; Tang, M.-X.; Liu, Q.; Zhou, G. Anti-PD-L1-modified and ATRA-loaded nanoparticles for immuno-treatment of oral dysplasia and oral squamous cell carcinoma. Nanomedicine 2020, 15, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Phung, C.D.; Nguyen, H.T.; Choi, J.Y.; Pham, T.T.; Acharya, S.; Timilshina, M.; Chang, J.-H.; Kim, J.-H.; Jeong, J.-H.; Ku, S.K.; et al. Reprogramming the T cell response to cancer by simultaneous, nanoparticle-mediated PD-L1 inhibition and immunogenic cell death. J. Control. Release 2019, 315, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, S.N.; Singh, M.P.; Patil, G.; Li, S.; Fiering, S.; Hoopes, P.J.; Guha, C.; Malayer, J.; Ranjan, A. Novel calreticulin-nanoparticle in combination with focused ultrasound induces immunogenic cell death in melanoma to enhance antitumor immunity. Theranostics 2020, 10, 3397–3412. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of inoperable sarcoma with the ’mixed toxins of erysipelas and bacillus prodigiosus.: Immediate and final results in one hundred and forty cases. J. Am. Med. Assoc. 1898, 31, 456–465. [Google Scholar] [CrossRef]
- Coley Nauts, H.; Swift, W.E.; Coley, B.L. The Treatment of Malignant Tumors by Bacterial Toxins as Developed by the Late William B. Coley, M.D., Reviewed in the Light of Modern Research. Cancer Res. 1946, 6, 205–216. [Google Scholar] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F. Conventional cancer therapy: Promise broken or promise delayed? Lancet 1998, 351, SII9–SII16. [Google Scholar] [CrossRef]
- Palumbo, M.O.; Kavan, P.; Miller, W.H.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol. 2013, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Hoption Cann, S.A.; Van Netten, J.P.; Van Netten, C. Dr William Coley and tumour regression: A place in history or in the future. Postgrad. Med. J. 2003, 79, 672–680. [Google Scholar]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.L. Biologic therapies: What and when? J. Clin. Pathol. 2007, 60, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell. Mol. Immunol. 2020, 18, 805–828. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Pelanda, R.; Piccirillo, C.A. Tolerance, immune regulation, and autoimmunity: Cells and cytokines that make a difference. Curr. Opin. Immunol. 2008, 20, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007, 121, 1–14. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Makkouk, A.; Weiner, G.J. Cancer immunotherapy and breaking immune tolerance: New approaches to an old challenge. Cancer Res. 2015, 75, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [Green Version]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Weiss, S. Bacteria in cancer therapy: Renaissance of an old concept. Int. J. Microbiol. 2016, 2016, 8451728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, M.T.Q.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, A.; Chang, S.; Kothari, A.; Snijders, A.M.; Mao, J.-H.; Wang, J.; Hernández, A.C.; Keasling, J.D.; Bivona, T.G. Overcoming the challenges of cancer drug resistance through bacterial-mediated therapy. Chronic Dis. Transl. Med. 2019, 5, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Lambin, P.; Theys, J.; Landuyt, W.; Rijken, P.; Van Der Kogel, A.; Van Der Schueren, E.; Hodgkiss, R.; Fowler, J.; Nuyts, S.; De Bruijn, E.; et al. Colonisation of Clostridium in the body is restricted to hypoxic and necrotic areas of tumours. Anaerobe 1998, 4, 183–188. [Google Scholar] [CrossRef]
- Malmgren, R.A.; Flanigan, C.C. Localization of the Vegetative Form of Clostridium tetani in Mouse Tumors Following Intravenous Spore Administration. Cancer Res. 1955, 15, 473–478. [Google Scholar]
- Pylaeva, E.; Lang, S.; Jablonska, J. The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Front. Immunol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sieow, B.F.L.; Wun, K.S.; Yong, W.P.; Hwang, I.Y.; Chang, M.W. Tweak to Treat: Reprograming Bacteria for Cancer Treatment. Trends Cancer 2020, 7, 447–464. [Google Scholar] [CrossRef]
- Holay, M.; Guo, Z.; Pihl, J.; Heo, J.; Park, J.H.; Fang, R.H.; Zhang, L. Bacteria-Inspired Nanomedicine. ACS Appl. Bio Mater. 2021, 4, 3830–3848. [Google Scholar] [CrossRef]
- Saltzman, D.A.; Heise, C.P.; Hasz, D.E.; Zebede, M.; Kelly, S.M.; Curtiss, R.; Leonard, A.S.; Anderson, P.M. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: A novel anti-tumor agent. Cancer Biother. Radiopharm. 1996, 11, 145–153. [Google Scholar] [CrossRef]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gurbatri, C.; Coker, C.; Hinchliffe, T.E.; Lia, I.; Castro, S.; Treuting, P.M.; Arpaia, N.; Danino, T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. bioRxiv 2019. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, A.G.; Xiang, R.; Becker, J.C.; Wodrich, H.; Pertl, U.; Karsten, G.; Eliceir, B.P.; Reisfeld, R.A. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat. Med. 2002, 8, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, X.; Chen, L.; Li, S.; Ren, D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol. Ther. 2008, 7, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.; Nguyen, V.H.; D’Alincourt, M.S.; Manuel, E.R.; Kaltcheva, T.; Tsai, W.; Blazar, B.R.; Diamond, D.J.; Melstrom, L.G. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth. Cancer Gene Ther. 2020, 27, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-Based Cancer Immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Yang, C.J.; Kuan, Y.D.; Wang, W.K.; Chang, W.W.; Lee, C.H. The inhibition of indoleamine 2, 3-dioxygenase 1 by connexin 43. Int. J. Med. Sci. 2017, 14, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.X.; Nguyen, V.H.; Duong, M.T.-Q.; Hong, Y.; Choy, H.E.; Min, J.-J. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol. Immunol. 2015, 59, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Kocijancic, D.; Leschner, S.; Felgner, S.; Komoll, R.; Frahm, M.; Pawar, V.; Weiss, S. Therapeutic benefit of Salmonella attributed to LPS and TNF-α is exhaustible and dictated by tumor susceptibility. Oncotarget 2017, 8, 36492–36508. [Google Scholar] [CrossRef] [Green Version]
- Kupz, A.; Curtiss, R., III; Bedoui, S.; Strugnell, R.A. In Vivo IFN-γ Secretion by NK Cells in Response to Salmonella Typhimurium Requires NLRC4 Inflammasomes. PLoS ONE 2014, 9, e97418. [Google Scholar] [CrossRef]
- Low, K.B.; Ittensohn, M.; Le, T.; Platt, J.; Sodi, S.; Amoss, M.; Ash, O.; Carmichael, E.; Chakraborty, A.; Fischer, J.; et al. Lipid a mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat. Biotechnol. 1999, 17, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Bettegowda, C.; Huso, D.L.; Kinzler, K.W.; Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 15155–15160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Zheng, M.; Ma, A.; Liu, L.; Cai, L. Cell/Bacteria-Based Bioactive Materials for Cancer Immune Modulation and Precision Therapy. Adv. Mater. 2021, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, T.; Diao, Z.; Blum, N.T.; Qiu, L.; Ma, A.; Huang, P. Engineering Bacteria and Bionic Bacterial Derivatives with Nanoparticles for Cancer Therapy. Small 2021, 18, 2104643. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, C.; Moreno, V.M.; Vallet-Regí, M. Bacteria-Assisted Transport of Nanomaterials to Improve Drug Delivery in Cancer Therapy. Nanomaterials 2022, 12, 288. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, L.; Song, X.; Tang, M.; Mo, C.; Li, X. Doxorubicin-conjugated Escherichia coli Nissle 1917 swimmers to achieve tumor targeting and responsive drug release. J. Control. Release 2017, 268, 390–399. [Google Scholar] [CrossRef]
- Park, W.; Cho, S.; Huang, X.; Larson, A.C.; Kim, D.H. Branched Gold Nanoparticle Coating of Clostridium novyi-NT Spores for CT-Guided Intratumoral Injection. Small 2017, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.H.; Huang, C.T.; Su, C.H.; Yeh, C.S. Bacteria-Mediated Hypoxia-Specific Delivery of Nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016, 16, 3493–3499. [Google Scholar] [CrossRef]
- Moreno, V.M.; Álvarez, E.; Izquierdo-Barba, I.; Baeza, A.; Serrano-López, J.; Vallet-Regí, M. Bacteria as Nanoparticles Carrier for Enhancing Penetration in a Tumoral Matrix Model. Adv. Mater. Interfaces 2020, 7, 1901942. [Google Scholar] [CrossRef]
- Dogra, N.; Izadi, H.; Vanderlick, T.K. Micro-motors: A motile bacteria based system for liposome cargo transport. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent Binding of Nanoliposomes to the Surface of Magnetotactic Bacteria for the Synthesis of Self-Propelled Therapeutic Agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De Lanauze, D.; Zhong Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.B.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Hosseinidoust, Z.; Park, B.W.; Yasa, O.; Sitti, M. Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery. ACS Nano 2017, 11, 9759–9769. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Qin, M.; Zhang, X.; Zhang, Z.; Sun, X.; Gu, Z. Bacteria-Driven Hypoxia Targeting for Combined Biotherapy and Photothermal Therapy. ACS Nano 2018, 12, 5995–6005. [Google Scholar] [CrossRef]
- Park, B.W.; Zhuang, J.; Yasa, O.; Sitti, M. Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery. ACS Nano 2017, 11, 8910–8923. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef]
- Stanton, M.M.; Simmchen, J.; Ma, X.; Miguel-López, A.; Sánchez, S. Biohybrid Janus Motors Driven by Escherichia coli. Adv. Mater. Interfaces 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Zoaby, N.; Shainsky-Roitman, J.; Badarneh, S.; Abumanhal, H.; Leshansky, A.; Yaron, S.; Schroeder, A. Autonomous bacterial nanoswimmers target cancer. J. Control. Release 2017, 257, 68–75. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, P.; Zhu, G.; Yang, Y.; Xu, Z.; Yan, L.T. Bacteria-Activated Janus Particles Driven by Chemotaxis. ACS Nano 2018, 12, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Chen, Y.; Li, Z.H.; Xu, L.; Li, C.X.; Li, B.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. Optically-controlled bacterial metabolite for cancer therapy. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in Living Systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, A.; Van Hest, J.C.M. Bioorthogonal chemistry in living organisms. Chem. Sci. 2014, 5, 2123–2134. [Google Scholar] [CrossRef]
- Darji, A.; Zur Lage, S.; Garbe, A.I.; Chakraborty, T.; Weiss, S. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol. Med. Microbiol. 2000, 27, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.D.; Falkow, S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996, 14, 533–561. [Google Scholar] [CrossRef]
- Naciute, M.; Kiwitt, T.; Kemp, R.A.; Hook, S. Bacteria biohybrid oral vaccines for colorectal cancer treatment reduce tumor growth and increase immune infiltration. Vaccine 2021, 39, 5589–5599. [Google Scholar] [CrossRef]
- Wei, B.; Pan, J.; Yuan, R.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021, 21, 4231–4240. [Google Scholar] [CrossRef]
- Nagata, S.; Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017, 17, 333–340. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci. Rep. 2018, 8, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Wang, J.W.; Wang, X.N.; Fan, J.X.; Liu, X.H.; Li, B.; Han, Z.Y.; Cheng, S.X.; Zhang, X.Z. Inhibition of Tumor Progression through the Coupling of Bacterial Respiration with Tumor Metabolism. Angew. Chem.-Int. Ed. 2020, 59, 21562–21570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189.e15. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, H.; Luo, Z.; Zhou, H.; Liang, R.; Pan, H.; Ma, Y.; Cai, L. In Situ Photocatalyzed Oxygen Generation with Photosynthetic Bacteria to Enable Robust Immunogenic Photodynamic Therapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2020, 30, 1910176. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Z.; Zhu, Y.; Qiao, N.; Zhang, Z.; Sun, X. Combination of Bacterial-Photothermal Therapy with an Anti-PD-1 Peptide Depot for Enhanced Immunity against Advanced Cancer. Adv. Funct. Mater. 2020, 30, 1906623. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, V.M.; Baeza, A. Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology. Pharmaceutics 2022, 14, 784. https://doi.org/10.3390/pharmaceutics14040784
Moreno VM, Baeza A. Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology. Pharmaceutics. 2022; 14(4):784. https://doi.org/10.3390/pharmaceutics14040784
Chicago/Turabian StyleMoreno, Víctor M., and Alejandro Baeza. 2022. "Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology" Pharmaceutics 14, no. 4: 784. https://doi.org/10.3390/pharmaceutics14040784
APA StyleMoreno, V. M., & Baeza, A. (2022). Bacteria as Nanoparticle Carriers for Immunotherapy in Oncology. Pharmaceutics, 14(4), 784. https://doi.org/10.3390/pharmaceutics14040784






