AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect
Abstract
:1. Introduction
2. Experimental
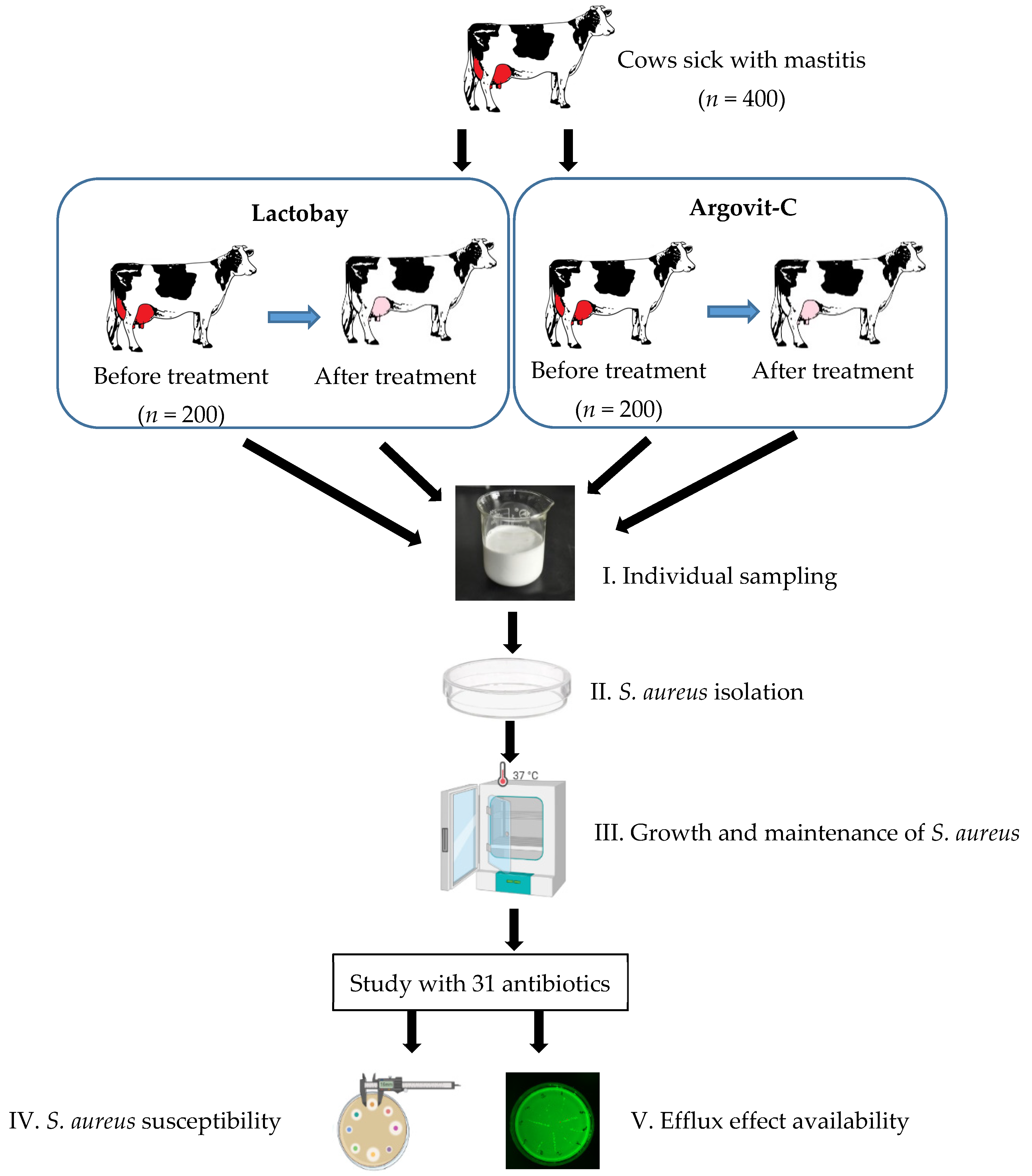
2.1. Sampling
2.2. Treatment Formulations
2.3. Isolation and Identification of S. aureus Bacteria
2.4. Antimicrobial Susceptibility and Efflux Testing
2.5. Statistical Analysis
3. Results
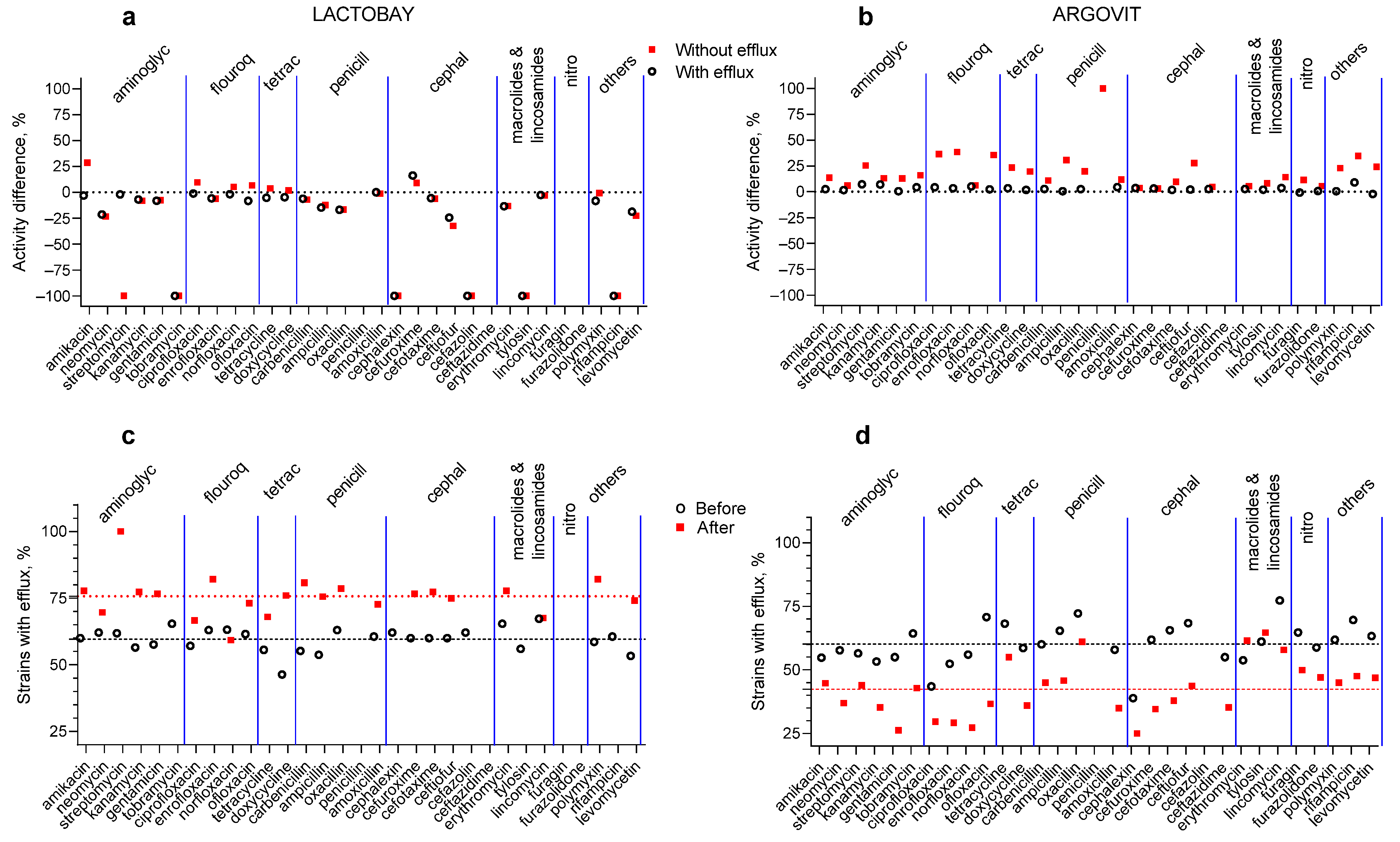
3.1. Antibiotic Susceptibility Changes after Treatments
3.2. Lactobay
3.3. Argovit-C
3.4. Changes in the Portion of Isolates with the Efflux Effect after Treatments
4. Discussion
- -
- P. aeruginosa: (1) ciprofloxacin resistant, (2) MDR isolates; (3) MDR and non-MDR;
- -
- E. coli: (1) (ATCC 700609), (2) biofilms of melioidosis pathogenic Ceftazidime-resistant (O157:H7), (3) ATCC 25922 and isolates of non-susceptible ofloxacin;
- -
- A. baumannii: (1) wild-type and MDR, (2) MDR, (3) 7865 (TNAB) and tigecycline susceptible 8010 (TSAB);
- -
- K. pneumoniae: (1) multidrug-resistant MGH78578 and (2) isolates (strain ATCC700603);
- -
- B. pseudomallei: (1) (1026b H777 and 316c)
- -
- E. cloacae: (1) clinical isolate (EspIMS6) and subsp. cloacae ATCC-13047.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, B.; Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Naghavi, M.; Lopez, A.D.; Zheng, P.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- ECDC/EMEA. The Bacterial Challenge: Time to React; European Center for Disease Prevention and Control: Stockholm, Sweden, 2009. [Google Scholar]
- Centers for Disease Control and Prevention, US Department of Health and Human Services. Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2013.
- WHO. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 8 February 2022).
- Vivas, R.; Teixeira Barbosa, A.A.; Santana Dolabela, S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resit. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Benkő, R.; Gajdács, M.; Matuz, M.; Bodó, G.; Lázár, A.; Hajdú, E.; Papfalvi, E.; Hannauer, P.; Erdélyi, P.; Pető, Z. Prevalence and Antibiotic Resistance of ESKAPE Pathogens Isolated in the Emergency Department of a Tertiary Care Teaching Hospital in Hungary: A 5-Year Retrospective Survey. Antibiotics 2020, 9, 624. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12, 454. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Majhi, R.K.; Goswami, L.; Goswami, C.; Mohapatra, H. Antibacterial Efficacy of Polysaccharide Capped Silver Nanoparticles Is Not Compromised by AcrAB-TolC Efflux Pump. Front Microbiol. 2018, 9, 823. [Google Scholar] [CrossRef]
- Nallathamby, P.D.; Lee, K.J.; Desai, T.; Xu, X.H. Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry 2010, 49, 5942–5953. [Google Scholar] [CrossRef] [Green Version]
- Abdolhosseini, M.; Zamani, H.; Salehzadeh, A. Synergistic antimicrobial potential of ciprofloxacin with silver nanoparticles conjugated to thiosemicarbazide against ciprofloxacin resistant Pseudomonas aeruginosa by attenuation of MexA-B efflux pump genes. Biologia 2019, 74, 1191–1196. [Google Scholar] [CrossRef]
- Madhi, M.; Hasani, A.; Shahbazi Mojarrad, J.; Ahangarzadeh Rezaee, M.; Zarrini, G.; Soodabeh, D.; Effat, A.; Vajihe, S. Impact of Chitosan and Silver Nanoparticles Laden with Antibiotics on Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Arch. Clin. Infect Dis. 2020, 15, e100195. [Google Scholar]
- Behdad, R.; Pargol, M.; Mirzaie, A.; Karizi, S.Z.; Noorbazargan, H.; Akbarzadeh, I. Efflux pump inhibitory activity of biologically synthesized silver nanoparticles against multidrug-resistant Acinetobacter baumannii clinical isolates. J. Basic Microbiol. 2020, 60, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Alhajjar, R. Comparative Study of Biocides and Nanoparticles on Bacterial Microorganisms. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2021. [Google Scholar]
- Stabryla, L.M.; Johnston, K.A.; Diemler, N.A.; Cooper, V.S.; Millstone, J.E.; Haig, S.J.; Gilbertson, L.M. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 2021, 16, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Srichaiyapol, O.; Thammawithan, S.; Siritongsuk, P.; Nasompag, S.; Daduang, S.; Klaynongsruang, S.; Kulchat, S.; Patramanon, R. Tannic Acid-Stabilized Silver Nanoparticles Used in Biomedical Application as an Effective Antimelioidosis and Prolonged Efflux Pump Inhibitor against Melioidosis Causative Pathogen. Molecules 2021, 26, 1004. [Google Scholar] [CrossRef]
- Pareek, V.; Devineau, S.; Sivasankaran, S.K.; Bhargava, A.; Panwar, J.; Srikumar, S.; Fanning, S. Silver Nanoparticles Induce a Triclosan-Like Antibacterial Action Mechanism in Multi-Drug Resistant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 183. [Google Scholar] [CrossRef]
- Aghigh, D.; Hassan, N.; Nazanin, K.; Pooria, M.; Naghmeh, Z.; Zahra, A.L.; Fatemeh, A. Ecofriendly biomolecule-capped Bifidobacterium bifidum-manufactured silver nanoparticles and efflux pump genes expression alteration in Klebsiella pneumoniae. Microb. Drug Resist. 2021, 27, 247–257. [Google Scholar]
- Chan, Y.Y.; Chua, K.L. The Burkholderia pseudomallei BpeAB-OprB efflux pump: Expression and impact on quorum sensing and virulence. J. Bacteriol. 2005, 187, 4707–4719. [Google Scholar] [CrossRef] [Green Version]
- Gopisetty, M.K.; Kovács, D.; Igaz, N.; Rónavári, A.; Bélteky, P.; Rázga, Z.; Kiricsi, M.; Venglovecz, V.; Bálint, C.; Imre, M.B.; et al. Endoplasmic reticulum stress: Major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Dongmei, J.; Wenping, S. Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. 2021, 93, 104937. [Google Scholar]
- Shkil, N.N.; Nefedova, E.V.; Burmistrov, V.A. Influence of silver nanoparticles of the drug Argovit on the antibiotic resistance of bacteria in the treatment of mastitis in cows. Polythematic Netw. Electron. Sci. J. Kuban State Agrar. Univ. 2018, 142, 57–67. [Google Scholar]
- Bardum, D.A.; Newbould, F.H.S. The use of the California mastitis test for the detection of bovine mastitis. Can. Vet. J. 1961, 2, 83. [Google Scholar]
- Buchanan, R.E.; Gibbons, N.E. Bergey’s Manual of Determinative Bacteriology, 8th ed.; Williams & Wilkins: Baltimore, MD, USA, 1974; p. 1268. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Disk Susceptibility Tests; M2-A10; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Martins, M.; Viveiros, M.; Couto, I. Identification of efflux pump-mediated multidrug-resistant bacteria by the ethidium bromide-agar cartwheel method. In Vivo 2011, 25, 171–178. [Google Scholar] [PubMed]
- Agrovetservis.ru. Available online: http://agrovetservis.ru/?mode=product&product_id=398598611 (accessed on 2 February 2022).
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Dashtbani-Roozbehani, A.; Brown, M.H. Efflux Pump Mediated Antimicrobial Resistance by Staphylococci in Health-Related Environments: Challenges and the Quest for Inhibition. Antibiotics 2021, 10, 1502. [Google Scholar] [CrossRef]
- Patel, D.; Kosmidis, C.; Seo, S.M.; Kaatz, G.W. Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5070–5073. [Google Scholar] [CrossRef] [Green Version]
- Tennent, J.M.; Lyon, B.R.; Midgley, M.; Jones, K.G.; Purewal, A.S.; Skurray, R.A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J. Gen. Microbiol. 1989, 135, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lekshmi, M.; Ammini, P.; Jones Adjei, L.M.S.; Shrestha, U.; Kumar, S.; Varela, M.F. Modulation of antimicrobial efflux pumps of the major facilitator superfamily in Staphylococcus aureus. AIMS Microbiol. 2018, 4, 1. [Google Scholar] [CrossRef]
- Wang, N.; Li, D.; Schwarz, S.; Qin, S.; Yao, H.; Du, X.-D. Novel Tet(L) Efflux Pump Variants Conferring Resistance to Tigecycline and Eravacycline in Staphylococcus Spp. Microbiol. Spectr. 2021, 9, e01310-21. [Google Scholar] [CrossRef]
- Matsuoka, M.; Inoue, M.; Endo, Y.; Nakajima, Y. Characteristic expression of three genes, msr(A), mph(C) and erm(Y), that confer resistance to macrolide antibiotics on Staphylococcus aureus. FEMS Microbiol. Lett. 2003, 220, 287–293. [Google Scholar] [CrossRef] [Green Version]
- LaBreck, P.T.; Bochi-Layec, A.C.; Stanbro, J.; Dabbah-Krancher, G.; Simons, M.P.; Merrell, D.S. Systematic analysis of efflux pump-mediated antiseptic resistance in Staphylococcus aureus suggests a need for greater antiseptic stewardship. mSphere 2020, 5, e00959-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitrofurans. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/VetInfo/VetAntibioResSen/LADDL/AntimicrobialClasses/otherantibiotics/Nitrofurans.pdf (accessed on 9 March 2022).
- Whitehouse, C.A.; Zhao, S.; Tate, H. Chapter One: Antimicrobial Resistance in Campylobacter Species: Mechanisms and Genomic Epidemiology. In Advances in Applied Microbiology; Sima, S., Geoffrey, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 103, pp. 1–47. [Google Scholar]
- Kang, X.Q.; Qiao, Y.; Lu, X.Y.; Jiang, S.P.; Li, W.S.; Wang, X.J.; Du, Y.Z.; Xu, X.-L.; Qi, J.; Xiao, Y.-H. Tocopherol Polyethylene Glycol Succinate-Modified Hollow Silver Nanoparticles for Combating Bacteria-Resistance. Biomater. Sci. 2019, 7, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Y.; Aly, U.F.; Abd El-Baky, R.M.; Waly, N.G.F.M. Effect of Titanium Dioxide Nanoparticles on the Expression of Efflux Pump and Quorum-Sensing Genes in MDR Pseudomonas aeruginosa Isolates. Antibiotics 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, I.; Giannousi, K.; Protonotariou, E.; Skoura, L.; Arsenakis, M.; Dendrinou-Samara, C. Cocktail of CuO, ZnO, or CuZn Nanoparticles and Antibiotics for Combating Multidrug-Resistant Pseudomonas aeruginosa via Efflux Pump Inhibition. ACS Appl. Nano Mater. 2021, 4, 9799–9810. [Google Scholar] [CrossRef]
- Arya, S.S.; Sharma, M.M.; Das, R.K.; Rookes, J.; Cahill, D.; Lenka, S.K. Vanillin mediated green synthesis and application of gold nanoparticles for reversal of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates. Heliyon 2019, 5, e02021. [Google Scholar] [CrossRef] [Green Version]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Singh, R.; Singh, K.P.; Chaicumpa, W.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]

| Microorganisms | Number of Isolates | % |
|---|---|---|
| S. aureus | 360 | 90 |
| S. epidermidis | 220 | 55 |
| S. dysgalactiae | 198 | 49.5 |
| S. agalactiae | 180 | 45 |
| S. pyogenes | 160 | 40 |
| E. coli | 51 | 12.7 |
| Activity Change Category | Isolates without the Efflux Effect | Isolates with the Efflux Effect | ||
|---|---|---|---|---|
| Number of Antibiotics | Average Change in Activity | Number of Antibiotics | Average Change in Activity | |
| Cumulative change | ||||
| Total activity change | 27 | −25.8% | 27 | −24.5% |
| Total activity change for 54 samples | −25.1% | |||
| Changes detailed | ||||
| Activity remains absent | 4 | 0 | 4 | 0 |
| Activity disappeared (−100%) | 6 | −100% | 5 | −100% |
| Activity appeared (+100%) | 0 | 0 | 0 | 0 |
| Activity decreased (−Δ%) | 14 | −11.5% | 20 | −9.1% |
| Activity increased (+Δ%) | 7 | +9.1% | 1 | +16.0% |
| Activity constant (0%) | 0 | 0 | 1 | 0 |
| Activity Change Category | Isolates without the Efflux Effect | Isolates with the Efflux Effect | ||
|---|---|---|---|---|
| Number of Antibiotics | Average Change in Activity | Number of Antibiotics | Average Change in Activity | |
| Cumulative change | ||||
| Total activity change | 30 | +19.9% | 29 | +2.9% |
| Total activity change for 59 samples | +11.4% | |||
| Changes detailed | ||||
| Remain absent | 1 | 0 | 2 | 0 |
| Disappeared (−100%) | 0 | 0 | 0 | 0 |
| Appeared (+100%) | 1 | +100% | 0 | 0 |
| Decreased (−Δ%) | 0 | 0 | 2 | −1.4% |
| Increased (+Δ%) | 29 | +17.1% | 27 | +3.2% |
| Constant (0%) | 0 | 0 | 0 | 0 |
| Biological System | Results Concerning the Efflux Effect | Reference |
|---|---|---|
| Ciprofloxacin-resistant P. aeruginosa. | AgNPs alone and with ciprofloxacin considerably decreased the expression of bacterial efflux pump MexA and MexB genes. | [14] |
| P. aeruginosa MDR isolates and A. baumannii Clinical antibiotic-resistance isolates | Efflux pump inhibitor activity of AgNPs was observed in 25 and 57% of isolates of Acinetobacter and Pseudomonas, respectively. | [15] |
| MDR, and non-MDR P. aeruginosa and wild-type and MDR A. baumannii | AgNPs had efflux pump inhibitor activity against A. baumannii and P. aeruginosa. | [20] |
| Multidrug-resistant A. baumannii | AdeA, AdeC, AdeS, AdeR, AdeI, AdeJ, and AdeK genes were downregulated considerably after AgNPs treatment. | [16] |
| E. coli (ATCC 700609) | Efflux pump inhibition led to enhancement of sensitivity to two antimicrobials. | [17] |
| Biofilms of melioidosis pathogenic Ceftazidime-resistant E. coli (O157:H7) and B. pseudomallei (1026b H777 and 316c) | AgNPs continue to exhibit a strong efflux pump inhibition against B. pseudomallei even after extended exposure for 30 passages with sublethal doses. | [19] |
| E. coli ATCC 25922, isolates of ofloxacin non-susceptible E. coli (N-E. coli), tigecycline non-susceptible A. baumannii 7865 (TNAB), and tigecycline susceptible A. baumannii 8010 (TSAB) | Surfactant modified AgNPs could decline the activity of efflux pumps AdeABC and AdeIJK in drug-resistant A. baumannii due to inhibition of the efflux pump genes ade B and ade J, in tigecycline-susceptible A. baumannii 8010 (TSAB). | [41] |
| Multidrug-resistant K. pneumoniae MGH78578 | Activation of efflux pumps after exposure to AgNPs. | [20] |
| K. pneumoniae isolates (strain ATCC700603) | Biosynthesized AgNPs reduce the expression of OxqAB efflux pump genes. | [21] |
| E. cloacae clinical isolate (EspIMS6) and E. cloacae subsp. cloacae ATCC-13047 | AgNPs addition reduced expression of functional AcrB protein in E. cloacae. | [12] |
| Bifidobacterium bifidum (probiotic) | Both biosynthesized and commercial AgNPs decreased the oxqAB gene expression levels. | [21] |
| The drug-resistant cancer MCF-7/KCR cell line (purchased from ATCC) was developed from MCF-7 under Doxorubicin (chemotherapy drug) in selection pressure from 10 nM to 1 μM | 75 nm AgNPs considerably inhibited P-glycoprotein efflux activity in DR breast cancer cells, while 5 nm AgNPs did not. | [23] |
| Planktonic cells and biofilms | AgNPs downregulated ERG1, ERG11, ERG25 and CDR2, decreased levels of membrane ergosterol and membrane fluidity, decreased membrane content of Cdr1p, Cdr2p, and consequently efflux pump activity. | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nefedova, E.; Shkil, N.; Luna Vazquez-Gomez, R.; Garibo, D.; Pestryakov, A.; Bogdanchikova, N. AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect. Pharmaceutics 2022, 14, 763. https://doi.org/10.3390/pharmaceutics14040763
Nefedova E, Shkil N, Luna Vazquez-Gomez R, Garibo D, Pestryakov A, Bogdanchikova N. AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect. Pharmaceutics. 2022; 14(4):763. https://doi.org/10.3390/pharmaceutics14040763
Chicago/Turabian StyleNefedova, Ekaterina, Nikolay Shkil, Roberto Luna Vazquez-Gomez, Diana Garibo, Alexey Pestryakov, and Nina Bogdanchikova. 2022. "AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect" Pharmaceutics 14, no. 4: 763. https://doi.org/10.3390/pharmaceutics14040763
APA StyleNefedova, E., Shkil, N., Luna Vazquez-Gomez, R., Garibo, D., Pestryakov, A., & Bogdanchikova, N. (2022). AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect. Pharmaceutics, 14(4), 763. https://doi.org/10.3390/pharmaceutics14040763








