Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Curcumin and Gentamicin Sulfate-Loaded Niosomes
2.2.2. Encapsulation Efficacy
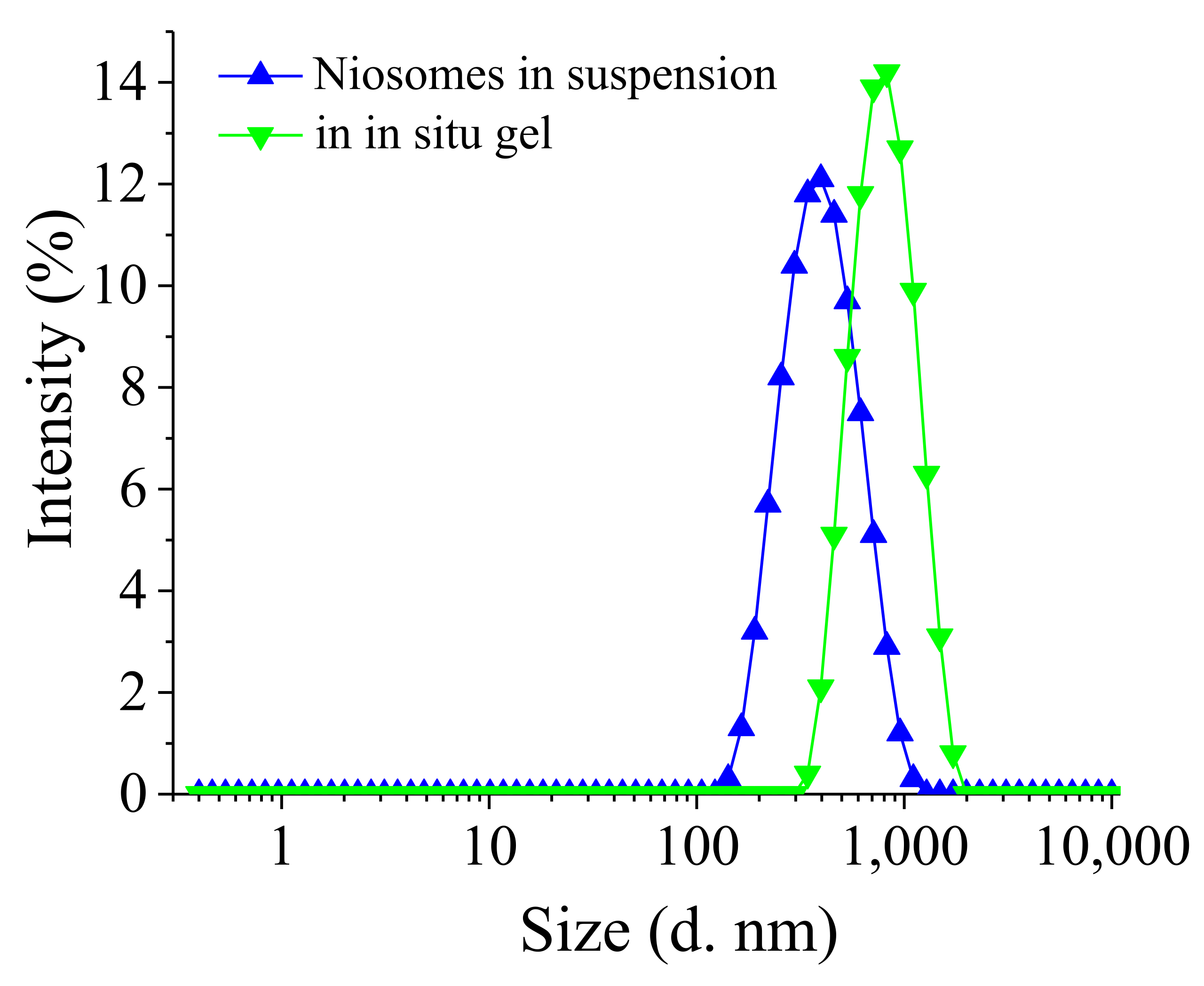
2.2.3. Size and Size Distribution
2.2.4. Zeta-Potential
2.2.5. Cryogenic Transmission Electron Microscopy (cryo-TEM) Measurements
2.3. Preparation and Characterization of Plain and Curc–GS Niosomal In Situ Gels
2.3.1. Preparation of In Situ Gels
2.3.2. Evaluation of Gelation Temperature
2.3.3. Gelling Time Determination
2.3.4. Gel Erosion Time
2.3.5. Rheological Study of Niosomal Thermoresponsive Gels
2.4. In Vitro Drug Release
2.5. Stability Evaluation
2.6. Evaluation of Antibacterial Activity
2.6.1. Bacterial Strains and Culture Conditions
2.6.2. Determination of Minimal Inhibitory Concentrations
2.6.3. Checkerboard Assay
2.6.4. Redox (Dehydrogenase) Activity Assay for Bacterial Cells
2.7. Evaluation of Cytotoxicity of Curcumin and Gentamicin-Loaded Niosomes and the In Situ Thermosensitive Gels Thereof
2.7.1. Cell Lines and Culture Conditions
2.7.2. MTT Colorimetric Assay
2.7.3. Statistical Methods
3. Results and Discussion
3.1. Preparation and Characterization of Curcumin and Gentamicin Simultaneously Loaded Niosomes
3.2. Cryo-TEM
3.3. Preparation and Characterization of Niosomal Thermo-Responsive In Situ Gels
3.3.1. Preliminary Evaluation of Gelling Properties of Plain and Hybrid Niosomal In Situ Gels
3.3.2. Rheological Studies of Selected Plain and Curc/GS-Loaded Niosomal In Situ Gels
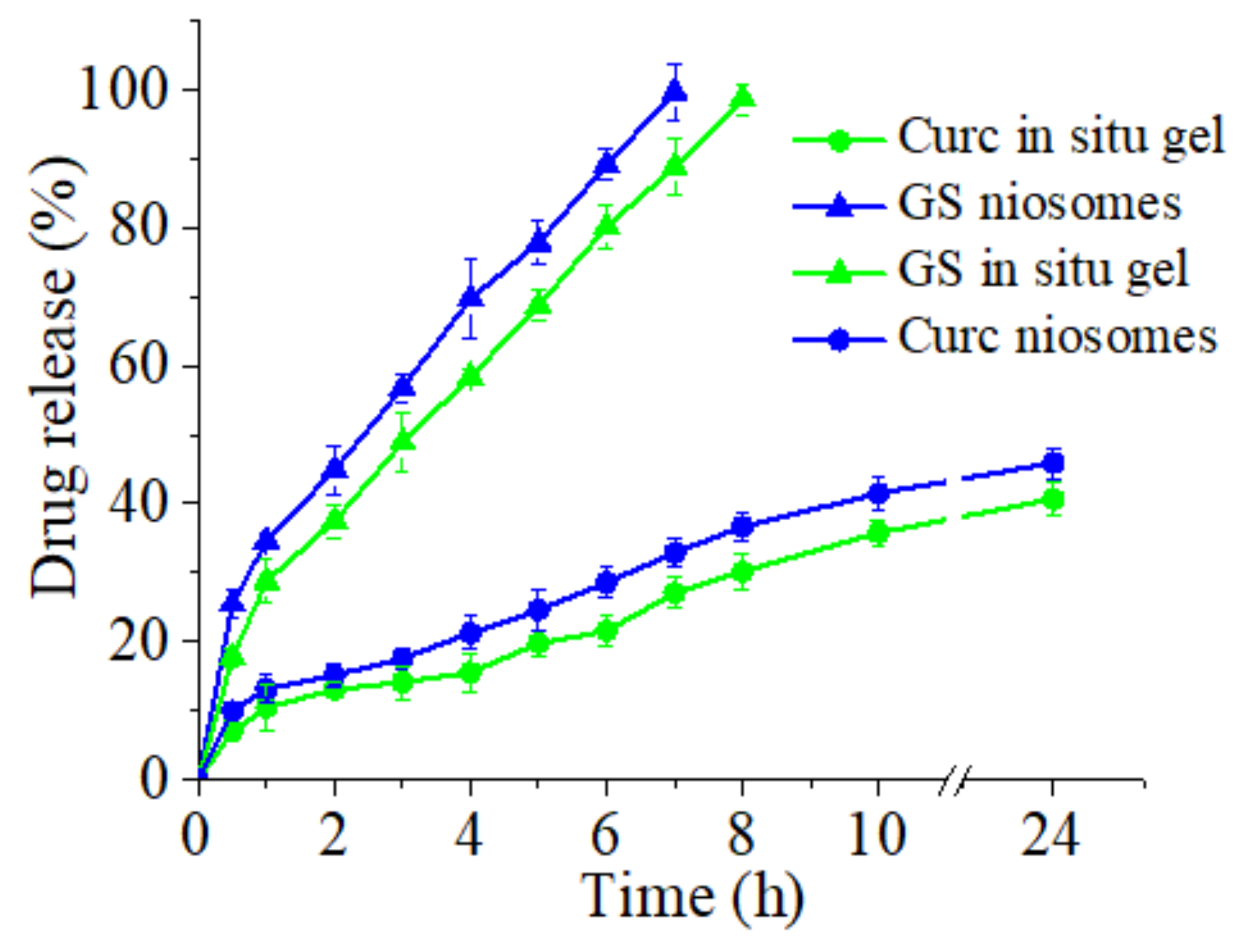
3.4. In Vitro Release of Curcumin and Gentamicin Sulfate from Niosomes and In Situ Gels
3.5. Stability Evaluation
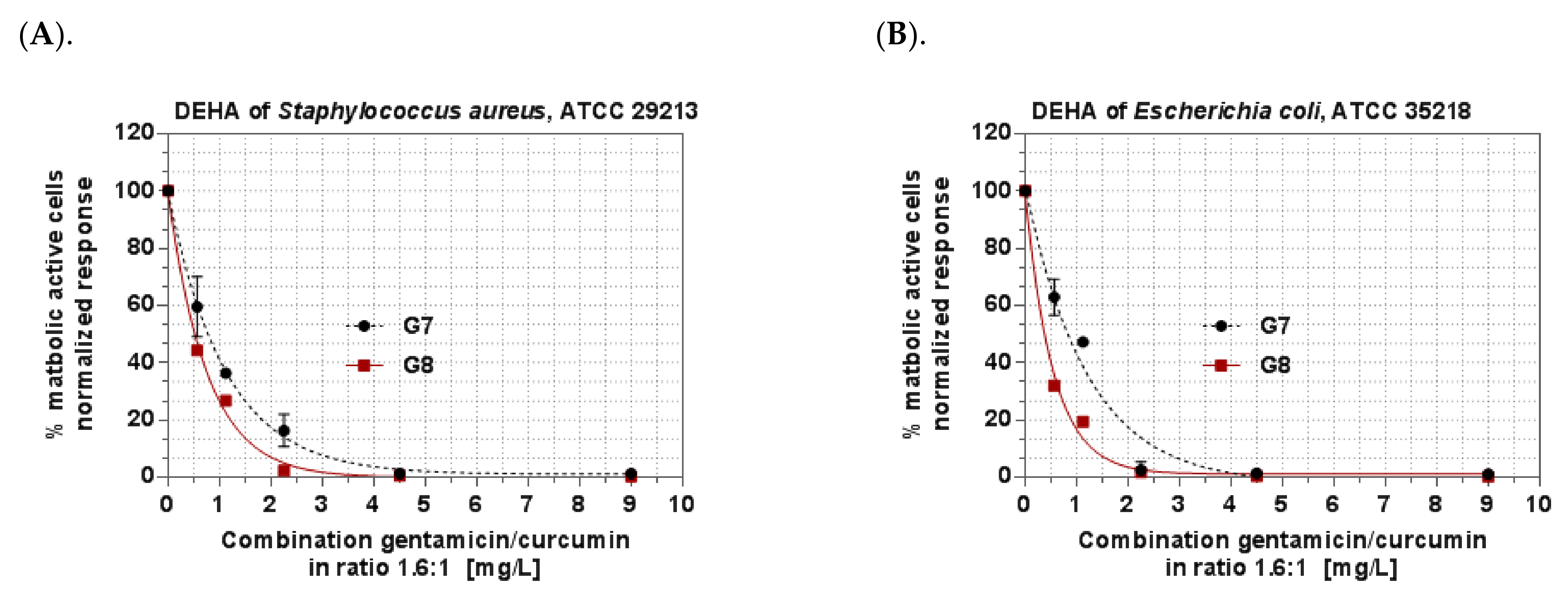
3.6. Antibacterial Activity of In Situ Gels
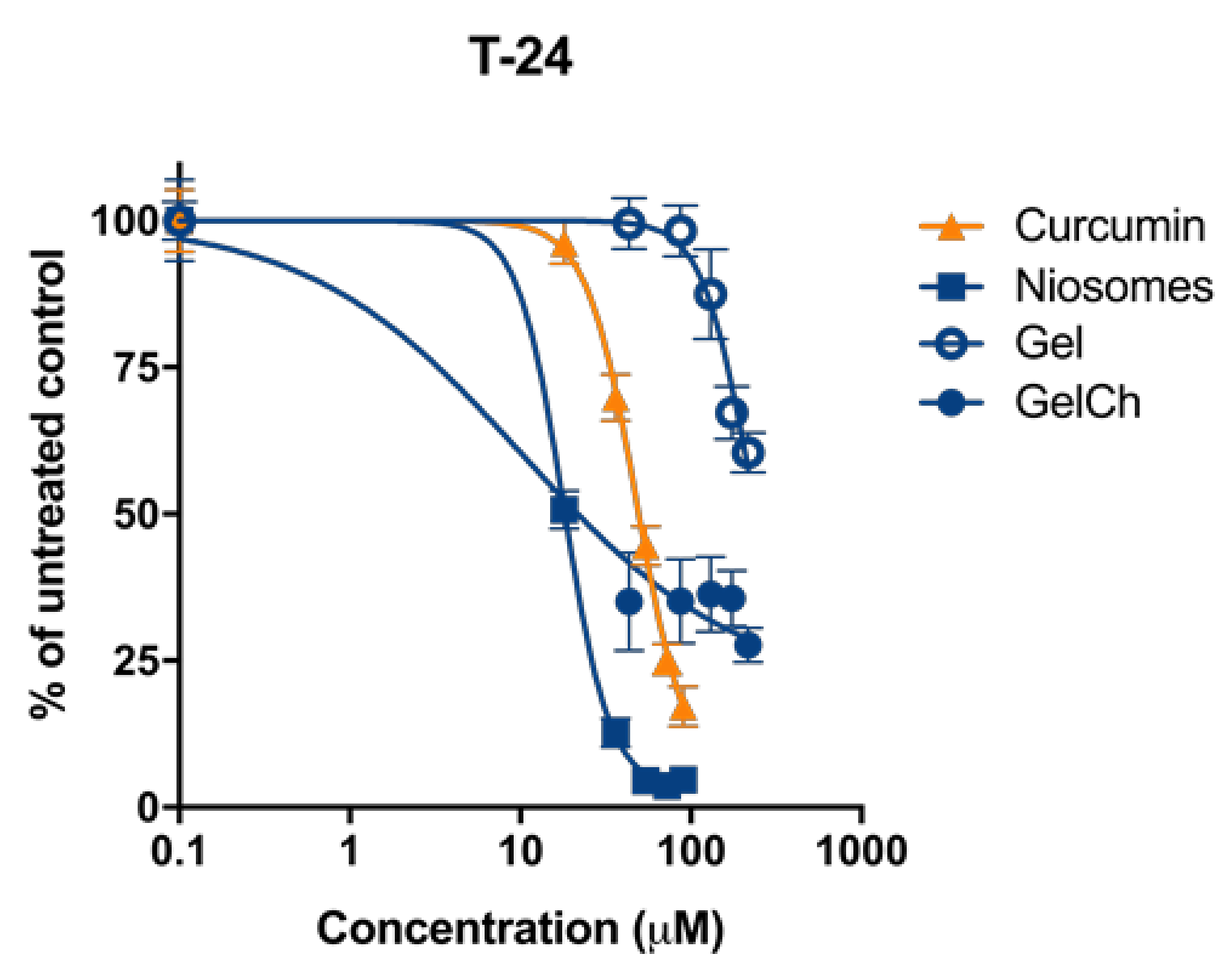
3.7. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Xiang, H.; Dong, P.; Zhang, T.; Lu, C.; Jin, T.; Chai, K.Y. Pegylated azelaic acid: Synthesis, tyrosinase inhibitory activity, antibacterial activity and cytotoxic studies. J. Mol. Struct. 2021, 1224, 129234. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral Protein Supraparticles for Tumor Suppression and Synergistic Immunotherapy—An enabling strategy for bioactive supramolecular chirality construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-Y.; Qi, J.-C.; Du, L. Intervention effect and mechanism of curcumin in chronic urinary tract infection in rats. Asian Pac. J. Trop. Med. 2017, 10, 594–598. [Google Scholar] [CrossRef]
- Sidaway, P. Intravesical gentamicin ameliorates recurrent UTI. Nat. Rev. Urol. 2017, 14, 391. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, F.; Xu, R.; Zhao, X.-K.; Zhong, Z.-H.; Zhang, L.; Jiang, H.-Y.; Yi, L.; Hou, Y.; Zhu, X. A Pilot Study on Intravesical Administration of Curcumin for Cystitis Glandularis. Evid. Based Complement. Altern. Med. 2013, 2013, 269745. [Google Scholar] [CrossRef]
- Falke, J.; Parkkinen, J.; Vaahtera, L.; Hulsbergen-van de Kaa, C.A.; Oosterwijk, E.; Witjes, J.A. Curcumin as Treatment for Bladder Cancer: A Preclinical Study of Cyclodextrin-Curcumin Complex and BCG as Intravesical Treatment in an Orthotopic Bladder Cancer Rat Model. BioMed Res. Int. 2018, 2018, 9634902. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, W.-R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.-B.; He, C.-W.; Chen, M.-W. Niosome Encapsulation of Curcumin: Characterization and Cytotoxic Effect on Ovarian Cancer Cells. J. Nanomater. 2016, 2016, 6365295. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [Green Version]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, J.; Saeedi, M.; Enayatifard, R.; Morteza-Semnani, K.; Hassan Hashemi, S.M.; Babaei, A.; Rahimnia, S.M.; Rostamkalaei, S.S.; Nokhodchi, A. Curcumin Niosomes (curcusomes) as an alternative to conventional vehicles: A potential for efficient dermal delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102035. [Google Scholar] [CrossRef]
- Pan, X.; Chen, S.; Li, D.; Rao, W.; Zheng, Y.; Yang, Z.; Li, L.; Guan, X.; Chen, Z. The Synergistic Antibacterial Mechanism of Gentamicin-Loaded CaCO3 Nanoparticles. Front. Chem. 2018, 5, 130. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Farouk, H.O.; Zaki, R.M. Fabrication and Appraisal of Simvastatin via Tailored Niosomal Nanovesicles for Transdermal Delivery Enhancement: In Vitro and In Vivo Assessment. Pharmaceutics 2021, 13, 138. [Google Scholar] [CrossRef]
- Barani, M.; Hajinezhad, M.R.; Sargazi, S.; Rahdar, A.; Shahraki, S.; Lohrasbi-Nejad, A.; Baino, F. In vitro and in vivo anticancer effect of pH-responsive paclitaxel-loaded niosomes. J. Mater. Sci. Mater. Med. 2021, 32, 147. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T. Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery. Pharmaceutics 2021, 13, 787. [Google Scholar] [CrossRef]
- Lai, W.-F.; Gui, D.; Wong, M.; Döring, A.; Rogach, A.L.; He, T.; Wong, W.-T. A self-indicating cellulose-based gel with tunable performance for bioactive agent delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102428. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Mahrous, G.M.; Alanazi, F.K. Novel in-situ gel for intravesical administration of ketorolac. Saudi Pharm. J. 2018, 26, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Ay Şenyiğit, Z.; Karavana, S.Y.; Ilem Ozdemir, D.; Caliskan, C.; Waldner, C.; Sen, S.; Bernkop-Schnürch, A.; Baloğlu, E. Design and evaluation of an intravesical delivery system for superficial bladder cancer: Preparation of gemcitabine HCl-loaded chitosan-thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers. Int. J. Nanomed. 2015, 10, 6493–6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO20776/1-2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases European Committee for Standardization (CEN), Technical Committee CEN/TC 140, Technical Committee ISO/TC 212:19. ISO: Geneva, Switzerland, 2006.
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, H.M.; Fayez, Y.M.; Tawakkol, S.M.; Fahmy, N.M.; Shehata, M.A.E. Evaluation of graphical and statistical representation of analytical signals of spectrophotometric methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 61–70. [Google Scholar] [CrossRef]
- Hemelryck, S.V.; Dewulf, J.; Niekus, H.; van Heerden, M.; Ingelse, B.; Holm, R.; Mannaert, E.; Langguth, P. In vitro evaluation of poloxamer in situ forming gels for bedaquiline fumarate salt and pharmacokinetics following intramuscular injection in rats. Int. J. Pharm. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Lou, J.; Hu, W.; Tian, R.; Zhang, H.; Jia, Y.; Zhang, J.; Zhang, L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 2014, 9, 2517–2525. [Google Scholar]
- Chaudhari, P.D.; Desai, U.S. Formulation and evaluation of niosomal in situ gel of prednisolone sodium phosphate for ocular drug delivery. Int. J. Appl. Pharm. 2019, 11, 97–116. [Google Scholar] [CrossRef] [Green Version]
- Goo, Y.T.; Yang, H.M.; Kim, C.H.; Kim, M.S.; Kim, H.K.; Chang, I.H.; Choi, Y.W. Optimization of a floating poloxamer 407-based hydrogel using the Box-Behnken design: In vitro characterization and in vivo buoyancy evaluation for intravesical instillation. Eur. J. Pharm. Sci. 2021, 163, 105885. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to Assess In Vitro Drug Release from Injectable Polymeric Particulate Systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef]
- Huang, W.; Czuba, L.C.; Isoherranen, N. Mechanistic PBPK Modeling of Urine pH Effect on Renal and Systemic Disposition of Methamphetamine and Amphetamine. J. Pharmacol. Exp. Ther. 2020, 373, 488–501. [Google Scholar] [CrossRef] [Green Version]
- EUCAST. Clinical Breakpoints—Breakpoints and Guidance. Retrieved. 2021. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 28 November 2021).
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, S.G.; Schuetz, A.N. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012, 87, 290–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Cheng, H.; Wang, F.; Wei, D.; Wang, X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J. Microbiol. Methods 2010, 82, 330–333. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ICS 11.100.20. International Organization for Standardization: Geneva, Switzerland, 2017.
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Essa, E. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J. Pharm. 2010, 4, 227–233. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Hisham, S.F.; Parkir, N. Effects of cholesterol and charging additives on stability of curcumin niosomes. J. Eng. Appl. Sci. 2017, 12, 8537–8541. [Google Scholar]
- Obeid, M.A.; Khadra, I.; Albaloushi, A.; Mullin, M.; Alyamani, H.; Ferro, V.A. Microfluidic manufacturing of different niosomes nanoparticles for curcumin encapsulation: Physical characteristics, encapsulation efficacy, and drug release. Beilstein J. Nanotechnol. 2019, 10, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Badria, F.A.; Fayed, H.A.; Ibraheem, A.K.; State, A.F.; Mazyed, E.A. Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics 2020, 12, 866. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of Poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Lin, T.; Wu, J.; Zhao, X.; Lian, H.; Yuan, A.; Tang, X.; Zhao, S.; Guo, H.; Hu, Y. In Situ Floating Hydrogel for Intravesical Delivery of Adriamycin Without Blocking Urinary Tract. J. Pharm. Sci. Technol. 2014, 103, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Chu, B. Formation of homogeneous gel-like phases by mixed triblock copolymer micelles in aqueous solution: FCC to BCC phase transition. J. Appl. Cryst. 2000, 33, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Nam, J.G.; Wilhellm, M.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear behavior of PEO-PPO-PEO triblock copolymer solutions. Rheol. Acta 2006, 45, 239–249. [Google Scholar] [CrossRef]
- Li, X.; Hyun, K. Rheological study of the effect of polyethylene oxide (PEO) homopolymer on the gelation of PEO-PPO-PEO triblock copolymer in aqueous solution. Korea-Aust. Rheol. J. 2018, 30, 109–125. [Google Scholar] [CrossRef]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Release Studies on Ciprofloxacin Loaded Non-ionic Surfactant Vesicles. Avicenna J. Med. Biotechnol. 2015, 7, 69–75. [Google Scholar]
- Haj-Ahmad, R.R.; Elkordy, A.A.; Chaw, C.S. In vitro characterisation of Span 65 niosomal formulations containing proteins. Curr. Drug Deliv. 2015, 12, 628–639. [Google Scholar] [CrossRef]
- Morsi, N.; Ghorab, D.; Refai, H.; Teba, H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int. J. Pharm. 2016, 506, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.M.; Kroumov, A.D.; Dimitrova, L.; Tsvetkova, I.; Trochopoulos, A.; Konstantinov, S.M. Micellar curcumin improves the antibacterial activity of the alkylphosphocholines erufosine and miltefosine against pathogenic Staphyloccocus aureus strains. Biotechnol. Biotechnol. Equip. 2019, 33, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.F.; Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic-pharmacodynamic model for gentamicin and its adaptive resistance with predictions of dosing schedules in newborn infants. Antimicrob. Agents Chemother. 2012, 56, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppi, B.; Bigucci, F.; Cerchiara, T.; Zecchi, V. Chitosan-based hydrogels for nasal drug delivery: From inserts to nanoparticles. Expert Opin. Drug Deliv. 2010, 7, 811–827. [Google Scholar] [CrossRef] [PubMed]
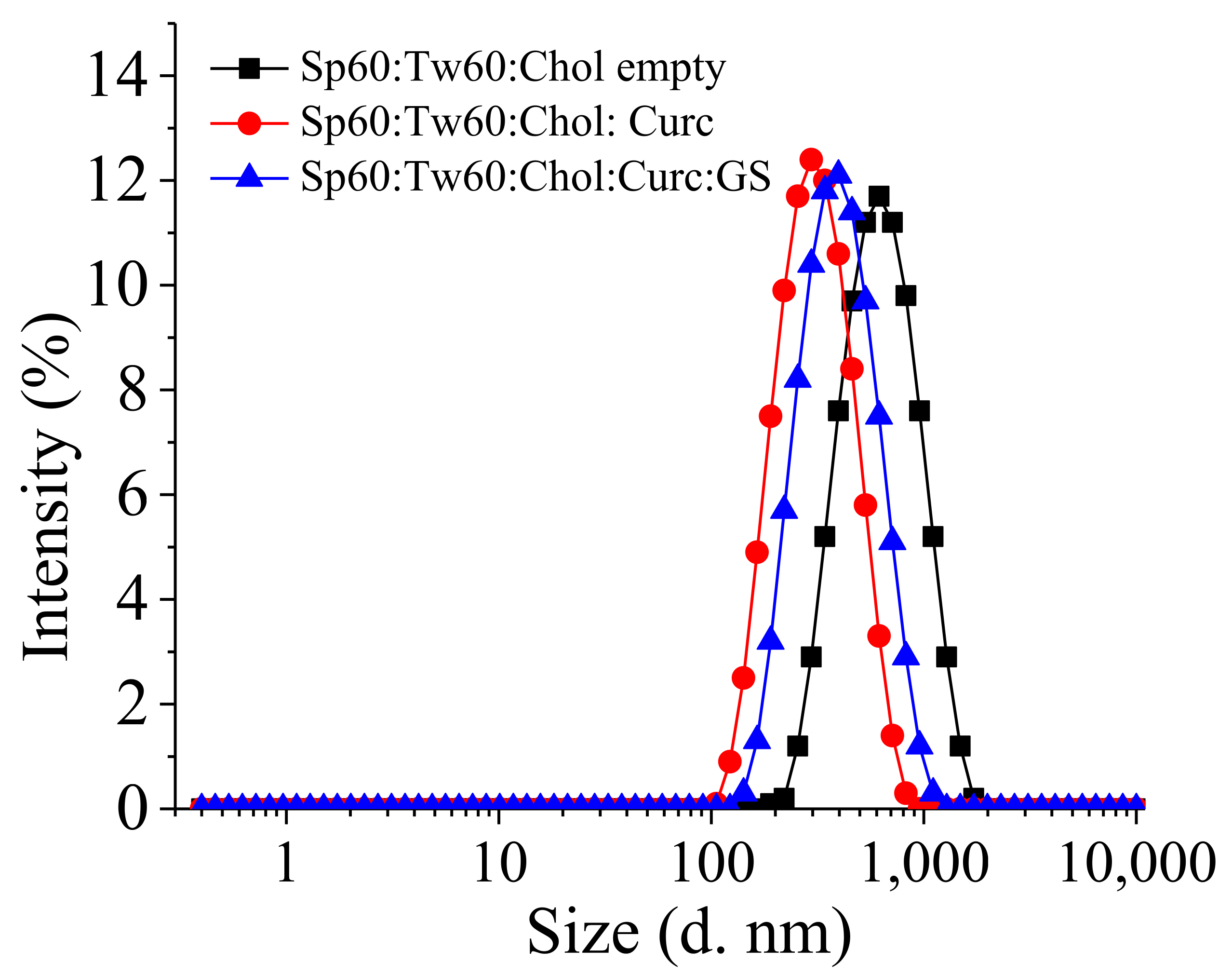
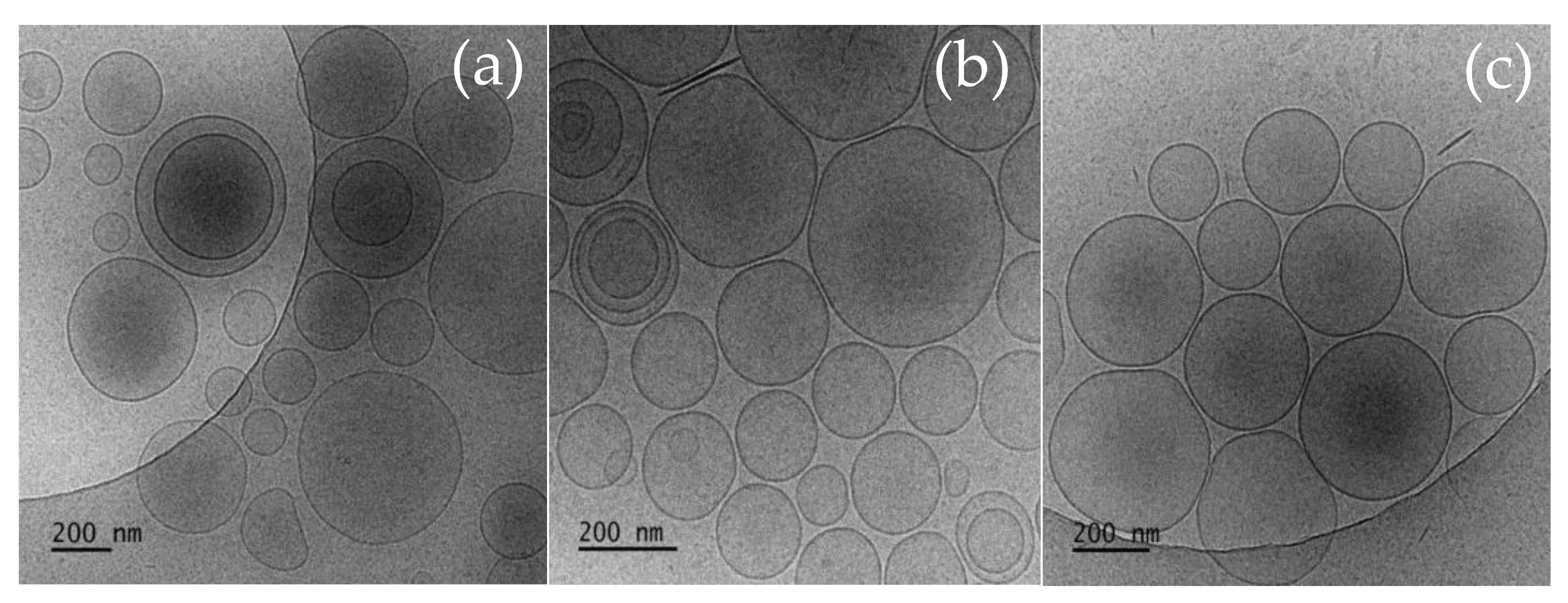

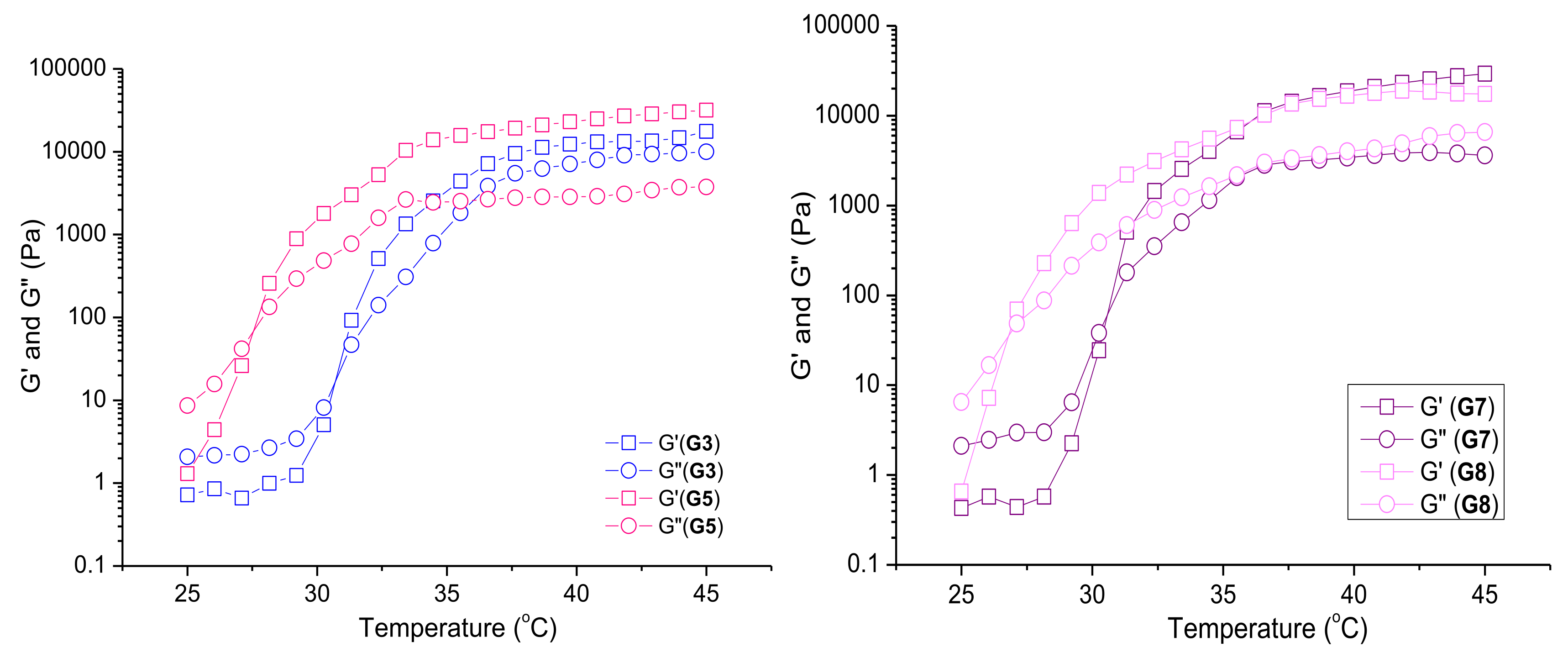

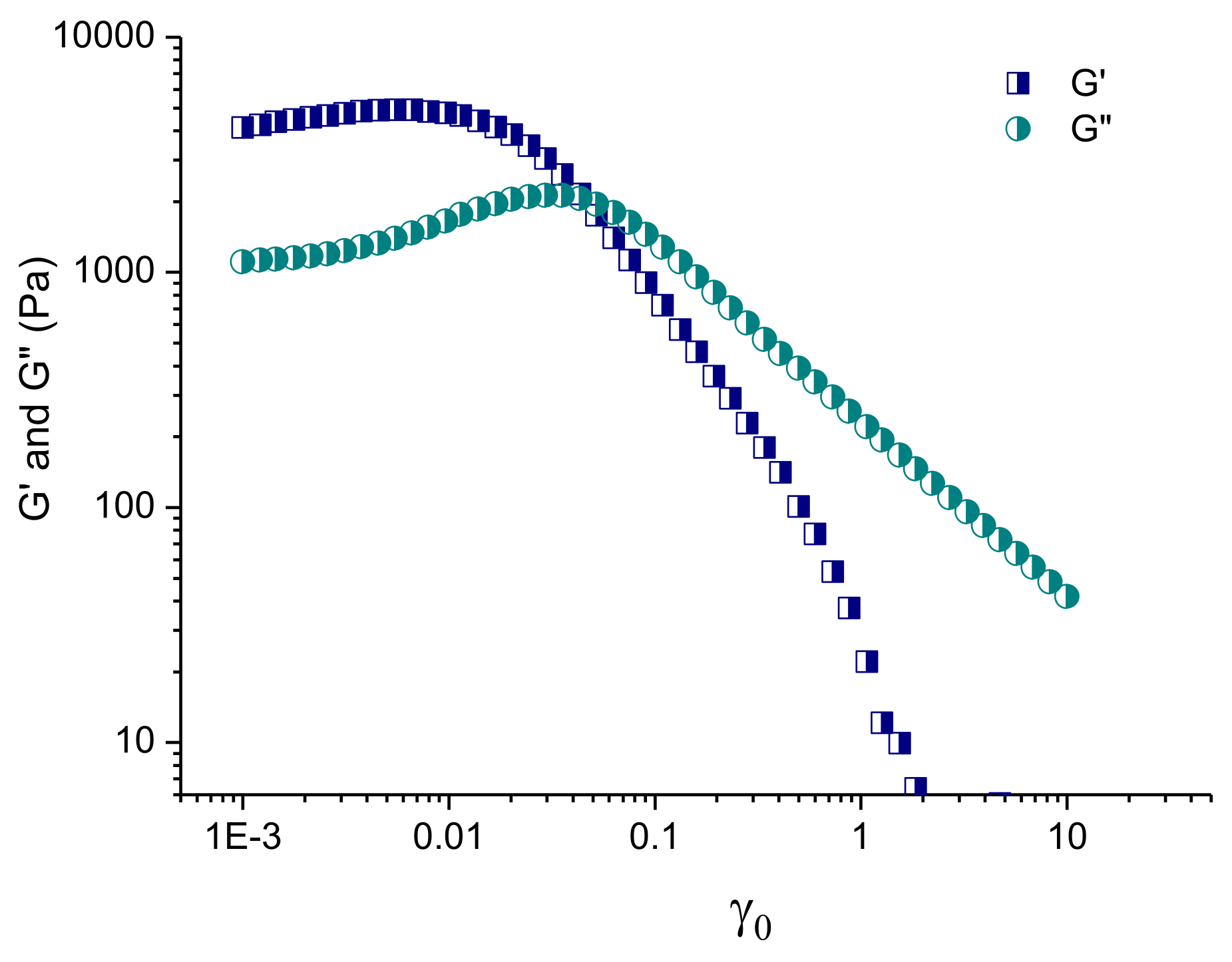

| Sample | SF:Chol (mol:mol) | CURC:SF (mol:mol) | HLB | dh (nm) ± SD | PDI ± SD | ζ-Potential (mV) ± SD | EE (%) ± SD | |
|---|---|---|---|---|---|---|---|---|
| Curc | GS | |||||||
| S1 | Sp80:Chol 7:3 | 1:10 | 4.3 | 264.3 ± 5.2 | 0.37 ± 0.04 | −10.7 ± 0.7 | 27 ± 1.6 | 10 ± 0.8 |
| S2 | Sp60:Chol 7:3 | 1:10 | 4.7 | 287.8 ± 3.9 | 0.41 ± 0.02 | −11.2 ± 1.4 | 24 ± 1.9 | 12 ± 1.1 |
| S3 | Tw60:Chol 7:3 | 1:10 | 14.9 | 25.6 ± 2.2 | 0.25 ± 0.05 | −10.2 ± 1.1 | - | - |
| S4 | Sp60:Tw60: Chol3.5:3.5:3 | 1:10 | 9.8 | 373.3 ± 9.4 | 0.44 ± 0.02 | −10.9 ± 2.5 | 65 ± 0.9 | 36 ± 1.7 |
| S5 | Tw60:Chol 1:1 | 1:10 | 14.9 | 362.1 ± 5.3 | 0.42 ± 0.03 | −12.2 ± 2.1 | 36 ± 2.1 | 39 ± 1.3 |
| S6 | Sp60: Tw60: Chol 3:3:4 | 1:10 | 9.8 | 595.5 ± 8.5 | 0.39 ± 0.05 | −10.2 ± 1.8 | 42 ± 0.3 | 34 ± 0.9 |
| S7 | Sp60: Tw60: Chol 3:3:4 | 1:15 | 9.8 | 496.5 ± 5.9 | 0.41 ± 0.06 | −11.7 ± 0.6 | 51 ± 2.1 | 34 ± 1.5 |
| S8 | Sp 60:Tw 60:Chol 3.5:3.5:3 | 1:20 | 9.8 | 401.4 ± 4.3 | 0.36 ± 0.03 | −11.1 ± 1.4 | 80 ± 1.9 | 36 ± 1.2 |
| S9 | Sp60: Tw60: Chol * 3.5:3.5:3 | 1:20 | 9.8 | 251.2 ± 5.8 | 0.32 ± 0.01 | −13.8 ± 1.3 | 81 ± 1.4 | - |
| S10 | Sp60: Tw60: Chol ** 3.5:3.5:3 | - | 9.8 | 489.1 ± 4.8 | 0.34 ± 0.06 | −12.3 ± 2.3 | - | - |
| Formulation Code | Water% | Niosomes *% (w/w) | P407% (w/w) | P188% (w/w) | Ch% (w/w) | Tsol–gel (°C) | Gelation Time (s) | Gel Erosion Time (h) |
|---|---|---|---|---|---|---|---|---|
| G1 | 75 | - | 25 | - | - | <22 # | - | - |
| G2 | 80 | - | 20 | - | - | <27 # | - | - |
| G3 | 72 | - | 20 | 8 | - | 33 ± 0.7 # 31 ± 0.2 ** | 10 ± 1.7 # | 4 ± 0.2 |
| G4 | 70 | - | 20 | 10 | - | 40 ± 1.1 # | 30 ± 2.1 # | - |
| G5 | 62 | - | 20 | 8 | 10 | 31 ± 0.8 # 27 ± 0.4 ** | 15 ± 1.0 # | 4.5 ± 0.1 |
| G6 | 52 | - | 20 | 8 | 20 | 30 ± 1.5 # | 30 ± 2.3 # | - |
| G7 | 10 | 62 | 20 | 8 | - | 33 ± 1.1 # 31 ± 0.8** | 10 ± 1.4 #35 ± 1.1 ** | 4 ± 0.2 |
| G8 | - | 62 | 20 | 8 | 10 | 30 ± 1.3 # 27 ± 0.3 ** | 15 ± 1.1 #33 ± 1.0 ** | 4.5 ± 0.1 |
| Formulation Code | Size (nm) ± SD | PDI ± SD | Tsol–gel (°C) ± SD | EE (%) ± SD | ||
|---|---|---|---|---|---|---|
| Curc | GS | |||||
| Niosomes in suspension (Formulation S8) | Freshly prepared | 401.4 ± 4.3 | 0.36 ± 0.03 | - | 80 ± 1.1 | 36 ± 1.2 |
| After storage | 431 ± 5.2 | 0.45 ± 0.05 | - | 77.9 ± 1.5 | 30.5 ± 1.4 | |
| Niosomes in gel (Formulation G7) | Freshly prepared | 465 ± 2.3 | 0.32 ± 0.03 | 33 ± 1.1 | 79.5 ± 1.4 | 36 ± 2.2 |
| After storage | 468 ± 2.2 | 0.38 ± 0.02 | 34 ± 0.9 | 79 ± 1.9 | 35.8 ± 2.1 | |
| Sample | Parameters | Staphylococcus aureus, ATCC 29213 | Escherichia coli, ATCC 35218 |
|---|---|---|---|
| Gentamicin, aqueous solution | MIC | 0.25 * mg/L | 2 * mg/L |
| Curcumin, ethanol solution | MIC | 50 mg/L | n. a. |
| GS-NGel | MIC | 1 mg/L | 4 mg/L |
| Curc-N Gel | MIC | 80 mg/L | 80 mg/L |
| Combination effect GS-NGel + CurcNGgel | MICC-GG | 0.5 mg/L | 2 mg/L |
| MICC-GC | 0.3 ÷ 5 mg/L | 0.3 ÷ 10 mg/L | |
| FICGG | 0.5 | 0.5 | |
| FICGC | 0.0038 | 0.0038 | |
| ∑FICGG/GC | 0.5038–synergistic | 0.5038–synergistic | |
| DEHAC | 1.70% | 0.55% | |
| GS-NGel-Ch | MIC | 1 mg/L | 4 mg/L |
| Curc-NGel-Ch | MIC | 40 mg/L | 80 mg/L |
| Combination effect GS-NGel-Ch + Curc-NGel-Ch | MICC-GChG | 0.5 mg/L | 2 mg/L |
| MICC-GChC | 0.3 ÷ 10 mg/L | 0.3 ÷ 20 mg/L | |
| FICGChG | 0.5 | 0.5 | |
| FICGChC | 0.0075 | 0.0038 | |
| ∑FICGChG/GChC | 0.5075–synergistic | 0.5038–synergistic | |
| DEHAC | 10.1% | 0.82% | |
| Curc/GS-NGel (GS:Curc = 80:50 mg/L = Curc:GS 1:1.6 wt:wt) | MIC | 0.5/0.3 mg/L | 2/1.25 mg/L |
| MBC | 4/2.5 mg/L | 4/2.5 mg/L | |
| Curc/GS-NGel–Ch (GS/Curc, 80:50 mg/L) | MIC | 0.5/0.3 mg/L | 1/0.625 mg/L |
| MBC | 4/2.5 mg/L | 2/1.25 mg/L |
| Cell Line Compound | T-24 |
|---|---|
| Free curcumin | 48.1 ± 5.1 |
| Curc/GS Niosomes | 17.9 ± 0.6 |
| Curc/GS NGel | 100.1 ± 6.9 |
| Curc/GS NGelCh | 10.3 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugleva, V.; Michailova, V.; Mihaylova, R.; Momekov, G.; Zaharieva, M.M.; Najdenski, H.; Petrov, P.; Rangelov, S.; Forys, A.; Trzebicka, B.; et al. Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate. Pharmaceutics 2022, 14, 747. https://doi.org/10.3390/pharmaceutics14040747
Gugleva V, Michailova V, Mihaylova R, Momekov G, Zaharieva MM, Najdenski H, Petrov P, Rangelov S, Forys A, Trzebicka B, et al. Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate. Pharmaceutics. 2022; 14(4):747. https://doi.org/10.3390/pharmaceutics14040747
Chicago/Turabian StyleGugleva, Viliana, Victoria Michailova, Rositsa Mihaylova, Georgi Momekov, Maya Margaritova Zaharieva, Hristo Najdenski, Petar Petrov, Stanislav Rangelov, Aleksander Forys, Barbara Trzebicka, and et al. 2022. "Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate" Pharmaceutics 14, no. 4: 747. https://doi.org/10.3390/pharmaceutics14040747
APA StyleGugleva, V., Michailova, V., Mihaylova, R., Momekov, G., Zaharieva, M. M., Najdenski, H., Petrov, P., Rangelov, S., Forys, A., Trzebicka, B., & Momekova, D. (2022). Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate. Pharmaceutics, 14(4), 747. https://doi.org/10.3390/pharmaceutics14040747













