Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases
Abstract
1. Introduction
2. Properties of MSCs in Autoimmune Diseases
2.1. Immunoregulatory and Immunosuppressive Potential
2.2. Regenerative Properties
2.3. MSC-EVs as Cell-Free Therapy
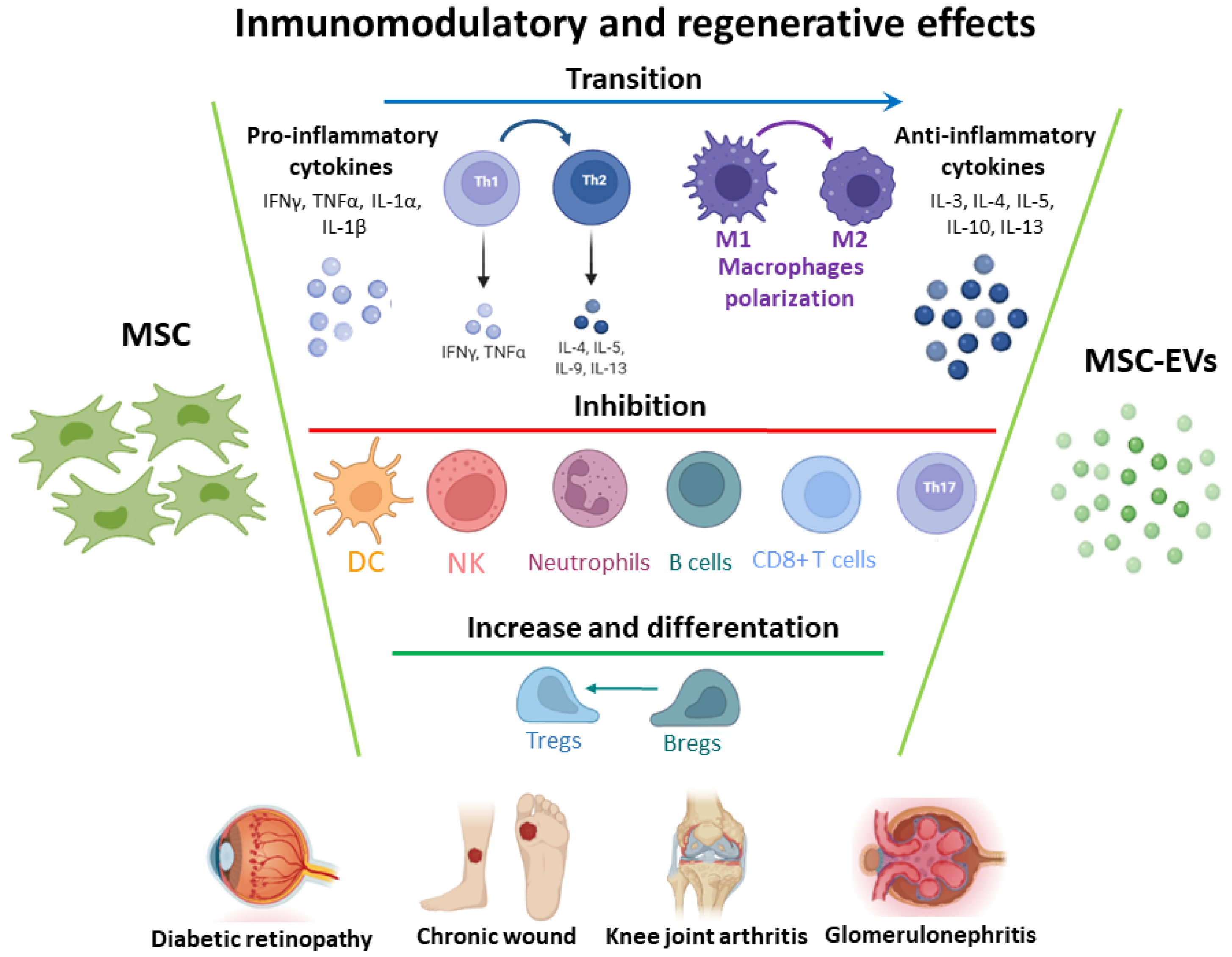
2.3.1. Immunomodulatory Properties
2.3.2. Regenerative Effect
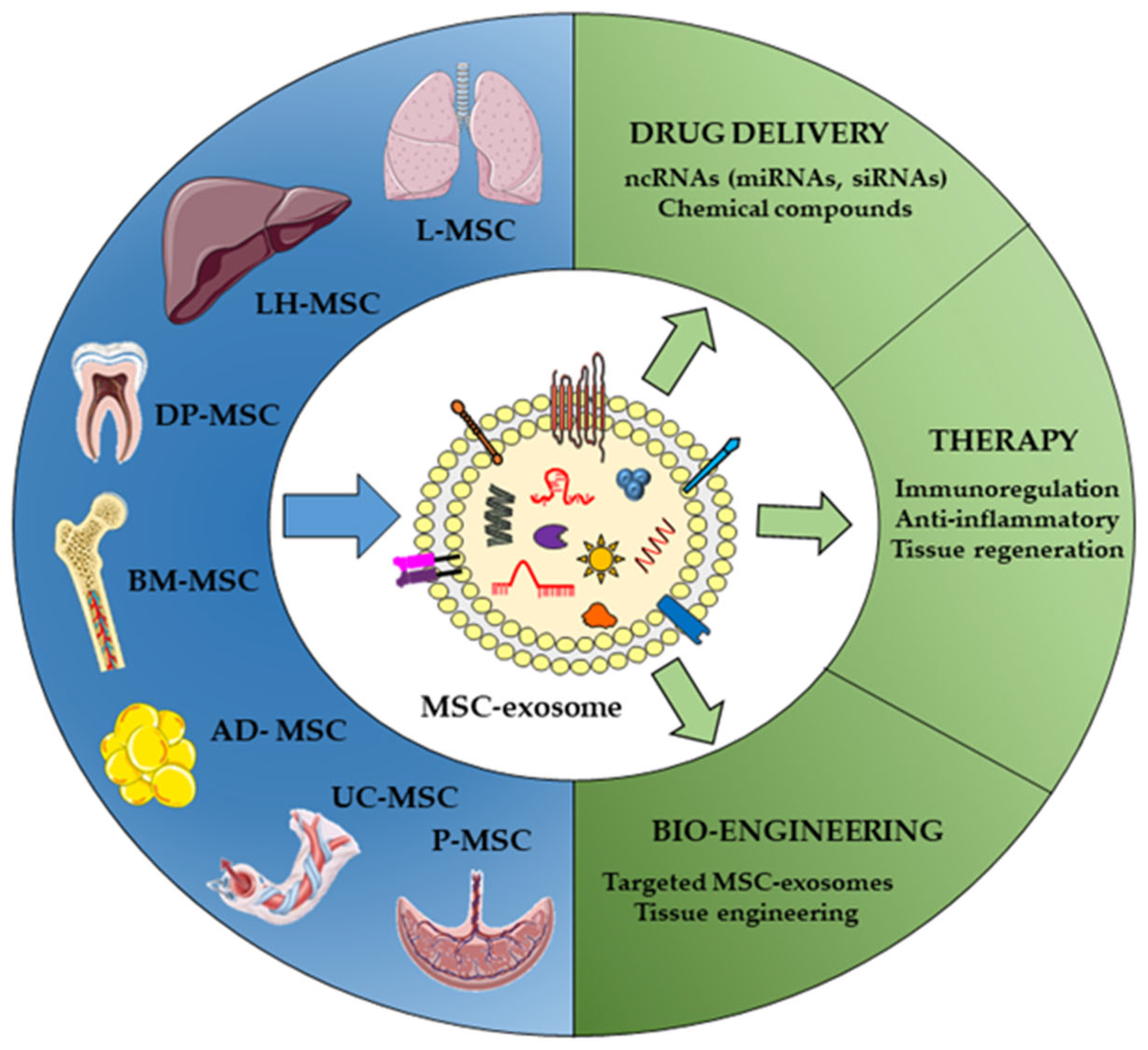
3. Key Points in MSC EVs as Non-Coding RNA Delivery Vehicles
3.1. Different Sources of MSCs
3.2. Non-Coding RNA Cargos
3.3. Separation Methods
3.4. Exosome Administration Routes
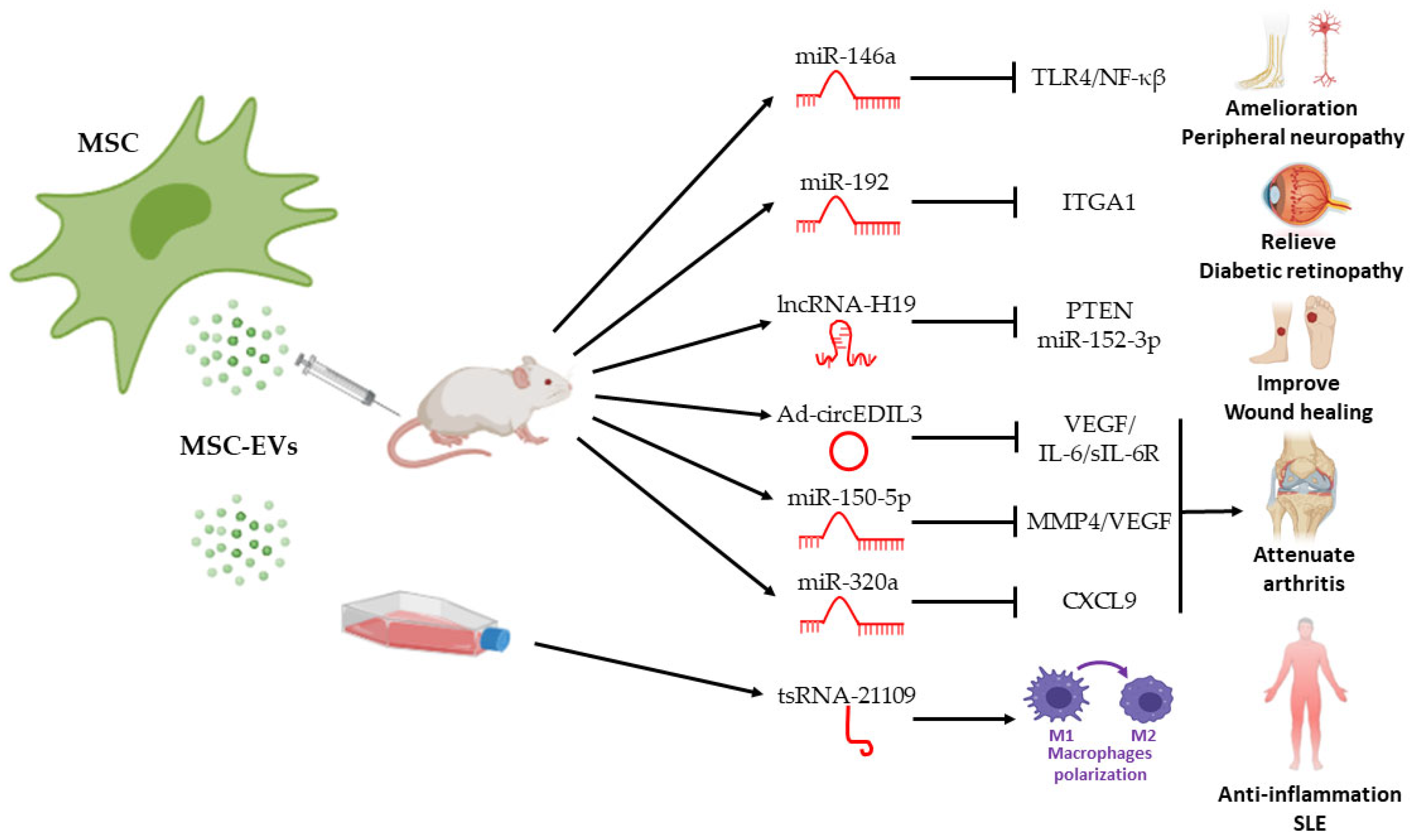
4. Therapeutic Effects of ncRNA in MSC EVs in Autoimmune Diseases
4.1. Rheumatoid Arthritis
4.2. Type I Diabetes Mellitus
4.3. Systemic Lupus Erythematosus
5. Conclusions, Challenges, and Limitations Associated with MSC-EVs and MSC-EVs ncRNA Cargos
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hayter, S.M.; Cook, M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Fugger, L.; Jensen, L.T.; Rossjohn, J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell 2020, 181, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Liu, Q.; Korner, H.; Wu, H.; Wei, W. Endoplasmic reticulum stress in autoimmune diseases. Immunobiology 2020, 225, 151881. [Google Scholar] [CrossRef]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to beta-Cell Dysfunction Through Loss of beta-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef]
- Berry, S.P.D.; Dossou, C.; Kashif, A.; Sharifinejad, N.; Azizi, G.; Hamedifar, H.; Sabzvari, A.; Zian, Z. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int. Immunopharmacol. 2022, 102, 108402. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Tummala, R.; Streicher, K.; Nogueira da Costa, A.; Brohawn, P.Z. The Pathogenesis, Molecular Mechanisms, and Therapeutic Potential of the Interferon Pathway in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 1286. [Google Scholar] [CrossRef]
- Roszkowski, L.; Ciechomska, M. Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis. Cells 2021, 10, 1860. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wu, X.; Zhou, J.; Meng, D.; Zhu, P. Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis. Front. Pharmacol. 2021, 12, 746396. [Google Scholar] [CrossRef]
- Scheiman-Elazary, A.; Duan, L.; Shourt, C.; Agrawal, H.; Ellashof, D.; Cameron-Hay, M.; Furst, D.E. The Rate of Adherence to Antiarthritis Medications and Associated Factors among Patients with Rheumatoid Arthritis: A Systematic Literature Review and Metaanalysis. J. Rheumatol. 2016, 43, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Hampton, J.M.; Valiente, G.R.; Wada, T.; Steigelman, H.; Young, M.C.; Spurbeck, R.R.; Blazek, A.D.; Bosh, S.; Jarjour, W.N.; et al. Therapeutic Development of Mesenchymal Stem Cells or Their Extracellular Vesicles to Inhibit Autoimmune-Mediated Inflammatory Processes in Systemic Lupus Erythematosus. Front. Immunol. 2017, 8, 526. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 1592. [Google Scholar] [CrossRef]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. Potential of stem cell-derived exosomes to regenerate beta islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell. Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J.; Tian, Y.; Li, H.; Zhang, L.; Yang, J.; Wang, J.; Zhang, J.; Yan, S.; Xu, D. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 714832. [Google Scholar] [CrossRef]
- Ansboro, S.; Roelofs, A.J.; De Bari, C. Mesenchymal stem cells for the management of rheumatoid arthritis: Immune modulation, repair or both? Curr. Opin. Rheumatol. 2017, 29, 201–207. [Google Scholar] [CrossRef]
- Che, N.; Li, X.; Zhou, S.; Liu, R.; Shi, D.; Lu, L.; Sun, L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell. Immunol. 2012, 274, 46–53. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef]
- Ma, D.; Xu, K.; Zhang, G.; Liu, Y.; Gao, J.; Tian, M.; Wei, C.; Li, J.; Zhang, L. Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int. Immunopharmacol. 2019, 74, 105687. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Fazekasova, H.; Lam, E.W.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.D.; Kosaka, Y.; Marcus, P.; Rashedi, I.; Keating, A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells Dev. 2019, 28, 933–943. [Google Scholar] [CrossRef]
- Maumus, M.; Jorgensen, C.; Noel, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef]
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef]
- Peng, H.; Ji, W.; Zhao, R.; Yang, J.; Lu, Z.; Li, Y.; Zhang, X. Exosome: A significant nano-scale drug delivery carrier. J. Mater. Chem. B 2020, 8, 7591–7608. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Kahmini, F.R.; Shahgaldi, S. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders. Exp. Mol. Pathol. 2021, 118, 104566. [Google Scholar] [CrossRef]
- Lee, O.J.; Luk, F.; Korevaar, S.S.; Koch, T.G.; Baan, C.C.; Merino, A.; Hoogduijn, M.J. The Importance of Dosing, Timing, and (in)Activation of Adipose Tissue-Derived Mesenchymal Stromal Cells on Their Immunomodulatory Effects. Stem Cells Dev. 2020, 29, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Nancarrow-Lei, R.; Mafi, P.; Mafi, R.; Khan, W. A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr. Stem Cell Res. Ther. 2017, 12, 601–610. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; El-Sherbiny, Y.; McGonagle, D.; Jones, E. Multipotent Mesenchymal Stromal Cells in Rheumatoid Arthritis and Systemic Lupus Erythematosus; From a Leading Role in Pathogenesis to Potential Therapeutic Saviors? Front. Immunol. 2021, 12, 643170. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Uccelli, A.; de Rosbo, N.K. The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Ann. N. Y. Acad. Sci. 2015, 1351, 114–126. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, I.; Gomez-Aristizabal, A.; Wang, X.H.; Viswanathan, S.; Keating, A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells 2017, 35, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Gomes, J.; Laranjeira, P.; Duarte, C.; Pedreiro, S.; Antunes, B.; Ribeiro, T.; Santos, F.; Martinho, A.; Fardilha, M.; et al. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J. Tissue Eng. Regen. Med. 2020, 14, 16–28. [Google Scholar] [CrossRef]
- Sun, L.; Akiyama, K.; Zhang, H.; Yamaza, T.; Hou, Y.; Zhao, S.; Xu, T.; Le, A.; Shi, S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009, 27, 1421–1432. [Google Scholar] [CrossRef]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2017, 14, 423–431. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Chen, X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front. Immunol. 2020, 11, 1843. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Yang, D.; Chen, A.; Tang, J.; Jing, Y.; Kang, D.; Jiang, P.; Dai, X.; Luo, L.; et al. CCL2 regulation of MST1-mTOR-STAT1 signaling axis controls BCR signaling and B-cell differentiation. Cell Death Differ. 2021, 28, 2616–2633. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Z.; Zhang, F.; Wang, Y.; Shen, B.; Liu, Y.; Guo, Y.; Fan, Y.; Qiu, J. Induction of Regulatory B-Cells by Mesenchymal Stem Cells is Affected by SDF-1alpha-CXCR7. Cell. Physiol. Biochem. 2015, 37, 117–130. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K. Human T follicular helper (Tfh) cells and disease. Immunol. Cell Biol. 2014, 92, 64–71. [Google Scholar] [CrossRef]
- Wheat, W.H.; Chow, L.; Kurihara, J.N.; Regan, D.P.; Coy, J.W.; Webb, T.L.; Dow, S.W. Suppression of Canine Dendritic Cell Activation/Maturation and Inflammatory Cytokine Release by Mesenchymal Stem Cells Occurs Through Multiple Distinct Biochemical Pathways. Stem Cells Dev. 2017, 26, 249–262. [Google Scholar] [CrossRef]
- Lu, Z.; Meng, S.; Chang, W.; Fan, S.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells activate Notch signaling to induce regulatory dendritic cells in LPS-induced acute lung injury. J. Transl. Med. 2020, 18, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, G.; Wang, Q.; Wang, R.; Zhang, M. The CD200/CD200R mechanism in mesenchymal stem cells’ regulation of dendritic cells. Am. J. Transl. Res. 2021, 13, 9607–9613. [Google Scholar] [PubMed]
- Mazlo, A.; Kovacs, R.; Miltner, N.; Toth, M.; Vereb, Z.; Szabo, K.; Bacskai, I.; Pazmandi, K.; Apati, A.; Biro, T.; et al. MSC-like cells increase ability of monocyte-derived dendritic cells to polarize IL-17-/IL-10-producing T cells via CTLA-4. iScience 2021, 24, 102312. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I.; Ringden, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003, 76, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Maggini, J.; Mirkin, G.; Bognanni, I.; Holmberg, J.; Piazzon, I.M.; Nepomnaschy, I.; Costa, H.; Canones, C.; Raiden, S.; Vermeulen, M.; et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 2010, 5, e9252. [Google Scholar] [CrossRef]
- Gao, S.; Mao, F.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, M.; Yan, Y.; Yang, T.; Zhang, J.; Zhu, W.; et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 2014, 239, 366–375. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef]
- Lopez-Garcia, L.; Castro-Manrreza, M.E. TNF-alpha and IFN-gamma Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9531. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Liu, F.X.; Wang, J.H.; Cheng, M.; Wang, S.F.; Xu, D.H. Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases. World J. Stem Cells 2020, 12, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Prins, H.J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng. Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Zaszczynska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, Electrospun and Composite Materials for Bone/Cartilage and Neural Tissue Engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Lv, X.; Sun, C.; Hu, B.; Chen, S.; Wang, Z.; Wu, Q.; Fu, K.; Xia, Z.; Shao, Z.; Wang, B. Simultaneous Recruitment of Stem Cells and Chondrocytes Induced by a Functionalized Self-Assembling Peptide Hydrogel Improves Endogenous Cartilage Regeneration. Front. Cell Dev. Biol. 2020, 8, 864. [Google Scholar] [CrossRef]
- Yang, J.; Jing, X.; Wang, Z.; Liu, X.; Zhu, X.; Lei, T.; Li, X.; Guo, W.; Rao, H.; Chen, M.; et al. In vitro and in vivo Study on an Injectable Glycol Chitosan/Dibenzaldehyde-Terminated Polyethylene Glycol Hydrogel in Repairing Articular Cartilage Defects. Front. Bioeng. Biotechnol. 2021, 9, 607709. [Google Scholar] [CrossRef]
- Jones, I.A.; Wilson, M.; Togashi, R.; Han, B.; Mircheff, A.K.; Vangsness, T.C., Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: A study protocol. BMC Musculoskelet. Disord. 2018, 19, 383. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Yao, M.; Yang, L.; Ao, L.; Hu, X.; Li, Z.; Wu, X.; Tan, Y.; Xing, W.; et al. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-gamma treatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 1298–1304. [Google Scholar] [CrossRef]
- Qu, Z.; Lou, Q.; Cooper, D.K.C.; Pu, Z.; Lu, Y.; Chen, J.; Ni, Y.; Zhan, Y.; Chen, J.; Li, Z.; et al. Potential roles of mesenchymal stromal cells in islet allo- and xenotransplantation for type 1 diabetes mellitus. Xenotransplantation 2021, 28, e12678. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, J.; Zhou, Q.; Xin, J.; Jiang, J.; Jiang, L.; Wu, T.; Li, J.; Ding, W.; Li, J.; et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 2017, 66, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Zhao, C.; Wang, D.; Ma, X.; Zhao, S.; Wang, S.; Niu, L.; Sun, L. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int. J. Rheum. Dis. 2017, 20, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Detry, O.; Vandermeulen, M.; Delbouille, M.H.; Somja, J.; Bletard, N.; Briquet, A.; Lechanteur, C.; Giet, O.; Baudoux, E.; Hannon, M.; et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J. Hepatol. 2017, 67, 47–55. [Google Scholar] [CrossRef]
- Suk, K.T.; Yoon, J.H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, F.; Xu, Y.; Yan, J.X.; Wu, Y.D.; Li, S.H.; Liao, X.; Liang, J.X.; Li, Z.H.; Liu, H.W. A novel role of angiotensin II in epidermal cell lineage determination: Angiotensin II promotes the differentiation of mesenchymal stem cells into keratinocytes through the p38 MAPK, JNK and JAK2 signalling pathways. Exp. Dermatol. 2019, 28, 59–65. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human Mesenchymal Stromal Cell-Derived Exosomes Promote In Vitro Wound Healing by Modulating the Biological Properties of Skin Keratinocytes and Fibroblasts and Stimulating Angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef]
- Feldman, D.S.; McCauley, J.F. Mesenchymal Stem Cells and Transforming Growth Factor-beta(3) (TGF-beta(3)) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing. J. Funct. Biomater. 2018, 9, 65. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, D.N.; Schwager, S.C.; Jeffries, L.A.; Bruce, A.; Corliss, B.A.; DeRosa, C.A.; Fraser, C.L.; Peirce, S.M.; Cottler, P.S. Oxygen-Sensing Biomaterial Construct for Clinical Monitoring of Wound Healing. Adv. Ski. Wound Care 2020, 33, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The Effects of Curcumin Nanoparticles Incorporated into Collagen-Alginate Scaffold on Wound Healing of Skin Tissue in Trauma Patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Laundos, T.L.; Vasques-Novoa, F.; Gomes, R.N.; Sampaio-Pinto, V.; Cruz, P.; Cruz, H.; Santos, J.M.; Barcia, R.N.; Pinto-do, O.P.; Nascimento, D.S. Consistent Long-Term Therapeutic Efficacy of Human Umbilical Cord Matrix-Derived Mesenchymal Stromal Cells After Myocardial Infarction Despite Individual Differences and Transient Engraftment. Front. Cell Dev. Biol. 2021, 9, 624601. [Google Scholar] [CrossRef]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef]
- Navas, A.; Magana-Guerrero, F.S.; Dominguez-Lopez, A.; Chavez-Garcia, C.; Partido, G.; Graue-Hernandez, E.O.; Sanchez-Garcia, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, Y.; Li, C.; Zhang, T.; Meng, F.; Zhang, J.; Li, J.; Chen, S.; Wang, Q.; Wang, Y.; et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022, 13, 18. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. 2021, 34, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Song, M.; Chai, C.; Wang, J.; Jin, C.; Wang, X.; Cheng, M.; Yan, S. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J. Cell. Physiol. 2019, 234, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Jorgensen, C.; Guilpain, P.; Noel, D. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNgamma-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Monguio-Tortajada, M.; Roura, S.; Galvez-Monton, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.R.; Bayes-Genis, A.; Borras, F.E. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Chung, H.; Moon, J.H.; Kang, S.J.; Park, C.G. Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunol. Lett. 2020, 225, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bolandi, Z.; Mokhberian, N.; Eftekhary, M.; Sharifi, K.; Soudi, S.; Ghanbarian, H.; Hashemi, S.M. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4(+) T cell. Life Sci. 2020, 259, 118218. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhuansun, Y.X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.J.; Bae, E.H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M.; et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013, 22, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Hyvarinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Kakela, R.; Laitinen, S.; Kerkela, E. Mesenchymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Cui, B.; Zhang, W.; Ma, W.; Zhao, G.; Xing, L. Exosomal miR-21 secreted by IL-1beta-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021, 264, 118658. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Herr, F.; Vernochet, A.; Mennesson, B.; Oberlin, E.; Durrbach, A. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019, 28, 44–55. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Mardpour, S.; Yousefi-Ahmadipour, A.; Allahverdi, A.; Izadpanah, M.; Daneshimehr, F.; Ai, J.; Banafshe, H.R.; Ebrahimi-Barough, S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J. Cell. Biochem. 2018, 119, 8048–8073. [Google Scholar] [CrossRef]
- Abreu, H.; Canciani, E.; Raineri, D.; Cappellano, G.; Rimondini, L.; Chiocchetti, A. Extracellular Vesicles in Musculoskeletal Regeneration: Modulating the Therapy of the Future. Cells 2021, 11, 43. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, Z.Y.; Lin, Y.H.; Chen, S.H.; Chou, P.Y.; Kao, H.K.; Lin, F.H. Extracellular Vesicles of Adipose-Derived Stem Cells Promote the Healing of Traumatized Achilles Tendons. Int. J. Mol. Sci. 2021, 22, 2373. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Casado-Diaz, A.; Quesada-Gomez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed. Res. Int. 2019, 2019, 9742765. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Vatsa, P.; Negi, R.; Ansari, U.A.; Khanna, V.K.; Pant, A.B. Insights of Extracellular Vesicles of Mesenchymal Stem Cells: A Prospective Cell-Free Regenerative Medicine for Neurodegenerative Disorders. Mol. Neurobiol. 2022, 59, 459–474. [Google Scholar] [CrossRef]
- Monguio-Tortajada, M.; Prat-Vidal, C.; Moron-Font, M.; Clos-Sansalvador, M.; Calle, A.; Gastelurrutia, P.; Cserkoova, A.; Morancho, A.; Ramirez, M.A.; Rosell, A.; et al. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact. Mater. 2021, 6, 3314–3327. [Google Scholar] [CrossRef]
- Nuzzi, R.; Buono, L.; Scalabrin, S.; De Iuliis, M.; Bussolati, B. Effect of Stem Cell-Derived Extracellular Vesicles on Damaged Human Corneal Endothelial Cells. Stem Cells Int. 2021, 2021, 6644463. [Google Scholar] [CrossRef]
- Rostami, Z.; Khorashadizadeh, M.; Naseri, M. Immunoregulatory properties of mesenchymal stem cells: Micro-RNAs. Immunol. Lett. 2020, 219, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Li, T.; Chu, X.; Ke, H.; Yu, Z.; Cao, L.; Bai, X.; Liu, D.; Wang, Z. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. 2020, 113, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Papait, A.; Perucca Orfei, C.; Silini, A.R.; Colombini, A.; Vigano, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: Anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical ‘’Mesenchymal Stem Cells’’ at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef]
- Menard, C.; Tarte, K. Immunoregulatory properties of clinical grade mesenchymal stromal cells: Evidence, uncertainties, and clinical application. Stem Cell Res. Ther. 2013, 4, 64. [Google Scholar] [CrossRef][Green Version]
- Kizilay Mancini, O.; Lora, M.; Cuillerier, A.; Shum-Tim, D.; Hamdy, R.; Burelle, Y.; Servant, M.J.; Stochaj, U.; Colmegna, I. Mitochondrial Oxidative Stress Reduces the Immunopotency of Mesenchymal Stromal Cells in Adults With Coronary Artery Disease. Circ. Res. 2018, 122, 255–266. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018, 27, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Dulong, J.; Roulois, D.; Hebraud, B.; Verdiere, L.; Pangault, C.; Sibut, V.; Bezier, I.; Bescher, N.; Monvoisin, C.; et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells 2020, 38, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Salton, G.D.; Furlan, J.M.; Schneider, N.; Angeli, M.H.; Laureano, A.M.; Silla, L.; Passos, E.P.; Paz, A.H. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy 2017, 19, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.N.; Fong, C.Y.; Subramanian, A.; Liu, W.; Feng, Y.; Choolani, M.; Biswas, A.; Rajapakse, J.C.; Bongso, A. Human Wharton’s Jelly Mesenchymal Stem Cells Show Unique Gene Expression Compared with Bone Marrow Mesenchymal Stem Cells Using Single-Cell RNA-Sequencing. Stem Cells Dev. 2019, 28, 196–211. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef]
- Rolandsson, S.; Andersson Sjoland, A.; Brune, J.C.; Li, H.; Kassem, M.; Mertens, F.; Westergren, A.; Eriksson, L.; Hansson, L.; Skog, I.; et al. Primary mesenchymal stem cells in human transplanted lungs are CD90/CD105 perivascularly located tissue-resident cells. BMJ Open Respir. Res. 2014, 1, e000027. [Google Scholar] [CrossRef]
- Sveiven, S.N.; Nordgren, T.M. Lung-resident mesenchymal stromal cells are tissue-specific regulators of lung homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L197–L210. [Google Scholar] [CrossRef]
- Herrera, M.B.; Bruno, S.; Buttiglieri, S.; Tetta, C.; Gatti, S.; Deregibus, M.C.; Bussolati, B.; Camussi, G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006, 24, 2840–2850. [Google Scholar] [CrossRef]
- Sancho-Bru, P.; Najimi, M.; Caruso, M.; Pauwelyn, K.; Cantz, T.; Forbes, S.; Roskams, T.; Ott, M.; Gehling, U.; Sokal, E.; et al. Stem and progenitor cells for liver repopulation: Can we standardise the process from bench to bedside? Gut 2009, 58, 594–603. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Mei, Q.; Chen, X.; Fang, Y.; Chen, L.; Li, Y.; Xiang, C. Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Juric, M.; Bosnjak, Z.J.; Dhanasekaran, A. Recent Insight on the Non-coding RNAs in Mesenchymal Stem Cell-Derived Exosomes: Regulatory and Therapeutic Role in Regenerative Medicine and Tissue Engineering. Front. Cardiovasc. Med. 2021, 8, 737512. [Google Scholar] [CrossRef]
- Fu, D.; Shi, Y.; Liu, J.B.; Wu, T.M.; Jia, C.Y.; Yang, H.Q.; Zhang, D.D.; Yang, X.L.; Wang, H.M.; Ma, Y.S. Targeting Long Non-coding RNA to Therapeutically Regulate Gene Expression in Cancer. Mol. Ther. Nucleic Acids 2020, 21, 712–724. [Google Scholar] [CrossRef]
- Yan, Y.; Chang, C.; Su, J.; Veno, M.T.; Kjems, J. Osteoblastogenesis Alters Small RNA Profiles in EVs Derived from Bone Marrow Stem Cells (BMSCs) and Adipose Stem Cells (ASCs). Biomedicines 2020, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, J.; Zhuang, X.; Yu, S.; Zhu, S.; Liu, Y.; Chen, X. Identification and Comparison of piRNA Expression Profiles of Exosomes Derived from Human Stem Cells from the Apical Papilla and Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jin, Q.; Ming-Yu, Y.; Yang, H.; Xu, T.; You-Xing, S.; Xu-Ting, B.; Wan, C.; Yun-Jiao, W.; Huan, W.; et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRalpha(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, J.; Byun, Y.; Kim, K.H.; An, S.H.; Kwon, K. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Protect Cardiomyocytes from Doxorubicin-Induced Cardiomyopathy by Upregulating Survivin Expression via the miR-199a-3p-Akt-Sp1/p53 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 7102. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, Y.; Zhang, H.; Zhao, Y.; Han, K.; Zhang, J.; Zhao, D.; Yu, Z.; Geng, Z.; Wang, L.; et al. hPMSCs-Derived Exosomal miRNA-21 Protects Against Aging-Related Oxidative Damage of CD4(+) T Cells by Targeting the PTEN/PI3K-Nrf2 Axis. Front. Immunol. 2021, 12, 780897. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1beta induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef]
- Raja, M.A.G.; Katas, H.; Amjad, M.W. Design, mechanism, delivery and therapeutics of canonical and Dicer-substrate siRNA. Asian J. Pharm. Sci. 2019, 14, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X. Adenovirus vector-attributed hepatotoxicity blocks clinical application in gene therapy. Cytotherapy 2021, 23, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Miron-Barroso, S.; Domenech, E.B.; Trigueros, S. Nanotechnology-Based Strategies to Overcome Current Barriers in Gene Delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology 2016, 91, 241e1–247e7. [Google Scholar] [CrossRef]
- Evers, M.J.W.; van de Wakker, S.I.; de Groot, E.M.; de Jong, O.G.; Gitz-Francois, J.J.J.; Seinen, C.S.; Sluijter, J.P.G.; Schiffelers, R.M.; Vader, P. Functional siRNA Delivery by Extracellular Vesicle-Liposome Hybrid Nanoparticles. Adv. Health Mater. 2021, 11, e2101202. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Xing, H.; Liang, C.; Xu, X.; Sun, H.; Ma, X.; Jiang, Z. Mesenchymal stroma/stem-like cells of GARP knockdown inhibits cell proliferation and invasion of mouse colon cancer cells (MC38) through exosomes. J. Cell. Mol. Med. 2020, 24, 13984–13990. [Google Scholar] [CrossRef]
- Huang, W.; Qu, M.; Li, L.; Liu, T.; Lin, M.; Yu, X. SiRNA in MSC-derived exosomes silences CTGF gene for locomotor recovery in spinal cord injury rats. Stem Cell Res. Ther. 2021, 12, 334. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Adelson, D.L. Evolutionary conservation and functional roles of ncRNA. Front. Genet. 2012, 3, 205. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Orom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef]
- Lodde, V.; Murgia, G.; Simula, E.R.; Steri, M.; Floris, M.; Idda, M.L. Long Noncoding RNAs and Circular RNAs in Autoimmune Diseases. Biomolecules 2020, 10, 1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.Z.; Sun, N.; Liu, J.H.; Chen, F.F.; Guan, X.L.; Li, A.; Wang, F.; Zhao, Q.F.; Wang, H.Y.; et al. Long noncoding RNA expression profile in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 227. [Google Scholar] [CrossRef]
- Xue, Z.; Cui, C.; Liao, Z.; Xia, S.; Zhang, P.; Qin, J.; Guo, Q.; Chen, S.; Fu, Q.; Yin, Z.; et al. Identification of LncRNA Linc00513 Containing Lupus-Associated Genetic Variants as a Novel Regulator of Interferon Signaling Pathway. Front. Immunol. 2018, 9, 2967. [Google Scholar] [CrossRef]
- Born, L.J.; Harmon, J.W.; Jay, S.M. Therapeutic potential of extracellular vesicle-associated long noncoding RNA. Bioeng. Transl. Med. 2020, 5, e10172. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 2018, 475, 3629–3638. [Google Scholar] [CrossRef]
- Cao, X.; Xue, L.D.; Di, Y.; Li, T.; Tian, Y.J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, S.; Li, W.; Yu, P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci. Rep. 2015, 5, 12453. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Yao, X.; Mao, Y.; Wu, D.; Zhu, Y.; Lu, J.; Huang, Y.; Guo, Y.; Wang, Z.; Zhu, S.; Li, X.; et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/beta-catenin axis. Cancer Lett. 2021, 512, 38–50. [Google Scholar] [CrossRef]
- Chang, L.; Kan, L. Mesenchymal Stem Cell-Originated Exosomal Circular RNA circFBXW7 Attenuates Cell Proliferation, Migration and Inflammation of Fibroblast-Like Synoviocytes by Targeting miR-216a-3p/HDAC4 in Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 6157–6171. [Google Scholar] [CrossRef]
- Mao, G.; Xu, Y.; Long, D.; Sun, H.; Li, H.; Xin, R.; Zhang, Z.; Li, Z.; Yang, Z.; Kang, Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021, 12, 389. [Google Scholar] [CrossRef]
- Sun, Y.H.; Lee, B.; Li, X.Z. The birth of piRNAs: How mammalian piRNAs are produced, originated, and evolved. Mamm. Genome 2021, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Al Hallak, M.N.; Philip, P.A.; Azmi, A.S.; Mohammad, R.M. Non-Coding RNAs in Pancreatic Cancer Diagnostics and Therapy: Focus on lncRNAs, circRNAs, and piRNAs. Cancers 2021, 13, 4161. [Google Scholar] [CrossRef]
- Mokarram, P.; Niknam, M.; Sadeghdoust, M.; Aligolighasemabadi, F.; Siri, M.; Dastghaib, S.; Brim, H.; Ashktorab, H. PIWI interacting RNAs perspectives: A new avenues in future cancer investigations. Bioengineered 2021, 12, 10401–10419. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wong, G. An old weapon with a new function: PIWI-interacting RNAs in neurodegenerative diseases. Transl. Neurodegener. 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, T.; Herczeg, K.; Onodi, Z.; Voszka, I.; Modos, K.; Marton, N.; Nagy, G.; Mager, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Johnson, T.K.; Wang, Y.; Thomas, M.; Huynh, K.; Yang, Q.; Bond, V.C.; Chen, Y.E.; Liu, D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res. Ther. 2020, 11, 162. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Reshi, Q.U.A.; Hasan, M.M.; Dissanayake, K.; Fazeli, A. Isolation of Extracellular Vesicles (EVs) Using Benchtop Size Exclusion Chromatography (SEC) Columns. Methods Mol. Biol. 2021, 2273, 201–206. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Choi, D.Y.; Park, J.N.; Paek, S.H.; Choi, S.C.; Paek, S.H. Detecting early-stage malignant melanoma using a calcium switch-enriched exosome subpopulation containing tumor markers as a sample. Biosens. Bioelectron. 2022, 198, 113828. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Mobius, W.; Simon, P.; Kramer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Martinez-Arroyo, O.; Forner, M.J.; Cortes, R. Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, S.; Cao, Y.; Liu, L.; Fang, Y.; Du, J.; Luo, L.; Chen, M.; Shen, B.; Zhang, Q. miRNA-221-3p in Endothelial Progenitor Cell-Derived Exosomes Accelerates Skin Wound Healing in Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 1259–1270. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Braun, R.K.; Chetty, C.; Balasubramaniam, V.; Centanni, R.; Haraldsdottir, K.; Hematti, P.; Eldridge, M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018, 503, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Xia, Y.; Yan, F.; Lu, Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J. Immunol. 2018, 201, 2472–2482. [Google Scholar] [CrossRef]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef]
- Zhdanova, D.Y.; Poltavtseva, R.A.; Svirshchevskaya, E.V.; Bobkova, N.V. Effect of Intranasal Administration of Multipotent Mesenchymal Stromal Cell Exosomes on Memory of Mice in Alzheimer’s Disease Model. Bull. Exp. Biol. Med. 2021, 170, 575–582. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.; Oliveira, M.C.; Broeren, M.G.; Bennink, M.B.; de Vries, M.; van Lent, P.L.; Koenders, M.I.; van den Berg, W.B.; van der Kraan, P.M.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Ma, Y.; Luo, L.; Chu, M.; Zhang, Z. Therapeutic Potential of Exosomal circRNA Derived from Synovial Mesenchymal Cells via Targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF Functional Module in Rheumatoid Arthritis. Int. J. Nanomed. 2021, 16, 7977–7994. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, L.; Iok In, I.; Chen, Y.; Jia, N.; Zhu, W. Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis. Int. Immunopharmacol. 2020, 78, 105985. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell. Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Luque-Campos, N.; Contreras-Lopez, R.A.; Jose Paredes-Martinez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Burbano, C.; Rojas, M.; Munoz-Vahos, C.; Vanegas-Garcia, A.; Correa, L.A.; Vasquez, G.; Castano, D. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci. Rep. 2018, 8, 17917. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noel, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Qiu, B. Exosomal MicroRNA-320a Derived From Mesenchymal Stem Cells Regulates Rheumatoid Arthritis Fibroblast-Like Synoviocyte Activation by Suppressing CXCL9 Expression. Front. Physiol. 2020, 11, 441. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Wang, X.; Cheng, W.; Hu, X.; Wang, Y.; Luo, B.; Huang, W.; Gu, J. miR-34a in extracellular vesicles from bone marrow mesenchymal stem cells reduces rheumatoid arthritis inflammation via the cyclin I/ATM/ATR/p53 axis. J. Cell. Mol. Med. 2021, 25, 1896–1910. [Google Scholar] [CrossRef]
- Meng, H.Y.; Chen, L.Q.; Chen, L.H. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet. Disord. 2020, 21, 150. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Ma, C.; Guan, C.; Ma, X.; Meng, S. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-kappaB pathway. J. Orthop. Surg. Res. 2021, 16, 116. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Huo, W.; Li, Y.; Zhang, Y.; Li, H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. FASEB J. 2020, 34, 13345–13360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-kappaB axis repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zou, F.; Xuan, R.; Lai, X.Y. Exosomes from mesenchymal stem cells expressing microribonucleic acid-125b inhibit the progression of diabetic nephropathy via the tumour necrosis factor receptor-associated factor 6/Akt axis. Endocr. J. 2021, 68, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Miao, J.; Liu, W.; Cai, K.; Huang, X.; Peng, L. Mesenchymal Stem Cell-Derived Exosomes Carry MicroRNA-125a to Protect Against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. Diabetes Metab. Syndr. Obes. 2021, 14, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef]
- Dou, R.; Zhang, X.; Xu, X.; Wang, P.; Yan, B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol. Immunol. 2021, 139, 106–114. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, R.; Zhou, W.; Cai, X.; Ye, Z. LncRNA NEAT1 regulates the proliferation and production of the inflammatory cytokines in rheumatoid arthritis fibroblast-like synoviocytes by targeting miR-204-5p. Hum. Cell 2021, 34, 372–382. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Hsieh, S.C.; Liu, C.W.; Lu, C.H.; Liao, H.T.; Chen, M.H.; Li, K.J.; Wu, C.H.; Shen, C.Y.; Kuo, Y.M.; et al. The Expression of Non-Coding RNAs and Their Target Molecules in Rheumatoid Arthritis: A Molecular Basis for Rheumatoid Pathogenesis and Its Potential Clinical Applications. Int. J. Mol. Sci. 2021, 22, 5689. [Google Scholar] [CrossRef]
- Topping, L.M.; Thomas, B.L.; Rhys, H.I.; Tremoleda, J.L.; Foster, M.; Seed, M.; Voisin, M.B.; Vinci, C.; Law, H.L.; Perretti, M.; et al. Targeting Extracellular Vesicles to the Arthritic Joint Using a Damaged Cartilage-Specific Antibody. Front. Immunol. 2020, 11, 10. [Google Scholar] [CrossRef]
- Khosravi-Maharlooei, M.; Madley, R.; Borsotti, C.; Ferreira, L.M.R.; Sharp, R.C.; Brehm, M.A.; Greiner, D.L.; Parent, A.V.; Anderson, M.S.; Sykes, M.; et al. Modeling human T1D-associated autoimmune processes. Mol. Metab. 2022, 56, 101417. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.R.; Lyon, A.W.; Hollenberg, M.D. Autoimmunity and the pathogenesis of type 1 diabetes. Crit. Rev. Clin. Lab. Sci. 2010, 47, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Hansen, M.P. Type 1 diabetes associated autoimmunity. Autoimmun. Rev. 2016, 15, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; La Cava, A. Adaptive immune regulation in autoimmune diabetes. Autoimmun. Rev. 2016, 15, 236–241. [Google Scholar] [CrossRef]
- Guay, C.; Kruit, J.K.; Rome, S.; Menoud, V.; Mulder, N.L.; Jurdzinski, A.; Mancarella, F.; Sebastiani, G.; Donda, A.; Gonzalez, B.J.; et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic beta Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019, 29, 348–361e346. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.D.; Dias, P. Exosomes or Microvesicles, a Secreted Subcellular Organelle Contributing to Inflammation and Diabetes. Diabetes 2018, 67, 2154–2156. [Google Scholar] [CrossRef]
- Abdi, R.; Fiorina, P.; Adra, C.N.; Atkinson, M.; Sayegh, M.H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 2008, 57, 1759–1767. [Google Scholar] [CrossRef]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef]
- Carlsson, P.O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumari, M.; Mishra, S.; Chaudhary, D.K.; Kumar, A.; Avni, B.; Tiwari, S. Exosomes Secreted by Umbilical Cord Blood-Derived Mesenchymal Stem Cell Attenuate Diabetes in Mice. J. Diabetes Res. 2021, 2021, 9534574. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Yin, G.; Xie, Q. Therapeutic prospects of MicroRNAs carried by mesenchymal stem cells-derived extracellular vesicles in autoimmune diseases. Life Sci. 2021, 277, 119458. [Google Scholar] [CrossRef]
- Tsukita, S.; Yamada, T.; Takahashi, K.; Munakata, Y.; Hosaka, S.; Takahashi, H.; Gao, J.; Shirai, Y.; Kodama, S.; Asai, Y.; et al. MicroRNAs 106b and 222 Improve Hyperglycemia in a Mouse Model of Insulin-Deficient Diabetes via Pancreatic beta-Cell Proliferation. EBioMedicine 2017, 15, 163–172. [Google Scholar] [CrossRef]
- Kubota, K.; Nakano, M.; Kobayashi, E.; Mizue, Y.; Chikenji, T.; Otani, M.; Nagaishi, K.; Fujimiya, M. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS ONE 2018, 13, e0204252. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605, quiz 586–605. [Google Scholar] [CrossRef]
- Pelizzo, G.; Avanzini, M.A.; Icaro Cornaglia, A.; Osti, M.; Romano, P.; Avolio, L.; Maccario, R.; Dominici, M.; De Silvestri, A.; Andreatta, E.; et al. Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: Pre-clinical study applicable in the pediatric surgical setting. J. Transl. Med. 2015, 13, 219. [Google Scholar] [CrossRef][Green Version]
- Walter, M.N.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Rui, B.Y.; Wang, Q.Y.; Zhou, D.; Zhang, Y.; Guo, S.C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018, 25, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Alarcon, G.S. Epidemiology of systemic lupus erythematosus. Expert. Rev. Clin. Immunol. 2017, 13, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.H. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, K.H.; Kim, H.S.; Kim, J.S.; Lee, J.H.; Ji, A.; Kim, K.S.; Lee, T.Y.; Chang, I.Y.; Bae, S.C.; et al. Effect of a Combination of Prednisone or Mycophenolate Mofetil and Mesenchymal Stem Cells on Lupus Symptoms in MRL.Fas(lpr) Mice. Stem Cells Int. 2018, 2018, 4273107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Myeong, S.H.; Lee, N.H.; Kim, H.; Son, H.J.; Chang, J.W.; Lee, N.K.; Na, D.L. Immunosuppressant Drugs Mitigate Immune Responses Generated by Human Mesenchymal Stem Cells Transplanted into the Mouse Parenchyma. Cell Transplant. 2021, 30, 9636897211019025. [Google Scholar] [CrossRef]
- Ranjbar, A.; Hassanzadeh, H.; Jahandoust, F.; Miri, R.; Bidkhori, H.R.; Monzavi, S.M.; Sanjar-Moussavi, N.; Matin, M.M.; Shariati-Sarabi, Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: Results of a phase I clinical trial. Curr. Res. Transl. Med. 2021, 70, 103324. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Chun, W.; Tian, J.; Zhang, Y. Transplantation of mesenchymal stem cells ameliorates systemic lupus erythematosus and upregulates B10 cells through TGF-beta1. Stem Cell Res. Ther. 2021, 12, 512. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.; Lou, Y.; Cai, Y.; Cui, W.; Wang, J.; Nie, P.; Chen, L.; Li, B.; Luo, P. Xenogeneic Transplantation of Human Placenta-Derived Mesenchymal Stem Cells Alleviates Renal Injury and Reduces Inflammation in a Mouse Model of Lupus Nephritis. BioMed. Res. Int. 2019, 2019, 9370919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, Y.L.; Pang, C.Y.; Lu, F.A.; Wang, Y.F. Transplantation of adipose tissue-derived stem cells ameliorates autoimmune pathogenesis in MRL/lpr mice: Modulation of the balance between Th17 and Treg. Z. Rheumatol. 2019, 78, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qin, X.; Wang, D.; Zhang, Z.; Tang, X.; Gao, X.; Chen, W.; Sun, L. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat. Commun. 2019, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Lee, M.; Song, J.W.; Shin, I.S.; Kim, S.J. Mesenchymal stem cell transplantation can restore lupus disease-associated miRNA expression and Th1/Th2 ratios in a murine model of SLE. Sci. Rep. 2016, 6, 38237. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H.Y.; Liao, C.; Lin, W.; Lin, S. Clinical Efficacy and Safety of Mesenchymal Stem Cells for Systemic Lupus Erythematosus. Stem Cells Int. 2020, 2020, 6518508. [Google Scholar] [CrossRef]
- Liu, F.; Chen, H.; Chen, T.; Lau, C.S.; Yu, F.X.; Chen, K.; Chen, H.P.; Pan, R.S.; Chan, G.C.; Zhang, X.Y.; et al. Immunotherapeutic effects of allogeneic mesenchymal stem cells on systemic lupus erythematosus. Lupus 2020, 29, 872–883. [Google Scholar] [CrossRef]
- Cortes, R.; Forner, M.J. Circular RNAS: Novel biomarkers of disease activity in systemic lupus erythematosus? Clin. Sci. 2019, 133, 1049–1052. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Forner, M.J.; Pinto, C.; Chaves, F.J.; Cortes, R.; Redon, J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PLoS ONE 2015, 10, e0138618. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Q.; Sun, H.; Zhang, X.; Yang, C.; Tao, Y.; Nong, G. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization to Attenuate Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage in Mice. Int. J. Stem Cells 2021, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef] [PubMed]
| MSC Source | ncRNA Cargo | Disease Model | Admin. Way | Mechanism/Effect | Ref. |
|---|---|---|---|---|---|
| BM-MSCs | miR-150-5p | FLS and HUVEC in vitro cells; CIA mice model | IP | Modulates MMP14 and VEGF | [213] |
| BM-MSCs | miR-320a | In vitro and CIA mice model | IV | Regulates RA FLS activation by suppressing CXCL9 expression | [227] |
| BM-MSCs | miR-34a | RA FLS in vitro model and rat model | IV | Reduces inflammation via the cyclin I/ATM/ATR/p53 axis | [228] |
| BM-MSCs | miR-192-5p | CIA rat model | IA | Delays the inflammatory response | [218] |
| BM-MSCs | miR-124a | MH7A cell line | - | Inhibits proliferation and migration of FLS cell line and promotes apoptosis | [229] |
| BM-MSCs | lncRNA HAND2-AS1 | Human synovial cell line MH7A | - | Impairs RA FLS activation through miR-143-3p/TNFAIP3/NF-κB pathway | [230] |
| BM-MSCs | circFBXW7 | human synovial cell line and rat model | ID | Attenuates cell proliferation, migration and inflammation of FLS by targeting miR-216a-3p/HDAC4 | [187] |
| Synovial-MSCs | Ad-circEDIL3 | CIA mice model | IA | Downregulates the expression of VEGF induced by the IL-6/sIL-6R complex | [217] |
| BM-MSCs | miR-17, miR-23a and miR-125b | db/db diabetic mice | IV | Ameliorates peripheral neuropathy through TLR4/NF-κB signalling pathway | [231] |
| UC-MSCs | miR-126 | STZ diabetic rats; HG-treated HRECs | IVT | Reduces retinal inflammation by downregulating the HMGB1 pathway | [219] |
| BM-MSCs | miR-21-5p | STZ diabetic rats and HG-treated CCSMCs | IV | Ameliorates erectile dysfunction through PDCD4 downregulation | [232] |
| AD-MSCs | miR-222 | STZ diabetic rabbits | IV, SC and IO | Retina regeneration | [220] |
| AD-MSCs | miR-192 | STZ diabetic rats | IVT | Relieves inflammatory response and angiogenesis ameliorating diabetic retinal damage through downregulation of ITGA1 | [221] |
| BM-MSCs | miR-486-3p | HG-treated Muller cells | - | Inhibits oxidative stress, inflammation and apoptosis in diabetic retinopathy via TLR4/NF-κB axis repression | [233] |
| BM-MSCs | miR-125b | Kidney epithelial cells HG-treated | - | Induces autophagy and inhibition of apoptosis in diabetic nephropathy via downregulation of TRAF6 | [234] |
| AD-MSCs | miR-125a | STZ diabetic rats and HG-treated rat glomerular mesangial cell (GMC) | IV | Protects against diabetic nephropathy by targeting Histone Deacetylase 1 and downregulating Endothelin-1 | [235] |
| UC-MSCs | miR-21 | Hypoxia on Beta cells (βTC-6) | - | Protects beta cells against apoptosis, alleviating ER stress and inhibiting p38 MAPK signalling | [236] |
| BM-MSCs | lncRNA SNHG7 | HRMECs in vitro model of diabetic retinopathy | - | Suppresses endothelial-mesenchymal transition and tube formation trough miR-34a-5p-XBP1 axis | [181] |
| BM-MSCs | miR-146a | db/db diabetic mice | IV | Suppresses peripheral blood inflammatory monocytes and activation of endothelial cells via inhibiting Toll-like receptor (TLR)-4/NF-κB signalling pathway in peripheral neuropathy | [237] |
| UC-MSCs | miR-let-7b | STZ diabetic rats | Topical | Macrophage polarization and resolution of chronic inflammation for wound healing | [209] |
| Myeloid-derived MSCs | lncRNA H19 | STZ diabetic mice | SI | Promotes wound healing in diabetic foot ulcers by upregulating PTEN via miR-152-3p | [208] |
| MSC | tsRNA-21109 | THP-1 cells differentiated to macrophages | - | Alleviates SLE by inhibiting macrophage M1 polarization | [238] |
| MSCs | MSC-EVs | |
|---|---|---|
| Advantages | Repair and regeneration of injured cells and tissues (i.e., cartilage, bone, skin…) | Maintainance of MSC regenerative potential |
| Immunoregulatory properties modulating B cells, T cells, NK cells, DCs, promoting macrophage polarization, etc… | Effectors of MSC immunoregulatory properties | |
| Low immunogenicity | Avoidance of tumorgeneity in transplanted chondrocytes | |
| Suppression of toxicity and immunogenicity in target organs/tissues (peripheral nerves, joints, eyes, skin…) | ||
| Absence of genetic mutability | ||
| Modification of EV surface for targeting specific organs/tissues (i.e., skin, eye…) | ||
| Allowing specific cargo loading for enhancing the regenerative power in wound healing, degenerated nerve or cartilage | ||
| Increase of immunomodulatory properties by pre-conditioning MSCs to enhance quantity of secreted EVs | ||
| Longer circulating half-life and more biocompatible compared to liposomes and polymeric nanoparticles | ||
| High stability and resistance to freeze–thaw cycles | ||
| Quick and effective sterilization | ||
| Ability to cross the blood–brain barrier and freely circulate through the microvasculature | ||
| Limitations | Poor cell survival | Absence of standarized methods for characterization |
| Immune rejection | Lacking scalable production and purification | |
| High cost production | Requiring an improvement in the targeting strategies | |
| Perpetuation of MSCs in the body after disease | Needing for a better knowledge of half-life biodistribution, side effects and mechanisms of action | |
| Loss of stemness induced by time/aging | ||
| Undesired differentiation that can produce ossification, calcification and tumorigenesis | ||
| Inability to cross the blood-brain barrier and trapping in organs such as liver or lung |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Arroyo, O.; Ortega, A.; Forner, M.J.; Cortes, R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases. Pharmaceutics 2022, 14, 733. https://doi.org/10.3390/pharmaceutics14040733
Martinez-Arroyo O, Ortega A, Forner MJ, Cortes R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases. Pharmaceutics. 2022; 14(4):733. https://doi.org/10.3390/pharmaceutics14040733
Chicago/Turabian StyleMartinez-Arroyo, Olga, Ana Ortega, Maria J. Forner, and Raquel Cortes. 2022. "Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases" Pharmaceutics 14, no. 4: 733. https://doi.org/10.3390/pharmaceutics14040733
APA StyleMartinez-Arroyo, O., Ortega, A., Forner, M. J., & Cortes, R. (2022). Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases. Pharmaceutics, 14(4), 733. https://doi.org/10.3390/pharmaceutics14040733








