Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Tubular Grafts (TGs)
2.2.1. Silk Fibroin (SF) Purification
2.2.2. Synthesis of ZnSr-Doped β-TCP Powders
2.2.3. Preparation of HRP-SF/ZnSr-β-TCP TGs
2.3. Physicochemical Characterization
2.3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) Analysis
2.3.2. Micro-Computed Tomography (Micro-CT)
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.4. X-ray Diffraction (XRD)
2.3.5. Mechanical Properties
2.4. Swelling Ratio and Degradation Profile
2.5. Bioactivity Assay in Simulated Body Fluid (SBF)
2.6. In Vitro Cell Studies
2.6.1. SaOs-2 Cell Culture and Expansion
2.6.2. Seeding of SaOs-2 in the TGs
2.6.3. Live/Dead Staining
2.6.4. Alamar Blue Assay
2.6.5. SEM Analysis
2.6.6. dsDNA Quantification
2.6.7. Alkaline Phosphatase (ALP) Quantification
2.6.8. Alizarin Red Staining
2.6.9. RNA Isolation and Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.7. Statistical Analysis
3. Results and Discussion
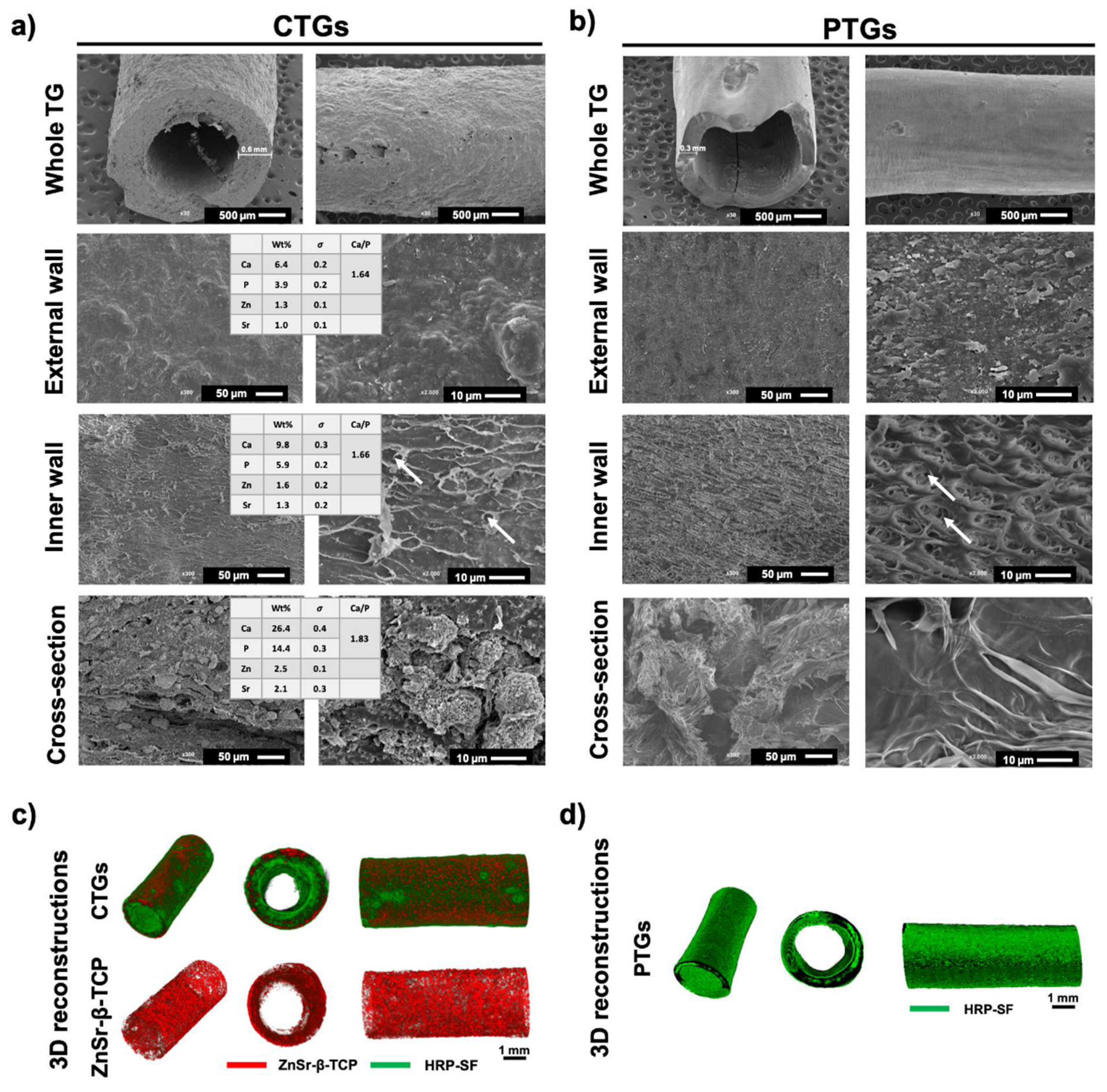
3.1. Morphology and Macro/Microstructure Characterization
3.2. Chemical Structure and Mechanical Properties
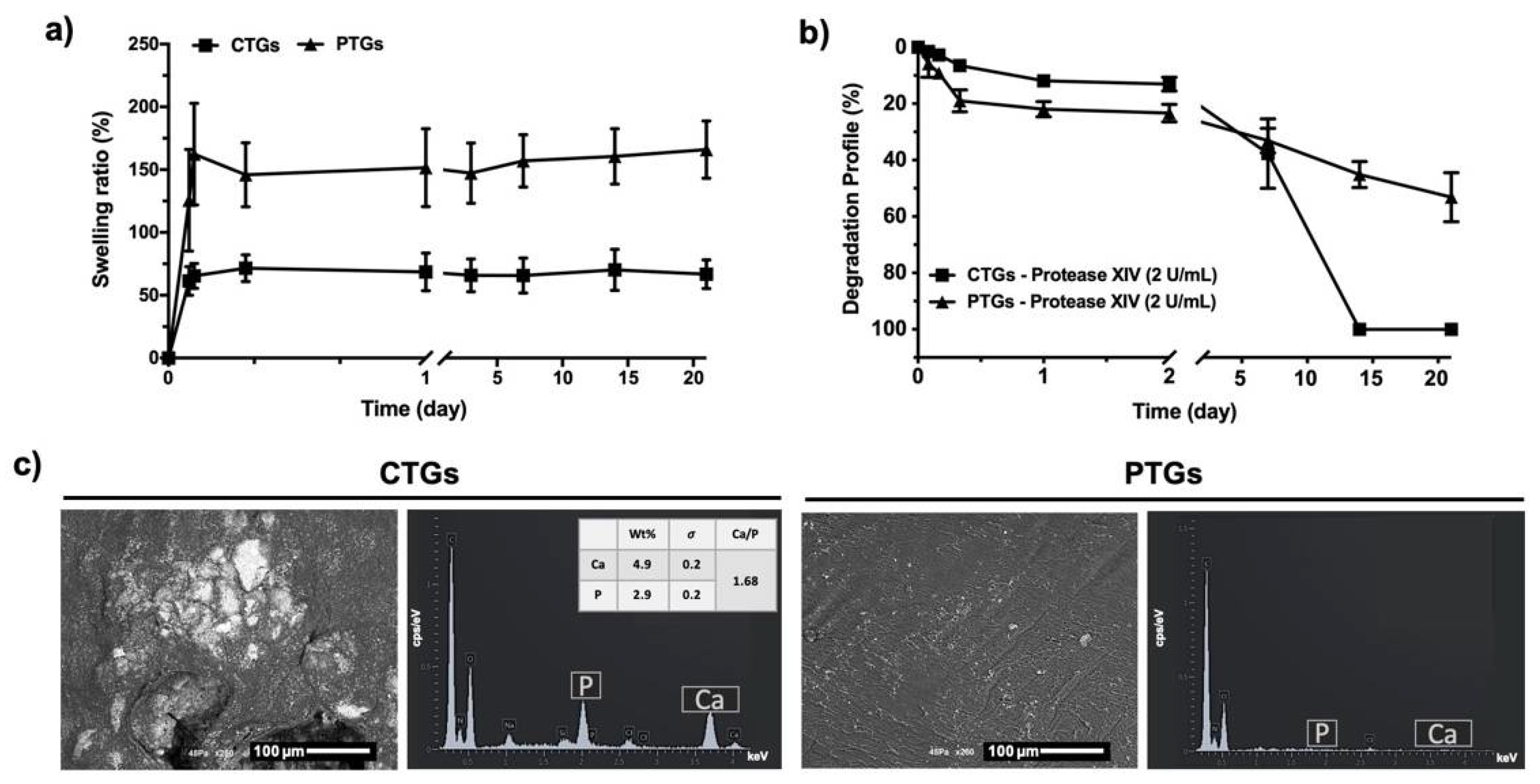
3.3. Swelling Ratio, Degradation Profile and Bioactivity Evaluation
3.4. Cell Viability, Proliferation and Morphological Profile
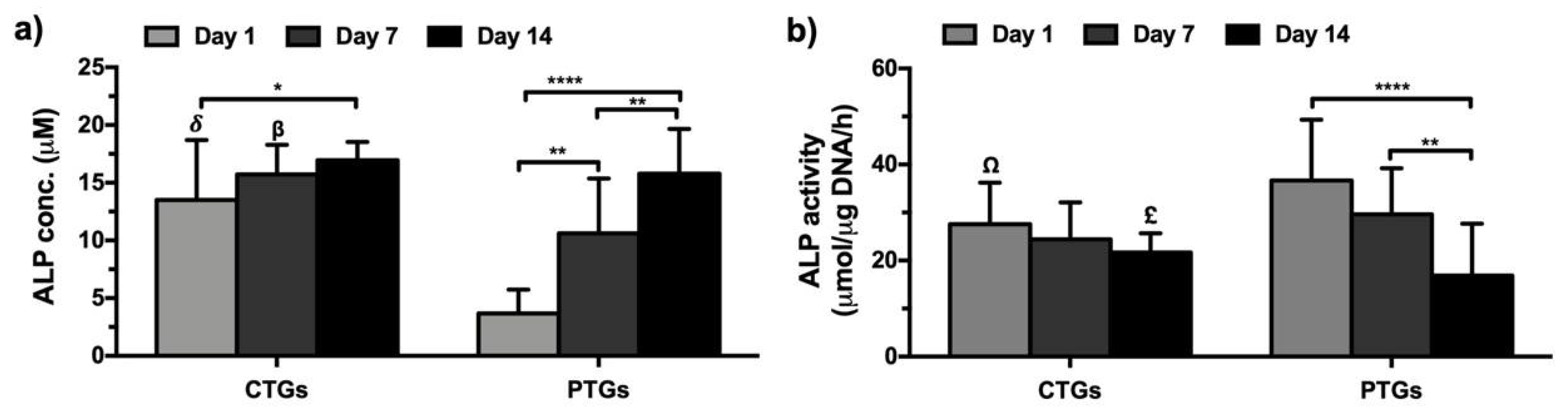
3.5. Biochemical Characterization and ECM Mineralization
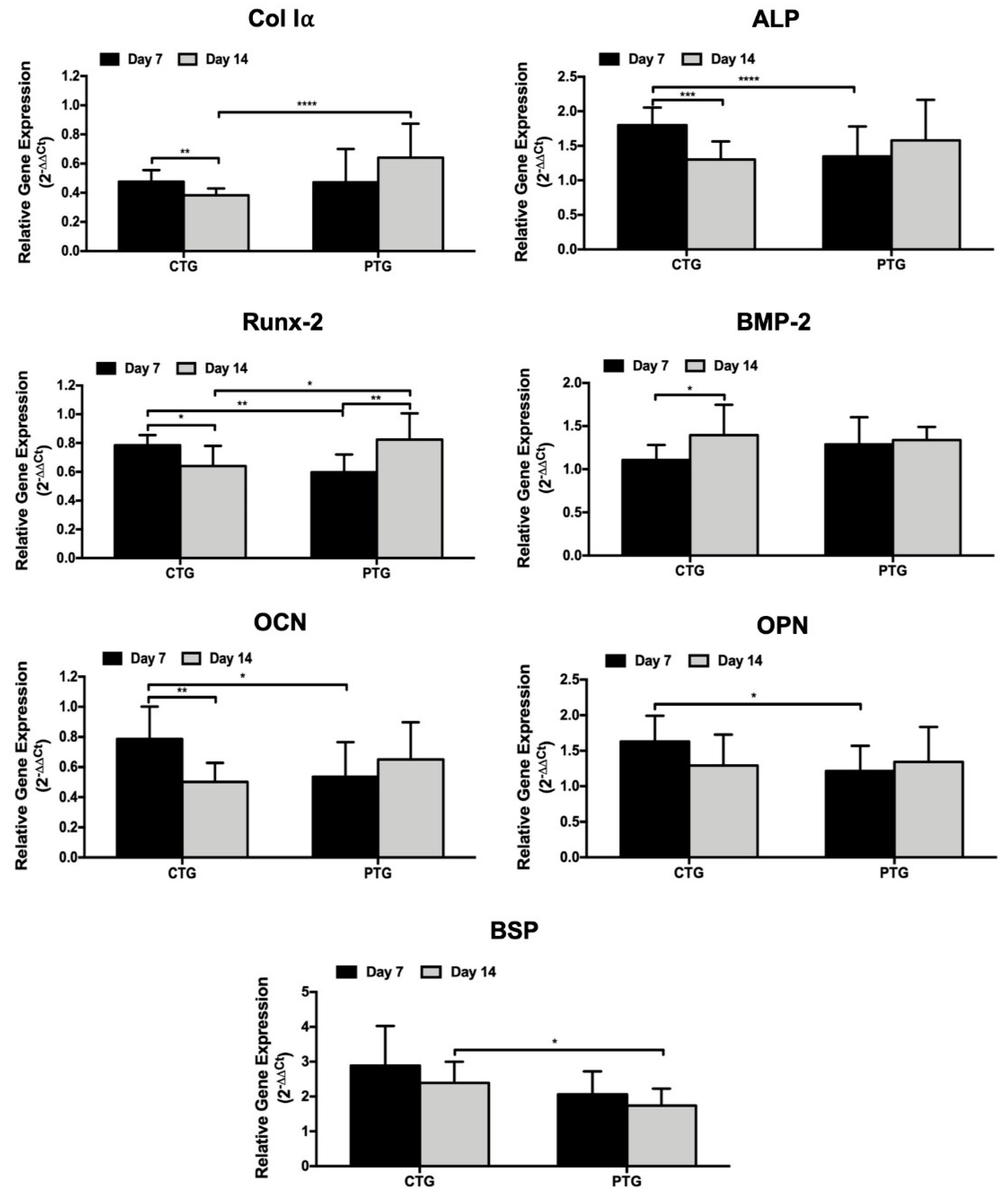
3.6. Osteogenic Genotype Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blaker, C.L.; Zaki, S.; Little, C.B.; Clarke, E.C. Long-term Effect of a Single Subcritical Knee Injury: Increasing the Risk of Anterior Cruciate Ligament Rupture and Osteoarthritis. Am. J. Sports Med. 2021, 49, 391–403. [Google Scholar] [PubMed]
- Ficek, K.; Rajca, J.; Stolarz, M.; Stodolak-Zych, E.; Wieczorek, J.; Muzalewska, M.; Wyleżoł, M.; Wróbel, Z.; Binkowski, M.; Błażewicz, S. Bioresorbable stent in anterior cruciate ligament reconstruction. Polymers 2019, 11, 1961. [Google Scholar]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current progress in tendon and ligament tissue engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [PubMed]
- Lansdown, D.A.; Riff, A.J.; Meadows, M.; Yanke, A.B.; Bach, B.R. What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin. Orthop. Relat. Res. 2017, 475, 2412–2426. [Google Scholar]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar]
- Bernardino, S. Various Prosthetic ACL Grafts: A Review of Literature. J. Dis. Disord. Treat. 2021, 101, 2–6. [Google Scholar]
- Elveos, M.M.; Drogset, J.O.; Engebretsen, L.; Brønn, R.; Lundemo, T.O.; Gifstad, T. Anterior cruciate ligament reconstruction using a Bone–Patellar Tendon–Bone graft with and without a ligament augmentation device: A 25-year follow-up of a prospective randomized controlled trial. Orthop. J. Sports Med. 2018, 6, 2325967118808778. [Google Scholar]
- Ageberg, E.; Roos, H.P.; Silbernagel, K.G.; Thomeé, R.; Roos, E.M. Knee extension and flexion muscle power after anterior cruciate ligament reconstruction with patellar tendon graft or hamstring tendons graft: A cross-sectional comparison 3 years post surgery. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 162–169. [Google Scholar]
- Giorgio, N.; Moretti, L.; Pignataro, P.; Carrozzo, M.; Vicenti, G.; Moretti, B. Correlation between fixation systems elasticity and bone tunnel widening after ACL reconstruction. Muscles Ligaments Tendons J. 2016, 6, 467. [Google Scholar] [PubMed]
- Musahl, V.; Abramowitch, S.D.; Gabriel, M.T.; Debski, R.E.; Hertel, P.; Fu, F.H.; Woo, S.L. Tensile properties of an anterior cruciate ligament graft after bone–patellar tendon–bone press-fit fixation. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 68–74. [Google Scholar]
- Edwards, J.H.; Jones, G.L.; Herbert, A.; Fisher, J.; Ingham, E. Integration and functional performance of a decellularised porcine superflexor tendon graft in an ovine model of anterior cruciate ligament reconstruction. Biomaterials 2021, 279, 121204. [Google Scholar]
- Kato, Y.; Chavez, J.; Yamada, S.; Hattori, S.; Takazawa, S.; Ohuchi, H. Beta-tricalcium phosphate block for donor site morbidity of the patella in anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft. Knee Surg. Relat. Res. 2019, 31, 113. [Google Scholar]
- Brian, K.T.; Vaughn, Z.D.; Lindsey, D.P.; Dragoo, J.L. Evaluation of a one-stage ACL revision technique using bone void filler after cyclic loading. Knee 2012, 19, 477–481. [Google Scholar]
- Zhu, C.; Qiu, J.; Thomopoulos, S.; Xia, Y. Augmenting Tendon-to-Bone Repair with Functionally Graded Scaffolds. Adv. Healthc. Mater. 2021, 10, 2002269. [Google Scholar]
- Park, S.H.; Choi, Y.-J.; Moon, S.W.; Lee, B.H.; Shim, J.-H.; Cho, D.-W.; Wang, J.H. Three-dimensional bio-printed scaffold sleeves with mesenchymal stem cells for enhancement of tendon-to-bone healing in anterior cruciate ligament reconstruction using soft-tissue tendon graft. Arthroscopy 2018, 34, 166–179. [Google Scholar] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar]
- Pollini, M.; Paladini, F. Bioinspired materials for wound healing application: The potential of silk fibroin. Materials 2020, 13, 3361. [Google Scholar]
- Zhang, W.; Yang, Y.; Zhang, K.; Luo, T.; Tang, L.; Li, Y. Silk-Poly (lactic-co-glycolic acid) Scaffold/Mesenchymal Stem Cell Composites for Anterior Cruciate Ligament Reconstruction in Rabbits. J. Biomater. Tissue Eng. 2017, 7, 571–581. [Google Scholar]
- Jiang, X.; Ren, Y.; Zhang, X.; You, T.; Ren, S.; Xie, X.; Zhou, R.; Li, C.; Zhang, W. Preparation of Silk Fibroin Nanofiber Scaffold and Its Application in Tendon-Bone Healing and Sports Rehabilitation. Sci. Adv. Mater. 2021, 13, 1374–1382. [Google Scholar]
- Fan, H.; Liu, H.; Toh, S.L.; Goh, J.C. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 2009, 30, 4967–4977. [Google Scholar] [PubMed]
- Teuschl, A.H.; Tangl, S.; Heimel, P.; Schwarze, U.Y.; Monforte, X.; Redl, H.; Nau, T. Osteointegration of a novel silk fiber–based ACL scaffold by formation of a ligament-bone interface. Am. J. Sports Med. 2019, 47, 620–627. [Google Scholar]
- Li, X.; He, J.; Bian, W.; Li, Z.; Zhang, W.; Li, D.; Snedeker, J.G. A novel silk-based artificial ligament and tricalcium phosphate/polyether ether ketone anchor for anterior cruciate ligament reconstruction–Safety and efficacy in a porcine model. Acta Biomater. 2014, 10, 3696–3704. [Google Scholar] [PubMed]
- Shi, P.; Teh, T.K.; Toh, S.L.; Goh, J.C. Variation of the effect of calcium phosphate enhancement of implanted silk fibroin ligament bone integration. Biomaterials 2013, 34, 5947–5957. [Google Scholar] [PubMed]
- Ruan, D.; Zhu, T.; Huang, J.; Le, H.; Hu, Y.; Zheng, Z.; Tang, C.; Chen, Y.; Ran, J.; Chen, X. Knitted silk-collagen scaffold incorporated with ligament stem/progenitor cells sheet for anterior cruciate ligament reconstruction and osteoarthritis prevention. ACS Biomater. Sci. Eng. 2019, 5, 5412–5421. [Google Scholar]
- Hu, Y.; Ran, J.; Zheng, Z.; Jin, Z.; Chen, X.; Yin, Z.; Tang, C.; Chen, Y.; Huang, J.; Le, H. Exogenous stromal derived factor-1 releasing silk scaffold combined with intra-articular injection of progenitor cells promotes bone-ligament-bone regeneration. Acta Biomater. 2018, 71, 168–183. [Google Scholar]
- Ribeiro, V.P.; da Silva Morais, A.; Maia, F.R.; Canadas, R.F.; Costa, J.B.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018, 72, 167–181. [Google Scholar] [PubMed]
- Ribeiro, V.P.; Pina, S.; Costa, J.o.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.d.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M. Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [PubMed]
- Ribeiro, V.P.; Pina, S.; Gheduzzi, S.; Araújo, A.C.; Reis, R.L.; Oliveira, J.M. Hierarchical HRP-crosslinked silk fibroin/ZnSr-TCP scaffolds for osteochondral tissue regeneration: Assessment of the mechanical and antibacterial properties. Front. Mater. 2020, 7, 49. [Google Scholar]
- Ribeiro, V.P.; Pina, S.C.A.; Canadas, R.F.; Morais, A.; Vilela, C.; Vieira, S.C.A.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. In vivo performance of hierarchical HRP-crosslinked silk fibroin/β-TCP scaffolds for osteochondral tissue regeneration. Regener. Med. Front. 2019, 7, 49. [Google Scholar]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast setting silk fibroin bioink for bioprinting of patient-specific memory-shape implants. Adv. Healthc. Mater. 2017, 6, 1701021. [Google Scholar]
- Carvalho, C.R.; Costa, J.B.; da Silva Morais, A.; López-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M. Tunable enzymatically cross-linked silk fibroin tubular conduits for guided tissue regeneration. Adv. Healthc. Mater. 2018, 7, 1800186. [Google Scholar]
- Ribeiro, V.P.; Silva-Correia, J.; Gonçalves, C.; Pina, S.; Radhouani, H.; Montonen, T.; Hyttinen, J.; Roy, A.; Oliveira, A.L.; Reis, R.L. Rapidly responsive silk fibroin hydrogels as an artificial matrix for the programmed tumor cells death. PLoS ONE 2018, 13, e0194441. [Google Scholar]
- Yan, L.-P.; Silva-Correia, J.; Ribeiro, V.P.; Miranda-Gonçalves, V.; Correia, C.; da Silva Morais, A.; Sousa, R.A.; Reis, R.M.; Oliveira, A.L.; Oliveira, J.M. Tumor growth suppression induced by biomimetic silk fibroin hydrogels. Sci. Rep. 2016, 6, 31037. [Google Scholar] [PubMed]
- Makaya, K.; Terada, S.; Ohgo, K.; Asakura, T. Comparative study of silk fibroin porous scaffolds derived from salt/water and sucrose/hexafluoroisopropanol in cartilage formation. J. Biosci. Bioeng. 2009, 108, 68–75. [Google Scholar] [PubMed]
- Tamada, Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules 2005, 6, 3100–3106. [Google Scholar]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [PubMed]
- Yan, L.-P.; Oliveira, J.M.; Oliveira, A.L.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012, 8, 289–301. [Google Scholar] [PubMed]
- Yan, L.-P.; Salgado, A.J.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. De novo bone formation on macro/microporous silk and silk/nano-sized calcium phosphate scaffolds. J. Bioact. Compat. Polym. 2013, 28, 439–452. [Google Scholar]
- Yan, L.-P.; Silva-Correia, J.; Correia, C.; Caridade, S.G.; Fernandes, E.M.; Sousa, R.A.; Mano, J.F.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Bioactive macro/micro porous silk fibroin/nano-sized calcium phosphate scaffolds with potential for bone-tissue-engineering applications. Nanomedicine 2013, 8, 359–378. [Google Scholar] [PubMed]
- Ma, P.; Chen, T.; Wu, X.; Hu, Y.; Huang, K.; Wang, Y.; Dai, H. Effects of bioactive strontium-substituted hydroxyapatite on osseointegration of polyethylene terephthalate artificial ligaments. J. Mater. Chem. B 2021, 9, 6600–6613. [Google Scholar] [PubMed]
- Sun, J.; Zhang, X.; Shi, Z.-Z.; Gao, X.-X.; Li, H.-Y.; Zhao, F.-Y.; Wang, J.-Q.; Wang, L.-N. Development of a high-strength Zn-Mn-Mg alloy for ligament reconstruction fixation. Acta Biomater. 2021, 119, 485–498. [Google Scholar] [PubMed]
- Pina, S.; Canadas, R.; Jiménez, G.; Perán, M.; Marchal, J.; Reis, R.; Oliveira, J. Biofunctional ionic-doped calcium phosphates: Silk fibroin composites for bone tissue engineering scaffolding. Cell. Tissues Organs 2017, 204, 150–163. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [PubMed]
- Rodriguez, M.; Kluge, J.A.; Smoot, D.; Kluge, M.A.; Schmidt, D.F.; Paetsch, C.R.; Kim, P.S.; Kaplan, D.L. Fabricating mechanically improved silk-based vascular grafts by solution control of the gel-spinning process. Biomaterials 2020, 230, 119567. [Google Scholar]
- Yue, L.; DeFroda, S.F.; Sullivan, K.; Garcia, D.; Owens, B.D. Mechanisms of bone tunnel enlargement following anterior cruciate ligament reconstruction. JBJS Rev. 2020, 8, e0120. [Google Scholar] [PubMed]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 17018. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar]
- Yao, S.; Xie, Y.; Xiao, L.; Cai, L.; Ma, Z. Porous and nonporous silk fibroin (SF) membranes wrapping for Achilles tendon (AT) repair: Which one is a better choice? J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 733–740. [Google Scholar]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar]
- Radu, I.-C.; Biru, I.-E.; Damian, C.-M.; Ion, A.-C.; Iovu, H.; Tanasa, E.; Zaharia, C.; Galateanu, B. Grafting versus crosslinking of silk Fibroin-g-PNIPAM via tyrosine-NIPAM bridges. Molecules 2019, 24, 4096. [Google Scholar]
- Liu, C.; Hua, J.; Ng, P.F.; Wang, Y.; Fei, B.; Shao, Z. Bioinspired Photo-Cross-Linking of Stretched Solid Silks for Enhanced Strength. ACS Biomater. Sci. Eng. 2022, 8, 484–492. [Google Scholar] [PubMed]
- Ghosh, S.; Parker, S.T.; Wang, X.; Kaplan, D.L.; Lewis, J.A. Direct-write assembly of microperiodic silk fibroin scaffolds for tissue engineering applications. Adv. Funct. Mater. 2008, 18, 1883–1889. [Google Scholar]
- Cheng, Y.; Koh, L.-D.; Li, D.; Ji, B.; Han, M.-Y.; Zhang, Y.-W. On the strength of β-sheet crystallites of Bombyx mori silk fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [PubMed]
- Gwiazda, M.; Kumar, S.; Świeszkowski, W.; Ivanovski, S.; Vaquette, C. The effect of melt electrospun writing fiber orientation onto cellular organization and mechanical properties for application in Anterior Cruciate Ligament tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103631. [Google Scholar] [PubMed]
- Thaunat, M.; Fayard, J.M.; Sonnery-Cottet, B. Hamstring tendons or bone-patellar tendon-bone graft for anterior cruciate ligament reconstruction? Orthop. Traumatol. Surg. Res. 2019, 105, S89–S94. [Google Scholar] [PubMed]
- Yari, S.S.; El Naga, A.N.; Patel, A.; Qadeer, A.A.; Shah, A. TightRope Versus Biocomposite Interference Screw for Fixation in Allograft ACL Reconstruction: Prospective Evaluation of Osseous Integration and Patient Outcomes. JBJS Open Access 2020, 5, e0057. [Google Scholar] [PubMed]
- Chandrashekar, N.; Mansouri, H.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J. Biomech. 2006, 39, 2943–2950. [Google Scholar]
- Ribeiro, V.P.; Silva-Correia, J.; Nascimento, A.I.; da Silva Morais, A.; Marques, A.P.; Ribeiro, A.S.; Silva, C.J.; Bonifácio, G.; Sousa, R.A.; Oliveira, J.M. Silk-based anisotropical 3D biotextiles for bone regeneration. Biomaterials 2017, 123, 92–106. [Google Scholar]
- Chvapil, M.; Speer, D.P.; Holubec, H.; Chvapil, T.A.; King, D.H. Collagen fibers as a temporary scaffold for replacement of ACL in goats. J. Biomed. Mater. Res. 1993, 27, 313–325. [Google Scholar] [PubMed]
- Yan, L.P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Core-shell silk hydrogels with spatially tuned conformations as drug-delivery system. J. Tissue Eng. Regen. Med. 2017, 11, 3168–3177. [Google Scholar] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar]
- Gupta, P.; Kumar, M.; Bhardwaj, N.; Kumar, J.P.; Krishnamurthy, C.; Nandi, S.K.; Mandal, B.B. Mimicking form and function of native small diameter vascular conduits using mulberry and non-mulberry patterned silk films. ACS Appl. Mater. Interfaces 2016, 8, 15874–15888. [Google Scholar]
- Xie, J.; Zhong, S.; Ma, B.; Shuler, F.D.; Lim, C.T. Controlled biomineralization of electrospun poly (ε-caprolactone) fibers to enhance their mechanical properties. Acta Biomater. 2013, 9, 5698–5707. [Google Scholar]
- DeVolder, R.J.; Kim, I.W.; Kim, E.-S.; Kong, H. Modulating the rigidity and mineralization of collagen gels using poly (lactic-co-glycolic acid) microparticles. Tissue Eng. Part A 2012, 18, 1642–1651. [Google Scholar]
- Castro, F.; Ribeiro, V.P.; Ferreira, A.; Oliveira, A.L.; Reis, R.L.; Teixeira, J.A.; Rocha, F. Continuous-flow precipitation as a route to prepare highly controlled nanohydroxyapatite: In vitro mineralization and biological evaluation. Mater. Res. Express 2016, 3, 075404. [Google Scholar]
- Li, H.; Fan, J.; Sun, L.; Liu, X.; Cheng, P.; Fan, H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016, 106, 180–192. [Google Scholar]
- Fan, J.; Sun, L.; Chen, X.; Qu, L.; Li, H.; Liu, X.; Zhang, Y.; Cheng, P.; Fan, H. Implementation of a stratified approach and gene immobilization to enhance the osseointegration of a silk-based ligament graft. J. Mater. Chem. B 2017, 5, 7035–7050. [Google Scholar]
- Santo, V.E.; Duarte, A.R.C.; Popa, E.G.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J. Control. Release 2012, 162, 19–27. [Google Scholar]
- Liu, J.; Zhao, L.; Ni, L.; Qiao, C.; Li, D.; Sun, H.; Zhang, Z. The effect of synthetic α-tricalcium phosphate on osteogenic differentiation of rat bone mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 1588. [Google Scholar] [PubMed]
- Hurle, K.; Maia, F.R.; Ribeiro, V.P.; Pina, S.; Oliveira, J.; Goetz-Neunhoeffer, F.; Reis, R. Osteogenic lithium-doped brushite cements for bone regeneration. Bioact. Mater. 2021, in press. [Google Scholar] [CrossRef]
- Kim, D.N.; Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Cho, K.H.; Park, K.D.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane enhances BMP-2-induced osteoblast differentiation in mesenchymal stem cells. Mol. Med. Rep. 2016, 14, 460–466. [Google Scholar] [PubMed][Green Version]
- Carvalho, M.S.; Cabral, J.; da Silva, C.L.; Vashishth, D. Bone matrix non-collagenous proteins in tissue engineering: Creating new bone by mimicking the extracellular matrix. Polymers 2021, 13, 1095. [Google Scholar] [PubMed]



| Gene | Sequences | Tm (°C) | |
|---|---|---|---|
| Forward (5′–3′) | Reverse (5′–3′) | ||
| GAPDH | ACAGTCAGCCGCATCTTCTT | GACAAGCTTCCCGTTCTCAG | 58.4 |
| Col Iα | CGAAGACATCCCACCAATCAC | GTCACAGATCACGTCATCCGC | 59.6 |
| ALP | CTCCTCGGAAGACACTCTG | AGACTGCGCCTGGTAGTTG | 60.0 |
| OPN | CCCACAGACCCTTCCAAGTA | GGGGACAACTGGAGTGAAAA | 58.4 |
| OCN | GTGCAGAGTCCAGCAAAGG | TCAGCCACTCGTCACAGC | 59.4 |
| Runx2 | TTCAGACCAGCAGCACTC | CAGCGTCAACACCATCATTC | 58.1 |
| BSP | ACTGAGCCTGTGTCTTGAAA | CTTCCAACAGCCAATCACTG | 56.2 |
| BMP-2 | TGAATCAGAATCCAAGCAGG | TCTTTTGTGGAGAGGATGCC | 56.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.P.; Costa, J.B.; Carneiro, S.M.; Pina, S.; Veloso, A.C.A.; Reis, R.L.; Oliveira, J.M. Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics 2022, 14, 697. https://doi.org/10.3390/pharmaceutics14040697
Ribeiro VP, Costa JB, Carneiro SM, Pina S, Veloso ACA, Reis RL, Oliveira JM. Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics. 2022; 14(4):697. https://doi.org/10.3390/pharmaceutics14040697
Chicago/Turabian StyleRibeiro, Viviana P., João B. Costa, Sofia M. Carneiro, Sandra Pina, Ana C. A. Veloso, Rui L. Reis, and Joaquim M. Oliveira. 2022. "Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction" Pharmaceutics 14, no. 4: 697. https://doi.org/10.3390/pharmaceutics14040697
APA StyleRibeiro, V. P., Costa, J. B., Carneiro, S. M., Pina, S., Veloso, A. C. A., Reis, R. L., & Oliveira, J. M. (2022). Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics, 14(4), 697. https://doi.org/10.3390/pharmaceutics14040697











