Gold Nanorods for Drug and Gene Delivery: An Overview of Recent Advancements
Abstract
:1. Introduction
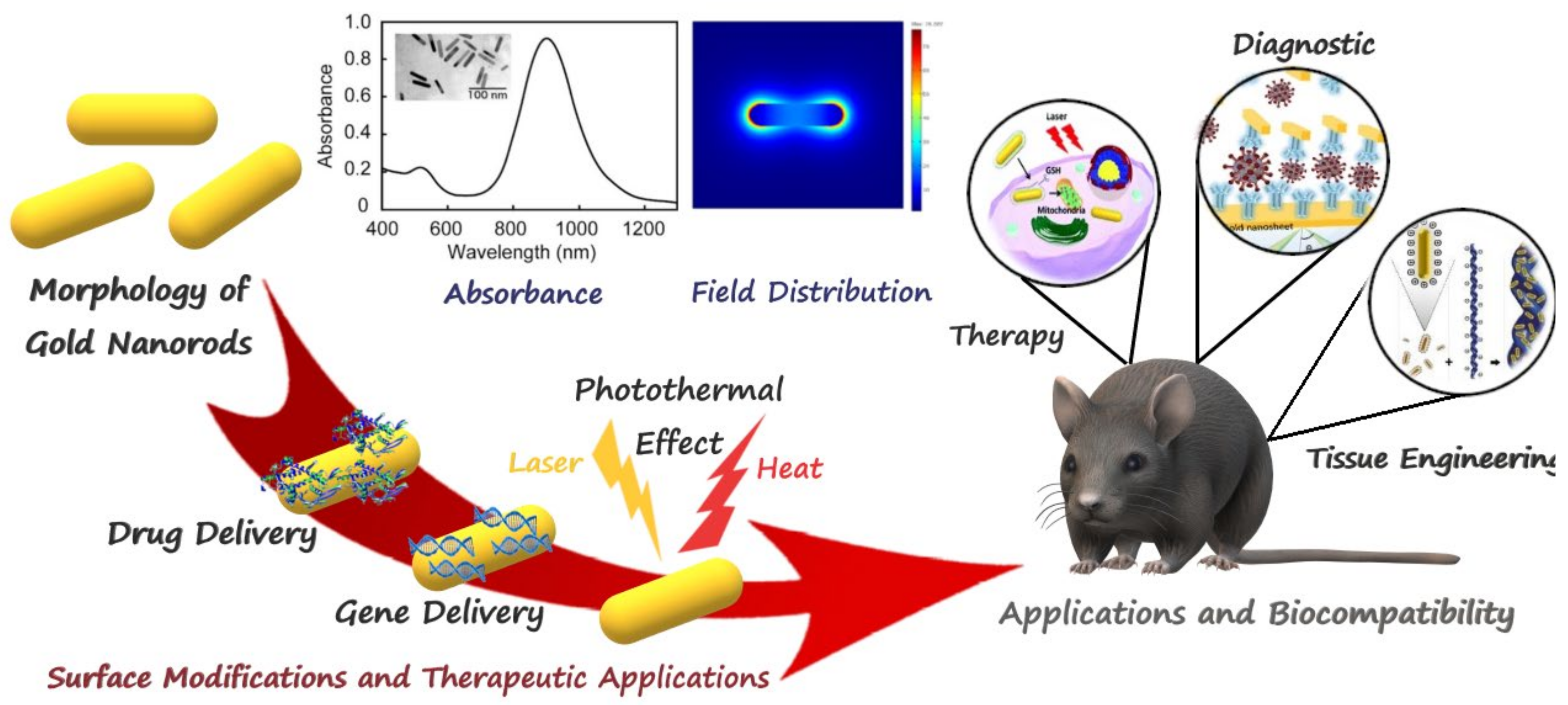
2. Properties and Synthesis of GNRs
2.1. GNR Synthesis Methods
2.2. Photothermal Effect
2.3. Toxicity Related to GNRs’ Surface Modifications
2.4. The GNRs–Proteins Interaction
3. GNRs for Therapeutic Applications
3.1. GNRs for Drug Delivery Systems
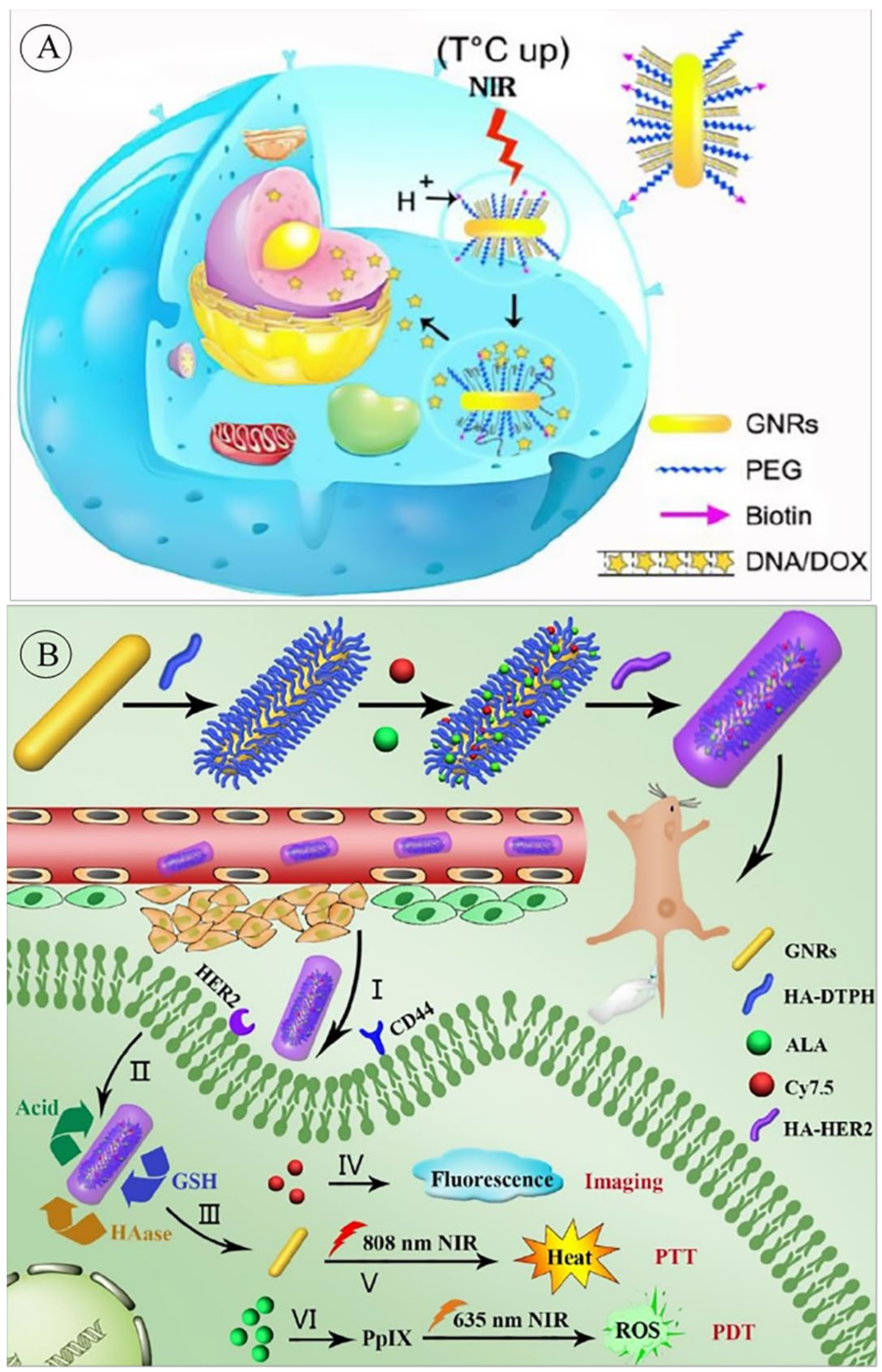
3.2. GNRs for Gene Delivery Systems
| Purpose | System’s Structure | Mechanism of Function | GNR’s Applied Feature | Ref. |
|---|---|---|---|---|
| RnD | Dendrimer coated GNRs | Delivery and enhanced expression of the brcaa1-shRNA to the targeted cells upon NIR irradiation | PT | [146] |
| DnD | Disulfide cross-linked polyethyleneimine-conjugated GNRs grafted by PEG and RGD peptide | Gene release in response to high glutathione concentration in target cells and NIR irradiation | PT | [147] |
| RnD | Layer-by-layer assembled chitosan-GNRs | Delivery of siRNAs, accumulation in tumor tissue, NIR-mediated photothermal ablation | PT | [140] |
| DnD | Cationic-charged surfactant and DNA modified GNRs | DNA release as a result of Photothermal hyperthermia | PT | [148] |
| Drug +siRNA co-delivery | DOX, YAP-siRNA and GNRs loaded cationic liposome | Targeted chemo, PT and gene combination therapy using NIR irradiation | PT | [131] |
| DnD | GNRs grafted with Poly(amidoamine) dendrimers and modified by GX1 peptide, FAM172A gene | DNA release and PTT as a result of Photothermal hyperthermia | PT | [142] |
3.3. GNRs for Photothermal/Photodynamic Therapy
4. GNRs for Theranostics; Combination of Diagnostic and Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GNR | Gold nanorods |
| NIR | Near-infrared |
| LSPR | Localized Surface Plasmon Resonance |
| nDDSs | nano-Drug delivery systems |
| BBB | Blood–brain barrier |
| CTAB | Cetyltrimethylammonium bromide |
| PE | Polyelectrolytes |
| PEG | Polyethylene glycol |
| DIC | Disseminated intravascular coagulopathy |
| DVT | Deep vein thrombosis |
| ROS | Reactive oxygen species |
| GNPs | Gold nanoparticles |
| PEI | Polyethylene imine |
| BSA | Bovine serum albumin |
| EPR | Permeability and retention |
| IFP | Interstitial fluid pressure |
| DOX | Doxorubicin |
| MDR | Multidrug-resistant |
| ALA | 5-Aminolevulinic acid |
| PT | Photothermal |
| DD | Drug delivery |
| PD | protein delivery |
| PTT | photothermal therapy |
| PA | Photoacoustic |
| Co | conductivity |
| Ca | Carrier |
| St | Stability |
| PDT | photodynamic therapy |
| Im | Imaging |
| GD | Gene delivery |
| Th | Theranostic |
| siRNA | small interfering RNA |
| YAP | Yes-associated protein |
| PLGA | Poly(lactic-co-glycolic acid) |
| CNTs | Carbon nanotubes |
| DnD | DNA delivery |
| RnD | RNA delivery |
| MNPs | Magnetic nanoparticles |
| AMF | Alternating magnetic field |
| ACE2 | Angiotensin-converting enzyme 2 |
| TD | Targeted delivery |
| PET | Positron emission tomography |
| TRK | Tracking |
| PL | Photoluminescence |
References
- Ranzoni, A.; Cooper, M.A. Chapter One—The Growing Influence of Nanotechnology in Our Lives. In Micro and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kar, M.; Simons, T.J.; Forsyth, M.; MacFarlane, D.R. Ionic liquid electrolytes as a platform for rechargeable metal–air batteries: A perspective. Phys. Chem. Chem. Phys. 2014, 16, 18658–18674. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Alexandridis, P. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bostrom, T. Application of Ionic Liquids in Solar Cells and Batteries: A Review. Curr. Org. Chem. 2015, 19, 556–566. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Jahangiri-Manesh, A.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Detection of hexanal gas as a volatile organic compound cancer biomarker using a nanocomposite of gold nanoparticles and selective polymers. J. Electroanal. Chem. 2021, 905, 115962. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly imprinted polymer-based chemiresistive sensor for detection of nonanal as a cancer related biomarker. Microchem. J. 2021, 173, 106988. [Google Scholar] [CrossRef]
- Su, X.; Fu, B.; Yuan, J. Gold nanocluster-coated gold nanorods for simultaneously enhanced photothermal performance and stability. Mater. Lett. 2016, 188, 111–114. [Google Scholar] [CrossRef]
- Naz, G.; Othaman, Z.; Shamsuddin, M.; Ghoshal, S.K. Gold nanorod libraries enhancement for optical imaging. Mater. Lett. 2016, 166, 63–66. [Google Scholar] [CrossRef]
- Yan, J.; Sun, H.; Li, J.; Qi, W.; Wang, H. A theranostic plaster combining photothermal therapy and photodynamic therapy based on chlorin e6/gold nanorods (Ce6/Au nrs) composite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 460–466. [Google Scholar] [CrossRef]
- Freitas, L.; Hamblin, M.; Anzengruber, F.; Perussi, J.R.; Ribeiro, A.; Martins, V.; Plepis, A. Zinc phthalocyanines attached to gold nanorods for simultaneous hyperthermic and photodynamic therapies against melanoma in vitro. J. Photochem. Photobiol. B Biol. 2017, 173, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Cao, Z.; Panwar, N.; Hu, R.; Wang, X.; Qu, J.; Tjin, S.C.; Xu, G.; Yong, K.-T. Functionalized gold nanorods for nanomedicine: Past, present and future. Coord. Chem. Rev. 2017, 352, 15–66. [Google Scholar] [CrossRef]
- Kilin, V.; Anton, H.; Anton, N.; Steed, E.; Vermot, J.; Vandamme, T.F.; Mely, Y.; Klymchenko, A.S. Counterion-enhanced cyanine dye loading into lipid nano-droplets for single-particle tracking in zebrafish. Biomaterials 2014, 35, 4950–4957. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.A.; Akhtari, Y.; Moghadam, T.T.; Ranjbar, B. Assembly of Gold Nanorods on HSA Amyloid Fibrils to Develop a Conductive Nanoscaffold for Potential Biomedical and Biosensing Applications. Sci. Rep. 2018, 8, 9333. [Google Scholar] [CrossRef]
- Bigham, A.; Rahimkhoei, V.; Abasian, P.; Delfi, M.; Naderi, J.; Ghomi, M.; Moghaddam, F.D.; Waqar, T.; Ertas, Y.N.; Sharifi, S.; et al. Advances in tannic acid-incorporated biomaterials: Infection treatment, regenerative medicine, cancer therapy, and biosensing. Chem. Eng. J. 2021, 432, 134146. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Amirmahani, N.; Mahmoodi, N.O.; Galangash, M.M.; Ghavidast, A. Advances in nanomicelles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Ould-Ouali, L.; Noppe, M.; Langlois, X.; Willems, B.; Riele, P.T.; Timmerman, P.; Brewster, M.E.; Ariën, A.; Préat, V. Self-assembling PEG-p(CL-co-TMC) copolymers for oral delivery of poorly water-soluble drugs: A case study with risperidone. J. Control. Release 2005, 102, 657–668. [Google Scholar] [CrossRef]
- Kipp, J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109–122. [Google Scholar] [CrossRef]
- Benita, S. Microencapsulation: Methods and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Pozo, D. Polysaccharide Colloids as Smart Vehicles in Cancer Therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef] [PubMed]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S. Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation. Adv. Sci. 2022, 9, 2102678. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Saebfar, H.; Gholami, M.H.; Hushmandi, K.; Zabolian, A.; Bikarannejad, P.; Hashemi, M.; Daneshi, S.; Mirzaei, S.; Sharifi, E.; et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: Stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin. Drug Deliv. 2022. accepted. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Demetzos, C. Advanced Drug Delivery Nanosystems: Perspectives and Regulatory Issues. GeNeDis 2014, 822, 195–198. [Google Scholar] [CrossRef]
- Nazarov, G.V.; Galan, S.E.; Nazarova, E.V.; Karkishchenko, N.N.; Muradov, M.M.; Stepanov, V.A. Nanosized forms of drugs (A Review). Pharm. Chem. J. 2009, 43, 163–170. [Google Scholar] [CrossRef]
- Arias, J.L. Porous Silica Nanoparticles for Drug Delivery and Controlled Release. In Nanotechnology and Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 301–338. [Google Scholar]
- Mohammadinejad, R.; Dehshahri, A.; Madamsetty, V.S.; Zahmatkeshan, M.; Tavakol, S.; Makvandi, P.; Khorsandi, D.; Pardakhty, A.; Ashrafizadeh, M.; Afshar, E.G.; et al. In Vivo gene delivery mediated by non-viral vectors for cancer therapy. J. Control. Release 2020, 325, 249–275. [Google Scholar] [CrossRef]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.; Ashrafizadeh, M.; Zare, E.; Agarwal, T.; Padil, V.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117–139. [Google Scholar] [CrossRef]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Hyperbranched polyglycerol coated on copper oxide nanoparticles as a novel core-shell nano-carrier hydrophilic drug delivery model. J. Mol. Liq. 2018, 250, 375–380. [Google Scholar] [CrossRef]
- Jahandar, M.; Zarrabi, A.; Shokrgozar, M.A.; Mousavi, H. Synthesis, characterization and application of polyglycerol coated Fe3O4nanoparticles as a nano-theranostics agent. Mater. Res. Express 2015, 2, 125002. [Google Scholar] [CrossRef]
- Mishra, A.K. Nanomedicine for Drug Delivery and Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, X.; Wang, S.; Zheng, X.; Lin, J.; Li, P.; Lin, L. Nab-paclitaxel (abraxane)-based chemotherapy to treat elderly patients with advanced non-small-cell lung cancer: A single center, randomized and open-label clinical trial. Chin. J. Cancer Res. 2015, 27, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Mumper, R.J. Paclitaxel nano-delivery systems: A comprehensive review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Kim, Y.-M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.-H.; Kim, B.-G.; Kim, S.-C.; Ryu, H.-S.; et al. An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203. [Google Scholar] [CrossRef]
- Docetaxel (Taxotere®). Available online: https://starpharma.com/drug_delivery/dep_docetaxel (accessed on 12 March 2022).
- Grippin, A.J.; Sayour, E.J.; Mitchell, D.A. Translational nanoparticle engineering for cancer vaccines. OncoImmunology 2017, 6, e1290036. [Google Scholar] [CrossRef] [Green Version]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Michaelis, M.; Kreuter, J.; Langer, K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Rowland, M.; Noe, C.R.; Smith, D.A.; Tucker, G.T.; Crommelin, D.J.; Peck, C.C.; Rocci, M.L., Jr.; Besançon, L.; Shah, V.P. Impact of the pharmaceutical sciences on health care: A reflection over the past 50 years. J. Pharm. Sci. 2012, 101, 4075–4099. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziara, J.M.; Whisman, T.R.; Tseng, M.T.; Mumper, R.J. In-Vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J. Control. Release 2006, 112, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Koziara, J.M.; Lockman, P.; Allen, D.D.; Mumper, R.J. Paclitaxel nanoparticles for the potential treatment of brain tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Gulyaev, A.E.; Gelperina, S.; Skidan, I.N.; Antropov, A.S.; Kivman, G.Y.; Kreuter, J. Significant Transport of Doxorubicin into the Brain with Polysorbate 80-Coated Nanoparticles. Pharm. Res. 1999, 16, 1564–1569. [Google Scholar] [CrossRef]
- Ljubimova, J.Y.; Sun, T.; Mashouf, L.; Ljubimov, A.V.; Israel, L.; Ljubimov, V.A.; Falahatian, V.; Holler, E. Covalent nano delivery systems for selective imaging and treatment of brain tumors. Adv. Drug Deliv. Rev. 2017, 113, 177–200. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Rupp, R.; Rosenthal, S.L.; Stanberry, L.R. VivaGel™(SPL7013 Gel): A candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2007, 2, 561. [Google Scholar]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Avasthi, A.; Paez-Muñoz, J.M.; Leal, M.P.; García-Martín, M.L. Passive targeting of high-grade gliomas via the EPR effect: A closed path for metallic nanoparticles? Biomater. Sci. 2021, 9, 7984–7995. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-H.; Le, Q.; Lan, S.; Liang, J.-X.; Tie, S.-L.; Xu, J.-L. Modifying the mechanical properties of gold nanorods by copper doping and triggering their cytotoxicity with ultrasonic wave. Colloids Surf. B Biointerfaces 2018, 163, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haine, A.T.; Niidome, T. Gold Nanorods as Nanodevices for Bioimaging, Photothermal Therapeutics, and Drug Delivery. Chem. Pharm. Bull. 2017, 65, 625–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Pulliam, B.; Sung, J.C.; Edwards, D.A. Design of nanoparticle-based dry powder pulmonary vaccines. Expert Opin. Drug Deliv. 2007, 4, 651–663. [Google Scholar] [CrossRef]
- Ragusa, A.; García, I.; Penadés, S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans. Nano Biosci. 2007, 6, 319–330. [Google Scholar] [CrossRef]
- Streicher, R.M.; Schmidt, M.; Fiorito, S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine 2007, 2, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Mwakwari, S.C.; Oyelere, A.K. Gold nanoparticles: From nanomedicine to nanosensing. Nanotechnol. Sci. Appl. 2008, 1, 45. [Google Scholar] [PubMed] [Green Version]
- Naderi, M.S.; Moghadam, T.T.; Khajeh, K.; Ranjbar, B. Improving the stability of chondroitinase ABC I via interaction with gold nanorods. Int. J. Biol. Macromol. 2018, 107, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ning, T.; Han, Y.; Sheng, Y.; Li, C.; Zhao, X.; Lu, Z.; Man, B.; Jiao, Y.; Jiang, S. Preparation, characterization, and nonlinear optical properties of hybridized graphene@ gold nanorods nanocomposites. Appl. Surf. Sci. 2018, 433, 45–50. [Google Scholar] [CrossRef]
- Stone, J.; Jackson, S.; Wright, D. Biological applications of gold nanorods. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 100–109. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, X.; Chen, M.; Sheng, J.; Ren, J.; Jiang, Z.; Cai, J.; Hu, Y. Fluorescence guided photothermal/photodynamic ablation of tumours using pH-responsive chlorin e6-conjugated gold nanorods. Colloids Surf. B Biointerfaces 2017, 160, 345–354. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chu, H.-C.; Kuo, T.-J.; Kuo, C.-L.; Huang, M.H. Seed-Mediated Synthesis of High Aspect Ratio Gold Nanorods with Nitric Acid. Chem. Mater. 2005, 17, 6447–6451. [Google Scholar] [CrossRef]
- Gao, F.; Bai, L.; Liu, S.; Zhang, R.; Zhang, J.; Feng, X.; Zheng, Y.; Zhao, Y. Rationally encapsulated gold nanorods improving both linear and nonlinear photoacoustic imaging contrast in vivo. Nanoscale 2016, 9, 79–86. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Shah, K.U.; Ur-Rehman, T.; Ud-Din, F. Gold nanorods: New generation drug delivery platform. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–84. [Google Scholar]
- Brown, K.R.; Natan, M.J. Hydroxylamine Seeding of Colloidal Au Nanoparticles in Solution and on Surfaces. Langmuir 1998, 14, 726–728. [Google Scholar] [CrossRef]
- Gao, J.; Bender, A.C.M.; Murphy, C.J. Dependence of the Gold Nanorod Aspect Ratio on the Nature of the Directing Surfactant in Aqueous Solution. Langmuir 2003, 19, 9065–9070. [Google Scholar] [CrossRef]
- Moghadam, T.T.; Ranjbar, B.; Khajeh, K. Conformation and activity of lysozyme on binding to two types of gold nanorods: A comparative study. Int. J. Biol. Macromol. 2012, 51, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L. (Ed.) Colloidal Synthesis of Plasmonic Nanometals; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Dalmau, J.; Vilches, C.; de Miguel, I.; Sanz, V.; Quidant, R. Optimum morphology of gold nanorods for light-induced hyperthermia. Nanoscale 2017, 10, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Marasini, R.; Pitchaimani, A.; Nguyen, T.D.T.; Comer, J.; Aryal, S. The influence of polyethylene glycol passivation on the surface plasmon resonance induced photothermal properties of gold nanorods. Nanoscale 2018, 10, 13684–13693. [Google Scholar] [CrossRef]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Alkilany, A.; Shatanawi, A.; Kurtz, T.; Caldwell, R. Toxicity and Cellular Uptake of Gold Nanorods in Vascular Endothelium and Smooth Muscles of Isolated Rat Blood Vessel: Importance of Surface Modification. Small 2012, 8, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- De La Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; Du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, Á.M.; da Silva, K.R.M.; Calado, C.M.S.; Saraiva, K.L.A.; Figueiredo, R.C.B.Q.; Leite, A.C.R.; Meneghetti, M.R. Evaluation of gold nanorods toxicity on isolated mitochondria. Toxicology 2019, 413, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Mansano, A.S.; Venturini, F.P.; Marangoni, V.S.; Lins, P.M.P.; Silva, B.P.C.; Dressler, B.; Zucolotto, V. Toxicity of gold nanorods on Ceriodaphnia dubia and Danio rerio after sub-lethal exposure and recovery. Environ. Sci. Pollut. Res. 2021, 28, 25316–25326. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, B.S.M. Acute and Developmental Toxicity of Gold Nanorods on Zebrafish (Danio Rerio) Embryos; University of Porto: Porto, Portugal, 2016. [Google Scholar]
- Pyshnaya, I.A.; Razum, K.; Poletaeva, Y.; Pyshnyi, D.; Zenkova, M.A.; Ryabchikova, E.I. Comparison of Behaviour in Different Liquids and in Cells of Gold Nanorods and Spherical Nanoparticles Modified by Linear Polyethyleneimine and Bovine Serum Albumin. BioMed. Res. Int. 2014, 2014, 908175. [Google Scholar] [CrossRef]
- Chakraborty, S.; Joshi, P.; Shanker, V.; Ansari, Z.A.; Singh, S.P.; Chakrabarti, P. Contrasting Effect of Gold Nanoparticles and Nanorods with Different Surface Modifications on the Structure and Activity of Bovine Serum Albumin. Langmuir 2011, 27, 7722–7731. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Wang, Y.; Wang, J.; Yu, Y.; Guo, S.; Chen, R.; Zhou, D. pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. J. Control. Release 2016, 232, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Haine, A.T.; Koga, Y.; Hashimoto, Y.; Higashi, T.; Motoyama, K.; Arima, H.; Niidome, T. Enhancement of transdermal protein delivery by photothermal effect of gold nanorods coated on polysaccharide-based hydrogel. Eur. J. Pharm. Biopharm. 2017, 119, 91–95. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2018, 83, 400–413. [Google Scholar] [CrossRef]
- Niidome, T.; Shiotani, A.; Mori, T.; Katayama, Y. Targeted delivery of gold nanorods modified with thermo-sensitive polymer. J. Control. Release 2010, 148, e65–e66. [Google Scholar] [CrossRef]
- Chen, J.; Han, X.; Deng, J.; Zhang, J.; Li, L.; Ni, J.; Huang, Y.; Xie, X.; Chen, S.; Ke, L.; et al. An injectable hydrogel based on phenylboronic acid hyperbranched macromer encapsulating gold nanorods and Astragaloside IV nanodrug for myocardial infarction. Chem. Eng. J. 2020, 413, 127423. [Google Scholar] [CrossRef]
- Shanmugam, V.; Chien, Y.-H.; Cheng, Y.-S.; Liu, T.-Y.; Huang, C.-C.; Su, C.-H.; Chen, Y.-S.; Kumar, U.; Hsu, H.-F.; Yeh, C.-S. Oligonucleotides—Assembled Au Nanorod-Assisted Cancer Photothermal Ablation and Combination Chemotherapy with Targeted Dual-Drug Delivery of Doxorubicin and Cisplatin Prodrug. ACS Appl. Mater. Interfaces 2014, 6, 4382–4393. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, W.; Peng, X.; Deng, X.; Sun, C.; Wu, H.; He, B. Near infrared light responsive hybrid nanoparticles for synergistic therapy. Biomaterials 2016, 100, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Yan, M.; Dong, S.; Hao, J. Near-Infrared-Light-Responsive Magnetic DNA Microgels for Photon- and Magneto-Manipulated Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 28185–28194. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Li, X.; Lv, Y.; Ma, T.; Guo, S.; Huang, Z.; Wang, X.; Xu, P. Dual targeting hyaluronic acid—RGD mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2017, 81, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Pandey, S.; Bhaisare, M.L.; Gedda, G.; Talib, A.; Wu, H.-F. Graphene oxide@gold nanorods for chemo-photothermal treatment and controlled release of doxorubicin in mice Tumor. Colloids Surf. B Biointerfaces 2017, 160, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Maiti, C.; Rajesh, Y.; Dey, K.K.; Pal, I.; Parekh, A.; Patra, R.; Dhara, D.; Dutta, P.K.; Mandal, M. Gold nanorod embedded reduction responsive block copolymer micelle-triggered drug delivery combined with photothermal ablation for targeted cancer therapy. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 3039–3052. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Shi, J.; Ma, T.; Zhang, Z.; Ma, H.; Cao, S. Hydroxyapatite/mesoporous silica coated gold nanorods with improved degradability as a multi-responsive drug delivery platform. Mater. Sci. Eng. C 2017, 83, 90–98. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Zhaoa, X.; Wub, Q.Q.; Zhub, H.; Maoa, Z.; Gaoa, C. Doxorubicin-conjugated pH-responsive gold nanorods for combined photothermal therapy and chemotherapy of cancer. Bioact. Mater. 2018, 3, 347–354. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Mahmoodzadeh, F.; Ghorbani, M.; Hamishehkar, H. A novel gold nanorods coated by stimuli-responsive ABC triblock copolymer for chemotherapy of solid tumors. Eur. Polym. J. 2019, 115, 313–324. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Xu, W.; Sun, T.; Wang, Y.; Wang, J.; Ji, L.; Suo, A. A novel pH-sensitive targeting polysaccharide-gold nanorod conjugate for combined photothermal-chemotherapy of breast cancer. Carbohydr. Polym. 2019, 212, 334–344. [Google Scholar] [CrossRef]
- Jiang, P.; Hu, Y.; Li, G. Biocompatible Au@Ag nanorod@ZIF-8 core-shell nanoparticles for surface-enhanced Raman scattering imaging and drug delivery. Talanta 2019, 200, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lim, K.; Kim, S.S.; Thien, L.X.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Chlorella-gold nanorods hydrogels generating photosynthesis-derived oxygen and mild heat for the treatment of hypoxic breast cancer. J. Control. Release 2019, 294, 77–90. [Google Scholar] [CrossRef]
- Alenezi, A.; Hulander, M.; Atefyekta, S.; Andersson, M. Development of a photon induced drug-delivery implant coating. Mater. Sci. Eng. C 2019, 98, 619–627. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Reis, C.A.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Optimization of gold core-mesoporous silica shell functionalization with TPGS and PEI for cancer therapy. Microporous Mesoporous Mater. 2019, 285, 1–12. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, J.; Zhang, Z.; Cao, Y.; Li, J.; Cao, S. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109889. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, S.; Moghadam, T.T.; Nikkhah, M.; Behmanesh, M. RGD-HK Peptide-Functionalized Gold Nanorods Emerge as Targeted Biocompatible Nanocarriers for Biomedical Applications. Nanoscale Res. Lett. 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tan, G.; Zhong, Y.; Jiang, Y.; Cai, L.; Yu, Z.; Liu, S.; Ren, F. Smart nanoplatform for sequential drug release and enhanced chemo-thermal effect of dual drug loaded gold nanorod vesicles for cancer therapy. J. Nanobiotechnol. 2019, 17, 44. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Z.; Fang, J.; Wang, B.; Lin, Z.; Chen, Z.-S.; Chen, Y.; Zhang, N.; Yang, X.; Gao, W. A multi-functionalized nanocomposite constructed by gold nanorod core with triple-layer coating to combat multidrug resistant colorectal cancer. Mater. Sci. Eng. C 2020, 107, 110224. [Google Scholar] [CrossRef]
- Puleio, R.; Licciardi, M.; Varvarà, P.; Scialabba, C.; Cassata, G.; Cicero, L.; Cavallaro, G.; Giammona, G. Effect of actively targeted copolymer coating on solid tumors eradication by gold nanorods-induced hyperthermia. Int. J. Pharm. 2020, 587, 119641. [Google Scholar] [CrossRef]
- Du, Z.; Yan, K.; Cao, Y.; Li, Y.; Yao, Y.; Yang, G. Regenerated keratin-encapsulated gold nanorods for chemo-photothermal synergistic therapy. Mater. Sci. Eng. C 2020, 117, 111340. [Google Scholar] [CrossRef]
- Chen, R.; Shi, J.; Zhu, B.; Zhang, L.; Cao, S. Mesoporous hollow hydroxyapatite capped with smart polymer for multi-stimuli remotely controlled drug delivery. Microporous Mesoporous Mater. 2020, 306, 110447. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, J.; Wu, Z.; Li, J.; Cao, S. Gold nanorods/polydopamine-capped hollow hydroxyapatite microcapsules as remotely controllable multifunctional drug delivery platform. Powder Technol. 2020, 372, 486–496. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Bagherifard, M.; Doroudian, M. Synthesis of micelles based on chitosan functionalized with gold nanorods as a light sensitive drug delivery vehicle. Int. J. Biol. Macromol. 2020, 149, 809–818. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Soni, S.; Sachdev, A.; Vikas; Mishra, S. Near-infrared stimulated hydrogel patch for photothermal therapeutics and thermoresponsive drug delivery. J. Photochem. Photobiol. B Biol. 2020, 210, 111960. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Vijay, J.; Ganesh, M.; Sundaramurthy, A. Multilayer capsules encapsulating nimbin and doxorubicin for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 582, 119350. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, D.; Zhang, X.; Xu, S.; Zhang, N. Targeted and triple therapy-based liposomes for enhanced treatment of rheumatoid arthritis. Int. J. Pharm. 2020, 586, 119642. [Google Scholar] [CrossRef]
- Guo, H.; Yi, S.; Feng, K.; Xia, Y.; Qu, X.; Wan, F.; Chen, L.; Zhang, C. In Situ formation of metal organic framework onto gold nanorods/mesoporous silica with functional integration for targeted theranostics. Chem. Eng. J. 2020, 403, 126432. [Google Scholar] [CrossRef]
- Guo, D.; Huang, Y.; Jin, X.; Zhang, C.; Zhu, X. A Redox-Responsive, In-Situ Polymerized Polyplatinum(IV)-Coated Gold Nanorod as An Amplifier of Tumor Accumulation for Enhanced Thermo-Chemotherapy. Biomaterials 2020, 266, 120400. [Google Scholar] [CrossRef]
- Darwish, W.M.; Abdoon, A.S.; Shata, M.S.; Elmansy, M. Vincristine-loaded polymeric corona around gold nanorods for combination (chemo-photothermal) therapy of oral squamous carcinoma. React. Funct. Polym. 2020, 151, 104575. [Google Scholar] [CrossRef]
- Cheng, D.; Ji, Y.; Wang, B.; Wang, Y.; Tang, Y.; Fu, Y.; Xu, Y.; Qian, X.; Zhu, W. Dual-responsive nanohybrid based on degradable silica-coated gold nanorods for triple-combination therapy for breast cancer. Acta Biomater. 2021, 128, 435–446. [Google Scholar] [CrossRef]
- Yin, M.; Qiao, Z.; Yan, D.; Yang, M.; Yang, L.; Wan, X.; Chen, H.; Luo, J.; Xiao, H. Ciprofloxacin conjugated gold nanorods with pH induced surface charge transformable activities to combat drug resistant bacteria and their biofilms. Mater. Sci. Eng. C 2021, 128, 112292. [Google Scholar] [CrossRef] [PubMed]
- Lihuang, L.; Qiuyan, G.; Yanxiu, L.; Mindan, L.; Jun, Y.; Yunlong, G.; Qiang, Z.; Benqiang, S.; Xiumin, W.; Liang-Cheng, L.; et al. Targeted combination therapy for glioblastoma by co-delivery of doxorubicin, YAP-siRNA and gold nanorods. J. Mater. Sci. Technol. 2020, 63, 81–90. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Sundaramoorthy, P.; Poudel, B.K.; Youn, Y.S.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Multimodal selenium nanoshell-capped Au@ mSiO2 nanoplatform for NIR-responsive chemo-photothermal therapy against metastatic breast cancer. NPG Asia Mater. 2018, 10, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Perry, H.L.; Wilton-Ely, J.D.E.T. Strategies for the functionalisation of gold nanorods to reduce toxicity and aid clinical translation. Nanotheranostics 2021, 5, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, M. Fast loading of PEG–SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760–17767. [Google Scholar] [CrossRef]
- Kinnear, C.; Dietsch, H.; Clift, M.J.; Endes, C.; Rothen-Rutishauser, B.; Petri-Fink, A. Gold nanorods: Controlling their surface chemistry and complete detoxification by a two-step place exchange. Angew. Chem. Int. Ed. 2013, 52, 1934–1938. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ma, Z.; Sun, H. Simple preparation of photothermal nanomaterial GNR@ SiO2 with enhanced drug loading content. IET Nanobiotechnol. 2018, 13, 257–261. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Ramasamy, T.; Munusamy, S.; Ruttala, H.B.; Kim, J.O. Smart Nanocarriers for the Delivery of Nucleic Acid-Based Therapeutics: A Comprehensive Review. Biotechnol. J. 2020, 16, 1900408. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.-W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomater. 2015, 25, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Fukushima, H.; Akiyama, Y.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. Controlled-release system of single-stranded DNA triggered by the photothermal effect of gold nanorods and its in vivo application. Bioorganic Med. Chem. 2011, 19, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, Y.; Mao, J.; Lei, X.; Yang, Q.; Cui, C. Dendrimer-modified gold nanorods as a platform for combinational gene therapy and photothermal therapy of tumors. J. Exp. Clin. Cancer Res. 2021, 40, 303. [Google Scholar] [CrossRef] [PubMed]
- Prabhune, M. RNAi vs. CRISPR: Guide to Selecting the Best Gene Silencing Method. Available online: https://www.synthego.com/blog/rnai-vs-crispr-guide#rn-ai-the-knockdown-pioneer (accessed on 30 September 2021).
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681. [Google Scholar] [CrossRef]
- Ni, S.; Zhu, J.; Mezour, M.A.; Lennox, R.B. Single wall carbon nanotube (SWCNT)–gold nanorod (AuNR) conjugates via thermally-mild reaction conditions. New J. Chem. 2017, 41, 12392–12396. [Google Scholar] [CrossRef]
- Cui, D.; Huang, P.; Zhang, C.; Ozkan, C.S.; Pan, B.; Xu, P. Dendrimer-modified gold nanorods as efficient controlled gene delivery system under near-infrared light irradiation. J. Control. Release 2011, 152, e137–e139. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Y.; Zhang, W.; Li, M.; Wang, Y.; Zhou, D.; Guo, S. Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod. J. Control. Release 2014, 196, 37–51. [Google Scholar] [CrossRef]
- Petralia, S.; Forte, G.; Aiello, M.; Nocito, G.; Conoci, S. Photothermal-triggered system for oligonucleotides delivery from cationic gold nanorods surface: A molecular dynamic investigation. Colloids Surf. B Biointerfaces 2021, 201, 111654. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Ruttala, R.R.T.; Tran, T.H.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Mitochondria-targeting multi-metallic ZnCuO nanoparticles and IR780 for efficient photodynamic and photothermal cancer treatments. J. Mater. Sci. Technol. 2021, 86, 139–150. [Google Scholar] [CrossRef]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Prospects for Gold Nanorod Particles in Diagnostic and Therapeutic Applications. Biotechnol. Genet. Eng. Rev. 2008, 25, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttala, H.B.; Ramasamy, T.; Poudel, B.K.; Ruttala, R.R.T.; Jin, S.G.; Choi, H.-G.; Ku, S.-K.; Yong, C.S.; Kim, J.O. Multi-responsive albumin-lonidamine conjugated hybridized gold nanoparticle as a combined photothermal-chemotherapy for synergistic tumor ablation. Acta Biomater. 2019, 101, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.-H.; et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Lal, S.; Verma, J.; Van Noorden, C.J. Nanoparticles for hyperthermic therapy: Synthesis strategies and applications in glioblastoma. Int. J. Nanomed. 2014, 9, 2863–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
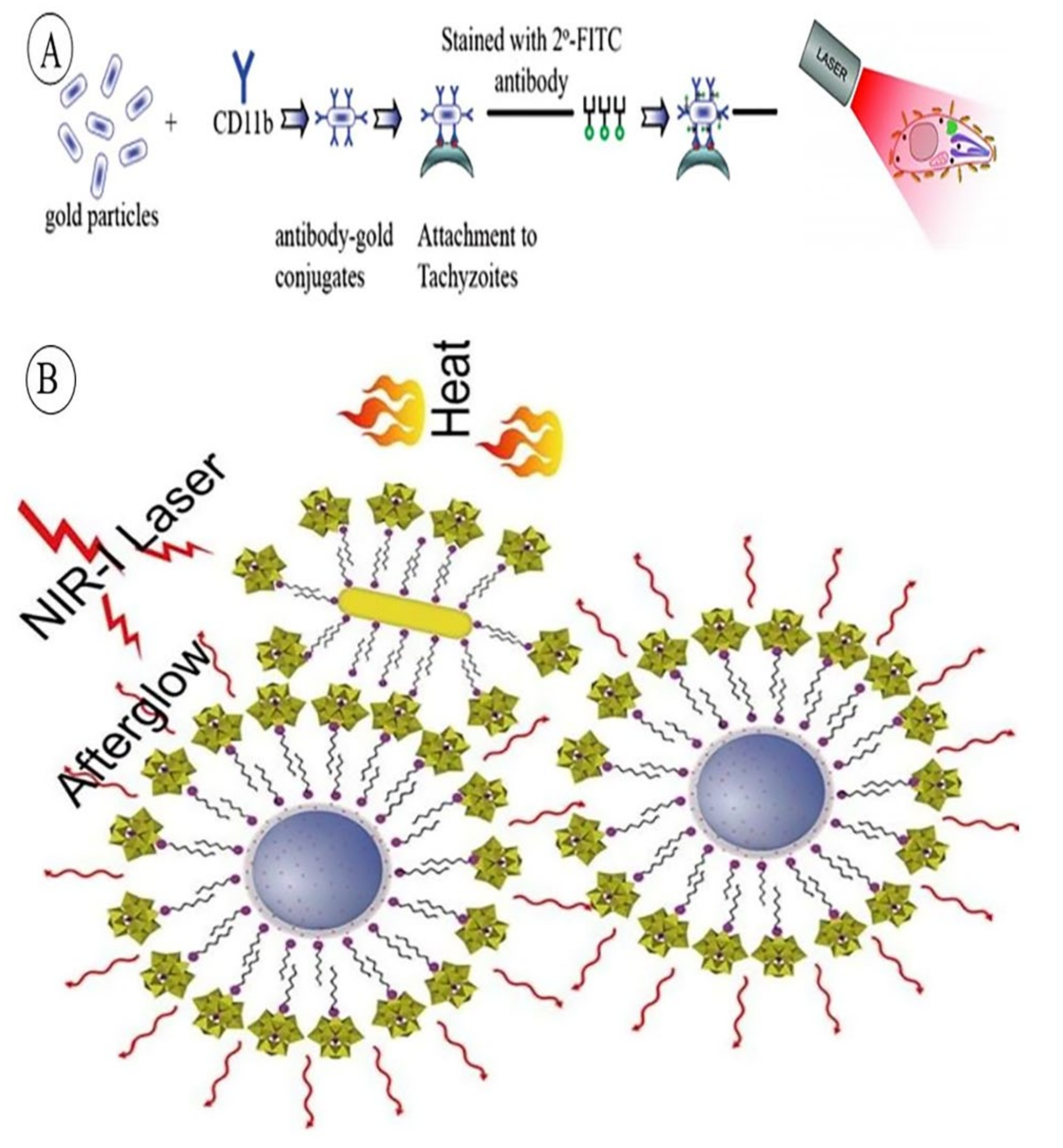
- Pissuwan, D.; Valenzuela, S.M.; Miller, C.M.; Cortie, M.B. A Golden Bullet? Selective Targeting of Toxoplasma gondii Tachyzoites Using Antibody-Functionalized Gold Nanorods. Nano Lett. 2007, 7, 3808–3812. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Youn, Y.S. Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Hooshmand, N.; Upreti, T.; El-Sayed, M.A. Localized Plasmonic Photothermal Therapy as a Life-saving Treatment Paradigm for Hospitalized COVID-19 Patients. Plasmonics 2021, 16, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, J.; Jiang, R.; Wang, S.; Zheng, L.; Wang, G.; Tian, X.; Zhu, H.; Yan, D.; Liu, C.; et al. Biocompatible PLNP-GNR composite nanoplatforms for monitoring deep-tissue photothermal therapy process. Appl. Surf. Sci. 2021, 562, 150189. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Q.; Beziere, N.; Song, L.; Zhang, Q.; Peng, D.; Chi, C.; Yang, X.; Guo, H.; Diot, G.; et al. DNA-Nanostructure-Gold-Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Adv. Mater. 2016, 28, 10000–10007. [Google Scholar] [CrossRef]
- Bhana, S.; O’Connor, R.; Johnson, J.; Ziebarth, J.D.; Henderson, L.; Huang, X. Photosensitizer-loaded gold nanorods for near infrared photodynamic and photothermal cancer therapy. J. Colloid Interface Sci. 2016, 469, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Paraiso, W.K.D.; Tanaka, H.; Sato, Y.; Shirane, D.; Suzuki, N.; Ogra, Y.; Tange, K.; Nakai, Y.; Yoshioka, H.; Harashima, H.; et al. Preparation of envelope-type lipid nanoparticles containing gold nanorods for photothermal cancer therapy. Colloids Surf. B Biointerfaces 2017, 160, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.P.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gür, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R.; et al. Enhanced targeting of invasive glioblastoma cells by peptide-functionalized gold nanorods in hydrogel-based 3D cultures. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacinto, T.A.; Rodrigues, C.F.; Moreira, A.F.; Miguel, S.A.P.; Costa, E.C.; Ferreira, P.; Correia, I.J. Hyaluronic acid and vitamin E polyethylene glycol succinate functionalized gold-core silica shell nanorods for cancer targeted photothermal therapy. Colloids Surf. B Biointerfaces 2020, 188, 110778. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lu, T.; Xu, M.; Yang, L.; Song, Y.; Li, N. SO2 prodrug doped nanorattles with extra-high drug payload for “collusion inside and outside” photothermal/pH triggered-gas therapy. Biomaterials 2020, 257, 120236. [Google Scholar] [CrossRef]
- Collini, E. Cooperative effects to enhance two-photon absorption efficiency: Intra-versus inter-molecular approach. Phys. Chem. Chem. Phys. 2012, 14, 3725–3736. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Ma, X.; Zha, Z.; Shi, L.; Tian, J.; Dai, Z. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials 2014, 35, 5795–5804. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Deng, Z.; Jing, L.; Li, X.; Dai, Z.; Li, C.; Huang, M. Prussian blue nanoparticles operate as a contrast agent for enhanced photoacoustic imaging. Chem. Commun. 2013, 49, 11029–11031. [Google Scholar] [CrossRef]
- Nima, Z.A.; Alwbari, A.M.; Dantuluri, V.; Hamzah, R.N.; Sra, N.; Motwani, P.; Arnaoutakis, K.; Levy, R.A.; Bohliqa, A.F.; Nedosekin, D.; et al. Targeting nano drug delivery to cancer cells using tunable, multi-layer, silver-decorated gold nanorods. J. Appl. Toxicol. 2017, 37, 1370–1378. [Google Scholar] [CrossRef]
- Zeng, J.-Y.; Zhang, M.-K.; Peng, M.-Y.; Gong, D.; Zhang, X.-Z. Porphyrinic Metal-Organic Frameworks Coated Gold Nanorods as a Versatile Nanoplatform for Combined Photodynamic/Photothermal/Chemotherapy of Tumor. Adv. Funct. Mater. 2017, 28. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Liu, J.; Yang, G.; Sun, Z.; Wan, J. Double functional aptamer switch probes based on gold nanorods for intracellular ATP detection and targeted drugs transportation. Sens. Actuators B Chem. 2016, 235, 655–662. [Google Scholar] [CrossRef]
- Newell, B.B.; Wang, Y.; Irudayaraj, J. Multifunctional gold nanorod theragnostics probed by multi-photon imaging. Eur. J. Med. Chem. 2012, 48, 330–337. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, B.; Chen, B.; Wang, J.; Xu, L.; Zhang, S.; Song, H. Multifunctional Au@ mSiO2/rhodamine B isothiocyanate nanocomposites: Cell imaging, photocontrolled drug release, and photothermal therapy for cancer cells. Small 2013, 9, 604–612. [Google Scholar] [CrossRef]
- Niidome, T.; Ohga, A.; Akiyama, Y.; Watanabe, K.; Niidome, Y.; Mori, T.; Katayama, Y. Controlled release of PEG chain from gold nanorods: Targeted delivery to tumor. Bioorganic Med. Chem. 2010, 18, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; You, Q.; Pang, X.; Tan, X.; Wang, J.; Liu, L.; Guo, F.; Tan, F.; Li, N. A photoresponsive and rod-shape nanocarrier: Single wavelength of light triggered photothermal and photodynamic therapy based on AuNRs-capped & Ce6-doped mesoporous silica nanorods. Biomaterials 2017, 122, 188–200. [Google Scholar] [PubMed]
- Choi, J.; Kim, S.Y. Photothermally enhanced photodynamic therapy based on glutathione-responsive pheophorbide a-conjugated gold nanorod formulations for cancer theranostic applications. J. Ind. Eng. Chem. 2020, 85, 66–74. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Ghosh, S.; Ponnusamy, V.K.; Chattopadhyay, S. Graphene oxide as broadband hyperthermic agent and chemo-photothermal dissolution of kidney-stone mimicking calcium oxalate crystals. J. Photochem. Photobiol. A Chem. 2020, 405, 112917. [Google Scholar] [CrossRef]
- Gong, B.; Shen, Y.; Li, H.; Li, X.; Huan, X.; Zhou, J.; Chen, Y.; Wu, J.; Li, W. Thermo-responsive polymer encapsulated gold nanorods for single continuous wave laser-induced photodynamic/photothermal tumour therapy. J. Nanobiotechnol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Luo, L.; Sun, W.; Feng, Y.; Qin, R.; Zhang, J.; Ding, D.; Shi, T.; Liu, X.; Chen, X.; Chen, H. Conjugation of a Scintillator Complex and Gold Nanorods for Dual-Modal Image-Guided Photothermal and X-ray-Induced Photodynamic Therapy of Tumors. ACS Appl. Mater. Interfaces 2020, 12, 12591–12599. [Google Scholar] [CrossRef] [PubMed]

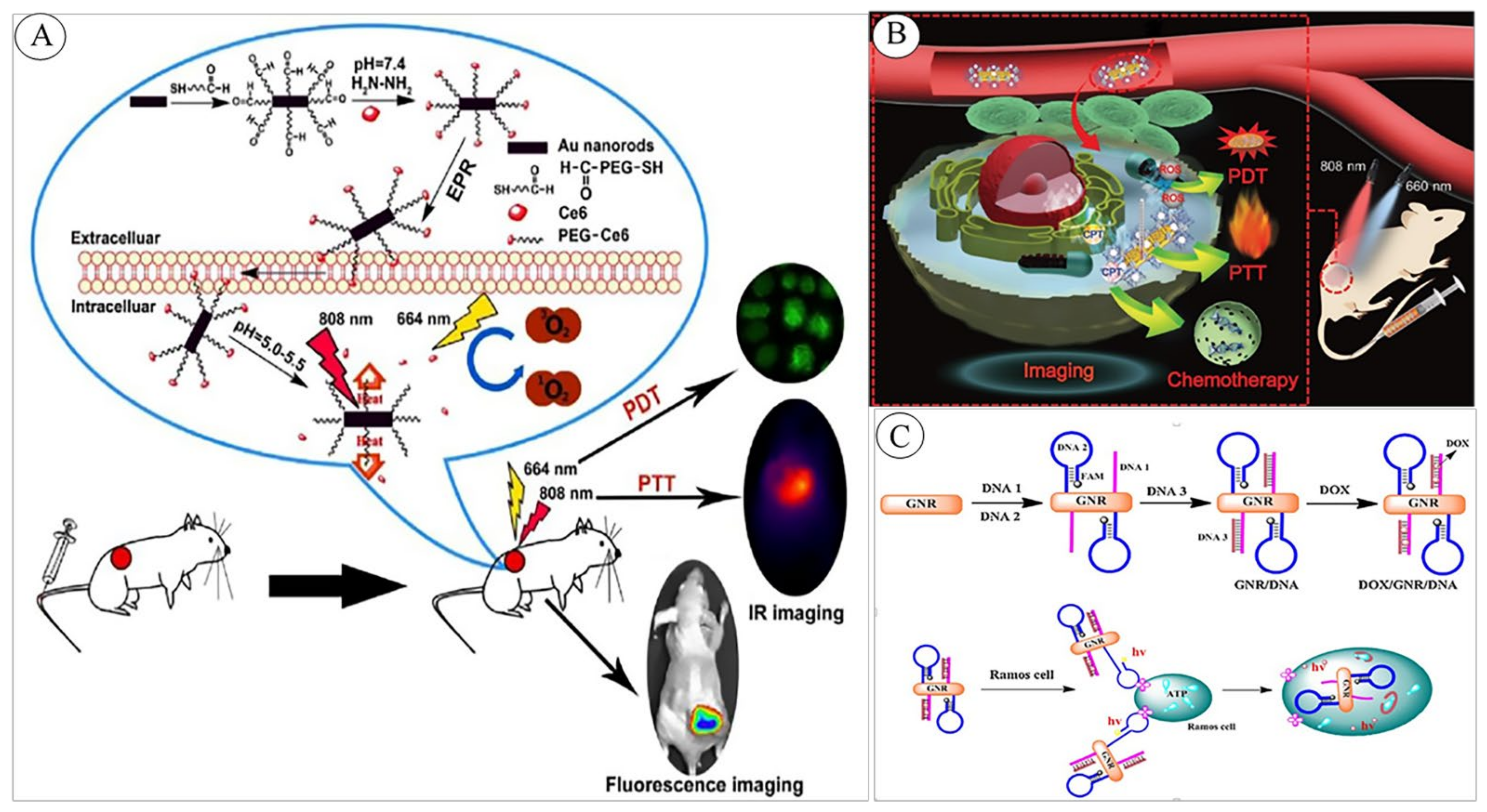
| Purpose | System’s Structure | Mechanism of Function | GNR’s Applied Feature | Ref. |
|---|---|---|---|---|
| DD | Folate targeted GNRs, cDNA and tether platinum [Pt(IV)] | Photothermal hyperthermia and dual drug release with the NIR-laser stimulation | PT | [100] |
| DD | NIR-responsive chromophore, b-cyclodextrins functionalized PEG, DOX-loaded GNRs | NIR-stimulated drug release | PT | [101] |
| DD | Biotin targeted DNA-conjugated GNRs loaded by DOX | NIR and pH-induced drug release | PT | [95] |
| DD | GNR core, magnetic ionic liquid, and DNA moieties shell | Photothermal hyperthermia, drug release | PT | [102] |
| DD, PTT | Hyaluronic acid and RGD (Arg-Gly-Asp)-conjugated silica-coated GNRs, loaded by DOX | Photothermal hyperthermia, pH-enzyme sensitivity, and NIR-triggered drug release | PT | [103] |
| PD | GNRs coated polysaccharide-based hydrogel loaded by fluorescein isothiocyanate-modified ovalbumin (FITC-OVA) | Photothermal hyperthermia and enhanced transdermal FITC-OVA delivery | PT | [96] |
| DD, PTT | Graphene oxide-conjugated GNRs loaded by DOX | Photothermal hyperthermia and NIR-stimulated drug release | PT | [104] |
| DD, PTT | GNRs with glutathione responsive diblock co-polymer micelles loaded by GW627368X | high glutathione concentration-responsive drug release, PTT-induced apoptosis, and necroptosis | PT | [105] |
| DD | GNR core, mesoporous silica/hydroxyapatite shell | NIR- and pH-induced drug release | PT | [106] |
| DD, PTT | DOX-loaded GNRs with a pH-sensitive linkage | pH responsive DOX release, Photothermal hyperthermia | PT | [107] |
| DD, PTT, PDT, Im | Hyaluronic acid (HA) functionalized GNRs, conjugating with 5-aminolevulinic acid (ALA), Cy7.5 and anti-HER2 antibody | HER2 and CD44 targeted system. pH, glutathione, and hyaluronidase-responsive drug release. NIR-stimulated PDT and PTT. | PT | [97] |
| DD | Stimuli-responsive ABC triblock co-polymer coated GNRs loaded by DOX | DOX release in response to pH and thermosensitive ABC triblock co-polymer | St | [108] |
| DD, PTT | Conjugate of dihydroxy phenyl/hydrazide bifunctionalized hydroxyethyl chitosan-GNR, decorated with a pH-sensitive oxidized hyaluronic acid and loaded by DOX | CD44 targeted, pH-responsive drug release, PTT | PT | [109] |
| DD, Im | Au@Ag core–shell nanorod coated by zeolitic imidazolate framework (ZIF-8) with 4-aminothiophenol (4-ATP) as reporter molecule, loaded by DOX | Cell entrance through endocytosis, Enhanced drug’s therapeutic effects | Ca | [110] |
| DD | DOX loaded Chlorella, GNRs and BSA-based Gel | Irradiation of 660 nm and 808 nm for oxygen and heat generation by Chlorella and GNR, respectively | PT | [111] |
| DD | GNRs incorporated with Poly (N-isopropylacrylamide)-co-acrylamide polymer, loaded with a model drug | Photothermal hyperthermia and local drug release | PT | [112] |
| DD | GNR core, mesoporous silica shell, functionalized with d-α tocopherol PEG 1000 succinate and PEI, loaded by DOX | NIR-activated drug release | PT | [113] |
| DD | PEGylated graphene oxide capped-GNR/SiO2 core–shell, loaded by DOX | Dual NIR- and Ph-activated drug release | PT | [114] |
| DD, GD | C(HK)4-mini PEG-RGD peptide-functionalized GNRs | αvβ3 integrin targeted, Cell uptake by RGD (Arg-Gly-Asp) peptide, HK (His-Lys) peptide-conjugated delivery | Ca | [115] |
| DD, PTT | cRGD peptide-modified GNRs loaded by paclitaxel and curcumin | αvβ3 integrin targeted by cRGD, NIR-responsive drug release and tumor growth inhibition by apoptosis | PT | [116] |
| DD, PTT | GNR @ silica coated/poly histidine/d-α-tocopherol PEG succinate/DOX-loaded PEGylated nanocomposite | NIR thermal-/pH-triggered drug release and PTT | PT | [117] |
| DD, PTT | GNRs coated by folate-α,β-poly(N-2 hydroxyethyl)-d,l-aspartamide, loaded by Antineoplastic drug irinotecan (Iri) | Folate-targeted drug release, Photothermal hyperthermia | PT | [118] |
| DD, PTT | GNRs@Kr (Keratine), loaded by DOX | NIR-triggered and redox-responsive drug release | PT | [119] |
| DD, PTT | Hollow H-apatite capped (poly(N-isopropylacrylamide-co-acrylic acid) GNRs-DOX loaded hybrids microcapsules | pH/NIR-responsive DOX release | PT | [120] |
| DD | GNR-polydopamine capped hollow h-apatite hybrid microcapsules | pH/NIR-responsive drug release | PT | [121] |
| DD | GNR-chitosan modified by poly(l-lactide), poly(N-isopropylacrylamide) and poly (acrylamide) micelles, loaded by paclitaxel | light triggered DD and PTT | PT | [122] |
| DD | Alginate/polyacrylamide hydrogel patch -hybridized by polyvinylpyrrolidone- graphene oxide@GNRs | NIR-stimulated thermoresponsive methotrexate and rhodamine B co-delivery | PT | [123] |
| DD | Multi-layer allylamine hydrochloride/poly methacrylic acid capsules –DOX/nimbin co-delivery | NIR-stimulated drugs release | PT | [124] |
| DD, PTT | Folate-modified liposomes encapsulating NF-κB decoy oligodeoxynucleotides, GNRs, and dexamethasone | Therapeutic effects by anti-inflammatory agents delivery and PTT | PT | [125] |
| DD, Th | Core–shell mesoporous silica-coated GNRs, deposited by the metal-organic framework, modified by hyaluronic acid, loaded by DOX | Laser irradiation-mediated imaging and drug release | PA/PT | [126] |
| DD, Im | GNRs coated by poly platinum (polyPt(IV)) | Photothermal hyperthermia, redox-triggered polyPt(IV) release | PA/PT | [127] |
| DD, PTT | PLGA-PEG polymeric corona loaded by vincristine encapsulating silica-coated GNRs | pH-responsive sustained drug release and PTT | PT | [128] |
| DD, PTT, PDT | Hyaluronic acid encapsulated, GNRs coated with mesoporous and degradable silica, loaded with DOX and IR820 (a photosensitizer) | Hyaluronidase and high glutathione concentration-targeted, triple therapy by NIR irradiation (PDT, PTT and chemotherapy) | PT | [129] |
| DD | GNRs decorated Polymethacrylate co-polymers and ciprofloxacin | Targeted delivery to bacteria and biofilms, PT effect and antibiotic delivery | PT | [130] |
| DD, GD | DOX, YAP-siRNA and GNRs loaded cationic liposome | Targeted chemo, PT and gene combination therapy using NIR irradiation | PT | [131] |
| DD | GNRs-doped hydrogel, made of hyaluronic acid, PEG diacrylate/4-vinyl phenylboronic acid and Astragaloside IV nanoparticles | Sustained drug release, as conductive as native myocardium | Co | [99] |
| DD, PTT | DOX, Mesoporous silica-capped GNRs covered with selenium nanoparticles shell (Se@Au@mSiO2) | Chemo-drug, selenium nanoparticles as anticancer agent, and PTT ability of GNRs as cancer therapy system | PT | [132] |
| Purpose | System’s Structure | Mechanism of Function | Ref. |
|---|---|---|---|
| TD, PTT | CD11b antibody @ GNRs | NIR-induced targeted protozoan cell (tachyzoites) death | [155] |
| PTT | BSA-coated GNRs-laden-macrophages | RAW 264.7 macrophages, hepatic satellite cells (HSCs), HepG2 photo-induced cell damage, leading to lower tumor recurrence | [159] |
| PTT, PDT | GNRs @ silicon 2,3-naphthalocyanine dihydroxide stabilized with alkylthiol-conjugated polyethylene glycol | PD & PT synergistic therapy, using a single specific laser wavelength | [160] |
| PTT | Encapsulated GNRs by lipid-based envelope | Good cellular uptake, enhanced NIR-induced PTT, caused cancer cells apoptosis | [161] |
| TD, PTT | Virus inspired therapeutic system (RVG 29-binded PEG-SiO2@ GNRs) | Rabies virus glycoprotein (RVG)-guided for BBB crossing, brain glioma targeted PTT | [156] |
| TD, Cancer PTT | GNRs combined with a Nestin binding peptide | PT-induced Nestin positive of Glioblastoma Multiform tumors cell apoptosis | [162] |
| PTT | Silica coated Au-TPGS (vitamin E polyethylene glycol succinate)-HA co-polymer | NIR-induced thermal cancer cell death | [163] |
| TD, PTT | ACE-2-functionalized GNRs | NIR-induced thermal SARS-CoV-2 cell damage | [157] |
| Gas synchronous PTT | GNR- polydopamine (PDA) core–shell nanostructure doped by Benzothiazole sulfinate (BTS) | pH triggered BTS (SO2 prodrug) release, resulting in deep tumor gas therapy and PTT | [164] |
| PTT | PLNP-GNR nanocomposite platforms consisting of ZGGO:Cr3+@CTAB@PW12 and GNR@CTAB, PW12- encapsulated | Persistent luminescent nanoparticles combined with GNRs as biocompatible platform for photo-induced therapy. | [158] |
| Purpose | System’s Structure | Mechanism of Function | GNR’s Applied Feature | Ref. |
|---|---|---|---|---|
| DD, TRK | GNRs@folic acid as the targeting ligand the anthracycline drug, DOX | Targeted drug delivery to cancerous cells (expressing folate receptor cells), fluorescence lifetime imaging, using photoluminescence of GNRs and the innate fluorescence of DOX | PL | [174] |
| DD, TRK | Au@mesoporous SiO2/rhodamine B isothiocyanate (Au@mSiO2/RBITC) nanocomposite | Monitoring the photothermal therapy, drug release, cell tracking using an 808 nm laser and a confocal laser scanning microscopy system | PT | [175] |
| DD, In situ ATP TRK | DNA-functionalized GNRs loaded DOX | Targeted drug delivery, drug release in response to aptamer–ATP interaction, tumor growth inhibition and increasing fluorescence emission of DOX proportional to ATP concentration | PT | [173] |
| PTT and Im | PEG-peptide-modified GNRs containing peptide substrate, overexpressed in malignant tumor cells | Formation of GNR aggregates in response to uPA activity and PEG-chains release and absorption reduction in tumor’s site | PT | [176] |
| PTT, PDT, Im | Ce6-doped mesoporous silica- nanorods (AuNRs-Ce6-MSNRs) | Generation of hyperthermia by GNRs (PTT) and singlet oxygen (1O2) production by Ce6-mediated PDT. Dual-imaging by the photoacoustic and NIR-induced fluorescence of AuNRs & Ce6 | PT/PA | [177] |
| Th | chlorin e6 (Ce6)-PEG-GNR | PTT and PDT, using pH-responsive Ce6 (fluorescent tag)-based local tumor tracking and dual IR imaging | PT/PDT | [72] |
| PTT, PDT, Im | AuNR@MOFs nanocomposite loaded CPT | photothermal-induced CPT release and photodynamic combinational therapy | PT/PDT/PA | [172] |
| Th (Im, PDT & PTT synergy/TD | Pheophorbide-responsive glutathione functionalized GNRs, conjugated folic acid-PEG co-polymer | Light triggered imaging and NIR-induced synergistic therapeutic | PT/PDT | [178] |
| Im, PTT | Graphene oxide (GO)-GNR contrasting thermal conductivity capacity and heating therapy. | NIR-induced imaging and thermal stone dissolution | PA/PT | [179] |
| Th (Im, PDT, PTT, TRK) | (PNIPAM) coated SiO2@GNR loaded ICG (nanocom-ICG), thermal-induced targeted release for tumor therapy | NIR-induced tracking and enhancing the thermal release of ICG-mediated photodynamic therapy | PA/PT | [180] |
| Th | Eu@SiO2@GNR complex as multiplexed PT agent, photo and radio sensitizer | Image-Guided Photothermal and X-ray-induced Photodynamic Therapy of Tumors | PT/PDT/PA | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangiri-Manesh, A.; Mousazadeh, M.; Taji, S.; Bahmani, A.; Zarepour, A.; Zarrabi, A.; Sharifi, E.; Azimzadeh, M. Gold Nanorods for Drug and Gene Delivery: An Overview of Recent Advancements. Pharmaceutics 2022, 14, 664. https://doi.org/10.3390/pharmaceutics14030664
Jahangiri-Manesh A, Mousazadeh M, Taji S, Bahmani A, Zarepour A, Zarrabi A, Sharifi E, Azimzadeh M. Gold Nanorods for Drug and Gene Delivery: An Overview of Recent Advancements. Pharmaceutics. 2022; 14(3):664. https://doi.org/10.3390/pharmaceutics14030664
Chicago/Turabian StyleJahangiri-Manesh, Atieh, Marziyeh Mousazadeh, Shirinsadat Taji, Abbas Bahmani, Atefeh Zarepour, Ali Zarrabi, Esmaeel Sharifi, and Mostafa Azimzadeh. 2022. "Gold Nanorods for Drug and Gene Delivery: An Overview of Recent Advancements" Pharmaceutics 14, no. 3: 664. https://doi.org/10.3390/pharmaceutics14030664
APA StyleJahangiri-Manesh, A., Mousazadeh, M., Taji, S., Bahmani, A., Zarepour, A., Zarrabi, A., Sharifi, E., & Azimzadeh, M. (2022). Gold Nanorods for Drug and Gene Delivery: An Overview of Recent Advancements. Pharmaceutics, 14(3), 664. https://doi.org/10.3390/pharmaceutics14030664








