Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure of Hot Melt Extrusion Runs
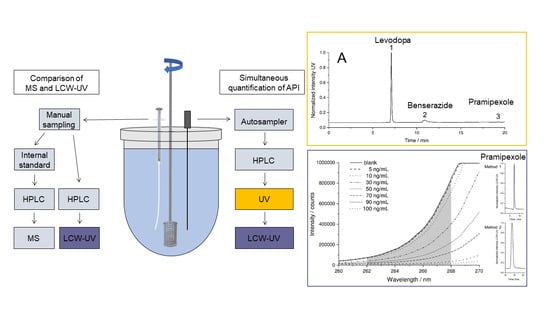
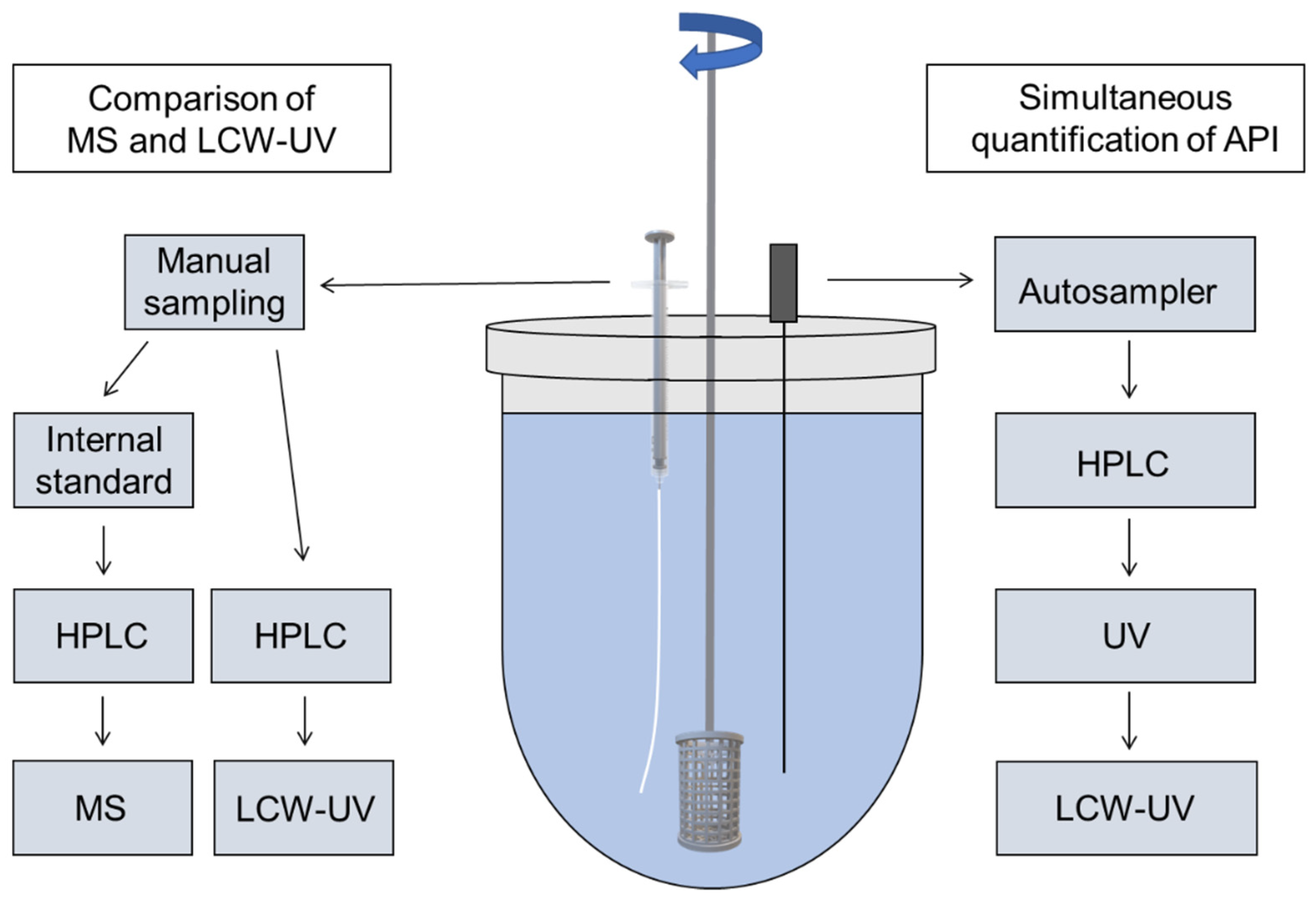
2.2. Dissolution Testing
2.3. LC-MS/MS Quantification of Pramipexole during Dissolution
2.4. Chromatographic Conditions for UV and LCW-UV Measurements
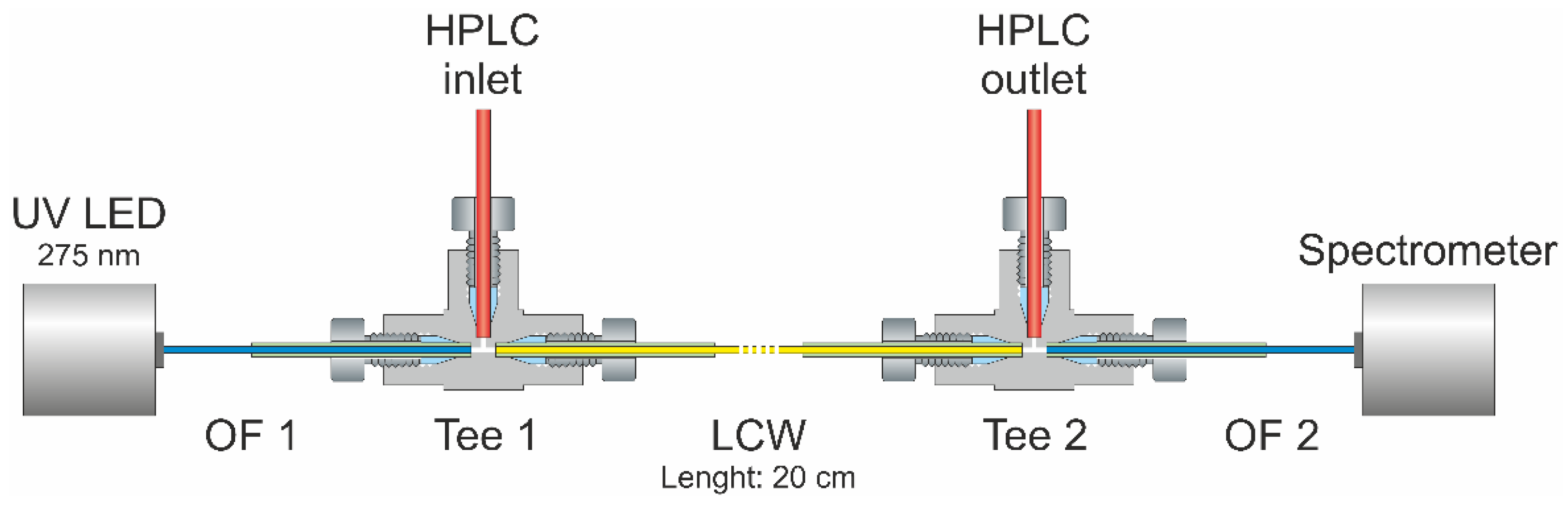
2.5. Liquid-Core Waveguide Ultraviolet Detection System (LCW-UV)
2.6. Mathematical Description
3. Results and Discussion
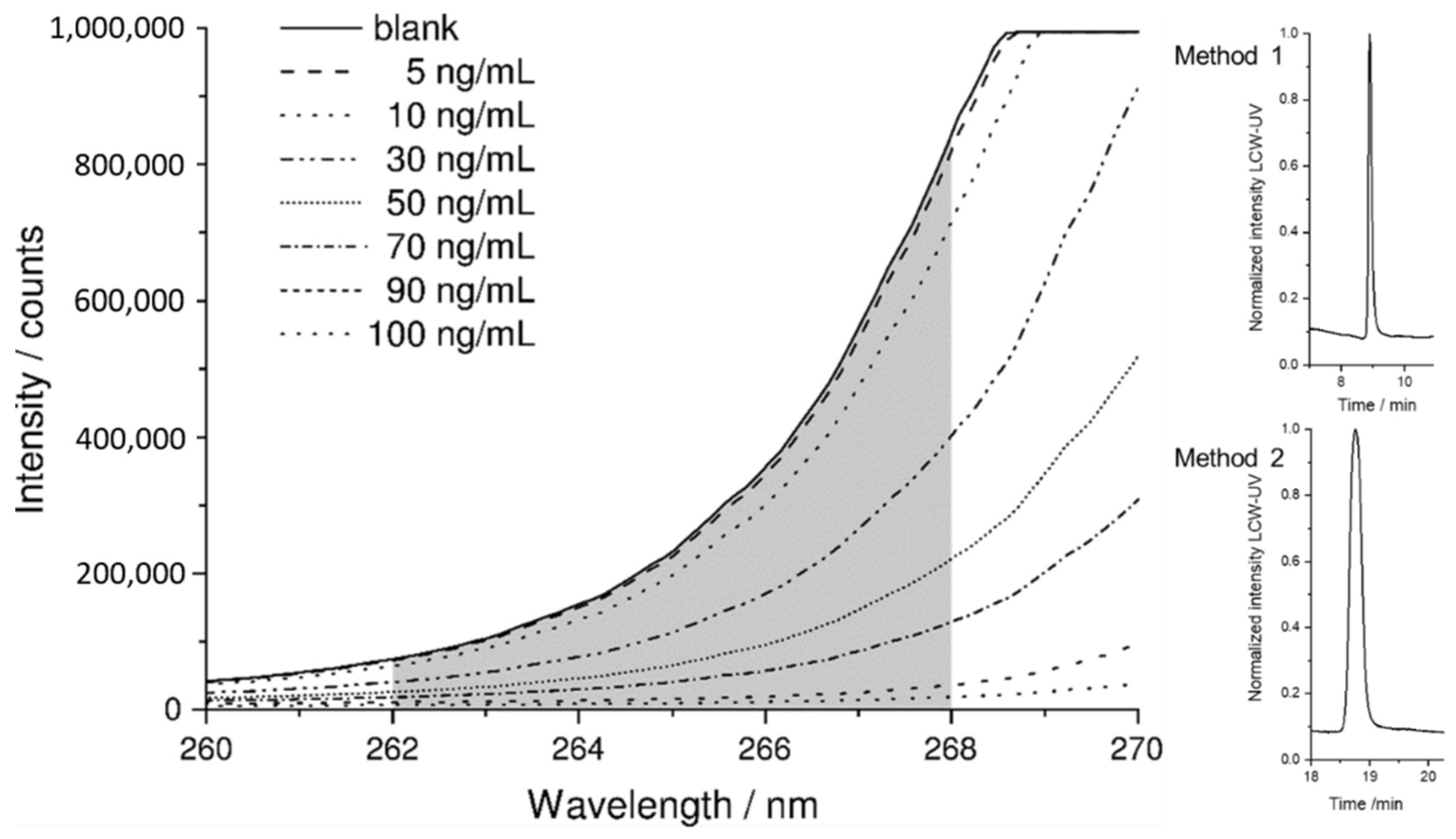
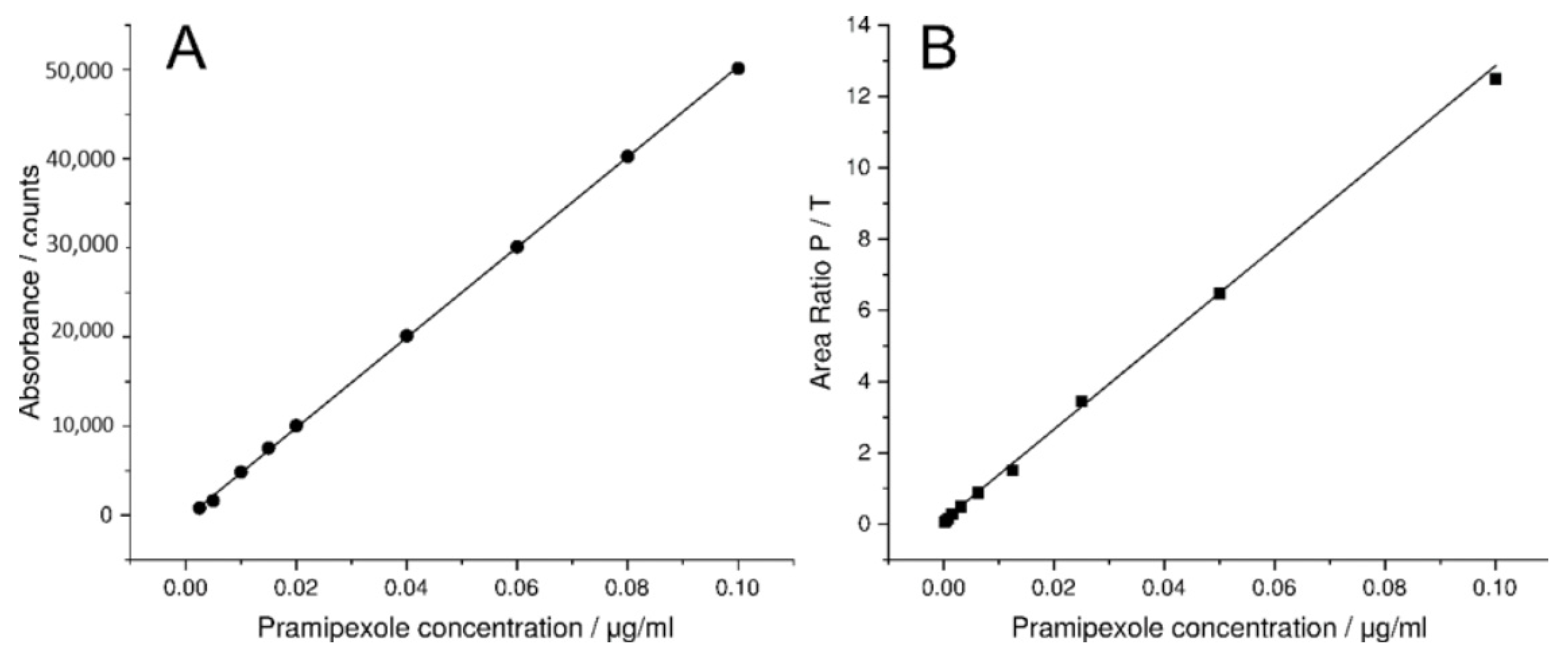
3.1. Spectroscopic Evaluation of LCW-UV Measurements
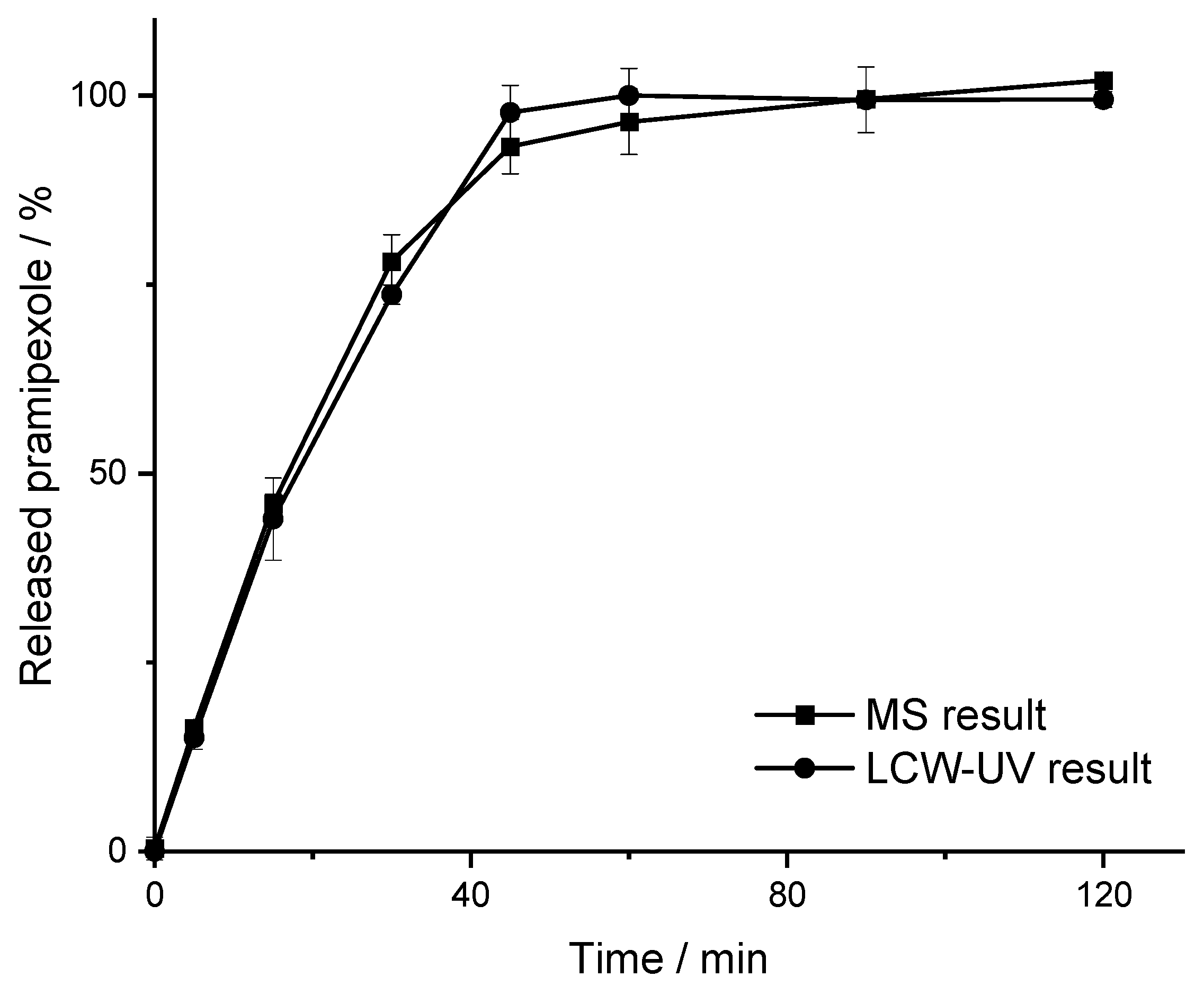
3.2. MS and LCW-UV Measurement in Comparison
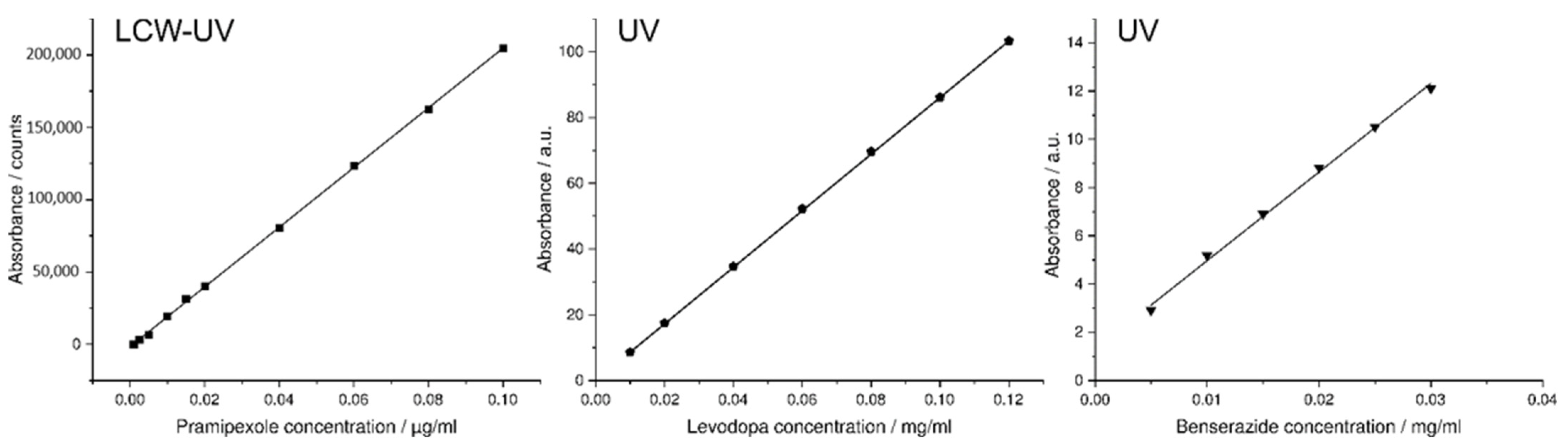
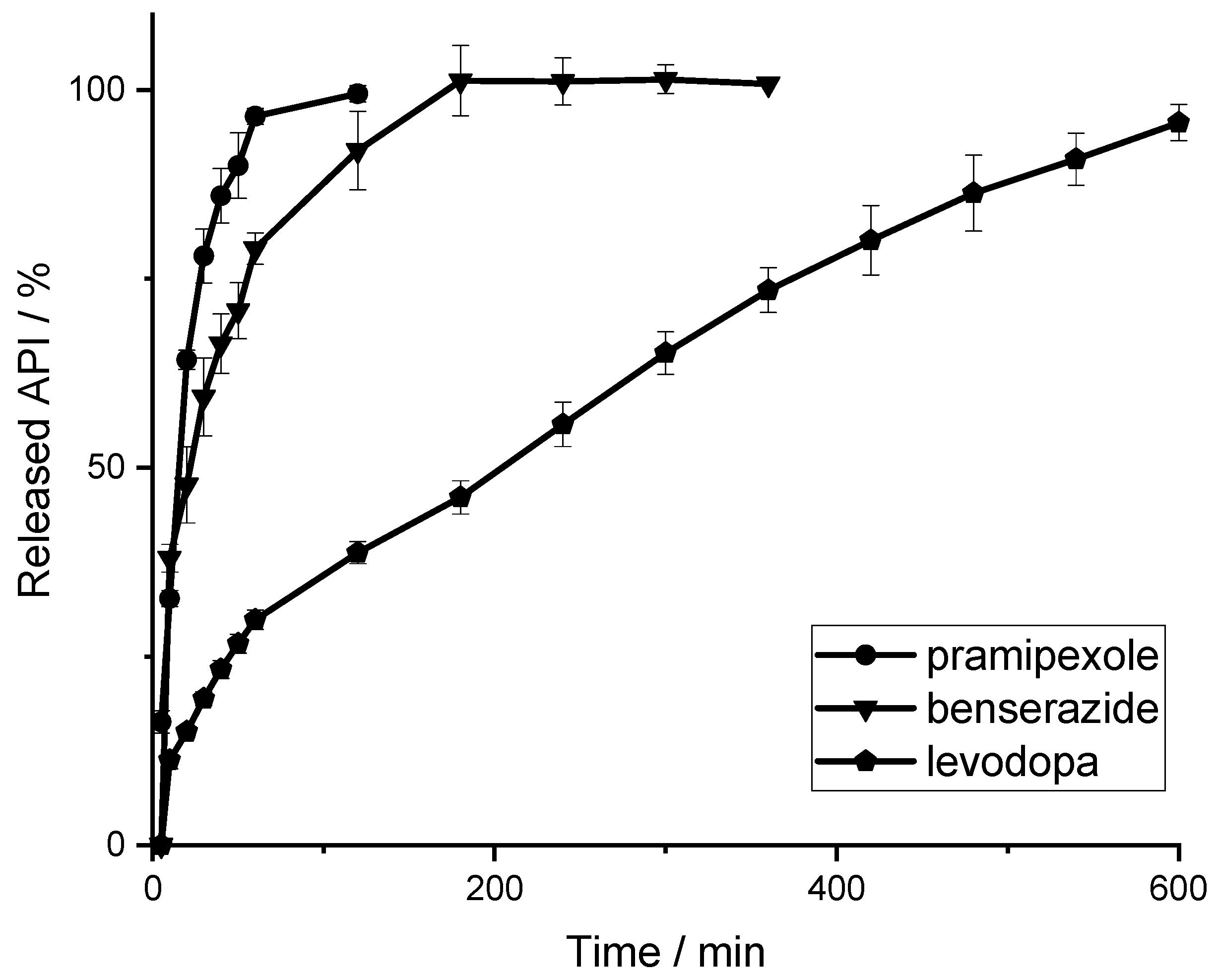
3.3. Simultaneous Quantification of Levodopa, Benserazide and Pramipexole in Dissolution Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | active pharmaceutical ingredient |
| BZ | benserazide |
| EVA | poly (ethylene-vinyl acetate)-copolymer |
| DAD | diode array detection |
| HME | hot melt extrusion |
| HPLC | high-performance liquid chromatography |
| LCW | liquid-core waveguide |
| LD | levodopa |
| LLOQ | lower limit of quantification |
| MDT | mean dissolution time |
| MS | mass spectrometry |
| P | pramipexole |
| PVA | polyvinyl alcohol |
| SD | standard deviation |
| S/N | signal-to-noise ratio |
| UV | ultraviolet |
| VA | poly (vinylpyrrolidone-vinyl acetate)-copolymer |
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Guglietta, A.; Guerrero, M. Issues to consider in the pharmaceutical development of a cardiovascular polypill. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Combination Products. Available online: https://www.fda.gov/combination-products/about-combination-products (accessed on 7 February 2022).
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single-and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Dose-independent Drug Release from 3D Printed Oral Medicines for Patient-specific Dosing to Improve Therapy Safety. Int. J. Pharm. 2022, 616, 121555. [Google Scholar] [CrossRef]
- Scheubel, E.; Lindenberg, M.; Beyssac, E.; Cardot, J.M. Small volume dissolution testing as a powerful method during pharmaceutical development. Pharmaceutics 2010, 2, 351–363. [Google Scholar] [CrossRef]
- Timed-Release Tablets and Capsules—In Vitro Test Procedure. In National Formulary XIV; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1975.
- Dissolution Methods for Drug Products Database, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2008. Available online: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults_Dissolutions.cfm?PrintAll=1 (accessed on 7 February 2022).
- European Pharmacopoeia Commission 5.17.1. Recommendations on Dissolution Testing. In European Pharmacopoeia; EDQM: Strasbourg, France, 2020; Volume 10.2, pp. 801–807. [Google Scholar]
- Pedersen, B.L.; Brøndsted, H.; Lennernäs, H.; Christensen, F.N.; Müllertz, A.; Kristensen, H.G. Dissolution of hydrocortisone in human and simulated intestinal fluids. Pharm. Res. 2000, 17, 183–189. [Google Scholar] [CrossRef]
- Wang, B.; Armenante, P.M. Experimental and computational determination of the hydrodynamics of mini vessel dissolution testing systems. Int. J. Pharm. 2016, 510, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Klein, S. The Mini Paddle Apparatus—A Useful Tool in the Early Development Stage? Experiences with Immediate-Release Dosage Forms. Dissolution Technol. 2006, 13, 6–11. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.E. Comparative LC–MS and HPLC analyses of selected antiepileptics and beta-blocking drugs. Il Farm. 2000, 55, 136–145. [Google Scholar] [CrossRef]
- Neilson, A.P.; Green, R.J.; Wood, K.V.; Ferruzzi, M.G. High-throughput analysis of catechins and theaflavins by high performance liquid chromatography with diode array detection. J. Chromatogr. 2006, 1132, 132–140. [Google Scholar] [CrossRef]
- Li, Q.; Morris, K.J.; Dasgupta, P.K.; Raimundo, I.M., Jr.; Temkin, H. Portable flow-injection analyzer with liquid-core waveguide based fluorescence, luminescence, and long path length absorbance detector. Anal. Chim. Acta 2003, 479, 151–165. [Google Scholar] [CrossRef]
- Kottke, D.; Burckhardt, B.B.; Breitkreutz, J.; Fischer, B. Application and validation of a coaxial liquid core waveguide fluorescence detector for the permeation analysis of desmopressin acetate. Talanta 2021, 226, 122145. [Google Scholar] [CrossRef]
- LeWitt, P.A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 2015, 30, 64–72. [Google Scholar] [CrossRef]
- Rinne, U.K.; Mölsä, P. Levodopa with benserazide or carbidopa in Parkinson disease. Neurology 1979, 29, 1584–1589. [Google Scholar] [CrossRef]
- Bennett, J.P., Jr.; Piercey, M.F. Pramipexole—A new dopamine agonist for the treatment of Parkinson’s disease. J. Neurol. Sci. 1999, 163, 25–31. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Shelor, C.P.; Kadjo, A.F.; Kraiczek, K.G. Flow-Cell-Induced Dispersion in Flow-through Absorbance Detection Systems: True Column Effluent Peak Variance. Anal. Chem. 2018, 90, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Tao, S. Intrinsic UV absorption spectrometry observed with a liquid core waveguide as a sensor technique for monitoring ozone in water. Analyst 2011, 136, 3335–3342. [Google Scholar] [CrossRef]
- Pan, J.-Z.; Yao, B.; Fang, Q. Hand-held Photometer Based on Liquid-Core Waveguide Absorption Detection for Nanoliter-scale Samples. Anal. Chem. 2010, 82, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Robles, T.; Paige, D.; Anastasio, C. Lens-coupled liquid core waveguide for ultraviolet-visible absorption spectroscopy. Rev. Sci. Instrum. 2006, 77, 073103. [Google Scholar] [CrossRef]
- Li, J.; Dasgupta, P.K. Selective Measurement of Gaseous Hydrogen Peroxide with Light Emitting Diode-Based Liquid-Core Waveguide Absorbance Detector. Anal. Sci. 2003, 19, 517–523. [Google Scholar] [CrossRef][Green Version]
- Byrne, R.H.; Kaltenbacher, E. Use of liquid core waveguides for long pathlength absorbance spectroscopy: Principles and practice. Limnol. Oceanogr. 2001, 46, 740–742. [Google Scholar] [CrossRef]
- D’Sa, E.J.; Steward, R.G.; Vodacek, A.; Blough, N.V.; Phinney, D. Determining optical absorption of colored dissolved organic matter in seawater with a liquid capillary waveguide. Limnol. Oceanogr. 1999, 44, 1142–1148. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ito, S. Application of waveguiding in solutions for absorption and fluorescence spectrometry. Trends Anal. Chem. 1991, 10, 184–190. [Google Scholar] [CrossRef]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2021, 1–19. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission 2.9.3. Dissolution Test for Solid Dosage Forms. In European Pharmacopoeia; EDQM: Strasbourg, France, 2020; Volume 10.2, pp. 326–333. [Google Scholar]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting Drug Release from 3D Printed Oral Medicines Based on the Surface Area to Volume Ratio of Tablet Geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Yordanov, Y.; Aluani, D.; Spassova, I.; Kovacheva, D. Development and In Vitro safety evaluation of pramipexole-loaded Hollow Mesoporous Silica (HMS) particles. Biotechnol. Equip. 2019, 33, 1204–1215. [Google Scholar] [CrossRef]
- Dinç, E.; Kaya, S.; Doganay, T.; Baleanu, D. Continuous wavelet and derivative transforms for the simultaneous quantitative analysis and dissolution test of levodopa–benserazide tablets. J. Pharm. Biomed. Anal. 2007, 44, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Krisai, K.; Charnvanich, D.; Chongcharoen, W. Increasing the solubility of levodopa and carbidopa using ionization approach. Thai. J. Pharm. Sci. 2020, 44, 251–255. [Google Scholar]
- Wang, W.; He, Q.; Wang, T.; Fen, M.; Liao, Y.; Ran, G. Absorbance study of liquid-core optical fibers in spectrophotometry. Anal. Chem. 1992, 64, 22–25. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Guidance for Industry—Dissolution Testing of Immediate Release Solid Oral Dosage Forms; FDA Center for Drug Evaluation and Research: Silver Spring, MD, USA, 1997; Volume 1, pp. 1–17.
- Chamberlain, R.; Windolf, H.; Geissler, S.; Quodbach, J.; Breitkreutz, J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics 2022, 14, 216. [Google Scholar] [CrossRef]
- Wollmer, E.; Klein, S. Development and validation of a robust and efficient HPLC method for the simultaneous quantification of levodopa, carbidopa, benserazide and entacapone in complex matrices. Int. J. Pharm. Sci. 2017, 20, 258–269. [Google Scholar] [CrossRef]

| APIs and Excipients | Concentration in % (w/w) | Function |
|---|---|---|
| Formulation 1 (PVA-P): | ||
| Pramipexole 2 HCl·H2O (P) | 0.5 | API |
| Polyvinyl alcohol (PVA) | 99.5 | matrix |
| Formulation 2 (EVA-LD-BZ): | ||
| Levodopa (LD) | 40 | API |
| Benserazide HCl (BZ) | 10 | API |
| Poly (ethylene-vinyl acetate)-copolymer (82:18) (EVA) | 35 | matrix |
| Poly (vinylpyrrolidone-vinyl acetate)-copolymer (60:40) (VA) | 15 | pore former |
| Temperature Profile in Zones 2–10/°C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| PVA-P formulation | 30 | 100 | 180 | 180 | 180 | 180 | 180 | 195 | 195 |
| EVA-LD-BZ formulation | 30 | 180 | 190 | 200 | 220 | 220 | 220 | 220 | 220 |
| Screw Configuration (Die–Gear) | |||||||||
| PVA-P/ EVA-LD-BZ formulation | die–10 CE 1 L/D–KZ 1: 5 × 60°–3 × 30°–5 CE 1 L/D–KZ 2: 4 × 90°–5 × 60°–3 × 30°–16 CE 1 L/D–2 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamberlain, R.; Windolf, H.; Burckhardt, B.B.; Breitkreutz, J.; Fischer, B. Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release. Pharmaceutics 2022, 14, 639. https://doi.org/10.3390/pharmaceutics14030639
Chamberlain R, Windolf H, Burckhardt BB, Breitkreutz J, Fischer B. Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release. Pharmaceutics. 2022; 14(3):639. https://doi.org/10.3390/pharmaceutics14030639
Chicago/Turabian StyleChamberlain, Rebecca, Hellen Windolf, Bjoern B. Burckhardt, Jörg Breitkreutz, and Björn Fischer. 2022. "Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release" Pharmaceutics 14, no. 3: 639. https://doi.org/10.3390/pharmaceutics14030639
APA StyleChamberlain, R., Windolf, H., Burckhardt, B. B., Breitkreutz, J., & Fischer, B. (2022). Embedding a Sensitive Liquid-Core Waveguide UV Detector into an HPLC-UV System for Simultaneous Quantification of Differently Dosed Active Ingredients during Drug Release. Pharmaceutics, 14(3), 639. https://doi.org/10.3390/pharmaceutics14030639