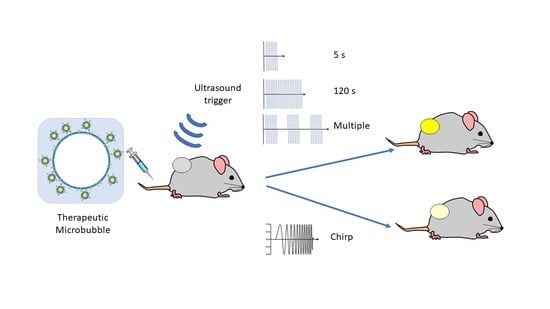
A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tumour Generation
2.3. Therapeutic Microbubble Generation
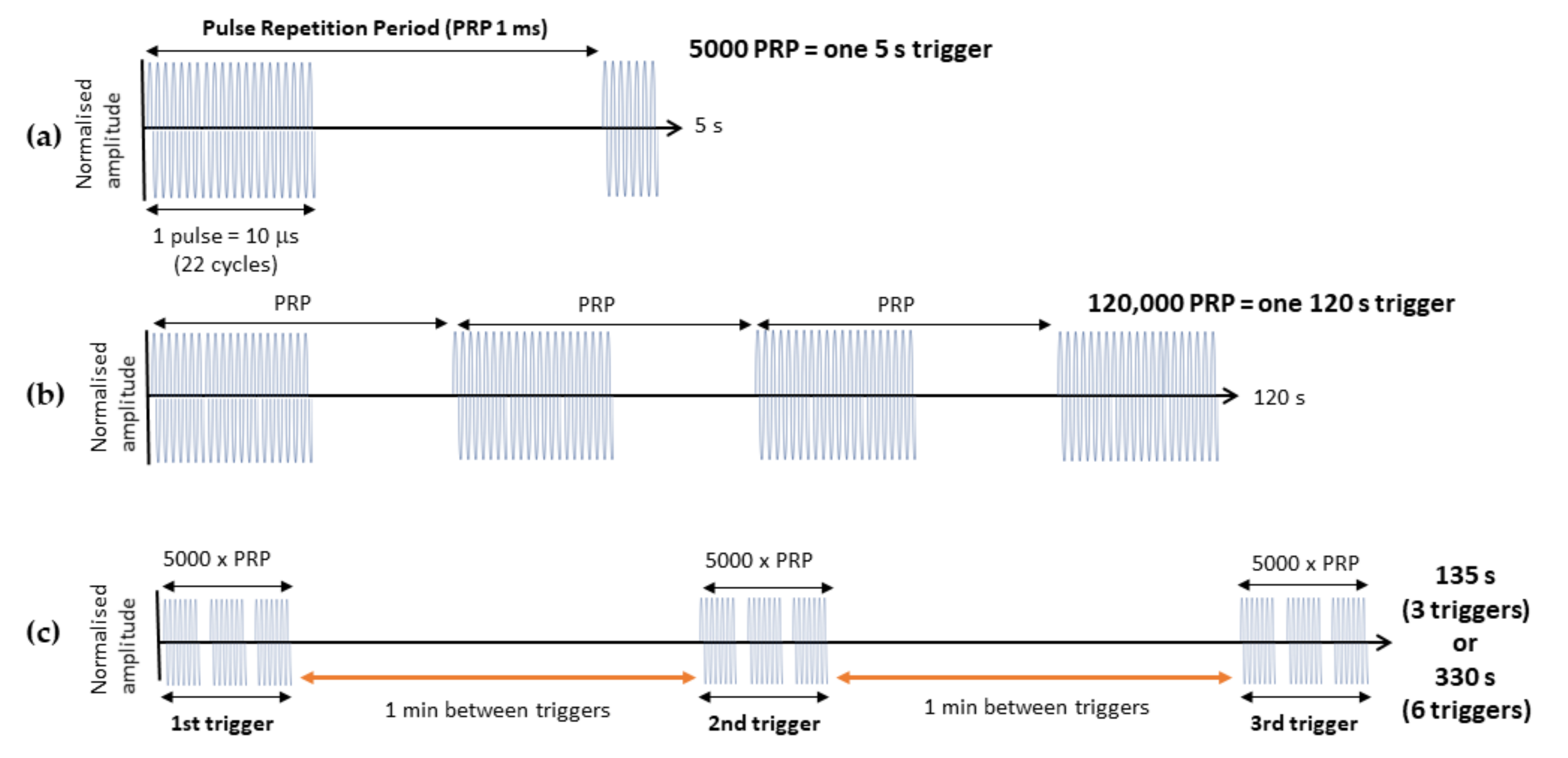
2.4. Delivery of thMBs and Ultrasound Pulses
2.5. Tissue Processing
2.6. Mass Spectrometry for Quantitating Drug in Tissue
2.7. Statistical Analyses
3. Results
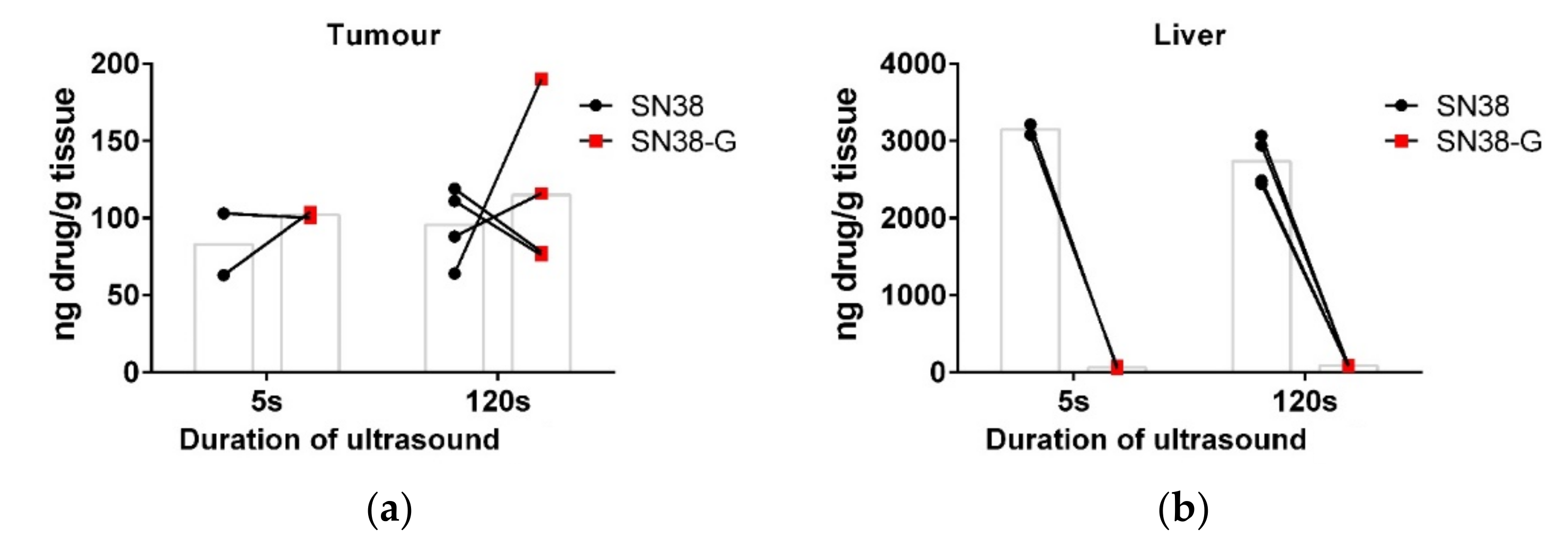
3.1. Increasing the Ultrasound Sonication Duration
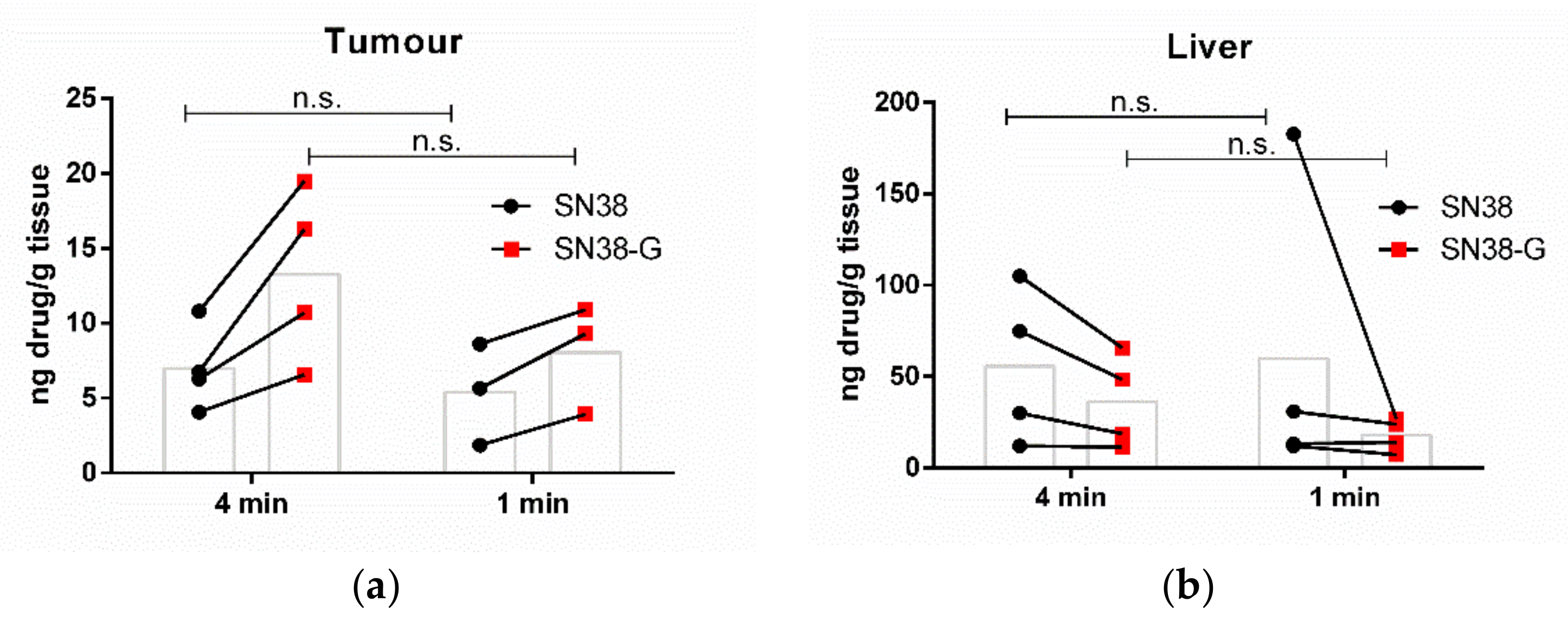
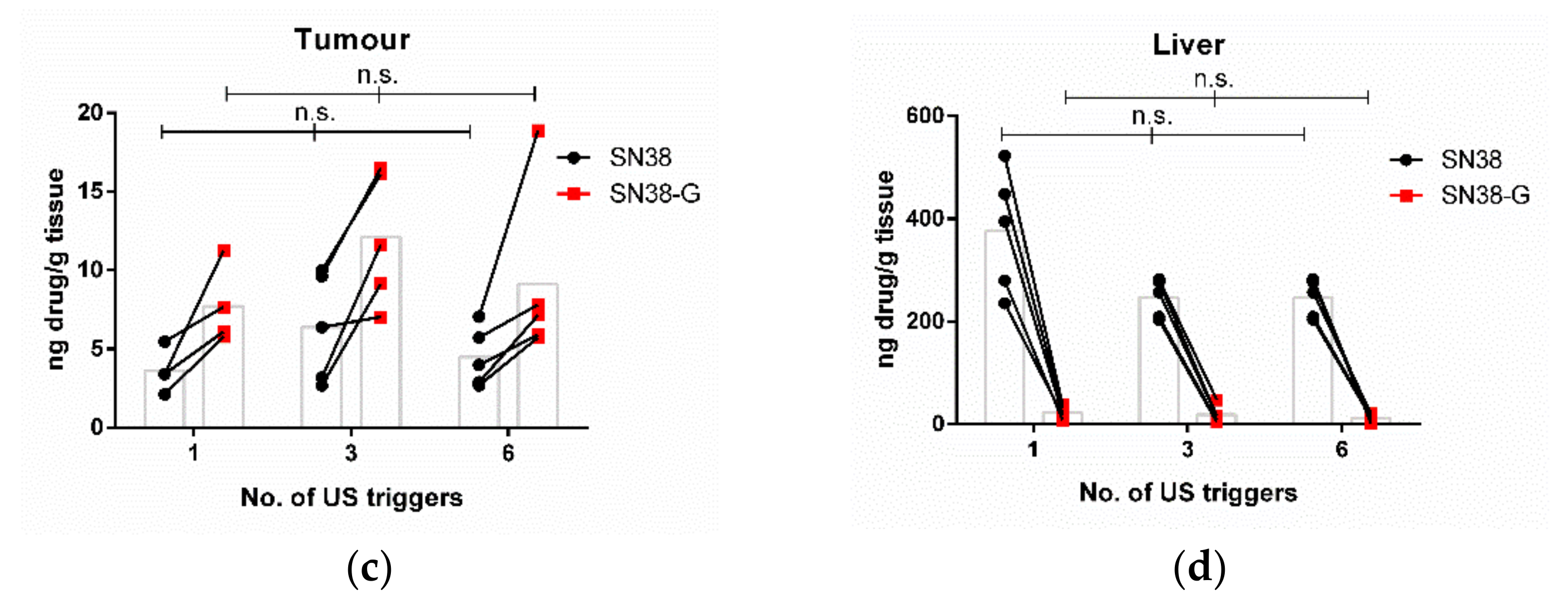
3.2. Increasing the Number of Ultrasound Triggers
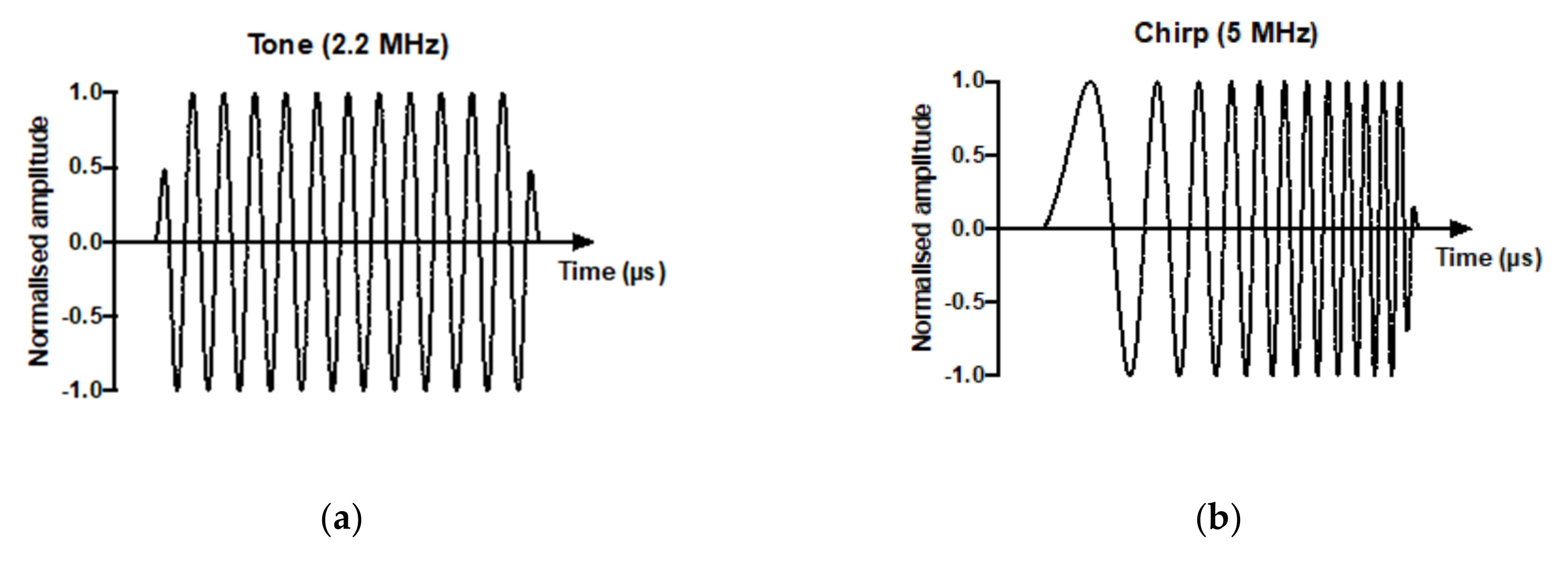
3.3. Drug Delivery and Metabolism Using a Tone Burst Trigger Versus a Chirp Trigger
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Rivory, L. Pharmacology of irinotecan. Drugs Today 1998, 34, 777–803. [Google Scholar] [CrossRef] [PubMed]
- Bala, V.; Rao, S.; Boyd, B.J.; Prestidge, C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J. Control Release 2013, 172, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Lajoinie, G.; Luan, Y.; Gelderblom, E.; Dollet, B.; Mastik, F.; Dewitte, H.; Lentacker, I.; de Jong, N.; Versluis, M. Non-spherical oscillations drive the ultrasound-mediated release from targeted microbubbles. Commun. Phys. 2018, 1, 22. [Google Scholar] [CrossRef]
- Luan, Y.; Lajoinie, G.; Gelderblom, E.; Skachkov, I.; van der Steen, A.F.; Vos, H.J.; Versluis, M.; De Jong, N. Lipid shedding from single oscillating microbubbles. Ultrasound Med. Biol. 2014, 40, 1834–1846. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Shih, R.; Bardin, D.; Martz, T.D.; Sheeran, P.S.; Dayton, P.A.; Lee, A.P. Flow-focusing regimes for accelerated production of monodisperse drug-loadable microbubbles toward clinical-scale applications. Lab Chip 2013, 13, 4816–4826. [Google Scholar] [CrossRef]
- Song, K.H.; Fan, A.C.; Brlansky, J.T.; Trudeau, T.; Gutierrez-Hartmann, A.; Calvisi, M.L.; Borden, M.A. High Efficiency Molecular Delivery with Sequential Low-Energy Sonoporation Bursts. Theranostics 2015, 5, 1419–1427. [Google Scholar] [CrossRef]
- Kamimura, H.A.; Wang, S.; Wu, S.Y.; Karakatsani, M.E.; Acosta, C.; Carneiro, A.A.; Konofagou, E.E. Chirp- and random-based coded ultrasonic excitation for localized blood-brain barrier opening. Phys. Med. Biol. 2015, 60, 7695–7712. [Google Scholar] [CrossRef]
- McLaughlan, J.; Ingram, N.; Smith, P.R.; Harput, S.; Coletta, P.L.; Evans, S.; Freear, S. Increasing the sonoporation efficiency of targeted polydisperse microbubble populations using chirp excitation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2511–2520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cool, S.K.; Geers, B.; Roels, S.; Stremersch, S.; Vanderperren, K.; Saunders, J.H.; De Smedt, S.C.; Demeester, J.; Sanders, N.N. Coupling of drug containing liposomes to microbubbles improves ultrasound triggered drug delivery in mice. J. Control Release 2013, 172, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Mannaris, C.; Geers, B.; Novell, A.; Lentacker, I.; Averkiou, M.; Bouakaz, A. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; Foppen-Harteveld, M.; van der Steen, A.F.; de Jong, N. Sonoporation of endothelial cells by vibrating targeted microbubbles. J. Control Release 2011, 154, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Dimcevski, G.; Gilja, O.H.; Hoem, D.; Postema, M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med. Phys. 2013, 40, 072902. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjanes, T.; Hoem, D.; Schjott, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control Release 2016, 243, 172–181. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Delalande, A.; Popa, M.; Mamaeva, V.; Dimcevski, G.; Gilja, O.H.; Postema, M.; Gjertsen, B.T.; McCormack, E. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol. Imaging Biol. 2014, 16, 53–62. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin. J. Cancer Res. 2018, 30, 553–563. [Google Scholar] [CrossRef]
- Morse, S.V.; Pouliopoulos, A.N.; Chan, T.G.; Copping, M.J.; Lin, J.; Long, N.J.; Choi, J.J. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology 2019, 291, 459–466. [Google Scholar] [CrossRef]
- Morse, S.V.; Mishra, A.; Chan, T.G.; de Rosales, R.T.M.; Choi, J.J. Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J. Control Release 2022, 341, 605–615. [Google Scholar] [CrossRef]
- Amate, M.; Goldgewicht, J.; Sellamuthu, B.; Stagg, J.; Yu, F.T.H. The effect of ultrasound pulse length on microbubble cavitation induced antibody accumulation and distribution in a mouse model of breast cancer. Nanotheranostics 2020, 4, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Maynard, J.; Peyman, S.A.; McLaughlan, J.R.; Fairclough, M.; Marston, G.; Valleley, E.M.A.; Jimenez-Macias, J.L.; et al. Ultrasound-triggered therapeutic microbubbles enhance the efficacy of cytotoxic drugs by increasing circulation and tumor drug accumulation and limiting bioavailability and toxicity in normal tissues. Theranostics 2020, 10, 10973–10992. [Google Scholar] [CrossRef] [PubMed]
- Ingram, N.; Macnab, S.A.; Marston, G.; Scott, N.; Carr, I.M.; Markham, A.F.; Whitehouse, A.; Coletta, P.L. The use of high-frequency ultrasound imaging and biofluorescence for in vivo evaluation of gene therapy vectors. BMC Med. Imaging 2013, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, R.H.; Peyman, S.A.; Johnson, B.R.; Marston, G.; Ingram, N.; Bushby, R.; Coletta, P.L.; Markham, A.F.; Evans, S.D. The influence of intercalating perfluorohexane into lipid shells on nano and microbubble stability. Soft Matter 2016, 12, 7223–7230. [Google Scholar] [CrossRef] [PubMed]
- Peyman, S.A.; Abou-Saleh, R.H.; McLaughlan, J.R.; Ingram, N.; Johnson, B.R.G.; Critchley, K.; Freear, S.; Evans, J.A.; Markham, A.F.; Coletta, P.L. Expanding 3D geometry for enhanced on-chip microbubble production and single step formation of liposome modified microbubbles. Lab Chip 2012, 12, 4544. [Google Scholar] [CrossRef]
- Fan, C.-H.; Ting, C.-Y.; Liu, H.-L.; Huang, C.-Y.; Hsieh, H.-Y.; Yen, T.-C.; Wei, K.-C.; Yeh, C.-K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013, 34, 2142–2155. [Google Scholar] [CrossRef]
- McEwan, C.; Kamila, S.; Owen, J.; Nesbitt, H.; Callan, B.; Borden, M.; Nomikou, N.; Hamoudi, R.A.; Taylor, M.A.; Stride, E.; et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials 2016, 80, 20–32. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Deng, Z.; Li, L.; Tan, G.; Liu, X.; Zheng, H.; Yan, F. Ultrasound-Triggered Drug Delivery for Breast Tumor Therapy Through iRGD-Targeted Paclitaxel-Loaded Liposome-Microbubble Complexes. J. Biomed. Nanotechnol. 2018, 14, 1384–1395. [Google Scholar] [CrossRef]
- Folarin, A.A.; Konerding, M.A.; Timonen, J.; Nagl, S.; Pedley, R.B. Three-dimensional analysis of tumour vascular corrosion casts using stereoimaging and micro-computed tomography. Microvasc. Res. 2010, 80, 89–98. [Google Scholar] [CrossRef]
- Gullino, P.M.; Grantham, F.H. The Vascular Space of Growing Tumors. Cancer Res. 1964, 24, 1727–1732. [Google Scholar]
- Doevendans, P.A.; Daemen, M.J.; de Muinck, E.D.; Smits, J.F. Cardiovascular phenotyping in mice. Cardiovasc. Res. 1998, 39, 34–49. [Google Scholar] [CrossRef]
- NC3Rs. Blood Sampling: Mouse. Available online: https://www.nc3rs.org.uk/mouse-decision-tree-blood-sampling (accessed on 13 January 2022).
- Bush, N.; Healey, A.; Shah, A.; Box, G.; Kirkin, V.; Kotopoulis, S.; Kvale, S.; Sontum, P.C.; Bamber, J. Therapeutic Dose Response of Acoustic Cluster Therapy in Combination With Irinotecan for the Treatment of Human Colon Cancer in Mice. Front. Pharmacol. 2019, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Olsman, M.; Sereti, V.; Andreassen, K.; Snipstad, S.; van Wamel, A.; Eliasen, R.; Berg, S.; Urquhart, A.J.; Andresen, T.L.; Davies, C.L. Ultrasound-mediated delivery enhances therapeutic efficacy of MMP sensitive liposomes. J. Control Release 2020, 325, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bourn, M.D.; Batchelor, D.V.B.; Ingram, N.; McLaughlan, J.R.; Coletta, P.L.; Evans, S.D.; Peyman, S.A. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. J. Control Release 2020, 326, 13–24. [Google Scholar] [CrossRef] [PubMed]
- De Cock, I.; Lajoinie, G.; Versluis, M.; De Smedt, S.C.; Lentacker, I. Sonoprinting and the importance of microbubble loading for the ultrasound mediated cellular delivery of nanoparticles. Biomaterials 2016, 83, 294–307. [Google Scholar] [CrossRef]
- Luan, Y.; Faez, T.; Gelderblom, E.; Skachkov, I.; Geers, B.; Lentacker, I.; van der Steen, T.; Versluis, M.; de Jong, N. Acoustical properties of individual liposome-loaded microbubbles. Ultrasound Med. Biol. 2012, 38, 2174–2185. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Shevchenko, T.I.; Raju, B.I.; Seip, R.; Chin, C.T. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: A tool for targeted drug delivery. J. Control Release 2010, 148, 13–17. [Google Scholar] [CrossRef]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J. Control Release 2005, 104, 213–222. [Google Scholar] [CrossRef]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef]
- Damioli, V.; Salvadori, A.; Beretta, G.P.; Ravelli, C.; Mitola, S. Multi-physics interactions drive VEGFR2 relocation on endothelial cells. Sci. Rep. 2017, 7, 16700. [Google Scholar] [CrossRef] [PubMed]
- Basagiannis, D.; Christoforidis, S. Constitutive Endocytosis of VEGFR2 Protects the Receptor against Shedding. J. Biol. Chem. 2016, 291, 16892–16903. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Batchelor, D.V.B.; Loadman, P.M.; McLaughlan, J.R.; Markham, A.F.; Evans, S.D.; Coletta, P.L. A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles. Pharmaceutics 2022, 14, 622. https://doi.org/10.3390/pharmaceutics14030622
Ingram N, McVeigh LE, Abou-Saleh RH, Batchelor DVB, Loadman PM, McLaughlan JR, Markham AF, Evans SD, Coletta PL. A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles. Pharmaceutics. 2022; 14(3):622. https://doi.org/10.3390/pharmaceutics14030622
Chicago/Turabian StyleIngram, Nicola, Laura E. McVeigh, Radwa H. Abou-Saleh, Damien V. B. Batchelor, Paul M. Loadman, James R. McLaughlan, Alexander F. Markham, Stephen D. Evans, and P. Louise Coletta. 2022. "A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles" Pharmaceutics 14, no. 3: 622. https://doi.org/10.3390/pharmaceutics14030622
APA StyleIngram, N., McVeigh, L. E., Abou-Saleh, R. H., Batchelor, D. V. B., Loadman, P. M., McLaughlan, J. R., Markham, A. F., Evans, S. D., & Coletta, P. L. (2022). A Single Short ‘Tone Burst’ Results in Optimal Drug Delivery to Tumours Using Ultrasound-Triggered Therapeutic Microbubbles. Pharmaceutics, 14(3), 622. https://doi.org/10.3390/pharmaceutics14030622