Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Factorial Design Approach
2.2. Particle Size Distribution and Zeta Potential
2.3. Entrapment Efficiency (EE)
2.4. In-Vitro Drug Release Studies
2.5. FTIR Study
2.6. DSC Study
2.7. X-RD Study
2.8. Animal Studies
Pharmacokinetic Evaluation
3. Results and Discussion
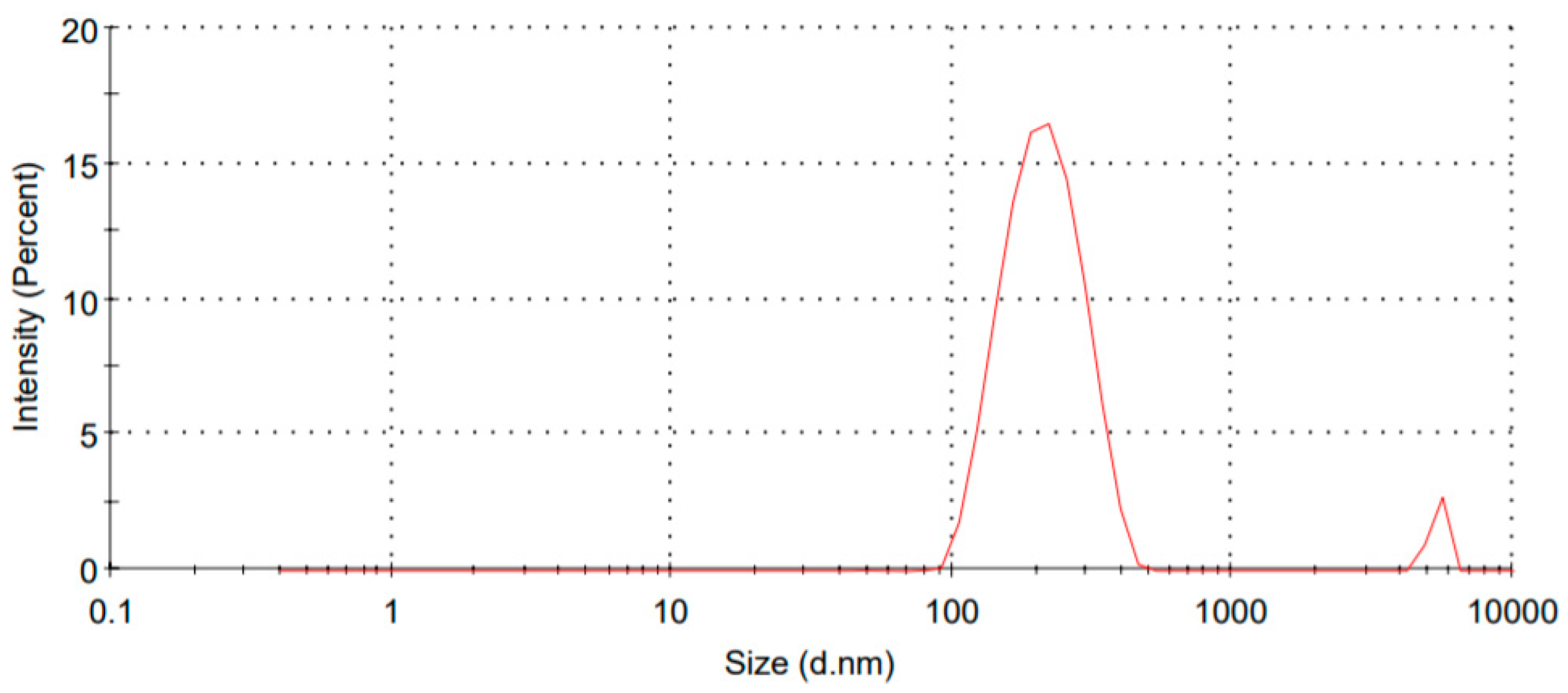
3.1. Particle Size and Zeta Potential
3.2. Encapsulation Efficiency
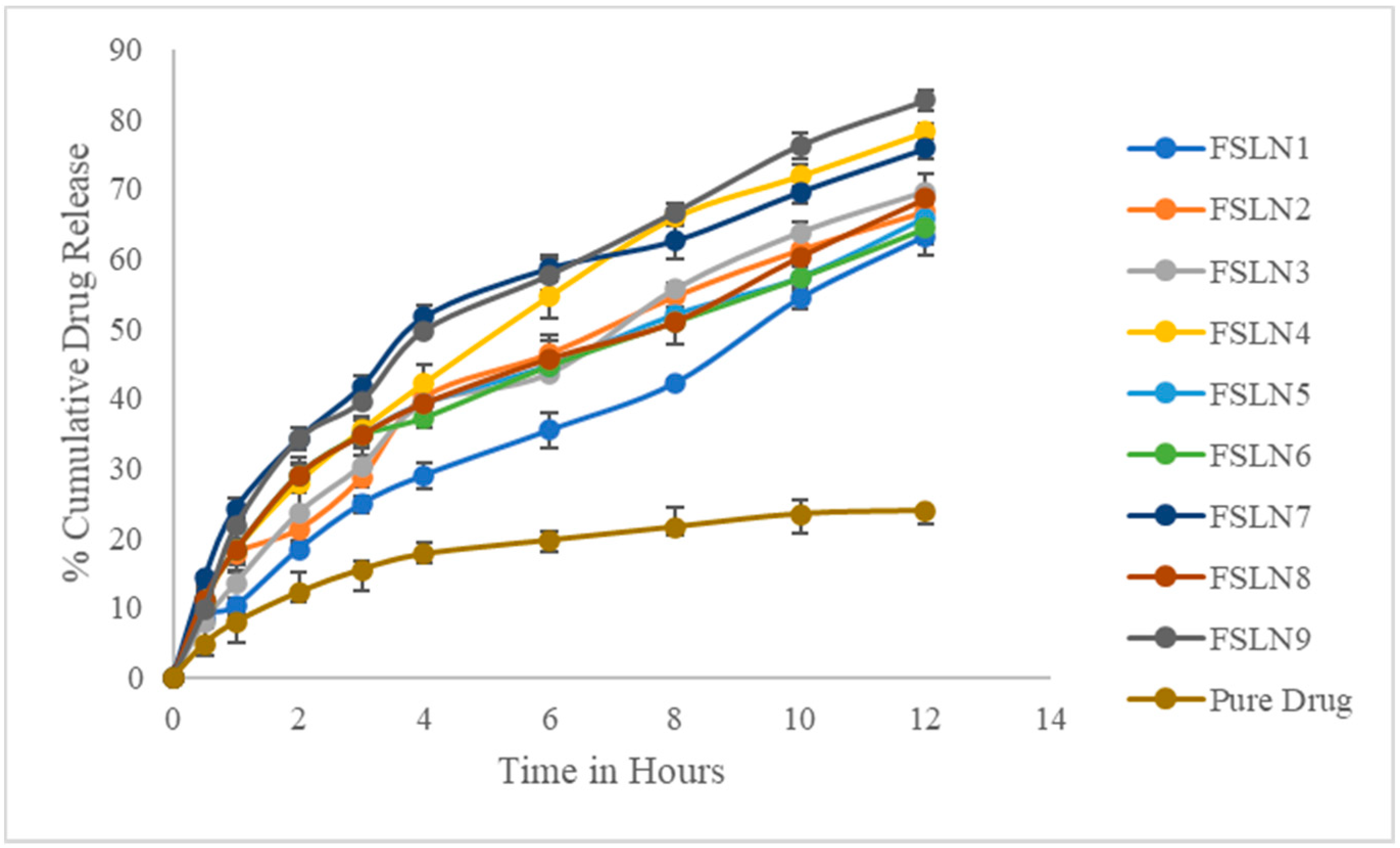
3.3. In Vitro Drug Release Studies
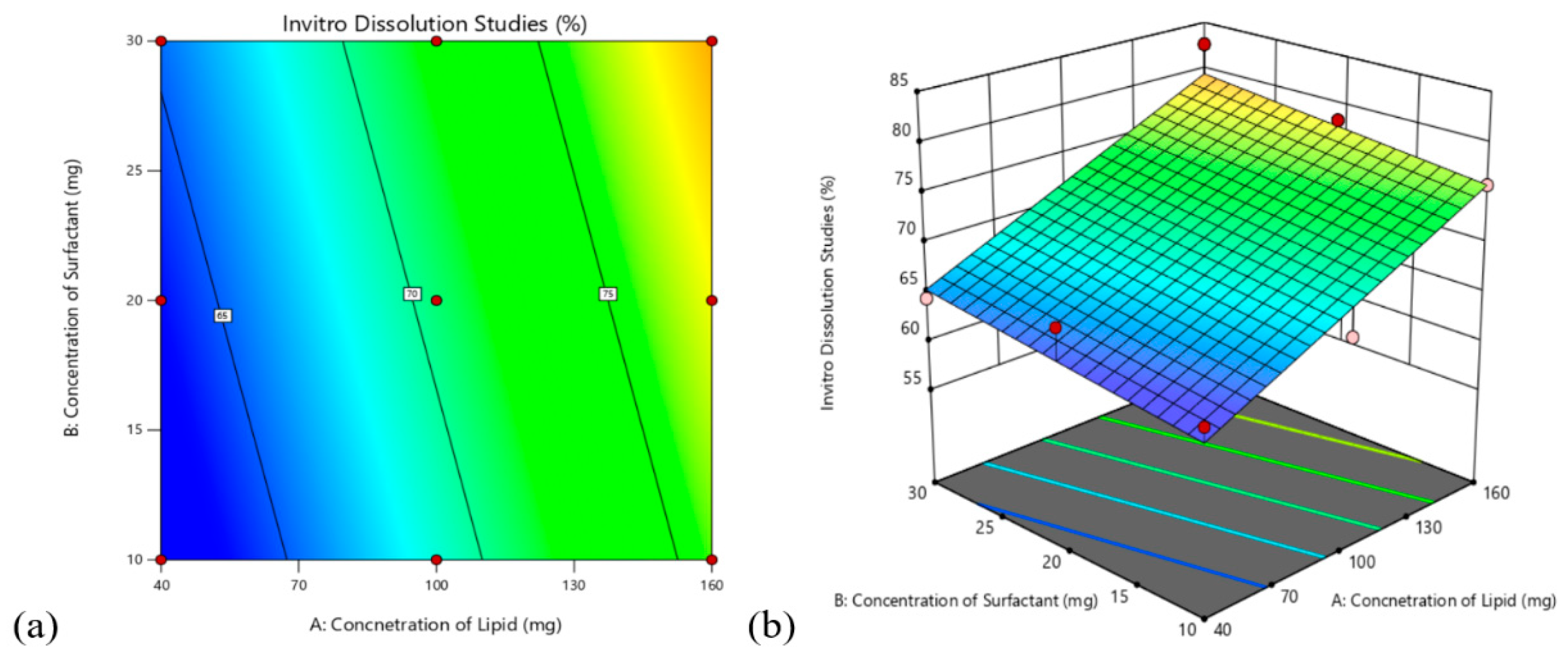
3.4. Design Analysis
3.4.1. Effects on Encapsulation Efficiency (Y1)
3.4.2. Effect on In-Vitro Drug Release Studies
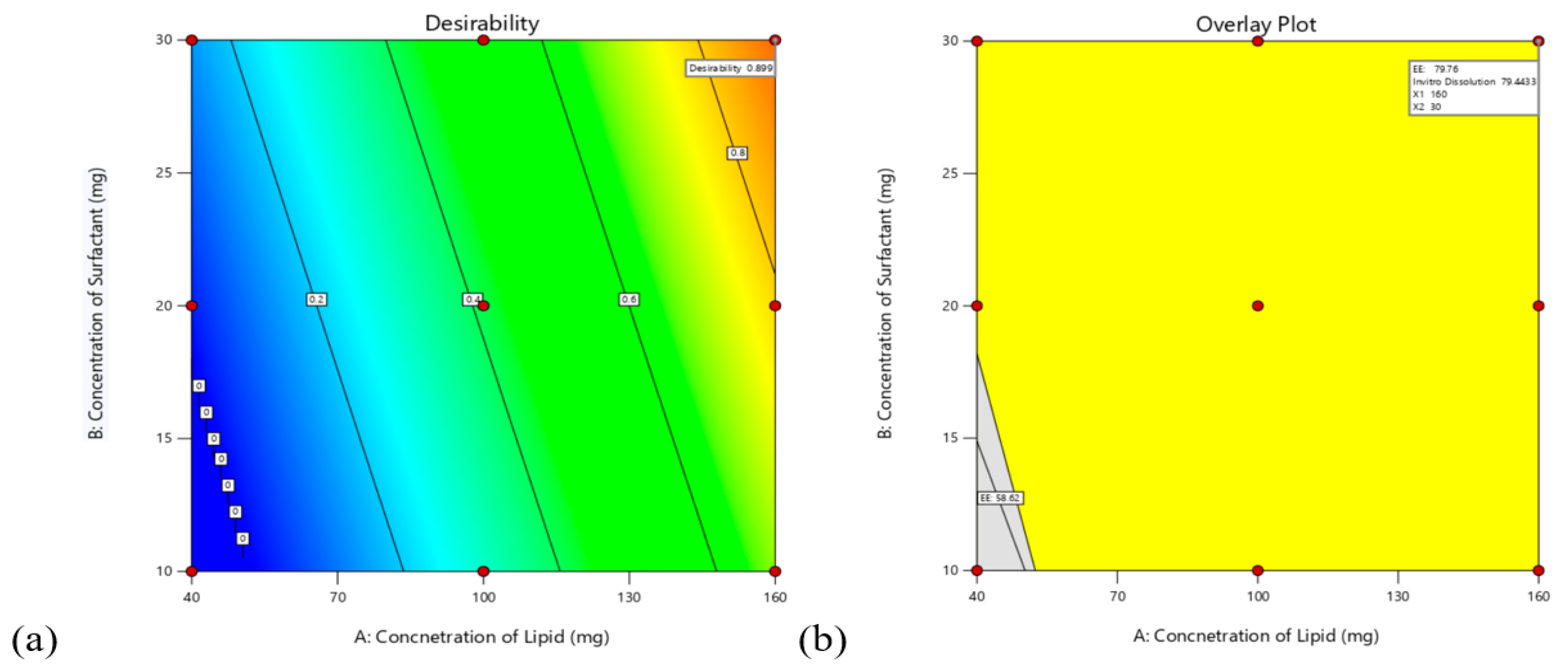
3.4.3. Validation of the Model
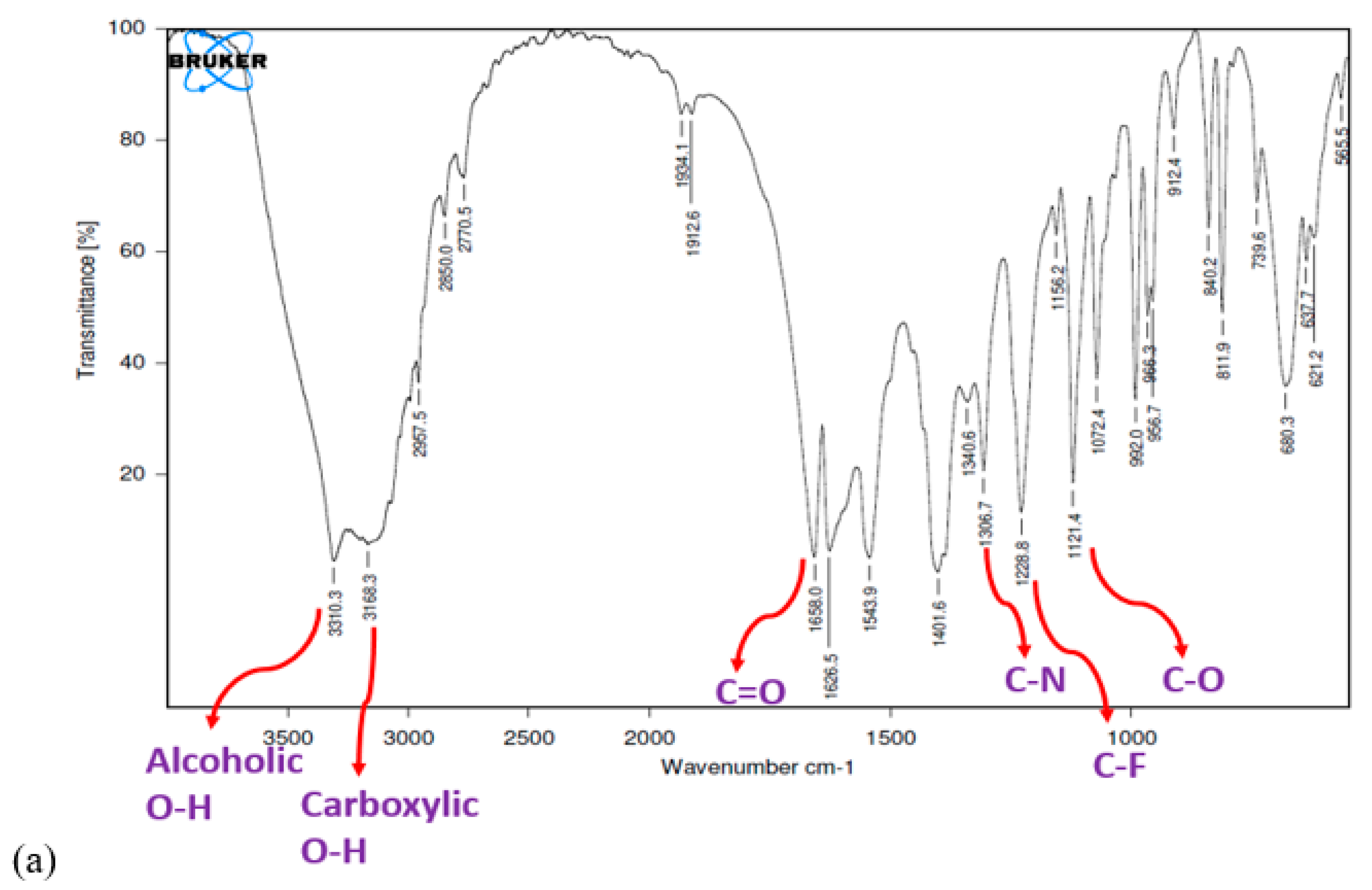
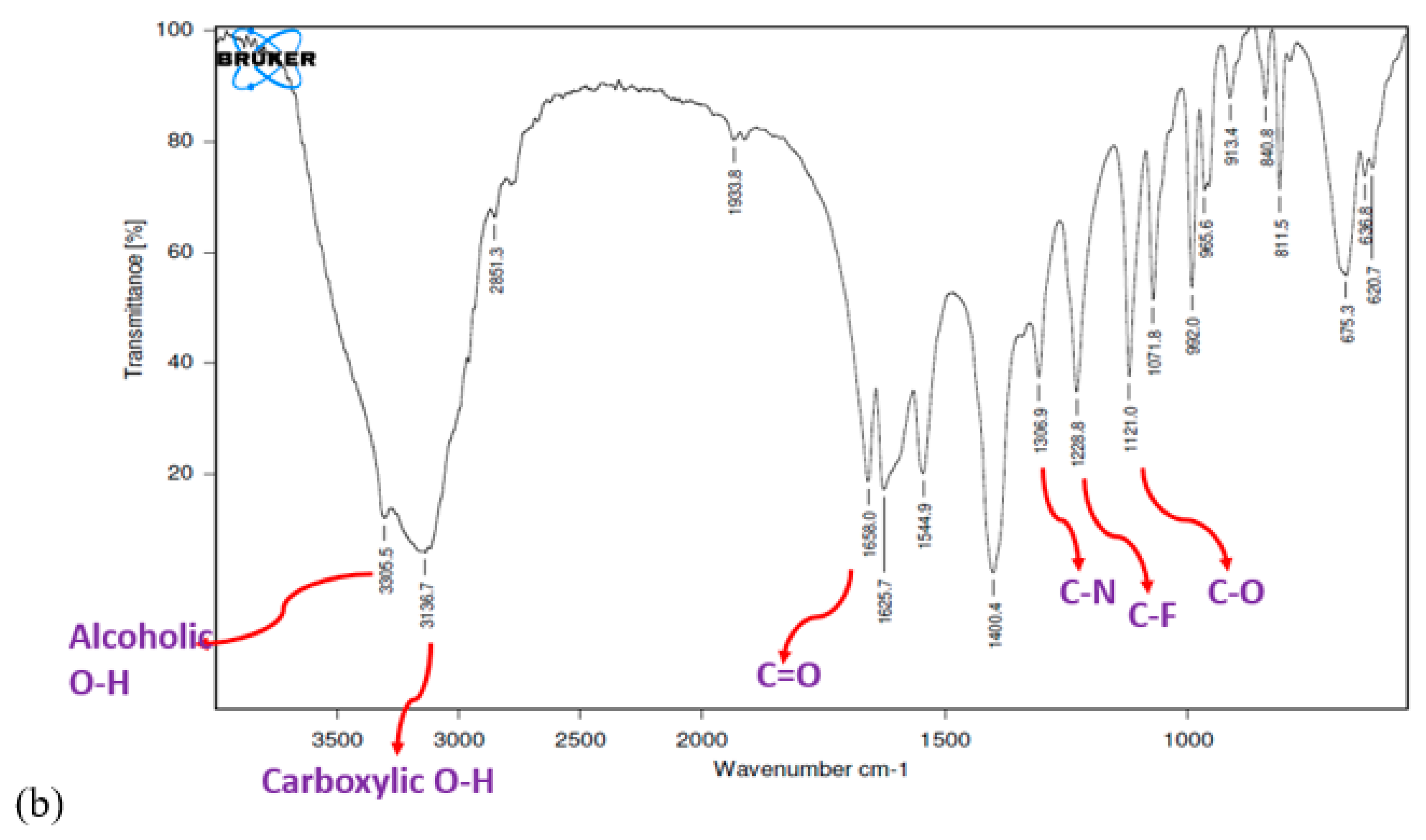
3.5. FTIR Study
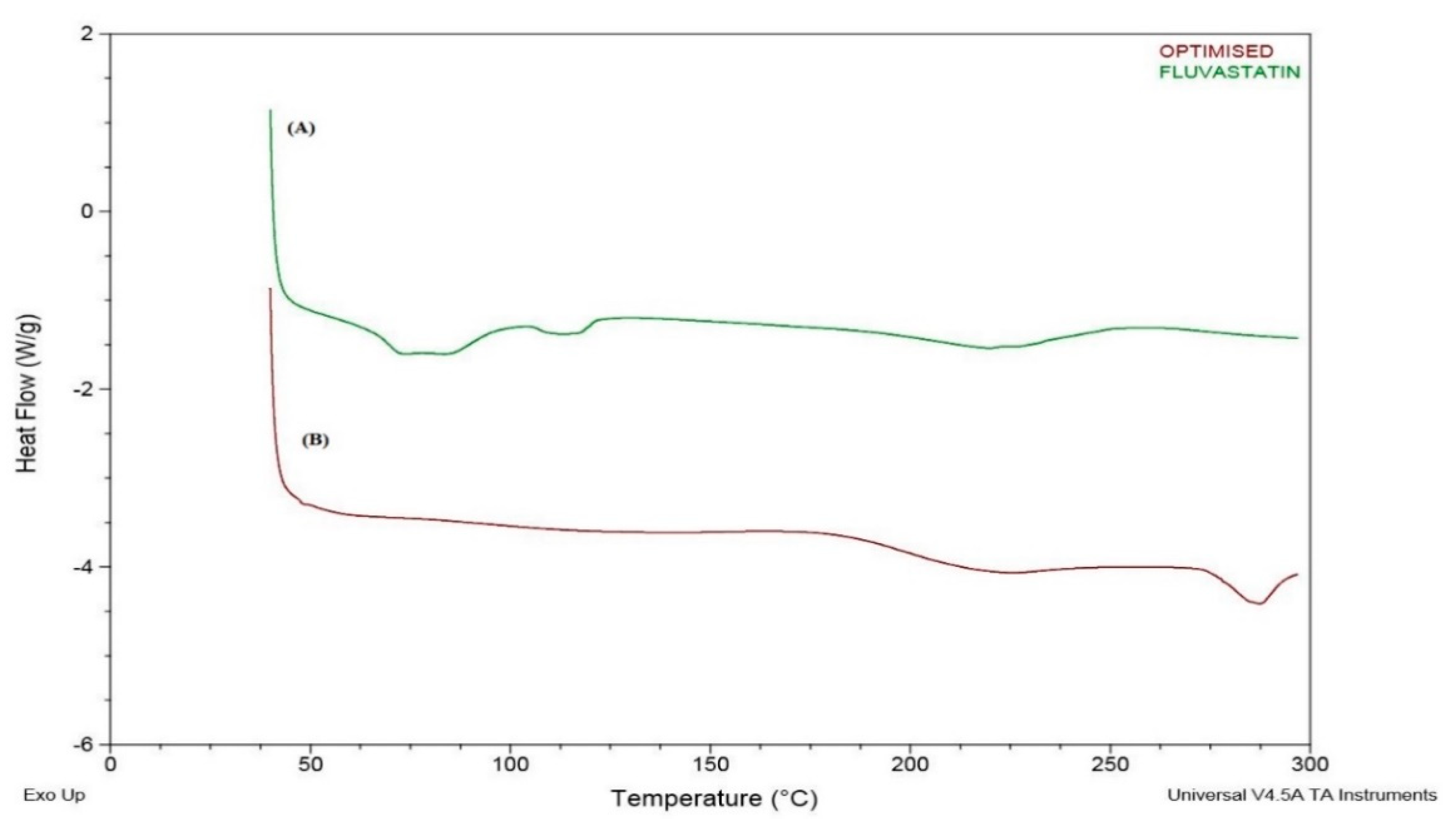
3.6. DSC Study
3.7. X-RD Study
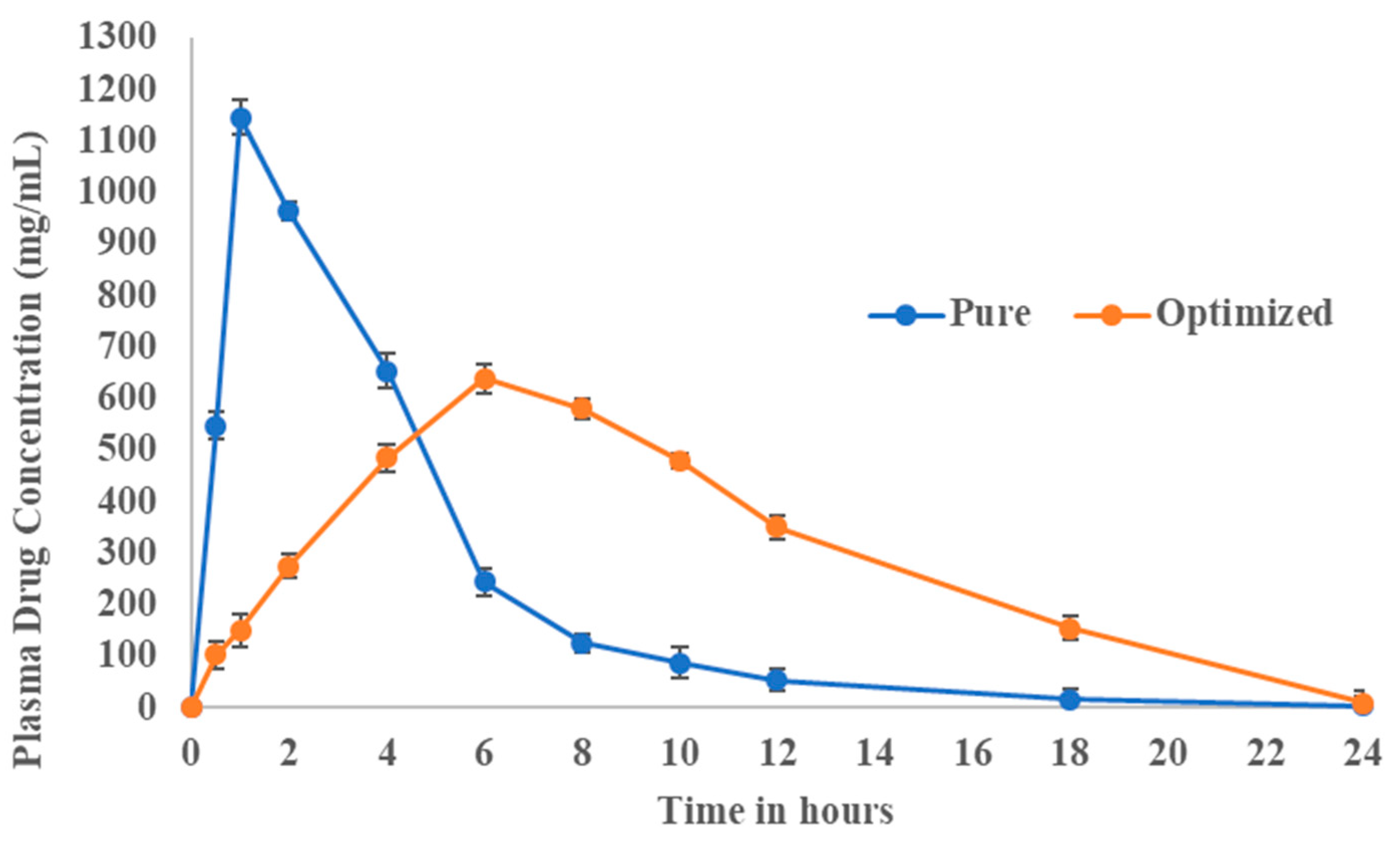
3.8. Pharmacokinetic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Yang, M.; Xu, W.R. Enhanced oral bioavailability of fluvastatin by using nanosuspensions containing cyclodextrin. Drug Des. Devel Ther. 2018, 12, 3491–3499. [Google Scholar] [PubMed] [Green Version]
- Podar, K.; Tai, Y.T.; Hideshima, T.; Vallet, S.; Richardson, P.G.; Anderson, K.C. Emerging therapies for multiple myeloma. Expert Opin. Emerg. Drugs 2009, 14, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Tonsberg, H.; Jorgensen, E.B.; Abedinpour, P.; Farsad, S.; Müllertz, A. Influence of bile on the absorption of halofantrine from lipid-based formulations. Eur. J. Pharm. Biopharm. 2012, 81, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Kim, S.; Ji, X.; Zhu, W.; Niroui, F.; Kong, J.; Chen, Y.A. High-Lift Micro-Aerial-Robot Powered by Low-Voltage and Long-Endurance Dielectric Elastomer Actuators. Adv. Mater. 2022, 34, 2106757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Hauss, D.J.; Fogal, S.E.; Ficorilli, J.V.; Price, C.A.; Roy, T.; Jayaraj, A.A. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J. Pharm. Sci. 1998, 87, 164–169. [Google Scholar] [CrossRef]
- Mokale, V.; Rajput, R.; Patil, J.; Yadava, S.; Naik, J. Formulation of metformin hydrochloride nanoparticles by using spray drying technique and in vitro evaluation of sustained release with 32-level factorial design approach. Dry Technol. 2016, 34, 1455–1461. [Google Scholar] [CrossRef]
- Verma, U.; Naik, J.B.; Patil, J.S.; Yadava, S.K. Screening of process variables to enhance the solubility of famotidine with 2-HydroxyPropyl–β-Cyclodextrin & PVP K-30 by using Plackett–Burman design approach. Mater. Sci. Eng. C 2017, 77, 282–292. [Google Scholar]
- Patil, J.S.; Patil, P.B.; Sonawane, P.; Naik, J.B. Design and development of sustained-release glyburide-loaded silica nanoparticles. Bull. Mater. Sci. 2017, 40, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Patil, J.; Rajput, R.; Nemade, R.; Naik, J. Preparation and characterization of artemether loaded solid lipid nanoparticles: A 32 factorial design approach. Mater. Technol. 2020, 35, 719–726. [Google Scholar] [CrossRef]
- Waghulde, M.; Rajput, R.; Mujumdar, A.; Naik, J. Production and evaluation of vildagliptin-loaded poly(dl-lactide) and poly(dl-lactide-glycolide) micro-/nanoparticles: Response surface methodology approach. Dry Technol. 2019, 37, 1265–1276. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. Biomed. Res. Int. 2017, 2017, 5984014. [Google Scholar] [CrossRef] [PubMed]
- Verma, U.; Rajput, R.; Naik, J.B. Development and characterization of Fast Dissolving Film of Chitosan embedded Famotidine Using 32 Full Factorial Design Approach. Mater. Today Proc. 2018, 5, 408–414. [Google Scholar] [CrossRef]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Attama, A.A.; Umeyor, C.E. The use of solid lipid nanoparticles for sustained drug release. Ther. Deliv. 2015, 6, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Krishnam Raju, K.; Sudhakar, B.; Murthy, K.V.R. Factorial Design Studies and Biopharmaceutical Evaluation of Simvastatin Loaded Solid Lipid Nanoparticles for Improving the Oral Bioavailability. Nanotechnology 2014, 2014, 951016. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.; Mujumdar, A.; Naik, J. Production of aceclofenac-loaded sustained release micro/nanoparticles using pressure homogenization and spray drying. Dry Technol. 2018, 36, 459–467. [Google Scholar] [CrossRef]
- Verma, U.; Mujumdar, A.; Naik, J. Preparation of Efavirenz resinate by spray drying using response surface methodology and its physicochemical characterization for taste masking. Dry Technol. 2020, 38, 793–805. [Google Scholar] [CrossRef]
- Desu, P.K.; Pasam, V.; Kotra, V. Implications of superporous hydrogel composites-based gastroretentive drug delivery systems with improved biopharmaceutical performance of fluvastatin. J. Drug Deliv. Sci. Technol. 2020, 57, 101668. [Google Scholar] [CrossRef]
- Gangane, P.S.; Pachpute, T.; Mahapatra, D.K.; Mahajan, N.M. HPMC Polymers and Xanthan Gum Assisted Development and Characterization of Stavudine Extended Release Floating Tablets. Indian J. Pharm. Edu. Res. 2021, 55, S681–S692. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, R.; Patil, P.R.; Gupta, R.; Mahor, A.; Bhardwaj, P. Preparation and Characterization of Transdermal Therapeutic System Containing Simvastatin: A Statistical Study. Indian J. Pharm. Edu. Res. 2022, 56, 112–120. [Google Scholar] [CrossRef]
- Honmane, S.M.; Chimane, S.M.; Bandgar, S.A.; Patil, S.S. Development and Optimization of Capecitabine loaded Nanoliposomal System for Cancer Delivery. Indian J. Pharm. Edu. Res. 2020, 54, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Dhat, S.; Pund, S.; Kokare, C.; Sharma, P.; Shrivastava, B. Mechanistic investigation of biopharmaceutic and pharmacokinetic characteristics of surface engineering of satranidazole nanocrystals. Eur. J. Pharm. Biopharm. 2016, 100, 109–118. [Google Scholar] [CrossRef]
- Nagaraju, B.; Anilkumar, K.V. Influence of Telmisartan on Pharmacodynamic and Pharmacokinetic Properties of Glimepiride-metformin Combination Using Rodent and Non-Rodent Models. Indian J. Pharm. Edu. Res. 2021, 55, 1060–1065. [Google Scholar] [CrossRef]


| Formulation Code | Variables in Coded Form | Response | ||||
|---|---|---|---|---|---|---|
| Amount of Lipid (A) | Amount of Surfactant (B) | |||||
| Coded Values | Actual Values (mg) | Coded Values | Actual Values (mg) | Encapsulation Efficiency (Y1) | In Vitro Drug Release (Y2) | |
| FSLN1 | −1 | 40 | −1 | 10 | 58.62 | 63.24 |
| FSLN2 | −1 | 40 | 0 | 20 | 61.32 | 66.84 |
| FSLN3 | 0 | 100 | 0 | 20 | 69.54 | 69.65 |
| FSLN4 | 1 | 160 | 0 | 20 | 76.2 | 78.28 |
| FSLN5 | 0 | 100 | −1 | 10 | 62.38 | 65.9 |
| FSLN6 | −1 | 40 | 1 | 30 | 61.38 | 64.44 |
| FSLN7 | 1 | 160 | −1 | 10 | 74.82 | 75.86 |
| FSLN8 | 0 | 100 | 1 | 30 | 71.54 | 68.62 |
| FSLN9 | 1 | 160 | 1 | 30 | 80.46 | 82.66 |
| Formulation Code | Particle Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| FSLN1 | 354.2 | 0.152 | −36.4 | 58.62 |
| FSLN2 | 324.8 | 0.168 | −32.2 | 61.32 |
| FSLN3 | 298.6 | 0.186 | −28.8 | 69.54 |
| FSLN4 | 265.8 | 0.182 | −26.6 | 76.2 |
| FSLN5 | 248.2 | 0.176 | −24.2 | 62.38 |
| FSLN6 | 243.8 | 0.168 | −21.5 | 61.38 |
| FSLN7 | 214.2 | 0.212 | −20.4 | 74.82 |
| FSLN8 | 189.5 | 0.168 | −18.6 | 71.54 |
| FSLN9 | 153.5 | 0.148 | −14.9 | 80.46 |
| Time | FSLN1 | FSLN2 | FSLN3 | FSLN4 | FSLN5 | FSLN6 | FSLN7 | FSLN8 | FSLN9 | Pure Drug |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 8.54 ± 1.54 | 10.65 ± 2.54 | 7.96 ± 1.12 | 10.86 ± 1.42 | 11.2 ± 1.58 | 11.2 ± 1.42 | 14.25 ± 0.68 | 11.2 ± 1.14 | 9.68 ± 1.54 | 4.8 ± 1.24 |
| 1 | 10.24 ± 1.24 | 17.65 ± 0.88 | 13.54 ± 2.54 | 18.24 ± 3.14 | 18.24 ± 2.98 | 18.24 ± 1.24 | 24.25 ± 1.37 | 18.24 ± 1.62 | 21.65 ± 1.84 | 7.9 ± 2.32 |
| 2 | 18.34 ± 0.84 | 21.24 ± 1.54 | 23.54 ± 3.24 | 27.96 ± 1.28 | 28.97 ± 1.46 | 28.97 ± 2.54 | 34.25 ± 1.68 | 28.97 ± 1.87 | 34.12 ± 0.24 | 12.18 ± 1.48 |
| 3 | 24.85 ± 1.24 | 28.63 ± 1.24 | 30.32 ± 2.48 | 35.42 ± 1.47 | 34.65 ± 2.88 | 34.65 ± 1.36 | 41.68 ± 1.47 | 34.65 ± 1.25 | 39.48 ± 0.86 | 15.42 ± 2.82 |
| 4 | 28.96 ± 1.86 | 40.19 ± 1.88 | 39.18 ± 1.84 | 42.17 ± 2.57 | 39.25 ± 1.34 | 37.25 ± 1.47 | 51.68 ± 1.62 | 39.25 ± 1.93 | 49.68 ± 0.14 | 17.64 ± 1.32 |
| 6 | 35.42 ± 2.45 | 46.45 ± 2.62 | 43.54 ± 1.24 | 54.68 ± 3.24 | 44.69 ± 1.58 | 44.69 ± 0.96 | 58.66 ± 187 | 45.69 ± 2.57 | 57.65 ± 2.24 | 19.64 ± 1.52 |
| 8 | 42.17 ± 0.54 | 54.65 ± 0.62 | 55.65 ± 0.98 | 65.98 ± 1.26 | 51.98 ± 1.21 | 50.98 ± 0.85 | 62.64 ± 2.68 | 50.98 ± 3.14 | 66.65 ± 1.36 | 21.54 ± 1.26 |
| 10 | 54.35 ± 1.68 | 61.35 ± 1.74 | 63.79 ± 1.42 | 71.94 ± 1.57 | 57.32 ± 2.76 | 57.32 ± 1.87 | 69.65 ± 1.58 | 59.32 ± 1.52 | 76.24 ± 1.87 | 23.42 ± 2.74 |
| 12 | 63.24 ± 2.68 | 66.84 ± 2.14 | 69.65 ± 2.66 | 78.28 ± 0.98 | 65.9 ± 1.92 | 64.44 ± 2.24 | 75.86 ± 1.58 | 68.62 ± 1.24 | 82.66 ± 1.48 | 23.87 ± 1.98 |
| Value | F-Value | p-Value | |
|---|---|---|---|
| |||
| Model | Linear | ||
| R2 | 0.9609 | 73.64 | <0.0001 |
| Adj iR2 | 0.9478 | ||
| Pred iR2 | 0.9099 | ||
| Adeq Precision | 21.8699 | ||
| |||
| Model | Linear | ||
| R2 | 0.8681 | 19.75 | <0.0023 |
| Adj iR2 | 0.8242 | ||
| Pred iR2 | 0.7006 | ||
| Adeq Precision | 10.79 | ||
| Pharmacokinetic Data | Pure Fluvastatin | Optimized Formulation |
|---|---|---|
| Cmax (ng/mL) | 1146 | 640 |
| tmax (h) | 1 | 6 |
| T1/2(h) | 2.9 | 2.9 |
| AUC(0–24) (mg/mL.h) | 5122.5 | 7298.2 |
| AUC(0–∞) (mg/mL.h) | 5135.2 | 7336.24 |
| AUMC(0–∞) (mg/mL.h2) | 20,696.90 | 66,578.74 |
| MRT (h) | 4.03 | 9.07 |
| KE (h−1) | 0.43 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, A.H.; Desu, P.K.; Alavala, R.R.; Rao, G.S.N.K.; Sreeharsha, N.; Meravanige, G. Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach. Pharmaceutics 2022, 14, 584. https://doi.org/10.3390/pharmaceutics14030584
Asif AH, Desu PK, Alavala RR, Rao GSNK, Sreeharsha N, Meravanige G. Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach. Pharmaceutics. 2022; 14(3):584. https://doi.org/10.3390/pharmaceutics14030584
Chicago/Turabian StyleAsif, Afzal Haq, Prasanna Kumar Desu, Rajasekhar Reddy Alavala, Gudhanti Siva Naga Koteswara Rao, Nagaraja Sreeharsha, and Girish Meravanige. 2022. "Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach" Pharmaceutics 14, no. 3: 584. https://doi.org/10.3390/pharmaceutics14030584
APA StyleAsif, A. H., Desu, P. K., Alavala, R. R., Rao, G. S. N. K., Sreeharsha, N., & Meravanige, G. (2022). Development, Statistical Optimization and Characterization of Fluvastatin Loaded Solid Lipid Nanoparticles: A 32 Factorial Design Approach. Pharmaceutics, 14(3), 584. https://doi.org/10.3390/pharmaceutics14030584










