Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture of OC
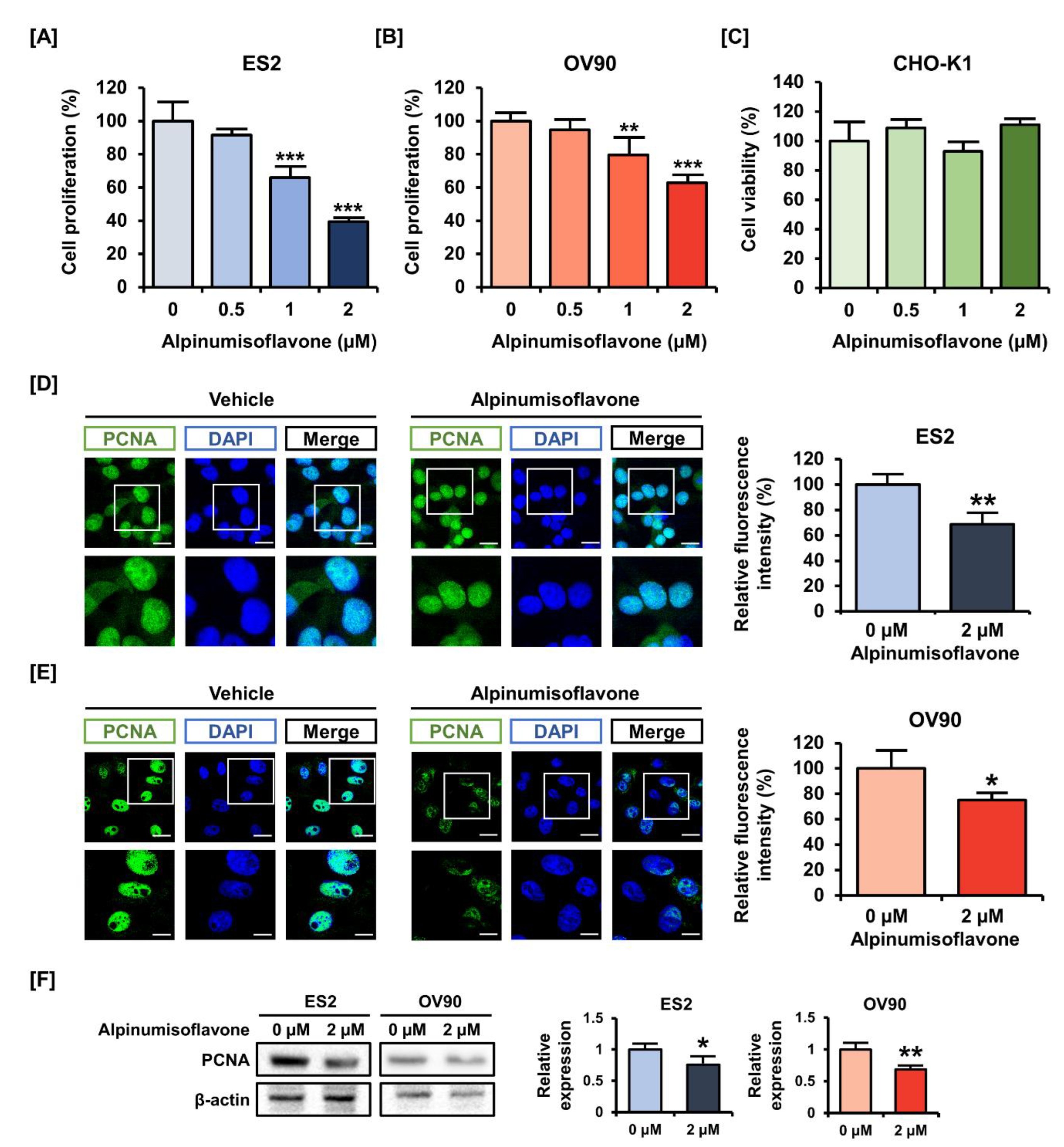
2.3. Cell Proliferation Assay and Viability Test
2.4. Immunofluorescence Analysis
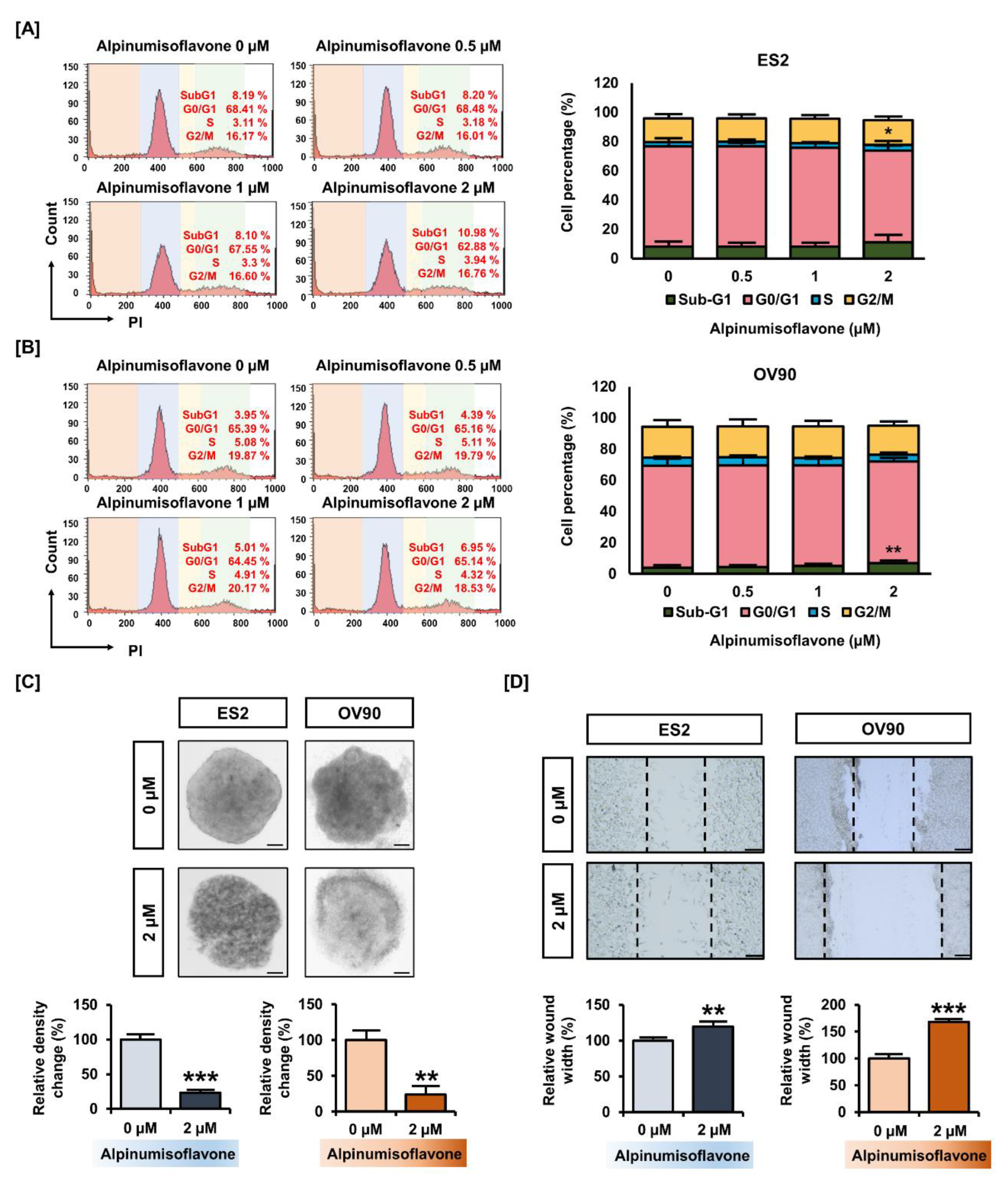
2.5. Spheroid Culture for OC
2.6. Migration Assay
2.7. Cell Cycle Assay
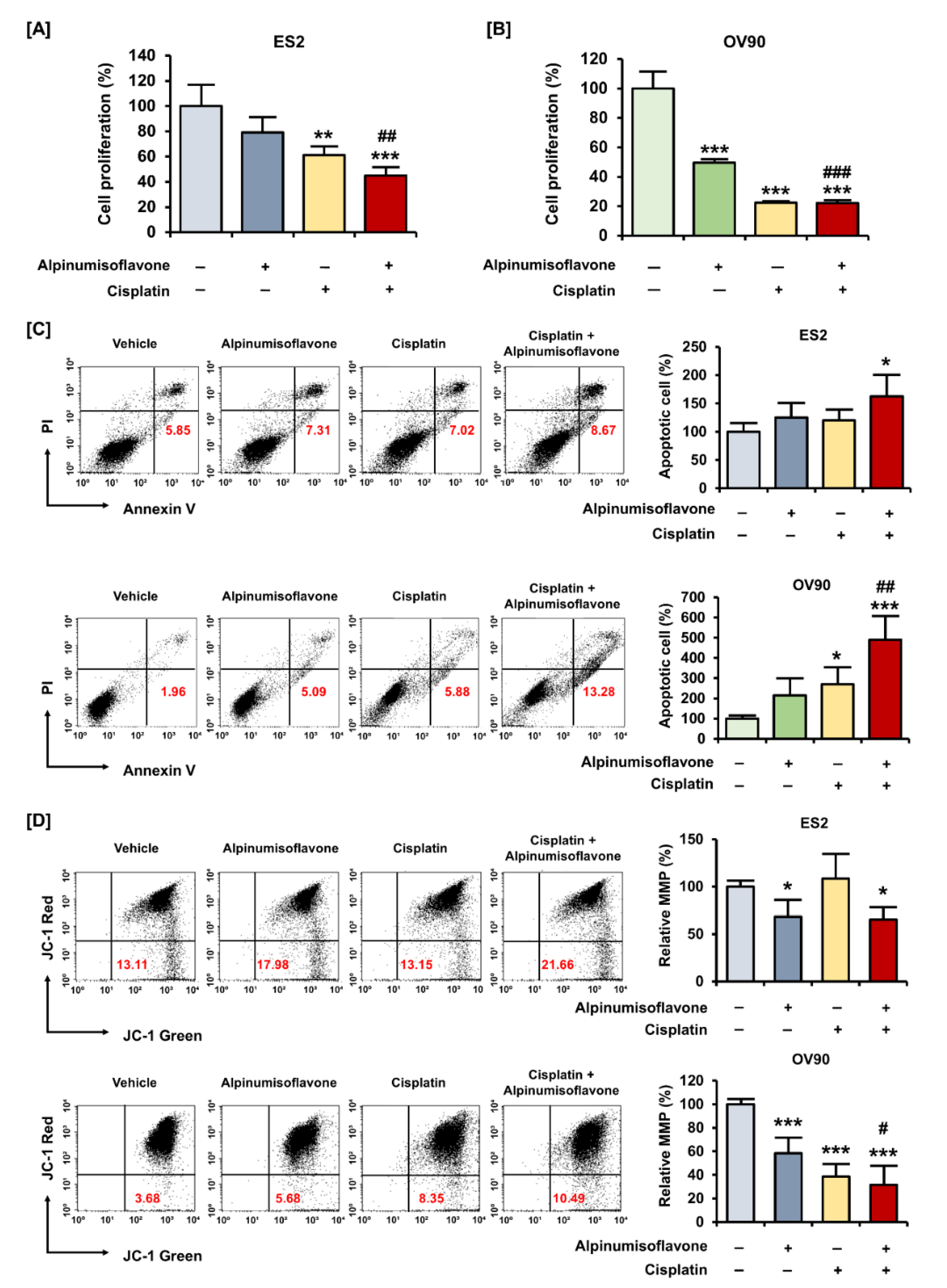
2.8. JC-1 Assay
2.9. Annexin V and PI Staining
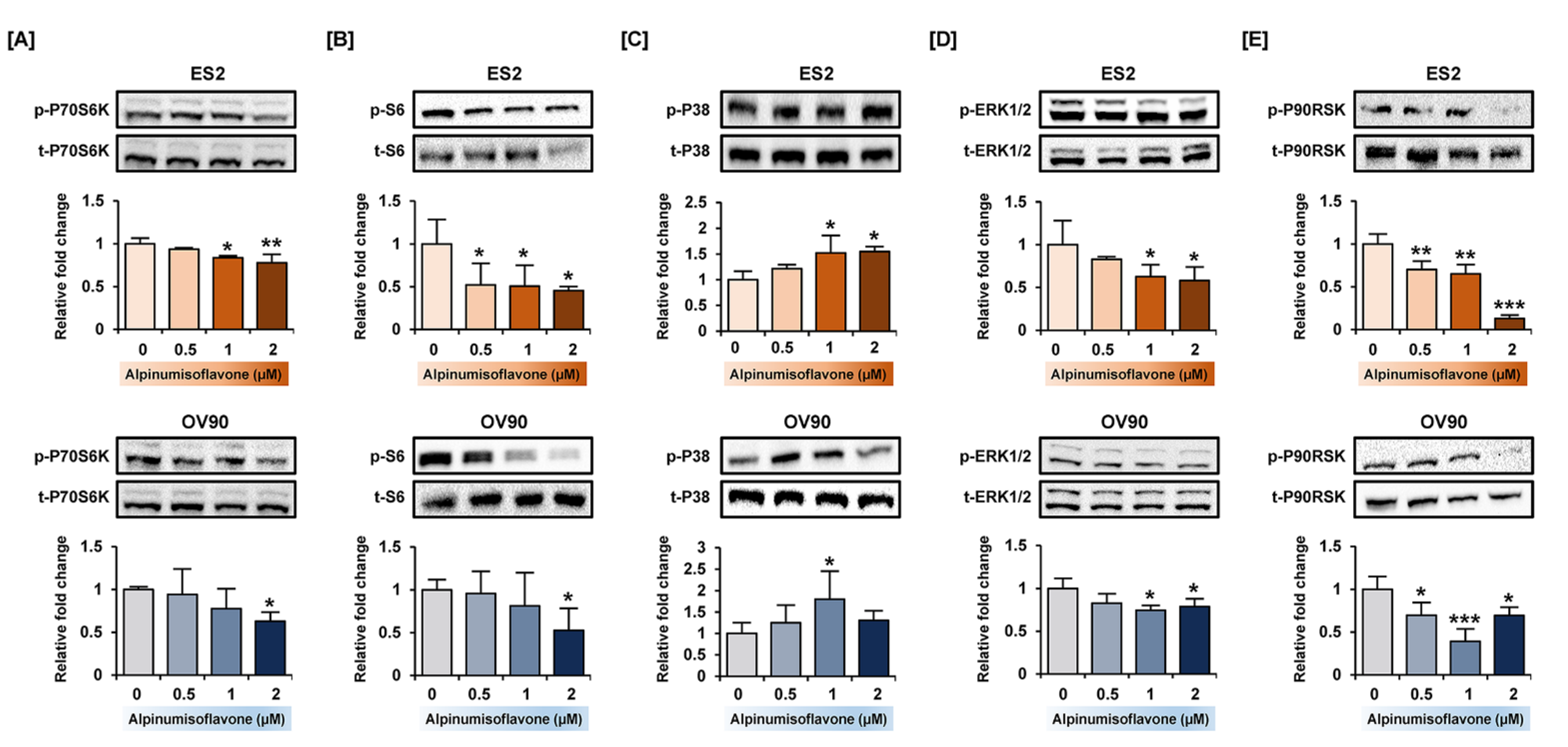
2.10. Western Blotting
2.11. Mitochondrial Respiration Measurement Using the Seahorse XFe24 Analyzer
2.12. Statistical Analysis
3. Results
3.1. Alpinumisoflavone Regulated the Proliferation of OC Cells
3.2. Alpinumisoflavone Inhibited Cell Growth and the Migration of ES2 and OV90 Cells
3.3. Alpinumisoflavone Induced Cell Death and the Depolarization of MMP (∆Ψm) in ES2 and OV90 Cells
3.4. Alpinumisoflavone Regulated Mitochondrial Respiration in OC Cells
3.5. Alpinumisoflavone Showed a Synergistic Effect with Cisplatin in Suppressing the Proliferation of OC Cells
3.6. Alpinumisoflavone Regulated Signaling Proteins Related to Proliferation and Endoplasmic Reticulum (ER) Stress in ES2 and OV90 Cells
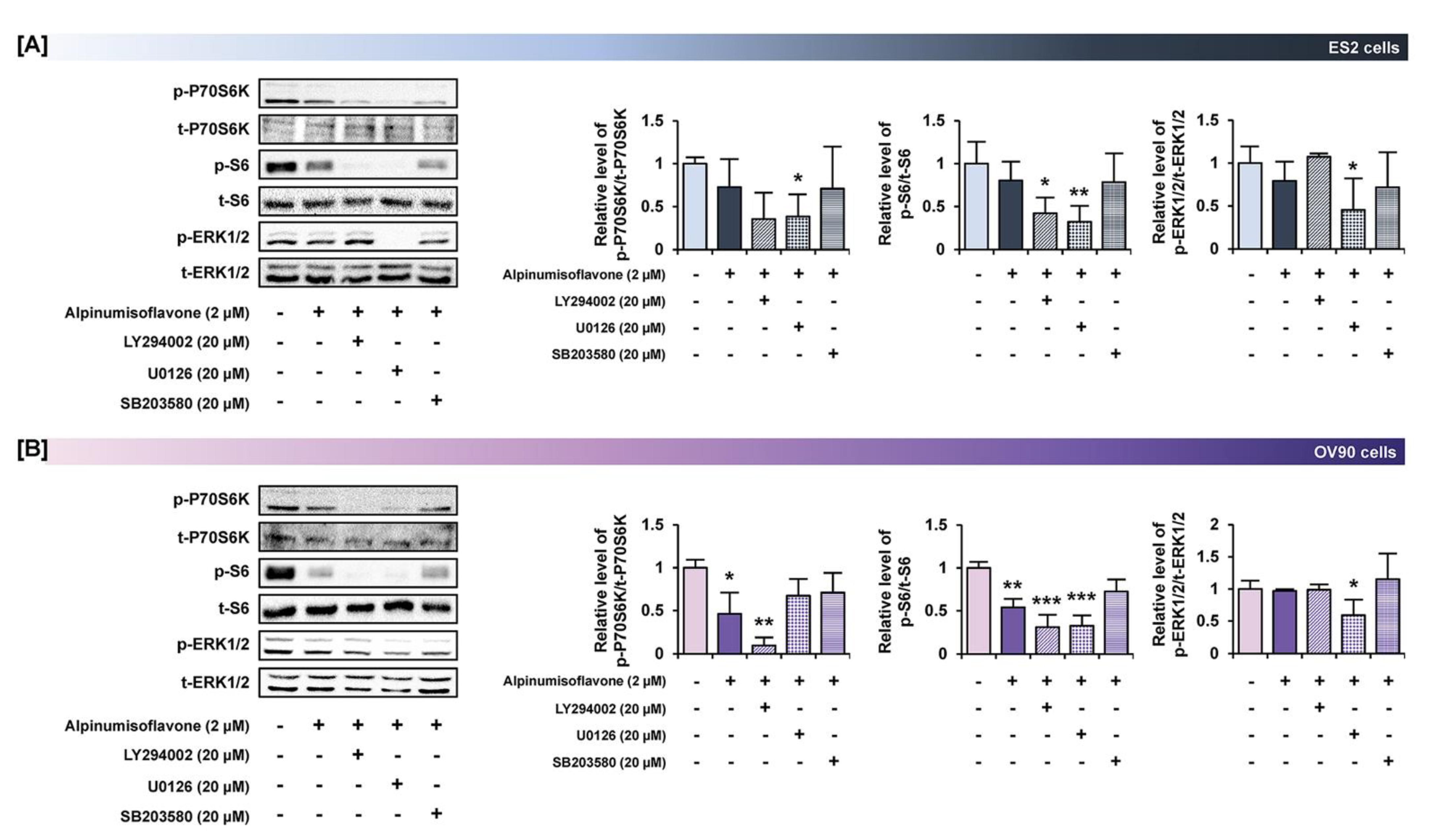
3.7. Alpinumisoflavone with Inhibitors of Target Pathways Induced Cell Apoptosis and Inhibited Intracellular Signaling
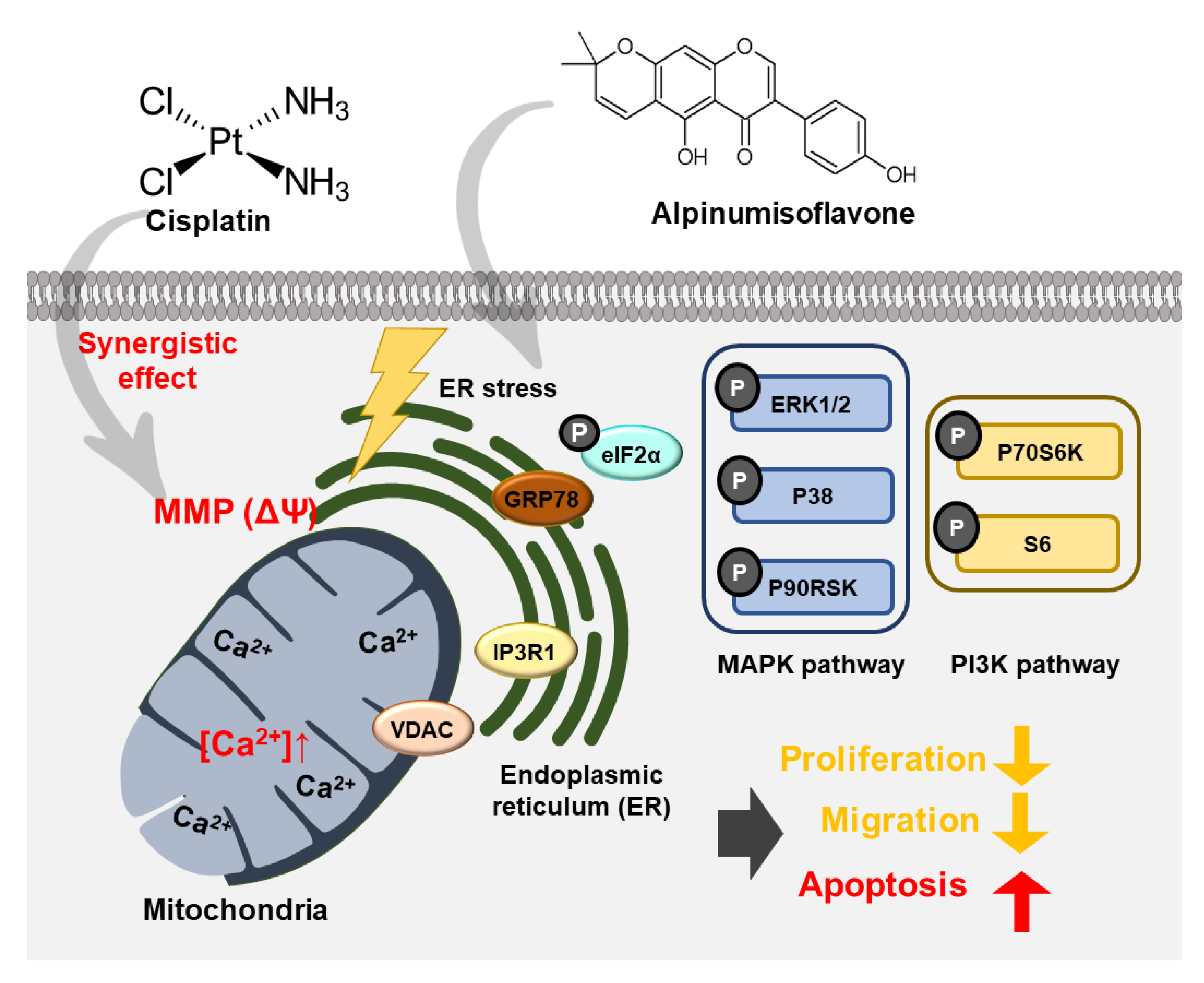
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- De Simone, F.I. The Need for Early Detection in Ovarian Cancer. Available online: https://www.mlo-online.com/disease/cancer/article/21222611/the-need-for-early-detection-in-ovarian-cancer (accessed on 19 May 2021).
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Physician 2009, 80, 609–616. [Google Scholar] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef]
- Ledermann, J.A. First-line treatment of ovarian cancer: Questions and controversies to address. Ther. Adv. Med. Oncol. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Dilruba, S.; Grondana, A.; Schiedel, A.C.; Ueno, N.T.; Bartholomeusz, C.; Cinatl, J., Jr.; McLaughlin, K.M.; Wass, M.N.; Michaelis, M.; Kalayda, G.V. Non-Phosphorylatable PEA-15 Sensitises SKOV-3 Ovarian Cancer Cells to Cisplatin. Cells 2020, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuniga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernandez-Tilapa, G.; Mendoza-Catalan, M.A.; Zacapala-Gomez, A.E.; Ortiz-Ortiz, J.; Ortuno-Pineda, C.; Navarro-Tito, N. Natural isoflavonoids in invasive cancer therapy: From bench to bedside. Phytother. Res. 2021, 35, 4092–4110. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S.; Song, Y.S. Genistein as a Potential Anticancer Agent against Ovarian Cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Fan, X.; Wang, Z.; Zhu, W.; Li, J. Alpinumisoflavone radiosensitizes esophageal squamous cell carcinoma through inducing apoptosis and cell cycle arrest. Biomed. Pharmacother. 2017, 95, 199–206. [Google Scholar] [CrossRef]
- Ateba, S.B.; Mvondo, M.A.; Djiogue, S.; Zingue, S.; Krenn, L.; Njamen, D. A Pharmacological Overview of Alpinumisoflavone, a Natural Prenylated Isoflavonoid. Front. Pharmacol. 2019, 10, 952. [Google Scholar] [CrossRef]
- Namkoong, S.; Kim, T.J.; Jang, I.S.; Kang, K.W.; Oh, W.K.; Park, J. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-kappaB pathways in lung tumor cells. Biol. Pharm. Bull. 2011, 34, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Masri, M.; Paliotti, K.; Tran, R.; Halaoui, R.; Lelarge, V.; Chatterjee, S.; Wang, L.T.; Moraes, C.; McCaffrey, L. Architectural control of metabolic plasticity in epithelial cancer cells. Commun. Biol. 2021, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Capoferri, D.; Grillo, E.; Silvestri, M.; Corsini, M.; Zanotti, L.; Todeschini, P.; Ravaggi, A.; Bignotti, E.; Odicino, F.; et al. The Claudin-Low Subtype of High-Grade Serous Ovarian Carcinoma Exhibits Stem Cell Features. Cancers 2021, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Lee, J.Y.; Yang, C.; Song, G.; Lim, W. Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis. Mar. Drugs 2020, 18, 45. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.S.; Kim, H.S.; Song, G.; Lim, W. Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 602–614. [Google Scholar] [CrossRef]
- Singh, K.K. Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann. N. Y. Acad. Sci. 2004, 1019, 260–264. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.; Lim, W.; Song, G. Therapeutic potential of alpha, beta-thujone through metabolic reprogramming and caspase-dependent apoptosis in ovarian cancer cells. J. Cell. Physiol. 2021, 236, 1545–1558. [Google Scholar] [CrossRef]
- Emmings, E.; Mullany, S.; Chang, Z.; Landen, C.N., Jr.; Linder, S.; Bazzaro, M. Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, S.; Hou, F.; Zhang, C.; Gao, J.; Wang, K. Inhibition of Ca(2+)-activated chloride channel ANO1 suppresses ovarian cancer through inactivating PI3K/Akt signaling. Int. J. Cancer 2019, 144, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, J.; Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 2014, 290, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.E.; Cullinane, C.; Hannan, K.M.; Wall, M.; Chan, J.; Barber, F.; Foo, J.; Cameron, D.; Neilsen, A.; Ng, P.; et al. Synergistic inhibition of ovarian cancer cell growth by combining selective PI3K/mTOR and RAS/ERK pathway inhibitors. Eur. J. Cancer 2013, 49, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Chaudhary, A.K.; Kumar, S.; O’Malley, J.; Inigo, J.R.; Kumar, R.; Yadav, N.; Chandra, D. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Luo, H.; Zhu, X. Targeting the GRP78 Pathway for Cancer Therapy. Front. Med. 2020, 7, 351. [Google Scholar] [CrossRef]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef] [Green Version]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Park, G.B.; Kim, Y.S.; Lee, H.K.; Song, H.; Cho, D.H.; Lee, W.J.; Hur, D.Y. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J. Immunol. 2010, 185, 7274–7284. [Google Scholar] [CrossRef]
- Hoogenboom, B.W.; Suda, K.; Engel, A.; Fotiadis, D. The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J. Mol. Biol. 2007, 370, 246–255. [Google Scholar] [CrossRef]
- Tsunoda, T.; Koga, H.; Yokomizo, A.; Tatsugami, K.; Eto, M.; Inokuchi, J.; Hirata, A.; Masuda, K.; Okumura, K.; Naito, S. Inositol 1,4,5-trisphosphate (IP3) receptor type1 (IP3R1) modulates the acquisition of cisplatin resistance in bladder cancer cell lines. Oncogene 2005, 24, 1396–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.; Xu, Y.; Gao, W.; Zhang, Y.; Su, J.; Liu, Y.; Guo, Y.; Dou, M.; Hu, K.; Sun, L. TATfused IP3Rderived peptide enhances cisplatin sensitivity of ovarian cancer cells by increasing ER Ca2+ release. Int. J. Mol. Med. 2018, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]




| Antibodies | Catalog No. | Supplier | Dilution |
|---|---|---|---|
| PCNA | sc-56 | Santa Cruz | 1:1000 |
| β-actin | sc-47778 | Santa Cruz | 1:1000 |
| p-P70S6K (Thr421/Ser424) | 9204 | Cell Signaling Technology (CST) | 1:1000 |
| p-S6 (Ser235/Ser236) | 2211 | CST | 1:1000 |
| p-P38 (Thr180/Tyr182) | 4511 | CST | 1:1000 |
| p-ERK1/2 (Thr202/Tyr204) | 9101 | CST | 1:1000 |
| p-P90RSK (Ser573) | 9346 | CST | 1:1000 |
| t-P70S6K | 9202 | CST | 1:1000 |
| t-S6 | 2217 | CST | 1:1000 |
| t-P38 | 9212 | CST | 1:1000 |
| t-ERK1/2 | 4695 | CST | 1:1000 |
| RSK1/RSK2/RSK3 | 9355 | CST | 1:1000 |
| p-eIF2α (Ser122) | 3398 | CST | 1:1000 |
| GRP78 | sc-13968 | Santa Cruz | 1:1000 |
| IP3R1 | PA1-901 | Invitrogen | 1:1000 |
| VDAC | 4661 | CST | 1:1000 |
| t-eIF2α | 5324 | CST | 1:1000 |
| TUBA | sc-32293 | Santa Cruz | 1:2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, T.; Ham, J.; Song, G.; Lim, W. Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer. Pharmaceutics 2022, 14, 564. https://doi.org/10.3390/pharmaceutics14030564
Hong T, Ham J, Song G, Lim W. Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer. Pharmaceutics. 2022; 14(3):564. https://doi.org/10.3390/pharmaceutics14030564
Chicago/Turabian StyleHong, Taeyeon, Jiyeon Ham, Gwonhwa Song, and Whasun Lim. 2022. "Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer" Pharmaceutics 14, no. 3: 564. https://doi.org/10.3390/pharmaceutics14030564
APA StyleHong, T., Ham, J., Song, G., & Lim, W. (2022). Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer. Pharmaceutics, 14(3), 564. https://doi.org/10.3390/pharmaceutics14030564








