1. Introduction
Conventional drug treatments aim to minimize the side-effects of drugs by targeting specific receptors or bodily compartments. For diseases with episodic, paroxysmal expression, another way to limit side-effects is to reduce exposure to active drugs inside the body by delivering the drug only when and where it is needed [
1]. Time-selective focal triggering of drug release has the potential to reduce side-effects by limiting drug exposure to the specific time period and location at which therapeutic effects occur [
2]. Such a possibility requires the development of on-demand drug delivery devices that sequester drugs so that they remain inert until required, and trigger release when necessary. We here report the electrophysiological effects of on-demand release of the gama aminobuturic acid (GABA) agonist, muscimol, from hollow gold nanoparticle-tethered liposomes (HGN-liposomes) and its effectiveness in attenuating seizures in experimental animal models.
Liposomes are phospholipid-based vesicles composed of a lipid bilayer, in which a wide range of drugs can be encapsulated [
3]. Drug release from liposomes can be triggered by external stimulation, such as hyperthermia [
4]. Earlier studies aimed at discharging the entire contents of the liposomes in a single release event [
5]. Later studies investigated the possibility of repeated release from liposomes over an extended life-time in the body. Our recently developed HGN-liposome system provides repetitive, on-demand release
ex vivo, with the temporal profile and quantity of release controlled by varying laser power and exposure duration or pulses of low-intensity, therapeutic ultrasound (US) [
6]. Here, we apply these technologies to release muscimol from liposomes within the extracellular matrix of the mammalian brain.
We used muscimol (3-hydroxy-5-aminomethylisoxazole) in the present study because it is a potent and selective GABA
A receptor agonist, used extensively in electrophysiological studies of GABAergic inhibitory neurotransmission. Muscimol potently and reversibly inhibits neuronal activity, and thus has been considered to have the potential to suppress an epileptic seizure [
7]. The metabolism of muscimol in both the brain and periphery is largely through the removal of an amino group by transamination [
8]. In the mouse, about 1/3 is excreted as muscimol, 1/3 as a cationic conjugate, and 1/3 as an oxidation product [
9]. The rapid clearance of muscimol in the periphery, and its slow passage across the blood–brain barrier (BBB), mean that high doses are needed when given intravenously, causing adverse effects, and making it unsuitable for systemic use in the treatment of epilepsy [
10,
11].
In contrast to systemic administration, when delivered transmeningeally in experimental animals, muscimol has antiepileptic effects, without the adverse effects associated with systemic delivery [
12,
13,
14,
15]. Direct injection of muscimol into the brain is orders-of-magnitude more effective than intravenous injection. For example, nanomomolar concentrations injected into brain produce similar effects to micromolar concentrations injected intravenously [
16,
17]. When injected locally into the brain in low μg quantities, muscimol produced no sedation or other central side-effects [
18,
19]. Similarly, studies of convection enhanced delivery of muscimol into the brain of non-human primates and patients with drug-resistant epilepsy, as well as other disorders, have shown that it is safe, with no adverse effects [
20,
21,
22]. Thus, muscimol is a potential anti-epileptic treatment with few side-effects, provided it can be delivered directly to the brain. Muscimol is, therefore, a suitable candidate for proof of concept of the HGN-liposome delivery system.
Here, we used muscimol-loaded HGN-liposomes to produce repetitive on-demand release of muscimol within the extracellular matrix of the brain. We aimed to determine whether release of muscimol from HGN-liposomes by laser or ultrasound stimulation in live brain tissue was effective in attenuating seizure activity. We used three well-known models of seizure activity: two
ex vivo models that rely on removal of Mg
2+ ions and repetitive stimulation [
23,
24,
25]; and
in vivo pentylenetetrazol (PTZ), to cause seizures that propagate to status epilepticus [
26]. We found that when muscimol-containing HGN-liposomes were present in brain slices, laser or US stimulation released muscimol on-demand and caused neural inhibition and arrest of seizure activity. Similarly, in whole animals, we found that US stimulation of the brain after intravenous injection of muscimol loaded HGN-liposomes was effective in reducing seizure activity.
2. Methods
2.1. Animals
A total of 11 mice and 58 rats were used in the research. Animals were handled in accordance with protocols approved by the Okinawa Institute of Science and Technology Animal Care and Use Committee (ex vivo hippocampal seizure model) and the University of Otago Animal Ethics Committee (ex vivo entorhinal cortex seizure model, and in vivo seizure model). In the ex vivo hippocampal experiments, brain slices were obtained from n = 6 male, 3 to 8-week-old Swiss Webster mice. Mice were group housed with littermates on reversed light cycle, with free access to standard chow and water. An additional n = 5 male 3 to 5-week-old Swiss Webster mice were used to test liposome ability to sequester contents in absence of stimulation. After injection, these mice were individually housed until perfused for histology. In the ex vivo entorhinal cortex experiments, brain slices were obtained from 40 male and female 4 to 8-week-old Wistar rats. In the in vivo experiments a total of n = 18 male Wistar rats were used, group housed in standard open top cages under reverse light cycle, and fed standard rat chow and water ad libitum. The targeted weight range was 250 to 300 g. These were allocated to four groups, unbiased by any animal-related factors (PTZ-only, n = 3; PTZ plus HGN-liposome, n = 3; US without liposomes, n = 4; and PTZ plus HGN-liposome plus US, n = 8).
2.2. HGN-Liposome Preparation
Liposomes and hollow gold nanoshells were prepared and assembled, as previously described [
6,
27,
28], by conjugation using a terminal thiol-derived phospholipid (DSPE-PEG2000-SH) to produce a biocompatible drug delivery system that could encapsulate muscimol, a GABA agonist. DSPE-PEG2000-SH was synthesized by combining 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000-NH
2) (100 mg; 35.8 μmol with 2-iminothiolane (10 mg, 73 μmol) in phosphate buffer (3 mmol L
−1; pH 9.5; 15 mL) for 30 min at room temperature. Sodium chloride (approx. 1 g) was dissolved in the reaction mixture, and the product was subsequently extracted into chloroform and dried over magnesium sulfate. The solvent was then removed by rotary evaporation, and the product was further dried under vacuum for 5 h.
Hollow gold nanoparticles were synthesized by the galvanic replacement of a silver nanoparticle template, as previously reported, resulting in a hydrodynamic diameter of approximately 30 nm and strong absorption in the visible to near-infrared region [
29]. HGN-liposomes were prepared using a phospholipid composition previously described by our group [
6,
27,
28]. Chloroform solutions of 1.2-distearoyl-sn-glycero-3-phosphcholine (DSPC), cholesterol, sphingomyelin, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000), DSPE-PEG2000-SH were combined in a molar ratio of 100:5:5:4:3.5. The solvent was removed under vacuum to form a thin lipid film, which was rehydrated using a phosphate-buffered solution (20 mmol L
−1 Na
2HPO
4; pH 5.5) containing either 100 mmol L
−1 muscimol or 25 mM kainic acid. The lipid suspension was then extruded through 400-nm polycarbonate membranes, to maximize the passive encapsulation of muscimol, producing uniformly sized liposomes for laser studies. However, as liposomes of smaller sizes are generally regarded as more suitable for intravenous administration, liposomes of 200 nm were prepared for ultrasound studies. The suspension of concentrated HGNs (Au concentration 6–10 mg mL
−1 by inductively coupled plasma mass spectrometry) was added incrementally to the liposome suspension, until a final HGN:liposome ratio of approximately 1:1 was reached (as determined by transmission electron microscopy (
Figure 1A). Approximately 200 μL of HGNs with an Au concentration of 7 mg mL
−1 to 1 mL of 200 nm liposomes, with a total lipid concentration of 10 mmol L
−1, or approximately ¼ the amount of gold was added to 400 nm liposomes, with an equivalent 10 mmol L
−1 phospholipid concentration. The HGN-liposome suspension was subsequently dialyzed against phosphate buffered saline (100 mmol L
−1 NaCl; 20 mmol L
−1 Na
2HPO
4; pH 7.4; 2 L) for 24 h to remove the unencapsulated muscimol.
We have previously reported on release measurements of dopamine, as well as carboxyfluorescein, from ultrasound and laser activated HGN liposomes [
6,
27]. In the present study, we were unable to do in vitro release studies for muscimol, because we were unable to identify a chemical, electrochemical, or spectroscopic assay that could distinguish between encapsulated and non-encapsulated muscimol and that was sensitive enough to measure its release in real time. Hence, we used the biological assays described below. Further details of the preparation and analysis of liposomal nanostructures and hollow gold nanoshells are given in
Supplementary Materials Figures S1–S5.
2.3. Ex Vivo Hippocampal Seizure Model
Mice were deeply anesthetized with isoflurane and decapitated, and the brain was rapidly removed. Horizontal slices, 300 mm thick, containing the hippocampus were cut on a vibratome (VT1200S, Leica Microsystems, Wetzlar, Germany) in cold cutting solution containing the following (in mM): 92.0 N-methyl-D-glucamine (NMDG), 2.5 KCl, 10.0 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 30.0 NaHCO3, 20.0 HEPES, 2.0 thiourea, 5.0 sodium ascorbate, 3.0 sodium pyruvate, and 25.0 glucose, and saturated with 95% O2—5% CO2, Slices were then incubated in oxygenated artificial cerebrospinal fluid (ACSF) maintained at a temperature of 36 °C for 1 h. The standard ACSF had the following composition (mM): 118.0 NaCl, 2.5 KCl, 2.0 CaCl2, 1.0 MgCl2, 26.0 NaHCO3, 1.25 NaH2PO4, 1.5 myo-inositol, 0.5 sodium ascorbate, 2.0 sodium pyruvate, and 10.0 Glucose. The composition of low Mg2+/high K+ ACSF was the same, except for (mM) 5.0 K+ and 0.5 Mg2+.
After incubation, a single slice was transferred to a recording chamber placed on the stage of an upright microscope, and perfused (3–4 mL/min) with oxygenated ACSF at 32 °C. HGN-liposomes were injected directly into the slice in the region of interest. The remaining slices were kept in a holding chamber containing oxygenated ACSF at room temperature until required.
The experimental setup for the hippocampal slice experiments is shown in
Figure 1A. Whole-cell recordings were made from CA1 pyramidal neurons using patch pipettes (4–6 MΩ) filled with internal solution containing the following (mM): 132.0 K gluconate, 6.0 KCL, 6 NaCl
2, 10.0 HEPES, 2 MgCL
2, 2.0 NaATP, 0.4 NaGTP, 0.5 EGTA; pH 7.2–7.4. Local field potentials (LFPs) were recorded in the same location using extracellular electrodes positioned in the CA1 stratum pyramidal layer of the subiculum. LFPs were measured using borosilicate glass pipettes (1–2 MΩ) filled with ACSF. Signals were amplified by MultiClamp 700B (Molecular Devices, Union City, CA, USA), digitized at 10,000 Hz, and band-pass filtered over 1–2000 Hz by pCLAMP 10 (Molecular Devices, Silicon Valley, CA, USA). Offline analysis was conducted using MATLAB (MathWorks, Natick, MA, USA).
Optical stimulation of liposomes was delivered using infrared (890 nm) femtosecond (fs) pulsed laser of a 2-photon microscope. Pulse duration was 100 fs, and repetition rate was 80 MHz. Laser pulses were transmitted through a 60× objective lens and made a 430-nm diameter spot in the brain slice. The light source (MaiTai, Coherent, Santa Clara, CA, USA) provided continuous laser power at the source of approximately 2 W, which was attenuated by an acousto-optic modulator. The laser stimulation was set using software (FluoView, Olympus, Tokyo, Japan) to a scan area of 211.14 µm × 211.14 µm and a sampling speed of 10 µs/pixel.
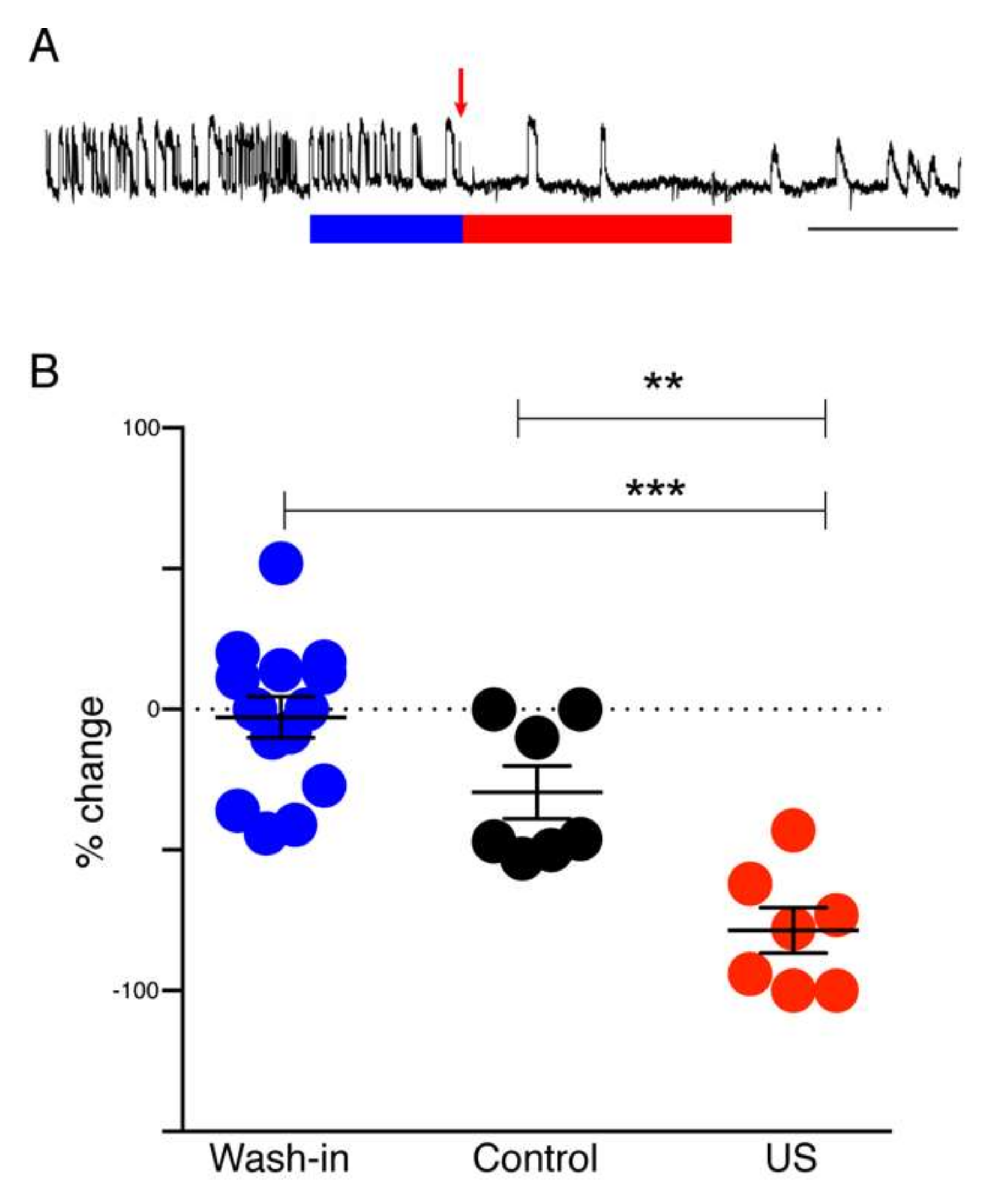
In the hippocampal slice model system, seizures were induced by perfusion with low Mg2+/high K+ ACSF. Spontaneous seizure-like events (SLEs) seldom occurred in response to this treatment alone. When SLEs did not occur spontaneously they were induced by repetitive electrical stimulation. Electrical stimulation (600–1200 µA, 100 µs, monophasic) was applied through a bipolar stimulating electrode placed in CA3, in order to stimulate Schaffer collaterals. The intensity of the stimulation for each slice was adjusted to a value that evoked SLEs in the CA1 area. After initial adjustment, the stimulation intensity remained fixed. To test the effect of muscimol release from liposomes, HGN-liposomes containing muscimol (100 mM, Tocris Bioscience, Tokyo, Japan), were pressure-injected directly into slices via a glass micropipette (tip diameter 50–100 µm) over a period of 1 s.
2.4. Ex Vivo Entorhinal Cortex Seizure Model
Experiments using an entorhinal cortex (EC) seizure model were performed on brain slice wedges obtained from 40 male and female 1–2 month-old Wistar rats. Electrophysiological recordings in the EC were made using methods described previously [
23]. Briefly, horizontal combined hippocampal EC slices (500 μm thick) were cut using a Vibroslice (Campden Instruments, Leicester, UK). From these slices, a wedge-shaped segment of the EC tissue, 2–3 mm wide, was dissected and transferred to a custom designed two-compartment grease gap chamber (see experimental setup in
Figure 1B) continuously perfused with ACSF at room temperature (~1.5 mL/min). The ACSF contained (in mM): NaCl 135, KCl 3, NaH
2PO
4 1.25, MgCl
2 2, CaCl
2 2, glucose 10, and NaHCO
3 26 (all from Sigma, NZ), saturated with 95% O
2/5% CO
2. For Mg
2+-free ACSF, the MgCl
2 was omitted. An HGN-liposome reservoir was plumbed in and out of the perfusion flow and an ultrasound probe was positioned at the base of the HGN-liposome reservoir to trigger release of muscimol.
Differential recordings were made across the grease gap using Ag/AgCl pellet electrodes (Harvard Apparatus, Waterbeach, UK) located in both chambers (
Figure 1B) with an NL102 amplifier (Neurolog, Welwyn Garden City, UK) (×100 gain, high-pass filter with 8.9 Hz cut-off) and a chart recorder (Semat, London, UK). After placing the slice on the grease gap, the slice was perfused with ACSF for 30 min, and thereafter with ACSF lacking Mg
2+. Removal of Mg
2+ from the ACSF led to the appearance of repetitive SLEs 40 to 120 min after the switch. A solution of muscimol-containing HGN-liposomes was applied for 15 min, after which the ultrasound (US) trigger was applied to the liposome reservoir (
Figure 1B). Slices were perfused with this ultrasonicated liposomal formulation for 30 min before being washed-out with Mg
2+-free ACSF and the frequency of seizure-like events (SLEs) and late recurrent discharges (LRDs) was measured before and during drug application.
2.5. In Vivo Seizure Model
Rats were anesthetized (urethane, 1500 to 1800 mg/kg) and mounted in a stereotaxic frame (
Figure 1C). Craniotomies were made on the superior surface of frontal bone (2.7 mm in diameter) and on the lateral side caudal to the left orbit (4.0 mm in diameter). A 4-mm collimator was inserted through the lateral craniotomy and pressed gently against the dura over the lateral aspect of the left frontal lobe, with a layer of acoustic coupling gel between. A silver wire epidural electroencephalogram (EEG) recording electrode was secured using dental cement in the uppermost craniotomy. Epileptiform EEG was induced using 60 mg/kg PTZ administered intravenously via a cannula in the left jugular vein. Animals were divided into four groups (PTZ-only,
n = 3; PTZ plus HGN-liposome,
n = 3; US without liposomes,
n = 4; and PTZ plus HGN-liposome plus US,
n = 8). In groups exposed to muscimol-loaded HGN-liposomes (90 mM), the formulation was administered intravenously via the jugular cannula and allowed to circulate for 5 min, after which ultrasound was delivered in bursts of 30 sec at 30% duty cycle at 1 MHz. No more than three applications of ultrasound occurred in any given 5 min recording interval.
For analysis, individual power spectra were constructed and normalized, such that the total area under the curve of each plot was 1. The fraction of total area in the EEG frequency bands 0–1 Hz, 1–3 Hz, and 3–5 Hz was calculated. For each rat mean post-PTZ values for the intervals 10–19, 20–29, 30–39, and 40–49 min were derived by averaging the values for the 1st and 2nd 5-min period in each interval. These averaged time epoch data for each rat were then normalized to the values for the 5-min period following PTZ application.
2.6. Test of Liposome Ability to Sequester Contents in Absence of Stimulation
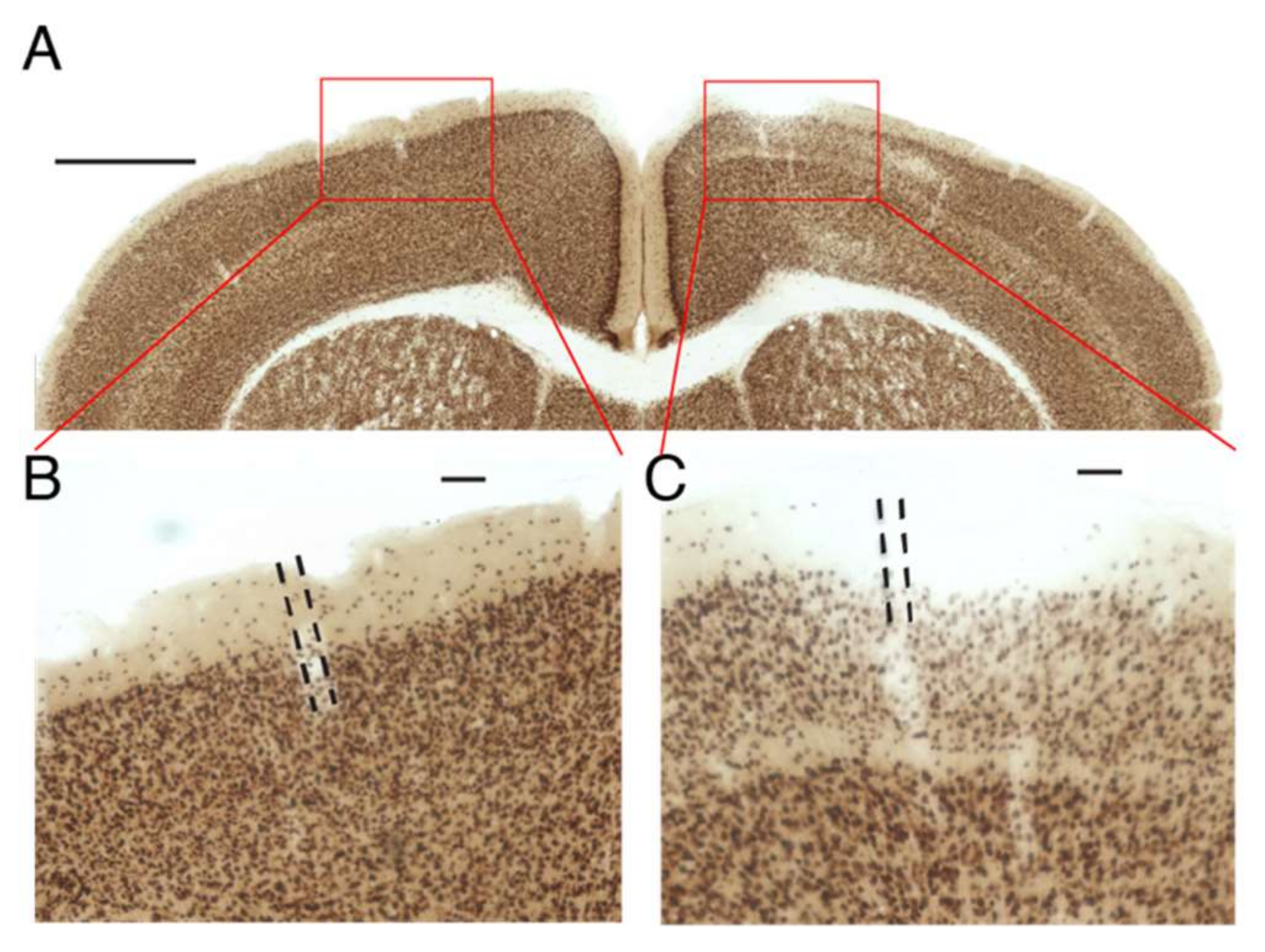
For this control experiment, designed to test whether a drug will remain in HGN-liposomes until released by an external trigger, HGN-liposomes containing kainic acid (KA) were injected into the primary somatosensory cortex of mice at stereotaxic coordinates (AP: −1.0 mm, ML: +/−1.5 mm, DV: 1.5 mm) in a volume of 1.0 µL. Positive control injections of KA directly into brain tissue were made in other mice, using a volume of 1 µL in a concentration of 10 nM. One week after the injection of HGN-liposomes, animals were perfused with 4% paraformaldehyde and were brains extracted and post-fixed in the same fixative. Coronal sections (80 µm) using a vibratome (VT1000S; Leica, Wetzlar, Germany) were prepared and sections divided into four vials. NeuN staining for neuronal nuclei was performed by Neu-N primary antibody (AB104224; Abcam, Tokyo, Japan) and a secondary antimouse IgG-conjugated biotin (Invitrogen, Tokyo, Japan). NeuN signals were enhanced by an avidin–biotin complex method (ABC Elite; Vector Laboratories, Tokyo, Japan) and visualized using a metal-enhanced DAB Substrate Kit (#34065; Thermo Scientific, Tokyo, Japan). Images of sections were obtained using a digital microscope (BZ-9000; Keyence, Osaka, Japan) and inspected for obvious qualitative signs of neuronal loss.
2.7. Statistical Analysis
For statistical analysis of group differences in the
ex vivo wedge experiments, we used one-way analysis of variance (ANOVA) to test for overall group differences and Tukey’s multiple comparisons post hoc test for contrasts. For statistical analysis of group differences in the
in vivo experiments, we used general linear model mixed model (GLMM) [
30,
31] analysis of data, further dissected by Fisher’s least significant difference post hoc analyses. In the
in vivo experiments GLMM analysis was used, due to the use of multiple control groups of smaller size.
4. Discussion
The main finding of this study was that on-demand release of muscimol using a HGN-liposome drug delivery system effectively reduced seizure activity in ex vivo and in vivo experimental models. In brain slices, patch-clamp recordings from hippocampal pyramidal cells showed that laser-triggered release of the GABA agonist muscimol from HGN-liposome caused hyperpolarization of the membrane potential and blockade of action potentials. This remotely-controlled release of muscimol also arrested seizure activity (SLEs, LRDs) in the hippocampus and entorhinal cortex wedges. In anaesthetized animals, in vivo US stimulation of previously intravenously injected HGN-liposomes caused attenuation of PTZ-induced SLEs, observed as increased power in the high frequency spectrum of the EEG. Finally, HGN-liposomes protected brain tissue from damage by intra-liposome neurotoxin for at least one week, demonstrating effective containment. Together, these findings demonstrate the potential for effective reduction of seizure activity in vivo, with reduced toxicity, by remotely controlled release of drugs sequestered in HGN-liposome.
We used muscimol in these proof-of-concept experiments, rather than approved medications, for several reasons. First, we were aiming for immediate seizure suppression, on demand, and focally at the site of seizure generation. Muscimol is both potent and rapidly-acting, thus is suitable for this approach. As shown in the present paper, and earlier studies [
12,
13,
14,
15], muscimol is immediately effective in attenuating seizures when applied locally at the site of the seizures. In contrast, the approved drugs are optimized for systemic treatment with minimal side effects, require several dosing cycles to make them effective at stopping seizures, and do not act as quickly when locally applied. Thus, because they are not optimal for local application and generally effective given systemically, there is less value in using them in HGN-liposome delivery. Second, about one-third of people with epilepsy have seizures refractory to systemic pharmacotherapy with approved medications [
32]. For these people in particular, new approaches are needed. Third, as noted by Gernert [
32], targeted intracranial delivery, by providing higher drug concentrations in localized target regions, and lower concentrations in other brain or peripheral areas, allows the use of drugs that are otherwise unsuitable for systemic administration because of their toxicity or poor uptake into the brain. Since intracranial delivery of muscimol, in small quantities, has been shown to be safe in previous studies [
11,
12,
13,
14,
15,
18,
19,
20], we used it as a test of the delivery system.
In the present study, we used laser stimulation to cause release of muscimol from HGN-liposomes in brain slices. Several previous studies have established that substances can be released from HGN-liposome nanostructures on a rapid timescale by laser stimulation in non-biological assays [
27,
33,
34,
35]. These studies demonstrated the feasibility of drug delivery on a rapid timescale using laser stimulation. We have also previously shown that release and dosage can be controlled by varying the number and intensity of femtosecond pulses of light [
27]; and, furthermore, that on-demand release of different neurochemicals and drugs from HGN-liposome in live brain tissue has rapid, repeatable, and reliable physiological effects [
28]. However, at present, laser-stimulated release from HGN-liposomes is not suitable for
in vivo use, because light does not penetrate far through the skull or brain parenchyma, and miniature femtosecond pulsed lasers are not available for chronic implantation. On the other hand, focused US can be transmitted through the skull and brain tissue. Recent work has demonstrated the feasibility of US-stimulated release of drugs from liposomes
in vivo [
2]. We have also shown that in vitro, US can evoke multiple release events of a constant amount over 25 individual applications [
6]. The present study extends this work, by showing that transcranial US-stimulation, both
ex vivo and
in vivo, can cause sufficient release of muscimol from HGN-liposomes, to arrest ongoing seizure activity.
Our intracellular recordings of the timecourse of the membrane potential hyperpolarization show that the timecourse of the inhibitory effect of muscimol after release from HGN-liposomes is extremely short, on the order of seconds. This finding is consistent with previous reports of rapid, subsecond to second clearance of other neurotransmitters such as dopamine [
27] and glutamate [
28], measured after release from laser-stimulated HGN-liposomes. The amount of release and, hence, peak concentration of the drug obtained with each stimulation is linearly related to duration and intensity for both laser [
27] and US [
6] stimulation. Since only a small percentage of content is released with each stimulus, dose can be titrated against clinical effect by increasing intensity or duration of stimulation, and terminated immediately by turning off stimulation. These properties of the drug delivery system provide a means for precise control over drug actions.
The very small quantities of muscimol contained in, and released from, HGN-liposomes are unlikely to cause adverse effects. The amount of muscimol needed to produce therapeutic effects by local application in the brain is small compared to the amount that would cause side effects after diffusion into the cerebrospinal fluid and distribution throughout the bloodstream. Studies in non-human primates showed that administration of muscimol into the subarachnoid space suppressed seizures locally, but otherwise led to no detectable levels of muscimol in blood or cisternal CSF [
15]. Delivery of 1.0–2.5 mM muscimol into the neocortex of rodent and nonhuman primate models has been shown to have powerful antiepileptic effects, without adverse effects on the animal’s behavior [
13,
36]. Muscimol is metabolized in the brain and periphery and excreted in the urine in roughly equal proportions as unchanged muscimol, a cationic conjugate, or an oxidation product [
9,
37]. Experiments with [3H] muscimol showed that it rapidly disappears from the blood [
8].
The biocompatibility, distribution and eventual fate of the liposome constituents and HGNs is less clear and possibly more of a concern than the distribution of muscimol. After intravenous injection liposomes in circulation might be sequestered in liver or spleen. They might also cause immune reactions peripherally or cellular changes after crossing the BBB. Some of the pitfalls have been reviewed recently [
38]. Gold, the constituent of the HGN component, has been used medically in ionic form in the treatment of human rheumatoid arthritis, and the literature concerning adverse reactions to ionic gold has been reviewed recently [
39]. In rats, gold nanoparticles were found to be biocompatible and relatively innocuous after intravenous injection, but the highest accumulation was in spleen and lowest in brain [
40]. Laser-synthesized gold nanoparticles are considered to be purer and safe for biomedical applications [
41], without causing liver damage. However, studies of gold nanoparticle effects in mice have revealed an increased rate of abortion and fetal abnormalities if given in the early pregnancy [
42]. Reviews of this topic highlight the limited available evidence and need for more knowledge concerning the toxicity of HGN after injection [
43]. Excretion of accumulated particles from the liver and spleen can take up to 3 to 4 months, indicating that further studies of the toxicity of HGNs are needed.
Even if biocompatibility issues can be overcome, clinical application of muscimol-containing HGN-liposomes will not be feasible until future technological developments provide a practical means to infiltrate them into the brain parenchyma. In the present study, HGN-liposomes may have gained access to the brain as a result of the seizure activity itself [
44], or by momentary disruption of the BBB by the US stimulation, allowing liposome penetration [
45]. The combination of systemic injection of HGN-liposomes with focal US stimulation might, thus, achieve a high local concentration of muscimol in the brain or brain vasculature, with relatively low concentration in the periphery, due to encapsulation within liposomes and dilution of the cerebrally released muscimol. However, procedures such as carotid or intracerebral injections, as used in the present study, are invasive neurosurgical procedures that might only be considered in intractable drug resistant epilepsy [
32]. For routine use, less invasive methods will be required to move HGN-liposomes across the BBB and into the brain. Trojan horse liposomes (THLs) may be a future possibility. THLs are pegylated liposomes with a receptor-specific monoclonal antibody targeted to receptors that can transport liposomes across the blood–brain barrier, such as the transferrin receptor [
46,
47]. The antibody is conjugated to the surface of the THL and the transferrin receptor ferries the liposome across the BBB. Further work is needed to determine if HGN-liposomes can be transported intact from the blood into the brain by hijacking existing transport mechanisms.
To be considered as a possible treatment, liposomes injected
in vivo must also retain the ability to release their contents over a useful time period following administration. Previous work measuring release in brain slices from previously injected animals has shown that liposomes retain their ability to repeatedly release drug after one week
in vivo [
28]. Further work is needed to determine the time course over which liposomal nanostructures remain intact and responsive to ultrasound stimulation after injection
in vivo. Another challenge is to minimize leakage from liposomes, while preserving the ability to trigger release. In the current work, we show that the HGN-liposomes successfully encapsulated KA and could be injected into the brain and remain there for one week, without causing damage to surrounding tissue. Leakage can be minimized by increasing the stability of the liposome formulation. However, there is a trade-off between the stability of liposomes and sensitivity to stimulation, which requires systematic study to arrive at optimal formulations.
Ideally, treatment for epilepsy should prevent seizures before they occur. However, in one third of patients existing treatments are not effective in preventing seizures, and treatment-resistant epilepsy is associated with significant morbidity and mortality [
48,
49]. Existing technology is not yet capable of predicting seizures with clinically useful reliability. However, the technology for seizure detection already exists. For example, Kim et al. [
50] concluded from a review of the literature that ‘… the state-of-the-art seizure detection system performance is sufficient to build a robust and reliable wearable device that could be used for daily seizure monitoring and classification.’ What is needed, therefore, is a means to deliver the drug immediately on the first sign of a seizure. Here we aimed to demonstrate proof of principle that seizures can be arrested almost instantaneously provided HGN-liposomes are preloaded in the brain. However, the practical use of this approach will require the future development of tools for applying US or laser stimulation in an ambulatory patient setting, as well as new technology for infiltrating the HGN-liposomes into the brain. Extensive studies of pharmacokinetics, pharmacodynamics, and toxicity will also be needed to determine the utility and safety of the technology in humans.
5. Conclusions
The present experiments demonstrated that the HGN-liposome formulation we have developed is able to encapsulate and contain muscimol, and release it in the brain in response to femtosecond laser or US stimulation. Release is rapid and immediate, causing fast and repeatable hyperpolarizations of neurons, similar to physiological inhibitory postsynaptic potentials. In ex vivo seizure models, stimulation of muscimol loaded HGN-liposomes caused immediate suppression of spontaneous and electrically-evoked seizure activity. Experiments also showed that ultrasound stimulation applied to the brain through the dura attenuated seizure activity induced by PTZ in rats given intravenous injection of muscimol-loaded HGN-liposomes. We also showed that intracerebrally injected HGN-liposomes loaded with toxic concentrations of KA did not cause damage to surrounding tissue. Thus, we demonstrated the feasibility of precise temporal control over exposure of neurons to the drug, potentially enabling therapeutic effects without continuous exposure. Overall, these findings suggest that HGN-liposomes combined with ultrasound triggering have potential for the development of innovative treatment strategies for neurological disorders, using on-demand release of pharmaceuticals.
The present study focused on epileptic seizures in particular, because of the challenges of long-term treatment with systemic antiepileptic drugs, and the large number of patients with drug-resistant epilepsy. The ability to deliver high concentrations of drug to target areas on demand, while keeping drug concentrations low at other sites and times may enable the use of drugs that are effective when applied locally, but unsuitable for systemic use, because of their effects on other systems. Muscimol is one example of such a drug, which has been found unsuitable for systemic application, but potentially effective when applied locally. For such applications, the development of technology to move HGN-liposomes across the BBB and anchor them with the brain parenchyma would be necessary. Further work is needed to determine the utility and safety of the technology in humans, particularly concerning the pharmacokinetics, pharmacodynamics, and toxicity of HGN-liposomes and their constituents. Technology for detection of seizures and application of US stimulation in ambulatory patients will also be needed. If these problems can be solved, HGN-liposomes have the potential to be developed into a new treatment for responsive forms of epilepsy.