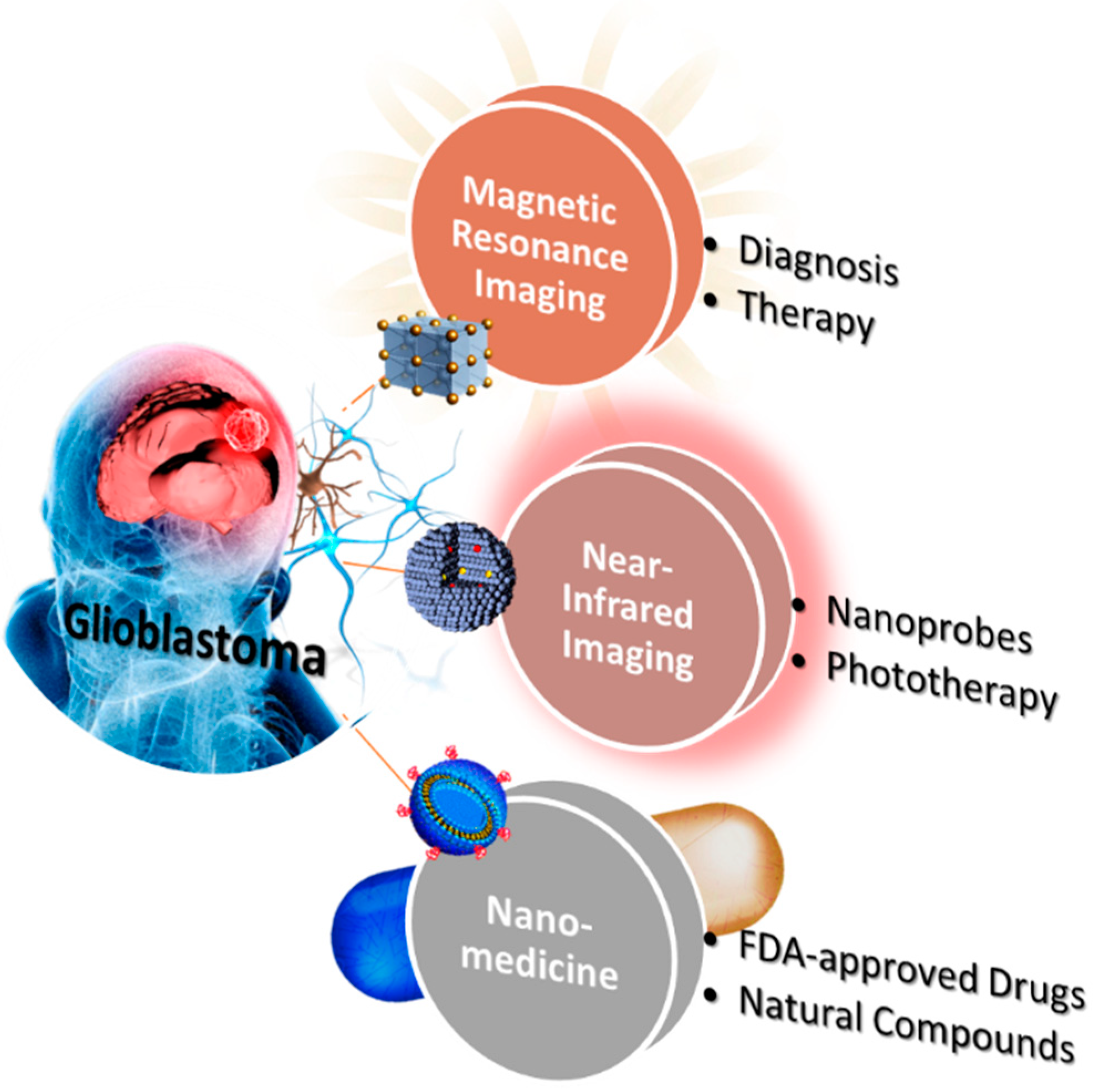
Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics
Abstract
1. Introduction of Brain Glioblastoma
2. Magnetic Resonance Imaging and Therapy of Brain Glioblastoma
2.1. Diagnosis
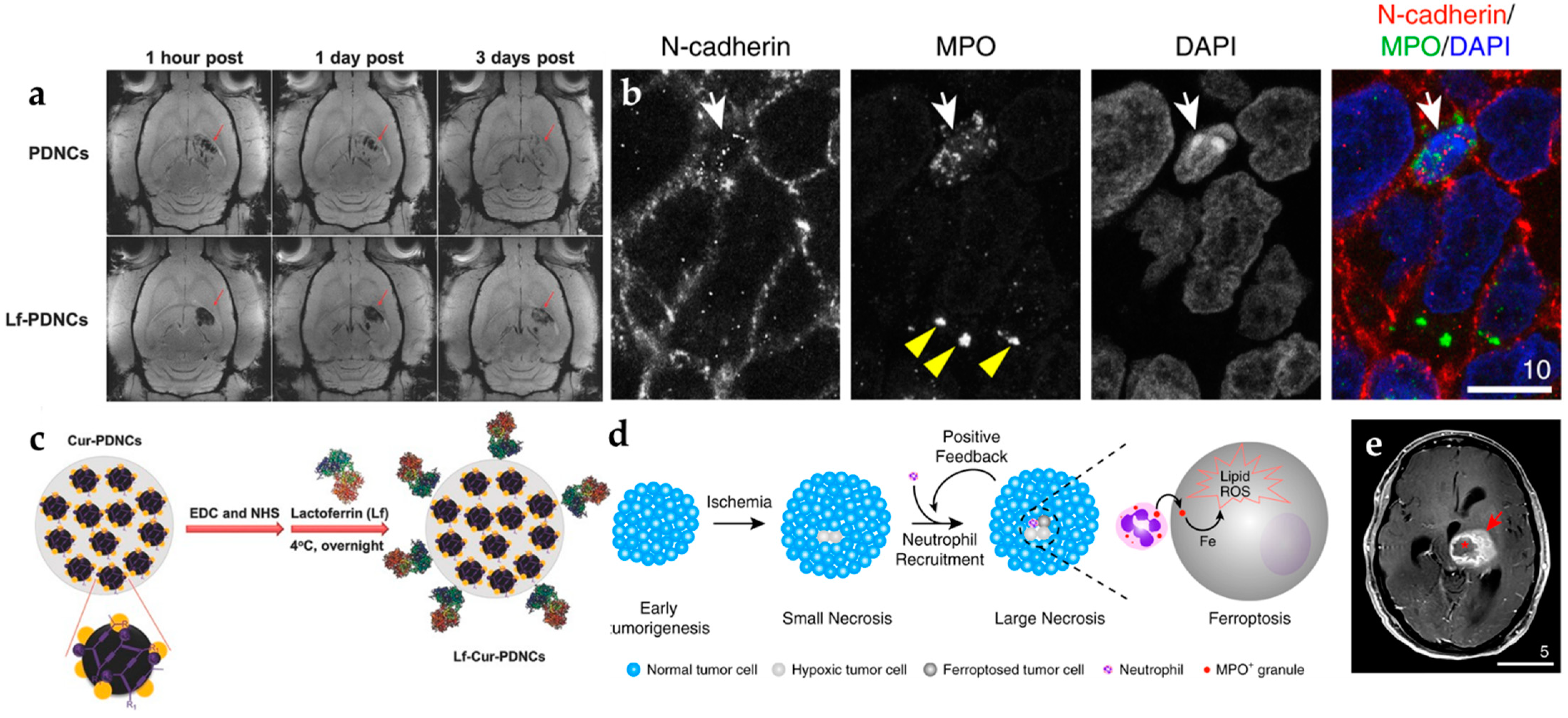
2.2. Therapy
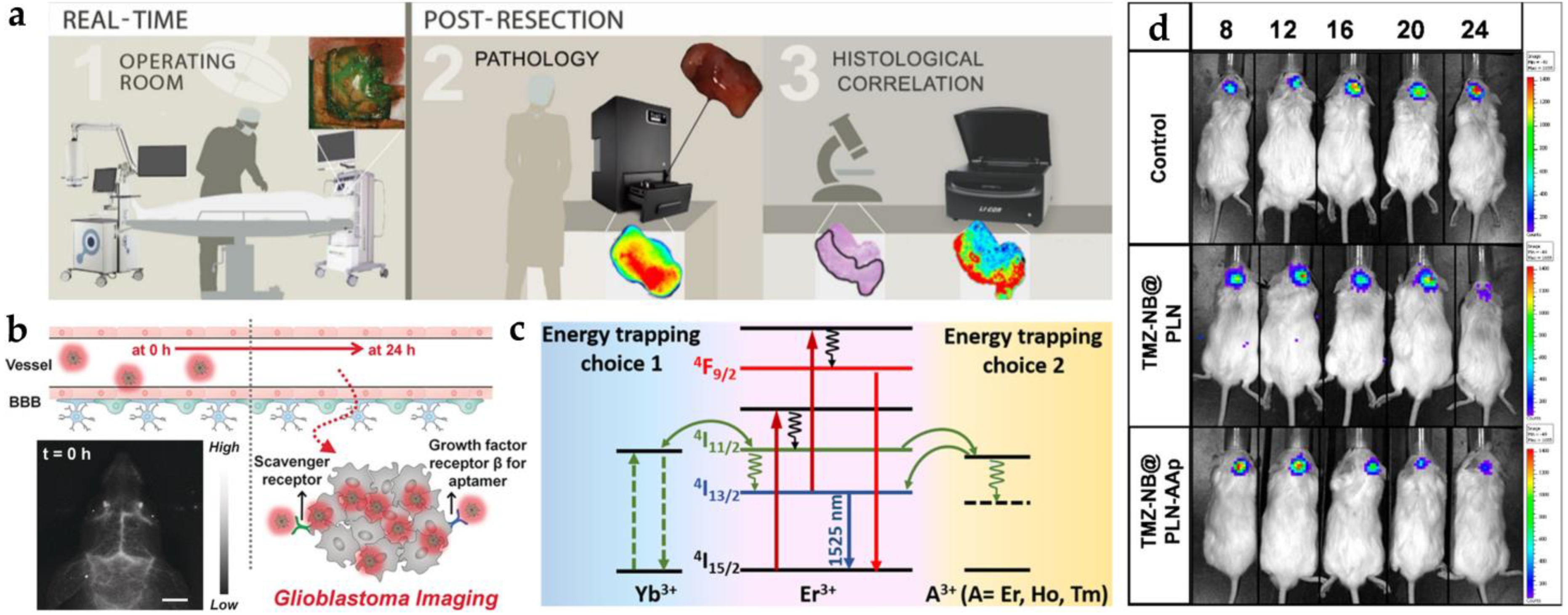
3. Near-Infrared Imaging and Therapy of Brain Glioblastoma
3.1. Diagnosis
3.1.1. Organic Dyes
3.1.2. Lanthanide-Doped Nanoparticles
3.1.3. Quantum Dots
3.1.4. Nanophosphors
3.1.5. Polymer Dots
3.2. Phototherapy and Other Therapies
3.2.1. Photothermal Therapy
3.2.2. Photodynamic Therapy
3.2.3. Other External Energies-Dependent Therapies
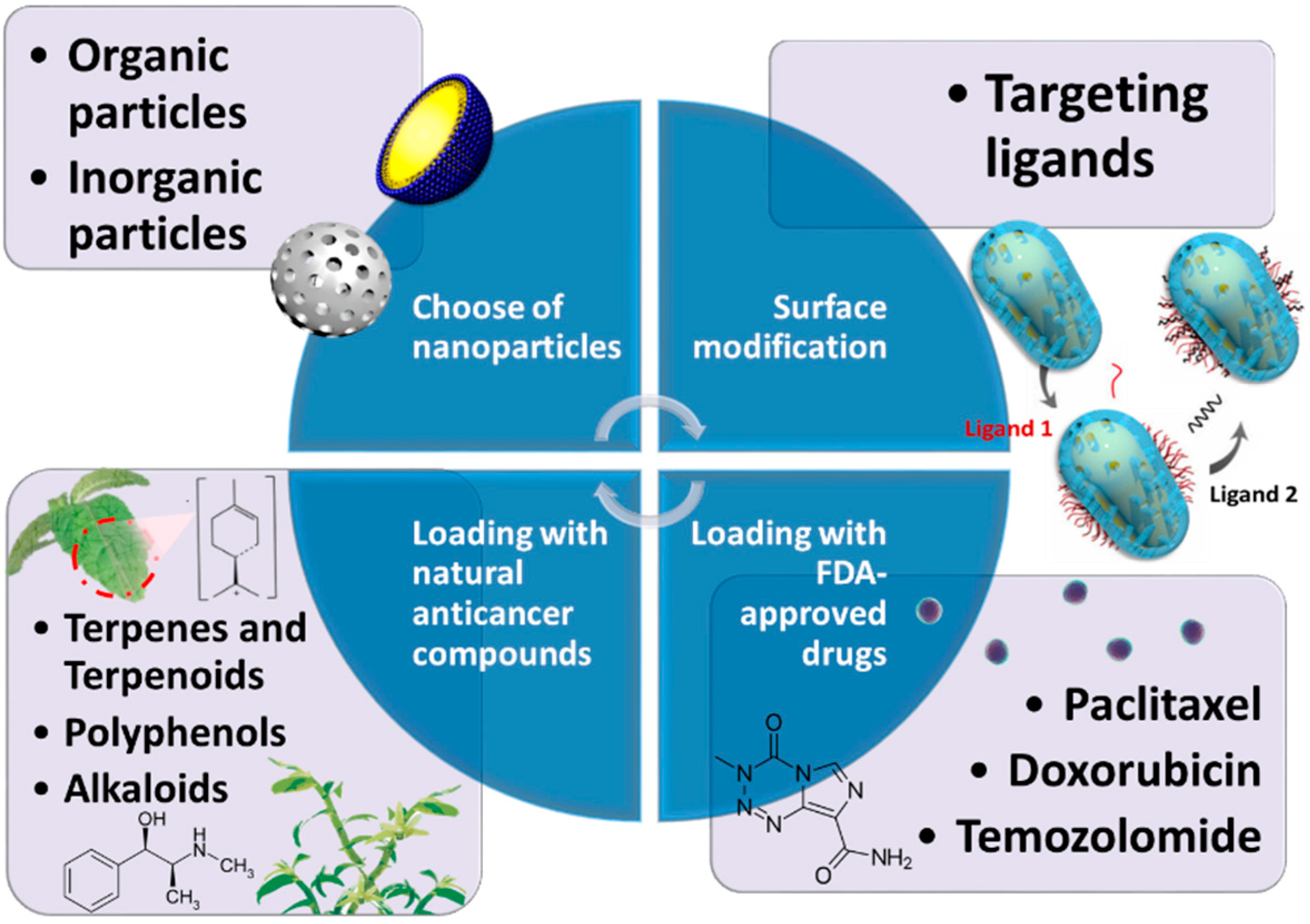
4. Nanocomposites Combined with Chemotherapy Applied in Curing Glioblastoma
4.1. Composition of Nanoparticles
4.1.1. Liposomes
4.1.2. Solid Lipid Nanoparticles
4.1.3. Cubosomes
4.1.4. Polymeric Nanoparticles
4.1.5. Silica Nanoparticles
4.2. Surface Modification
4.2.1. Folate Receptor (FR) Targeted Ligand
4.2.2. Transferrin Receptor (TfR) Targeted Ligand
4.2.3. Glucose Transporter Targeted Ligand
4.3. Nanoformulations with FDA-Approved Drugs
4.3.1. Paclitaxel
4.3.2. Doxorubicin
4.3.3. Temozolomide
4.4. Nanoformulations with Natural Compounds
4.4.1. Terpenes and Terpenoids
4.4.2. Polyphenols
4.4.3. Alkaloids
4.5. Drugs and Nanoparticles Complex Platform
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsu, J.F.; Chu, S.M.; Liao, C.C.; Wang, C.J.; Wang, Y.S.; Lai, M.Y.; Wang, H.C.; Huang, H.R.; Tsai, M.H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sherwani, S.; Khan, S.; Alouffi, S.; Alam, M.; Al-Motair, K.; Khan, S. Insights into Multifunctional Nanoparticle-Based Drug Delivery Systems for Glioblastoma Treatment. Molecules 2021, 26, 2262. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, L.; Gu, Y.Q.; Yang, H.S.; Du, B.X.; Liu, H.M.; Li, Y.L. Increased the TMZ Concentration in Brain by Poly(2-ethyl-2-oxazoline) Conjugated Temozolomide Prodrug Micelles for Glioblastoma Treatment. Eur. Polym. J. 2021, 145, 110232. [Google Scholar] [CrossRef]
- Helal, D.O.; Rouatbi, N.; Han, S.P.; Wang, J.T.W.; Walters, A.A.; Abdel-Mottaleb, M.M.A.; Kamel, A.O.; Geneidi, A.S.; Awad, G.A.S.; Al-Jamal, K.T. A Natural Protein Based Platform for the Delivery of Temozolomide Acid to Glioma Cells. Eur. J. Pharm. Biopharm. 2021, 169, 297–308. [Google Scholar] [CrossRef]
- Rui, Y.; Green, J.J. Overcoming Delivery Barriers in Immunotherapy for Glioblastoma. Drug Deliv. Transl. Res. 2021, 11, 2302–2316. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Chiarelli, P.A.; Revia, R.A.; Wang, K.; Kievit, F.; Dayringer, C.; Jeon, M.; Ellenbogen, R.; Zhang, M.Q. Time-Resolved MRI Assessment of Convection-Enhanced Delivery by Targeted and Nontargeted Nanoparticles in a Human Glioblastoma Mouse Model. Cancer Res. 2019, 79, 4776–4786. [Google Scholar] [CrossRef]
- Grippin, A.J.; Wummer, B.; Wildes, T.; Dyson, K.; Triyedi, V.; Yang, C.L.; Sebastian, M.; Mendez-Gomez, H.R.; Padala, S.; Grubb, M.; et al. Dendritic Cell-Activating Magnetic Nanoparticles Enable Early Prediction of Antitumor Response with Magnetic Resonance Imaging. ACS Nano 2019, 13, 13884–13898. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xi, K.Y.; Fu, X.; Sun, H.F.; Wang, H.; Yu, D.X.; Li, Z.W.; Ma, Y.; Liu, X.J.; Huang, B.; et al. Versatile Metal-Phenolic Network Nanoparticles for Multitargeted Combination Therapy and Magnetic Resonance Tracing in Glioblastoma. Biomaterials 2021, 278, 121163. [Google Scholar] [CrossRef]
- Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yague, B.; Caverzan, M.D.; Chesta, C.A.; Palacios, R.E.; Lopez-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; Mu, Q.; Stephen, Z.R.; Yu, Y.; Zhou, S.; Zhang, M. Mesoporous Carbon Nanoshells for High Hydrophobic Drug Loading, Multimodal Optical Imaging, Controlled Drug Release, and Synergistic Therapy. Nanoscale 2017, 9, 1434–1442. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Anthracycline Nano-Delivery Systems to Overcome Multiple Drug Resistance: A Comprehensive Review. Nano Today 2013, 8, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mackey, M.A.; El-Sayed, M.A.; Bellamkonda, R.V. Remote Triggered Release of Doxorubicin in Tumors by Synergistic Application of Thermosensitive Liposomes and Gold Nanorods. ACS Nano 2011, 5, 4919–4926. [Google Scholar] [CrossRef] [PubMed]
- Moras, A.M.; Henn, J.G.; Reinhardt, L.S.; Lenz, G.; Moura, D.J. Recent Developments in Drug Delivery Strategies for Targeting DNA Damage Response in Glioblastoma. Life Sci. 2021, 287, 120128. [Google Scholar] [CrossRef] [PubMed]
- Ndemazie, N.B.; Inkoom, A.; Morfaw, E.F.; Smith, T.; Aghimien, M.; Ebesoh, D.; Agyare, E. Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS PharmSciTech 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Lv, Q.; Li, L.M.; Tang, X.J.; Li, F.Z.; Hu, Y.L.; Han, M. Glioma Targeting and Blood-Brain Barrier Penetration by Dual-Targeting Doxorubincin Liposomes. Biomaterials 2013, 34, 5628–5639. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Pourgholi, F.; Farhad, J.-N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Fang, J.Q.; Xue, W.; Zhang, J.F.; Tong, H.P.; Liu, H.; Guo, Y.; Yang, Y.Z.; Zhang, W.G. Non-Invasive Monitoring of the Kinetic Infiltration and Therapeutic Efficacy of Nanoparticle-Labeled Chimeric Antigen Receptor T Cells in Glioblastoma via 7.0-Tesla Magnetic Resonance Imaging. Cytotherapy 2021, 23, 211–222. [Google Scholar] [CrossRef]
- Sanz-Ortega, L.; Rojas, J.M.; Barber, D.F. Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting. Pharmaceutics 2020, 12, 812. [Google Scholar] [CrossRef]
- Spencer, D.; Yu, D.; Morshed, R.A.; Li, G.N.; Pituch, K.C.; Gao, D.X.; Bertolino, N.; Procissi, D.; Lesniak, M.S.; Balyasnikova, I.V. Pharmacologic Modulation of Nasal Epithelium Augments Neural Stem Cell Targeting of Glioblastoma. Theranostics 2019, 9, 2071–2083. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.Y.; Schuette, A.J.; Chen, H.W.; Wu, X.Y.; Mao, H. EGFRvIII Antibody-Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.W.; Hsu, C.H.; Li, Z.; Kim, T.S.; Hwang, L.P.; Lin, Y.C.; Lin, Y.Y. Magnetic Resonance Nano-Theranostics for Glioblastoma Multiforme. Curr. Pharm. Design. 2015, 21, 5256–5266. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.R.; Jia, Q.J.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.B.; Galla, H.J.; Gao, M.Y. Receptor-Mediated Delivery of Magnetic Nanoparticles across the Blood-Brain Barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Luque-Michel, E.; Lemaire, L.; Blanco-Prieto, M.J. SPION and doxorubicin-loaded polymeric nanocarriers for glioblastoma theranostics. Drug Deliv. Transl. Res. 2021, 11, 515–523. [Google Scholar] [CrossRef]
- Fang, J.H.; Chiu, T.L.; Huang, W.C.; Lai, Y.H.; Hu, S.H.; Chen, Y.Y.; Chen, S.Y. Dual-Targeting Lactoferrin-Conjugated Polymerized Magnetic Polydiacetylene-Assembled Nanocarriers with Self-Responsive Fluorescence/Magnetic Resonance Imaging for In Vivo Brain Tumor Therapy. Adv. Healthc. Mater. 2016, 5, 688–695. [Google Scholar] [CrossRef]
- Yee, P.P.; Wei, Y.J.; Kim, S.Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.L.; Liu, Z.J.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-Induced Ferroptosis Promotes Tumor Necrosis in Glioblastoma Progression. Nat. Commun. 2020, 11, 5424. [Google Scholar] [CrossRef]
- Li, Y.J.; Teng, X.C.; Wang, Y.J.; Yang, C.R.; Yan, X.P.; Li, J.H. Neutrophil Delivered Hollow Titania Covered Persistent Luminescent Nanosensitizer for Ultrosound Augmented Chemo/Immuno Glioblastoma Therapy. Adv. Sci. 2021, 8, 2004381. [Google Scholar] [CrossRef]
- Shin, H.J.; Jeong, E.A.; Lee, J.Y.; An, H.S.; Jang, H.M.; Ahn, Y.J.; Lee, J.; Kim, K.E.; Roh, G.S. Lipocalin-2 Deficiency Reduces Oxidative Stress and Neuroinflammation and Results in Attenuation of Kainic Acid-Induced Hippocampal Cell Death. Antioxidants 2021, 10, 100. [Google Scholar] [CrossRef]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and Immunoregulation in Glioblastoma and Brain Metastases: Recent Developments in Imaging Approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The Role of LAT1 in F-18-DOPA Uptake in Malignant Gliomas. J. Neuro-Oncol. 2013, 111, 11–18. [Google Scholar] [CrossRef]
- An, Z.J.; Hu, Y.; Bai, Y.; Zhang, C.; Xu, C.; Kang, X.; Yang, S.B.; Li, W.B.; Zhong, X.S. Antitumor Activity of the Third Generation EphA2 CAR-T Cells Against Glioblastoma is Associated with Interferon Gamma Induced PD-L1. Oncoimmunology 2021, 10, 1960728. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Chen, W.; Li, C.H.; Fang, C.Y.; Chang, Y.C.; Wei, D.H.; Liu, R.S.; Hsiao, M. An Advanced In Situ Magnetic Resonance Imaging and Ultrasonic Theranostics Nanocomposite Platform: Crossing the Blood-Brain Barrier and Improving the Suppression of Glioblastoma Using Iron-Platinum Nanoparticles in Nanobubbles. ACS Appl. Mater. Interfaces 2021, 13, 26759–26769. [Google Scholar] [CrossRef] [PubMed]
- Persano, S.; Vicini, F.; Poggi, A.; Fernandez, J.L.C.; Rizzo, G.M.R.; Gavilan, H.; Silvestri, N.; Pellegrino, T. Elucidating the Innate Immunological Effects of Mild Magnetic Hyperthermia on U87 Human Glioblastoma Cells: An In Vitro Study. Pharmaceutics 2021, 13, 1668. [Google Scholar] [CrossRef] [PubMed]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.N.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic Hyperthermia Therapy for the Treatment of Glioblastoma: A Review of the Therapy’s History, Efficacy and Application in Humans. Int. J. Hyperther. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Raoufi, N.J.; Karimi, M.R.; Shakeri-Zadeh, A. Development of a Magnetic Nano-Graphene Oxide Carrier for improved Glioma-Targeted Drug Delivery and Imaging: In vitro and In Vivo Evaluations. Chem-Biol. Interact. 2018, 295, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, D. Evolution of Magnetic Hyperthermia for Glioblastoma Multiforme Therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef]
- Li, Z.; Ding, X.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Recent Advances on Inorganic Lanthanide-Doped NIR-II Fluorescence Nanoprobes for Bioapplication. J. Lumin. 2020, 228, 117627. [Google Scholar] [CrossRef]
- Yuan, L.; Jin, Y.; Zhu, D.; Mou, Z.; Xie, G.; Hu, Y. Ni2+-Doped Yttrium Aluminum Gallium Garnet Phosphors: Bandgap Engineering for Broad-Band Wavelength-Tunable Shortwave-Infrared Long-Persistent Luminescence and Photochromism. ACS Sustain. Chem. Eng. 2020, 8, 6543–6550. [Google Scholar] [CrossRef]
- Chan, M.H.; Huang, W.T.; Wang, J.; Liu, R.S.; Hsiao, M. Next-Generation Cancer-Specific Hybrid Theranostic Nanomaterials: MAGE-A3 NIR Persistent Luminescence Nanoparticles Conjugated to Afatinib for In Situ Suppression of Lung Adenocarcinoma Growth and Metastasis. Adv. Sci. 2020, 7, 1903741. [Google Scholar] [CrossRef]
- Moreno, M.J.; Ling, B.; Stanimirovic, D.B. In Vivo Near-Infrared Fluorescent Optical Imaging for CNS Drug Discovery. Expert Opin. Drug Discov. 2020, 15, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near Infrared Fluorescent Imaging of Brain Tumor with IR780 Dye Incorporated Phospholipid Nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef]
- Polikarpov, D.M.; Campbell, D.H.; McRobb, L.S.; Wu, J.; Lund, M.E.; Lu, Y.; Deyev, S.M.; Davidson, A.S.; Walsh, B.J.; Zvyagin, A.V. Near-Infrared Molecular Imaging of Glioblastoma by Miltuximab®-IRDye800CW as a Potential Tool for Fluorescence-Guided Surgery. Cancers 2020, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Vider, J.; Kovar, J.L.; Olive, D.M.; Mellinghoff, I.K.; Mayer-Kuckuk, P.; Kircher, M.F.; Blasberg, R.G. Integrin αvβ3-Targeted IRDye 800CW Near-Infrared Imaging of Glioblastoma. Clin. Cancer Res. 2012, 18, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Reichel, D.; Sagong, B.; Teh, J.; Zhang, Y.; Wagner, S.; Wang, H.; Chung, L.W.; Butte, P.; Black, K.L.; Yu, J.S. Near Infrared Fluorescent Nanoplatform for Targeted Intraoperative Resection and Chemotherapeutic Treatment of Glioblastoma. ACS Nano 2020, 14, 8392–8408. [Google Scholar] [CrossRef]
- Wu, J.B.; Shi, C.; Chu, G.C.-Y.; Xu, Q.; Zhang, Y.; Li, Q.; John, S.Y.; Zhau, H.E.; Chung, L.W. Near-Infrared Fluorescence Heptamethine Carbocyanine Dyes Mediate Imaging and Targeted Drug Delivery for Human Brain Tumor. Biomaterials 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Feng, J.; Li, S.; Fan, H.-J.; Lin, Y.; Lu, Y. Dendritic Polylysine Based ανβ3 Integrin Targeted Probe for Near-Infrared Fluorescent Imaging of Glioma. Colloids Surf. B 2019, 178, 146–152. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H. First-In-Human Intraoperative Near-Infrared Fluorescence Imaging of Glioblastoma Using Cetuximab-IRDye800. J. Neuro-Oncol. 2018, 139, 135–143. [Google Scholar] [CrossRef]
- Burley, T.A.; Mączyńska, J.; Shah, A.; Szopa, W.; Harrington, K.J.; Boult, J.K.; Mrozek-Wilczkiewicz, A.; Vinci, M.; Bamber, J.C.; Kaspera, W. Near-Infrared Photoimmunotherapy Targeting EGFR—Shedding New Light on Glioblastoma Treatment. Int. J. Cancer Res. 2018, 142, 2363–2374. [Google Scholar] [CrossRef]
- Kim, S.M.; Jeong, C.H.; Woo, J.S.; Ryu, C.H.; Lee, J.-H.; Jeun, S.-S. In Vivo Near-Infrared Imaging for the Tracking of Systemically Delivered Mesenchymal Stem Cells: Tropism for Brain Tumors and Biodistribution. Int. J. Nanomed. 2016, 11, 13. [Google Scholar] [CrossRef]
- Zhou, H.; Luby-Phelps, K.; Mickey, B.E.; Habib, A.A.; Mason, R.P.; Zhao, D. Dynamic Near-Infrared Optical Imaging of 2-Deoxyglucose Uptake by Intracranial Glioma of Athymic Mice. PLoS ONE 2009, 4, e8051. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Deng, G.; Sun, Z.; Peng, X.; Li, J.; Gong, P.; Zhang, P.; Cai, L. Scaffolds Biomimicking Macrophages for a Glioblastoma NIR-Ib Imaging Guided Photothermal Therapeutic Strategy by Crossing Blood-Brain Barrier. Biomaterials 2019, 211, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Li, X.; Ma, X.; Wang, S.; Kang, F.; Yang, W.; Ma, W.; Wang, J. Near-infrared Fluorescent Peptides with High Tumor Selectivity: Novel Probes for Image-Guided Surgical Resection of Orthotopic Glioma. Mol. Pharm. 2018, 16, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Shao, C.; Li, S.; Wang, Z.; Qu, Y.; Gu, W.; Yu, C.; Ye, L. Cy5. 5 Conjugated MnO Nanoparticles for Magnetic Resonance/Near-Infrared Fluorescence Dual-Modal Imaging of Brain Gliomas. J. Colloid. Interf. Sci. 2015, 457, 27–34. [Google Scholar] [CrossRef]
- Lee, C.; Kim, G.R.; Yoon, J.; Kim, S.E.; Yoo, J.S.; Piao, Y. In Vivo Delineation of Glioblastoma by Targeting Tumor-Associated Macrophages with Near-Infrared Fluorescent Silica Coated Iron Oxide Nanoparticles in Orthotopic Xenografts for Surgical Guidance. Sci. Rep. 2018, 8, 11122. [Google Scholar] [CrossRef]
- Huang, M.; Xiong, C.; Lu, W.; Zhang, R.; Zhou, M.; Huang, Q.; Weinberg, J.; Li, C. Dual-Modality Micro-Positron Emission Tomography/Computed Tomography and Near-Infrared Fluorescence Imaging of EphB4 in Orthotopic Glioblastoma Xenograft Models. Mol. Imaging Biol. 2014, 16, 74–84. [Google Scholar] [CrossRef][Green Version]
- Tomanek, B.; Iqbal, U.; Blasiak, B.; Abulrob, A.; Albaghdadi, H.; Matyas, J.R.; Ponjevic, D.; Sutherland, G.R. Evaluation of Brain Tumor Vessels Specific Contrast Agents for Glioblastoma Imaging. Neuro-Oncol. 2012, 14, 53–63. [Google Scholar] [CrossRef][Green Version]
- Patil, R.; Galstyan, A.; Sun, T.; Shatalova, E.S.; Butte, P.; Mamelak, A.N.; Carico, C.; Kittle, D.S.; Grodzinski, Z.B.; Chiechi, A. Polymalic Acid Chlorotoxin Nanoconjugate for Near-Infrared Fluorescence Guided Resection of Glioblastoma Multiforme. Biomaterials 2019, 206, 146–159. [Google Scholar] [CrossRef]
- Lee, J.Y.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Chen, X.; Tian, J. Fluorescence Molecular Imaging and Tomography of Matrix Metalloproteinase-Activatable Near-Infrared Fluorescence Probe and Image-Guided Orthotopic Glioma Resection. Mol. Imaging Biol. 2018, 20, 930–939. [Google Scholar] [CrossRef]
- Battogtokh, G.; Gotov, O.; Kang, J.H.; Hong, E.J.; Shim, M.S.; Shin, D.; Ko, Y.T. Glycol Chitosan-Coated Near-Infrared Photosensitizer-Encapsulated Gold Nanocages for Glioblastoma Phototherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kurbegovic, S.; Juhl, K.; Chen, H.; Qu, C.; Ding, B.; Leth, J.M.; Drzewiecki, K.T.; Kjaer, A.; Cheng, Z. Molecular Targeted NIR-II Probe for Image-Guided Brain Tumor Surgery. Bioconjug. Chem. 2018, 29, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C.; Chen, J.; Lian, X.; Xiong, C.; Tian, R.; Hu, L.; Xiong, X.; Tian, J. uPAR Targeted Phototheranostic Metal-Organic Framework Nanoprobes for MR/NIR-II Imaging-Guided Therapy and Surgical Resection of Glioblastoma. Mater. Des. 2021, 198, 109386. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, L.; Chao, Z.; Shao, C.; Chen, Z.; Wei, Z.; Lu, J.; Huang, Y.; Li, L.; Liu, Q. Organic Spherical Nucleic Acids for the Transport of a NIR-II-Emitting Dye Across the Blood–Brain Barrier. Angew. Chem. Int. Ed. 2020, 132, 9789–9797. [Google Scholar] [CrossRef]
- Jin, J.F.; Xu, Z.H.; Zhang, Y.; Gu, Y.J.; Lam, M.H.W.; Wong, W.T. Upconversion Nanoparticles Conjugated with Gd3+-DOTA and RGD for Targeted Dual-Modality Imaging of Brain Tumor Xenografts. Adv. Healthc. Mater. 2013, 2, 1501–1512. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, F.; Zhang, H.; Yuan, Q.; Jiang, Z.; Liu, H.; Sun, Q.; Li, Z. Boosting Often Overlooked Long Wavelength Emissions of Rare-Earth Nanoparticles for NIR-II fluorescence Imaging of Orthotopic Glioblastoma. Biomaterials 2019, 219, 119364. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Fu, Q.; Ye, J.; Su, L.; Ge, X.; Chen, L.; Song, J.; Yang, H. Neodymium (3+)-Coordinated Black Phosphorus Quantum Dots with Retrievable NIR/X-Ray Optoelectronic Switching Effect for Anti-Glioblastoma. Small 2021, 18, 2105160. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.; Lu, L.; Li, X.; Wang, W.; Zhao, M.; Hu, L.; El-Toni, A.M.; Li, Q.; Zhang, F. Kinetics-Mediate Fabrication of Multi-Model Bioimaging Lanthanide Nanoplates with Controllable Surface Roughness for Blood Brain Barrier Transportation. Biomaterials 2017, 141, 223–232. [Google Scholar] [CrossRef]
- Ren, F.; Liu, H.; Zhang, H.; Jiang, Z.; Xia, B.; Genevois, C.; He, T.; Allix, M.; Sun, Q.; Li, Z. Engineering NIR-IIb Fluorescence of Er-based Lanthanide Nanoparticles for Through-Skull Targeted Imaging and Imaging-Guided Surgery of Orthotopic Glioma. Nano Today 2020, 34, 100905. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.-P. Fabrication of Vascular Endothelial Growth Factor Antibody Bioconjugated Ultrasmall Near-Infrared Fluorescent Ag2S Quantum Dots for Targeted Cancer Imaging In Vivo. Chem. Commun. 2013, 49, 3324–3326. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Wang, M.; Zhang, Y.; Chen, G.; Li, L.; Wu, D.; Wang, Q. In Vivo Real-Time Visualization of Tissue Blood Flow and Angiogenesis Using Ag2S Quantum Dots in the NIR-II Window. Biomaterials 2014, 35, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, L.; Zhang, Y.; Yi, P.; Wang, M.; Tan, B.; Deng, Z.; Wu, D.; Wang, Q. Preoperative Detection and Intraoperative Visualization of Brain Tumors for More Precise Surgery: A New Dual-Modality MRI and NIR Nanoprobe. Small 2015, 11, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, Q.; Wang, K.; Revia, R.A.; Yen, C.; Gu, X.; Tian, B.; Liu, J.; Zhang, M. Nitrogen and Boron Dual-Doped Graphene Quantum Dots for Near-Infrared Second Window Imaging and Photothermal Therapy. Appl. Mater. Today 2019, 14, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Chan, M.H.; Feng, S.J.; Hsiao, M.; Liu, R.S. Long-Term Near-Infrared Signal Tracking of the Therapeutic Changes of Glioblastoma Cells in Brain Tissue with Ultrasound-Guided Persistent Luminescent Nanocomposites. ACS Appl. Mater. Interfaces 2021, 13, 6099–6108. [Google Scholar] [CrossRef]
- Chen, S.; Miao, H.; Jiang, X.; Sun, P.; Fan, Q.; Huang, W. Starlike Polymer Brush-Based Ultrasmall Nanoparticles with Simultaneously Improved NIR-II Fluorescence and Blood Circulation for Efficient Orthotopic Glioblastoma Imaging. Biomaterials 2021, 275, 120916. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through Scalp and Skull NIR-II Photothermal Therapy of Deep Orthotopic Brain Tumors with Precise Photoacoustic Imaging Guidance. Adv. Mater. 2018, 30, 1802591. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, M.; Zeng, X.; Xiao, Y.; Wu, H.; Zhou, H.; Ding, B.; Qu, C.; Hou, W.; Er-Bu, A. Novel Bright-Emission Small-Molecule NIR-II Fluorophores for In Vivo Tumor Imaging and Image-Guided Surgery. Chem. Sci. 2017, 8, 3489–3493. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S. Brain-Targeted Aggregation-Induced-Emission Nanoparticles with Near-Infrared Imaging at 1550 nm Boosts Orthotopic Glioblastoma Theranostics. Adv. Mater. 2021, 34, 2106082. [Google Scholar] [CrossRef]
- Lu, S.; Ke, J.; Li, X.; Tu, D.; Chen, X. Luminescent Nano-Bioprobes Based on NIR Dye/Lanthanide Nanoparticle Composites. Aggregate 2021, 2, e59. [Google Scholar] [CrossRef]
- Ni, D.; Zhang, J.; Bu, W.; Xing, H.; Han, F.; Xiao, Q.; Yao, Z.; Chen, F.; He, Q.; Liu, J. Dual-Targeting Upconversion Nanoprobes Across the Blood–Brain Barrier for Magnetic Resonance/Fluorescence Imaging of Intracranial Glioblastoma. ACS Nano 2014, 8, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, B.; Yang, P.; Lin, J.; Dai, Y. Progress in Light-Responsive Lanthanide Nanoparticles Toward Deep Tumor Theranostics. Adv. Funct. Mater. 2021, 31, 2104325. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Liu, H.; Ren, F.; Qiu, W.; Sun, Q.; Yan, F.; Zheng, H.; Li, Z.; Gao, M. Second Near-Infrared Photodynamic Therapy and Chemotherapy of Orthotopic Malignant Glioblastoma with Ultra-Small Cu 2−x Se Nanoparticles. Nanoscale 2019, 11, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Chan, M.H.; Chen, X.; Hsiao, M.; Liu, R.S. Theranostic Nanobubble Encapsulating A Plasmon-Enhanced Upconversion Hybrid Nanosystem for Cancer Therapy. Theranostics 2020, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Yuan, Z. Polymer Dots for Precision Photothermal Therapy of Brain Tumors in the Second Near-Infrared Window: A Mini-Review. ACS Appl. Polym. Mater. 2020, 2, 4319–4330. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, Z.; Ji, L.; Zhang, J.; Wang, Q.; Guo, T.; Ni, S.; Cai, R.; Mu, X. Recent Progress on NIR-II Photothermal Therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, S.P.; Chen, C.W.; Chan, Y.C.; Lin, R.J.; Tsai, D.P.; Hsiao, M.; Chung, R.J.; Chen, X.; Liu, R.S. Single 808 nm Laser Treatment Comprising Photothermal and Photodynamic Therapies by Using Gold Nanorods Hybrid Upconversion Particles. J. Phys. Chem. C 2018, 122, 2402–2412. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Zhang, F.; Xu, H.; Chen, W.; Jiang, T. A Novel Nanocomposite Based on Fluorescent Turn-on Gold Nanostars for Near-Infrared Photothermal Therapy and Self-Theranostic Caspase-3 Imaging of Glioblastoma Tumor Cell. Colloids Surf. B 2018, 170, 303–311. [Google Scholar] [CrossRef]
- Qian, M.; Du, Y.; Wang, S.; Li, C.; Jiang, H.; Shi, W.; Chen, J.; Wang, Y.; Wagner, E.; Huang, R. Highly Crystalline Multicolor Carbon Nanodots for Dual-Modal Imaging-Guided Photothermal Therapy of Glioma. ACS Appl. Mater. Interfaces 2018, 10, 4031–4040. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G. Fe3O4@ Au Composite Magnetic Nanoparticles Modified with Cetuximab for Targeted Magneto-Photothermal Therapy of Glioma Cells. Int. J. Nanomed. 2018, 13, 2491. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Manganese-Doped Magnetic Nanoclusters for Hyperthermia and Photothermal Glioblastoma Therapy. ACS Appl. Nano Mater. 2020, 3, 2026–2037. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, A.; Sofi, R.; Smith, S.J.; Rahman, R.; Teschemacher, A.G.; Kasparov, S. Feasibility of Photodynamic Therapy for Glioblastoma with the Mitochondria-Targeted Photosensitizer Tetramethylrhodamine Methyl Ester (TMRM). Biomedicines 2021, 9, 1453. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional Mesoporous Silica-Coated Graphene Nanosheet Used for Chemo-Photothermal Synergistic Targeted Therapy of Glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Lin, L.; Hao, K.; Chen, J.; Tian, H.; Chen, X. Cyanine-Assisted Exfoliation of Covalent Organic Frameworks in Nanocomposites for Highly Efficient Chemo-Photothermal Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39503–39512. [Google Scholar] [CrossRef]
- Maziukiewicz, D.; Grześkowiak, B.F.; Coy, E.; Jurga, S.; Mrówczyński, R. NDs@ PDA@ ICG Conjugates for Photothermal Therapy of Glioblastoma Multiforme. Biomimetics 2019, 4, 3. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.S.; Wang, P. Hypocrellin-Based Multifunctional Phototheranostic Agent for NIR-Triggered Targeted Chemo/Photodynamic/Photothermal Synergistic Therapy against Glioblastoma. ACS Appl. Bio Mater. 2020, 3, 3817–3826. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Vijayaraghavan, P.; Chiang, W.H.; Chen, H.H.; Liu, T.I.; Shen, M.Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.C. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics 2018, 8, 1435. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Ding, Y.; Wang, K.; Xing, Z.; Sun, X.; Guo, W.; Hong, X.; Zhu, X.; Liu, Y. Enhanced Photothermal-Photodynamic Therapy for Glioma Based on Near-Infrared Dye Functionalized Fe3O4 Superparticles. Chem. Eng. J. 2020, 381, 122693. [Google Scholar] [CrossRef]
- Sinek, J.; Frieboes, H.; Zheng, X.; Cristini, V. Two-Dimensional Chemotherapy Simulations Demonstrate Fundamental Transport and Tumor Response Limitations Involving Nanoparticles. Biomed. Microdevices 2004, 6, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic Acid-Conjugated Liposome Nanoparticles for Targeted Delivery to CD44 Overexpressing Glioblastoma Cells. Oncotarget 2016, 7, 34158. [Google Scholar] [CrossRef] [PubMed]
- McNeeley, K.M.; Annapragada, A.; Bellamkonda, R.V. Decreased Circulation Time Offsets Increased Efficacy of PEGylated Nanocarriers Targeting Folate Receptors of Glioma. Nanotechnology 2007, 18, 385101. [Google Scholar] [CrossRef]
- Fan, R.; Wang, H.; Zhang, L.; Ma, T.; Tian, Y.; Li, H. Nanocrystallized Oleanolic Acid Better Inhibits Proliferation, Migration and Invasion in Intracranial Glioma via Caspase-3 Pathway. J. Cancer 2020, 11, 1949. [Google Scholar] [CrossRef]
- Tadokoro, A.; Kanaji, N.; Liu, D.; Yokomise, H.; Haba, R.; Ishii, T.; Takagi, T.; Watanabe, N.; Kita, N.; Kadowaki, N. Vimentin Regulates Invasiveness and Is a Poor Prognostic Marker in Non-Small Cell Lung Cancer. Anticancer Res. 2016, 36, 1545–1551. [Google Scholar]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid Lipid Nanoparticles: A Review. Nanosci. Nanotechnol. Res. 2017, 2, 67–72. [Google Scholar]
- Kuo, Y.C.; Liang, C.T. Inhibition of Human Brain Malignant Glioblastoma Cells Using Carmustine-Loaded Catanionic Solid Lipid Nanoparticles with Surface Anti-Epithelial Growth Factor Receptor. Biomaterials 2011, 32, 3340–3350. [Google Scholar] [CrossRef]
- Barriga, H.M.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome Dispersions as Delivery Systems for Percutaneous Administration of Indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.P.; Soucé, M.; Perse, X.; Vial, F.; Perrier, T.; Yvergnaux, F.; Chourpa, I.; Munnier, E. Lipid-Based Submicron Capsules as a Strategy to Include High Concentrations of a Hydrophobic Lightening Agent in a Hydrogel. Int. J. Cosmet. Sci. 2017, 39, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Flak, D.K.; Adamski, V.; Nowaczyk, G.; Szutkowski, K.; Synowitz, M.; Jurga, S.; Held-Feindt, J. AT101-Loaded Cubosomes as an Alternative for Improved Glioblastoma Therapy. Int. J. Nanomed. 2020, 15, 7415. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.L.; Hamid, Z.A.; Mariatti, M.; Yahaya, B.; Lim, K.; Bee, S.T.; Sin, L.T. Approaches to Improve Therapeutic Efficacy of Biodegradable PLA/PLGA Microspheres: A Review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Crotts, G.; Park, T.G. Protein Delivery from Poly(lactic-co-glycolic acid) Biodegradable Microspheres: Release Kinetics and Stability Issues. J. Microencapsul. 1998, 15, 699–713. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol. Rev. 1999, 51, 691–744. [Google Scholar]
- Nance, E.; Zhang, C.; Shih, T.Y.; Xu, Q.; Schuster, B.S.; Hanes, J. Brain-Penetrating Nanoparticles Improve Paclitaxel Efficacy in Malignant Glioma Following Local Administration. ACS Nano 2014, 8, 10655–10664. [Google Scholar] [CrossRef]
- He, Y.; Wu, C.; Duan, J.; Miao, J.; Ren, H.; Liu, J. Anti-Glioma Effect with Targeting Therapy Using Folate Modified Nano-Micelles Delivery Curcumin. J. Biomed. Nanotechnol. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic Dual-Ligand Doxorubicin Liposomes Improve Targeting and Therapeutic Efficacy of Brain Glioma in Animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef]
- Guerin, C.; Laterra, J.; Hruban, R.H.; Brem, H.; Drewes, L.R.; Goldstein, G.W. The Glucose Transporter and Blood-Brain Barrier of Human Brain Tumors. Ann. Neurol. 1990, 28, 758–765. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, Blood-Brain Barrier, and Brain Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.L.; Bode, C.J.; Georg, G.I.; Himes, R.H. Understanding Tubulin–Taxol Interactions: Mutations that Impart Taxol Binding to Yeast Tubulin. Proc. Natl. Acad. Sci. USA 2003, 100, 6394–6397. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Hosseini Najafabadi, M.R.; Webster, T.J.; Hadjighassem, M.R.; Sadroddiny, E.; Ghanbari, H.; Khosravani, M.; Adabi, M. Effect of Paclitaxel/etoposide Co-Loaded Polymeric Nanoparticles on Tumor Size and Survival Rate in a Rat Model of Glioblastoma. Int. J. Pharm. 2021, 604, 120722. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/Methotrexate Co-Loaded PLGA Nanoparticles in Glioblastoma Treatment: Formulation Development and In Vitro Antitumor Activity Evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H. A Convergent Synthetic Platform for Anticancer Drugs Formulation with Nanoparticle Delivery for the Treatment and Nursing Care of Glioma Cancer. Process Biochem. 2021, 111, 172–180. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, J.; Feng, S.S. Targeted Co-Delivery of Docetaxel, Cisplatin and Herceptin by Vitamin E TPGS-cisplatin Prodrug Nanoparticles for Multimodality Treatment of Cancer. J. Control. Release 2013, 169, 185–192. [Google Scholar] [CrossRef]
- Mujokoro, B.; Adabi, M.; Sadroddiny, E.; Adabi, M.; Khosravani, M. Nano-Structures Mediated Co-Delivery of Therapeutic Agents for Glioblastoma Treatment: A Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1092–1102. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Cameotra, S.S.; Makkar, R.S. Recent Applications of Biosurfactants as Biological and Immunological Molecules. Curr. Opin. Microbiol. 2004, 7, 262–266. [Google Scholar] [CrossRef]
- Chen, J.; Song, X.; Zhang, H.; Qu, Y. Production, Structure Elucidation and Anticancer Properties of Sophorolipid from Wickerhamiella Domercqiae. Enzym. Microb. Technol. 2006, 39, 501–506. [Google Scholar] [CrossRef]
- Dhar, S.; Reddy, E.M.; Prabhune, A.; Pokharkar, V.; Shiras, A.; Prasad, B.L.V. Cytotoxicity of Sophorolipid-Gellan Gum-Gold Nanoparticle Conjugates and Their Doxorubicin Loaded Derivatives towards Human Glioma and Human Glioma Stem Cell Lines. Nanoscale 2011, 3, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, C.; Cheng, R.; Cheng, L.; Meng, F.; Liu, Z.; Zhong, Z. cRGD-directed, NIR-Responsive and Robust AuNR/PEG-PCL Hybrid Nanoparticles for Targeted Chemotherapy of Glioblastoma In Vivo. J. Control. Release 2014, 195, 63–71. [Google Scholar] [CrossRef] [PubMed]
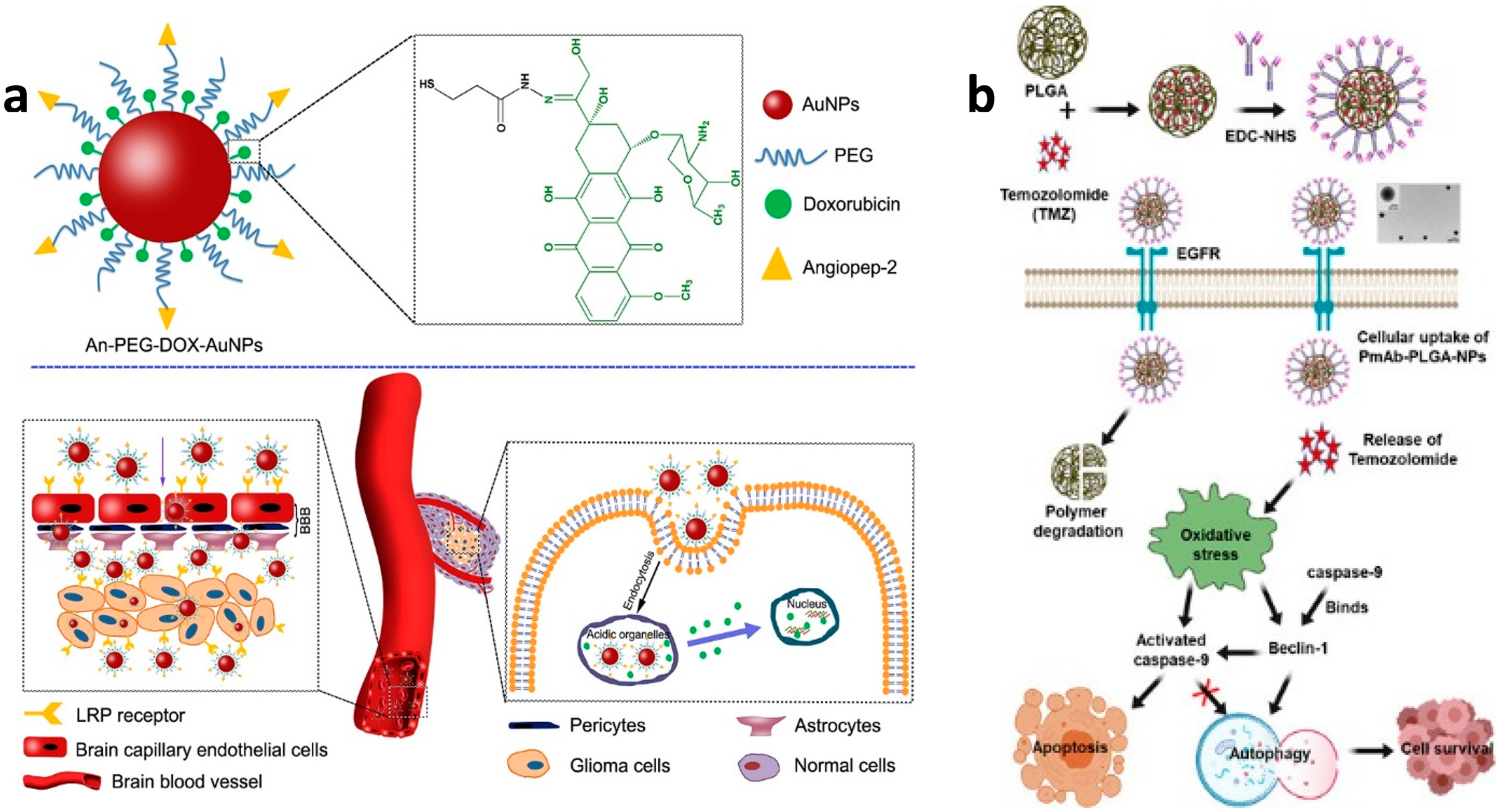
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor Microenvironment Sensitive Doxorubicin Delivery and Release to Glioma Using Angiopep-2 Decorated Gold Nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
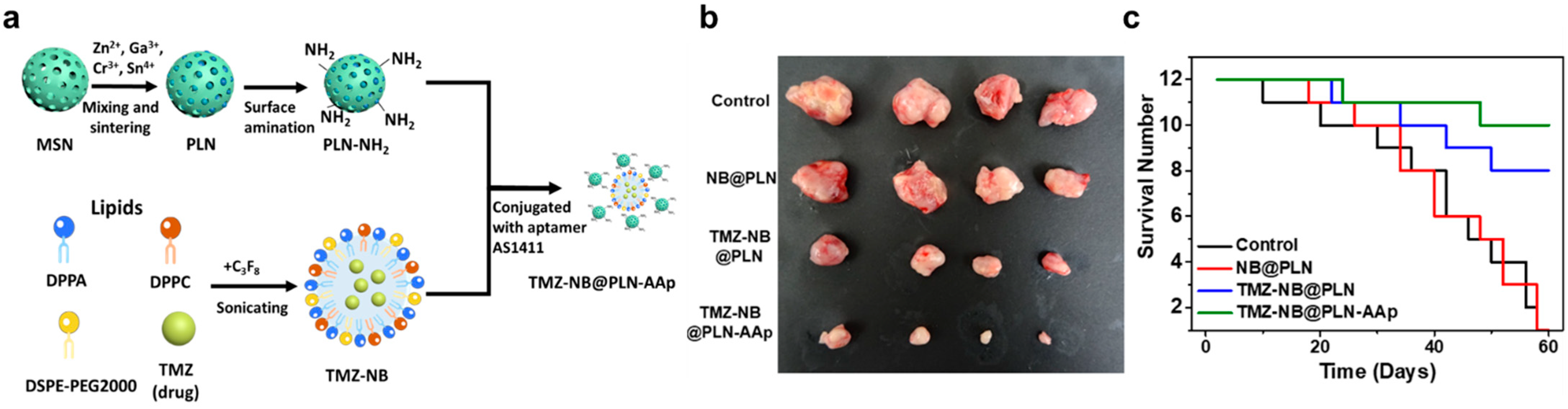
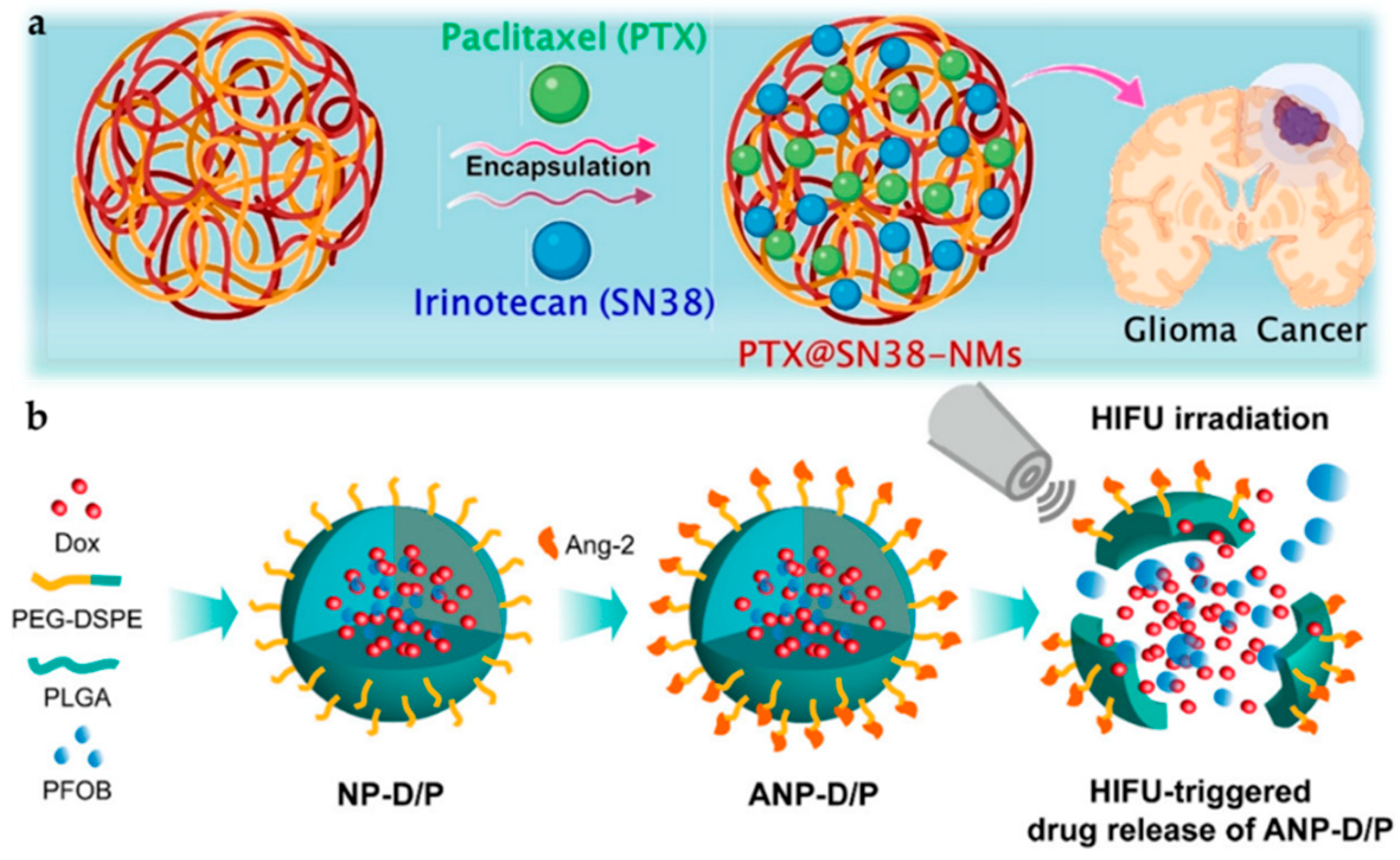
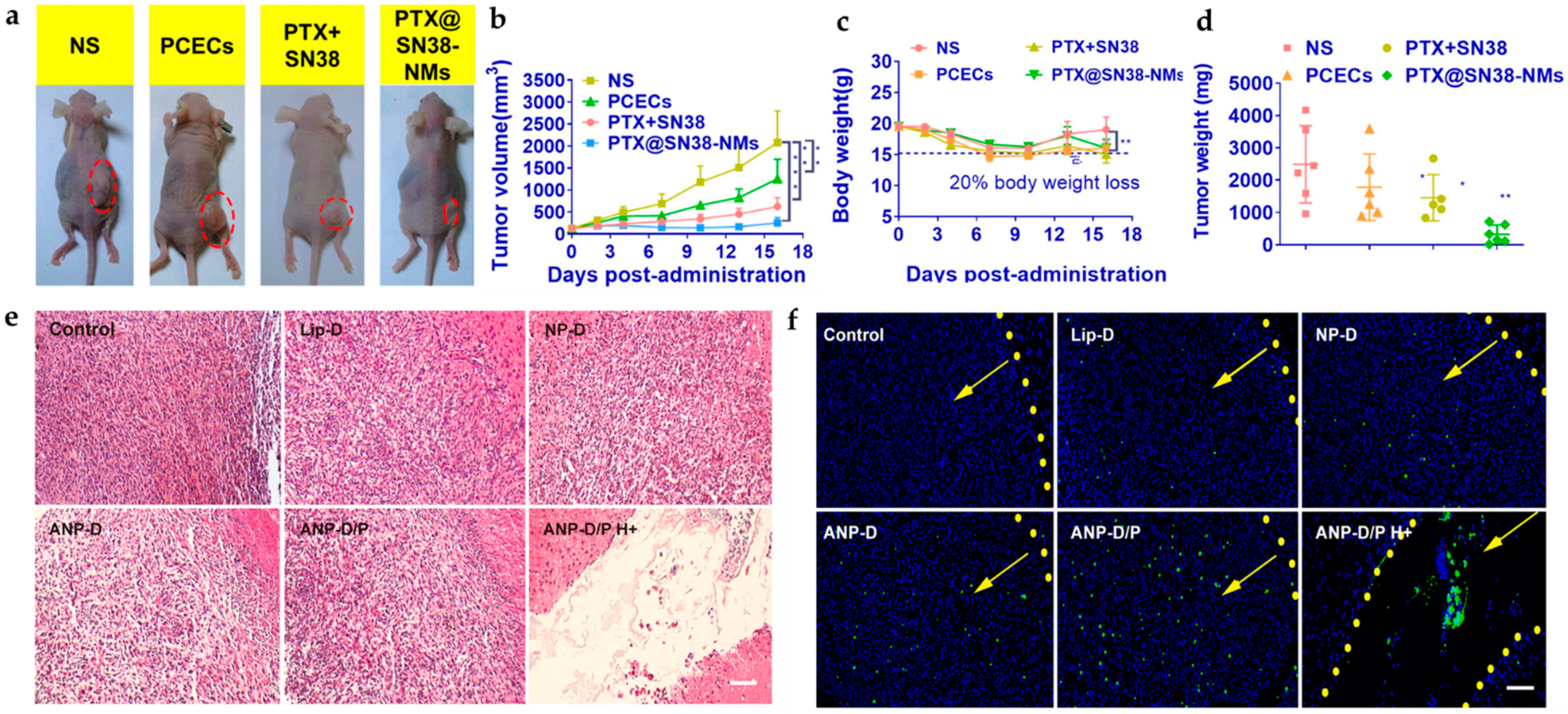
- Luo, Z.; Jin, K.; Pang, Q.; Shen, S.; Yan, Z.; Jiang, T.; Zhu, X.; Yu, L.; Pang, Z.; Jiang, X. On-Demand Drug Release from Dual-Targeting Small Nanoparticles Triggered by High-Intensity Focused Ultrasound Enhanced Glioblastoma-Targeting Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31612–31625. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Sahoo, S.K. Enhanced Accumulation of Curcumin and Temozolomide Loaded Magnetic Nanoparticles Executes Profound Cytotoxic Effect in Glioblastoma Spheroid Model. Eur. J. Pharm. Biopharm. 2013, 85, 452–462. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Ruzicka, L. The Isoprene Rule and the Biogenesis of Terpenic Compounds. Experientia 1953, 9, 357–367. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Komohara, Y.; Kudo, R.; Tsurushima, K.; Ohnishi, K.; Ikeda, T.; Takeya, M. Oleanolic Acid Inhibits Macrophage Differentiation into the M2 Phenotype and Glioblastoma Cell Proliferation by Suppressing the Activation of STAT3. Oncol. Rep. 2011, 26, 1533–1537. [Google Scholar]
- Zhang, Y.; Xu, H.; Wang, H.; Yu, W.; Zhao, X.; Xue, Y. Fluorine-18-Deoxyglucose Positron Emission Tomography/Computed Tomography with Ki67 and GLUT-1 Immunohistochemistry for Evaluation of the Radiosensitization Effect of Oleanolic Acid on C6 Rat Gliomas. Nucl. Med. Commun. 2015, 36, 21–27. [Google Scholar] [CrossRef]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a Potential Anticancer Compound for Breast Cancer Therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Xu, X.; Guan, S.; Zhang, R.; Zhang, X.; Kang, Y.; Wu, J. Nanodrug Carrier Based on Poly (Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv. Funct. Mater. 2020, 30, 1907857. [Google Scholar] [CrossRef]
- Feng, X.M.; Su, X.L. Anticancer Effect of Ursolic Acid via Mitochondria-Dependent Pathways. Oncol. Lett. 2019, 17, 4761–4767. [Google Scholar] [CrossRef]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer Activity and Concurrent Analysis of Ursolic Acid, β-sitosterol and Lupeol in Three Different Hibiscus Species (Aerial Parts) by Validated HPTLC Method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef]
- Paik, K.J.; Jeon, S.S.; Chung, H.Y.; Lee, K.H.; Kim, K.W.; Chung, J.K.; Kim, N.D. Induction of Differentiation of the Cultured Rat Mammary Epithelial Cells by Triterpene Acids. Arch. Pharmacal Res. 1998, 21, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D.; Vadgama, J.V. Sensitization to Docetaxel in Prostate Cancer Cells by Green Tea and Quercetin. J. Nutr. Biochem. 2015, 26, 408–415. [Google Scholar] [CrossRef]
- Forrest, G. Studies on the Polyphenol Metabolism of Tissue Cultures Derived from the Tea Plant (Camellia sinensis L.). Biochem. J. 1969, 113, 765–772. [Google Scholar] [CrossRef]
- Davies, R.; Coulson, C.; Lewis, D. Polyphenols in Plant, Humus, and Soil: Iv. Factors Leading to Increase in Biosynthesis of Polyphenol in Leaves and Their Relationship to Mull and Mor Formation. J. Soil Sci. 1964, 15, 310–318. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M. New Insights on the Anticancer Properties of Dietary Polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X. Resveratrol Suppresses Colon Cancer Growth by Targeting the AKT/STAT3 Signaling Pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol Inhibits the Proliferation and Induces the Apoptosis in Ovarian Cancer Cells via Inhibiting Glycolysis and Targeting AMPK/mTOR Signaling Pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Xu, H.; Jia, F.; Singh, P.K.; Ruan, S.; Zhang, H.; Li, X. Synergistic Anti-Glioma Effect of a Coloaded Nano-Drug Delivery System. Int. J. Nanomed. 2017, 12, 29. [Google Scholar] [CrossRef]
- Banerjee, S.; Gnanamani, E.; Lynch, S.R.; Zuñiga, F.Z.; Jiménez-Vargas, J.M.; Possani, L.D.; Zare, R.N. An Alkaloid from Scorpion Venom: Chemical Structure and Synthesis. J. Nat. Prod. 2018, 81, 1899–1904. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a Harmful Cyanobacterial Neurotoxin from the Environmental Scale to the Molecular Target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Mekky, H.; Al-Sabahi, J.; Abdel-Kreem, M. Potentiating Biosynthesis of the Anticancer Alkaloids Vincristine and Vinblastine in Callus Cultures of Catharanthus Roseus. S. Afr. J. Bot. 2018, 114, 29–31. [Google Scholar] [CrossRef]
- Kato, G.; Tan, E.; Yung, J. Acetylcholinesterase: Kinetic Studies on the Mechanism of Atropine Inhibition. J. Biol. Chem. 1972, 247, 3186–3189. [Google Scholar] [CrossRef]
- Schultz, A.G. Camptothecin. Chem. Rev. 1973, 73, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.G.; Handler, P. Metabolism of Trigonelline. J. Biol. Chem. 1962, 237, 3185–3188. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, Y.; Wang, X.Q.; Liu, L.H.; Hu, J.J.; Luo, G.F.; Chen, W.H.; Rong, L.; Zhang, X.Z. One-Pot Construction of Functional Mesoporous Silica Nanoparticles for the Tumor-Acidity-Activated Synergistic Chemotherapy of Glioblastoma. ACS Appl. Mater. Interfaces 2013, 5, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; De, K.; Mukherjee, D.; Dey, G.; Chattopadhyay, S.; Mukherjee, M.; Mandal, M.; Bandyopadhyay, A.K.; Gupta, A.; Ganguly, S.; et al. Paclitaxel-Loaded Solid Lipid Nanoparticles Modified with Tyr-3-octreotide for Enhanced Anti-Angiogenic and Anti-Glioma Therapy. Acta Biomater. 2016, 38, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Yakati, V.; Shankar, G.; Jaggarapu, M.M.C.S.; Moku, G.; Madhusudana, K.; Banerjee, R.; Ramkrishna, S.; Srinivas, R.; Chaudhuri, A. Amphetamine Decorated Cationic Lipid Nanoparticles Cross the Blood–Brain Barrier: Therapeutic Promise for Combating Glioblastoma. J. Mater. Chem. B 2020, 8, 4318–4330. [Google Scholar] [CrossRef]
- Mallick, D.J.; Eastman, A. AT101 [(-)-Gossypol] Selectively Inhibits MCL1 and Sensitizes Carcinoma to BH3 Mimetics by Inducing and Stabilizing NOXA. Cancers 2020, 12, 2298. [Google Scholar] [CrossRef]
- Householder, K.T.; DiPerna, D.M.; Chung, E.P.; Wohlleb, G.M.; Dhruv, H.D.; Berens, M.E.; Sirianni, R.W. Intravenous Delivery of Camptothecin-Loaded PLGA Nanoparticles for the Treatment of Intracranial Glioma. Int. J. Pharm. 2015, 479, 374–380. [Google Scholar] [CrossRef]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and Its Derivatives are Known to Target Topoisomerase I (Top1) as Their Mechanism of Action: Did We Miss Something in CPT Analogue Molecular Targets for Treating Human Disease Such as Cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar]
- Rezaei, T.; Hejazi, M.; Mansoori, B.; Mohammadi, A.; Amini, M.; Mosafer, J.; Rezaei, S.; Mokhtarzadeh, A.; Baradaran, B. MicroRNA-181a Mediates the Chemo-Sensitivity of Glioblastoma to Carmustine and Regulates Cell Proliferation, Migration, and Apoptosis. Eur. J. Pharmacol. 2020, 888, 173483. [Google Scholar] [CrossRef]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Wilson Miller, G.; Suk, J.S.; Hanes, J.; Price, R.J. MR Image-Guided Delivery of Cisplatin-Loaded Brain-Penetrating Nanoparticles to Invasive Glioma with Focused Ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-Modified Docetaxel-Loaded Nanoparticles for Brain-Targeted Glioma Therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J. Preclinical Mechanisms of Action of Docetaxel and Docetaxel Combinations in Prostate Cancer. Semin. Oncol. 2001, 28, 3–7. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual Functionalized Liposomes for Efficient Co-Delivery of Anti-Cancer Chemotherapeutics for the Treatment of Glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Chapter Four—Erlotinib. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 45, pp. 93–117. [Google Scholar]
- Gu, G.; Gao, X.; Hu, Q.; Kang, T.; Liu, Z.; Jiang, M.; Miao, D.; Song, Q.; Yao, L.; Tu, Y.; et al. The Influence of the Penetrating Peptide iRGD on the Effect of Paclitaxel-Loaded MT1-AF7p-conjugated Nanoparticles on Glioma Cells. Biomaterials 2013, 34, 5138–5148. [Google Scholar] [CrossRef]
- Lu, V.M.; Jue, T.R.; McDonald, K.L. Cytotoxic Lanthanum Oxide Nanoparticles Sensitize Glioblastoma Cells to Radiation Therapy and Temozolomide: An In Vitro Rationale for Translational Studies. Sci. Rep. 2020, 10, 18156. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Xu, H.; Li, X.; Yan, H.; Tang, H.; Wen, C.; Li, Y. The Construction of the Multifunctional Targeting Ursolic Acids Liposomes and Its Apoptosis Effects to C6 Glioma Stem Cells. Oncotarget 2017, 8, 64129. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Jayalakshmi, J.; Gopi, M.; Shajahan, A.; Barathikannan, K.; Kalaichelvan, P.T.; Wang, M.-H. Trigonelline-Loaded Chitosan Nanoparticles Prompted Antitumor Activity on Glioma Cells and Biocompatibility with Pheochromocytoma Cells. Int. J. Biol 2020, 163, 36–43. [Google Scholar] [CrossRef]
- Banstola, A.; Duwa, R.; Emami, F.; Jeong, J.-H.; Yook, S. Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(lactic-co-glycolic acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells. Mol. Pharm. 2020, 17, 4386–4400. [Google Scholar] [CrossRef]


| Type | NIR Windows | Nanoprobe | Dose | Emission | Ref. |
|---|---|---|---|---|---|
| Organic dye | NIR-I | IR dyes | 1 μM * | 650–950 nm | [42,43,44,45,46,47,48,49,50,51,52] |
| Cy5.5 | 2 mg/mL | 690–750 nm | [53,54,55,56,57] | ||
| ICG | 200 nmol/kg | 750–950 nm | [58,59] | ||
| MMP | 20 nM | 750 nm | [60] | ||
| SiNC | 1 mg/kg | 600–800 nm | [61] | ||
| NIR-II | New designed IR dyes | 10 mM | 900–1400 nm | [62,63,64] | |
| Lanthanide-doped nanoparticle | NIR-I | Tm-doped | 15 mg/kg | 800 nm | [65] |
| NIR-II | Nd-doped | 1 mg/mL | 1060 and 1340 nm | [66,67,68] | |
| NIR-III | Er-doped | 5 mg/mL | 1525 nm | [69] | |
| Quantum dot | NIR-I | Ag2S | 1 mg/mL * | 650–840 nm | [70] |
| NIR-II | 1000–1400 nm | [71,72,73] | |||
| N,B-doped graphene quantum dot | 1 mg/mL | 950–1100 nm | [74] | ||
| Nanophosphor | NIR-I | Cr3+-doped | 250 mg/mL * | 650–850 nm | [75] |
| Polymer dot | NIR-II | Aggregation-induced emission (AIE) | 10 mg/kg * | 800–1600 nm | [76,77,78,79] |
| Therapy | Type | Photosensitizer | Excitation Wavelength | Dose | Approach | Ref. |
|---|---|---|---|---|---|---|
| Photothermal | gold nanomaterial | gold nanostar | 808 nm laser (2 W/cm2) | 10 nM | ΔT 1 ~ 30 °C | [88] |
| two-dimensional materials | mesoporous silica-coated graphene nanosheet | 808 nm laser (6 W/cm2) | 50 μg/mL | ΔT ~ 30 °C | [95] | |
| quantum dot | carbon nanodot | 808 nm laser (2 W/cm2) | 5 mg/mL | ΔT ~ 50 °C; η 2 = 42% | [89] | |
| N,B-doped graphene quantum dot | 808 nm laser (1.5 W/cm2) | 1 mg/mL | η = 32% | [74] | ||
| metal oxide materials | Fe3O4@Au | 635 nm laser (0.3 W/cm2) | 0.5 mg/mL | ΔT ~ 20 °C | [90] | |
| Mn-doped magnetic nanoclusters | 750 nm laser | 250 μg/mL | ΔT ~ 16 °C | [91] | ||
| organic dye | COF@IR783 | 808 nm laser (0.75 W/cm2) | 2.5 mg/kg | ΔT ~ 20 °C | [96] | |
| D–A structured molecular | 1064/808 nm (1 W/cm2) | 0.05 mg/kg | η = 30% | [77] | ||
| ICG | 808 nm (2 W/cm2) | 40 μg/mL | η = 45% | [97] | ||
| ApoE-Ph (AIE) | 808 nm laser (0.5 W/cm2) | 10 mg/kg | η = 34% | [79] | ||
| Photodynamic | quantum dots | Cu2−xSe | 1064 nm laser (0.75 W/cm2) | 5 mg/kg | n/a | [83] |
| organic dye | dicysteamine-modified hypocrellin derivative (DCHB) | 721 nm laser (0.5 W/cm2) | 0.5 mM | η = 33%; quantum yield of 0.51 | [98] | |
| inorganic nanoparticle and organic dye | ANG-IMNPs | PTT: 808 nm laser (0.36 W/cm2) PDT: 980 nm laser (0.8 W/cm2) | 1.1 mg/kg | ΔT ~ 18 °C; cell viability ~ 28% | [99] | |
| Fe3O4-IR806 | 808 nm laser (3 W/cm2) | 10 mg/kg | η = 42%; cell viability ~ 19.2% | [100] |
| Nanoparticles (NP) | Medicine | Diagnostic Methods | Dose | Targeted Cellular Pathway | Ref. | |
|---|---|---|---|---|---|---|
| Lipid NP | Dioleoyl phosphatidylcholine (DOPC), Cholesterol (Chol), 16-DCL, PD-L1siRNA, and DSPE-PEG(2000)amine | PTX | Liquid chromatography-mass spectrometry (LC-MS) | 20 μM | n/a | [166] |
| Cubosomes | Glyceryl monooleate and Pluronic F-127 | AT101 | UV/Vis/NIR spectrophotometry and qRT-PCR | 10 wt% | Akt-signaling pathway | [167] |
| Polymer NP | PLGA | Camptothecin | H&E staining and IHC staining | 180 mg/kg | DNA damaging | [168,169] |
| Catanionic solid lipid NP | Anti-epithelial growth factor receptor (EGFR) | Carmustine | HPLC-UV system and enzyme-linked immunosorbent assay (ELISA) | 1.5 mM | PI3K/ AKT signaling pathway | [109,170] |
| Brain penetrating NP | Polyaspartic acid (PAA) and PEG | Cisplatin | MRI and ultrasound | 40 wt% | TNFα pathway | [171,172] |
| Polymer | PLGA and PEG | Docetaxel | ELISA, LC-MS, and flow cytometry | 1500 μg/mL | Arrestment of G2 and M phase | [173,174] |
| Liposomes | Transferrin and penetratin | Doxorubicin | HPLC, H&E staining | 15.2 μmoles/kg | n/a | [175] |
| Erlotinib | HPLC and H&E staining | n/a | [175,176] | |||
| Hybrid NP | Angiopep-2, PLGA, and perfluorooctyl bromide (PFOB) | Doxorubicin | High intensity focused ultra-sound (HIFU), fluorescence imaging, and flow cytometry | 50 μg/mL | n/a | [134] |
| Magnetic silica NP | Transferrin, PLGA, and mesoporous silica nanoparticle (MSN) | Doxorubicin | Flow cytometry | 400 μg/mL | n/a | [177] |
| Paclitaxel | Non-invasive bioluminescence imaging and H&E staining | [177] | ||||
| Lanthanum oxide (La2O3) NPs | La2O3 | Temozolomide | Western blotting | 100 µg/mL | Arrestment of G2/M phase | [178] |
| Lipid NP | Cholesterol | oleanolic | RT-PCR | 17.5 mM/kg | Caspase-3 pathway | [106] |
| Cubosomes | DiI | UA EGCG | LC-MS | 5 mg/kg | Hindrance of mitotic spindle | [179] |
| Polymer NP | NH2-PEG2000-DSPE | TMZ resveratrol | CLSM | 500 μg/mL | Inhibition of the p-Akt expression | [156] |
| Catanionic solid lipid NP | mPEG-PCL | Curcumin | flow cytometry | 50 mg/kg | Suppression of neovascularization | [118] |
| Brain penetrating NP | mPEG-PLA | Trigonelline | fluorescence microscope flow cytometry analysis | 34 μg/mL | Downregulation of the Nrf2 | [180] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.-H.; Huang, W.-T.; Satpathy, A.; Su, T.-Y.; Hsiao, M.; Liu, R.-S. Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics. Pharmaceutics 2022, 14, 456. https://doi.org/10.3390/pharmaceutics14020456
Chan M-H, Huang W-T, Satpathy A, Su T-Y, Hsiao M, Liu R-S. Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics. Pharmaceutics. 2022; 14(2):456. https://doi.org/10.3390/pharmaceutics14020456
Chicago/Turabian StyleChan, Ming-Hsien, Wen-Tse Huang, Aishwarya Satpathy, Ting-Yi Su, Michael Hsiao, and Ru-Shi Liu. 2022. "Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics" Pharmaceutics 14, no. 2: 456. https://doi.org/10.3390/pharmaceutics14020456
APA StyleChan, M.-H., Huang, W.-T., Satpathy, A., Su, T.-Y., Hsiao, M., & Liu, R.-S. (2022). Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics. Pharmaceutics, 14(2), 456. https://doi.org/10.3390/pharmaceutics14020456







