Novel Dermal Delivery Cargos of Clobetasol Propionate: An Update
Abstract
1. Introduction
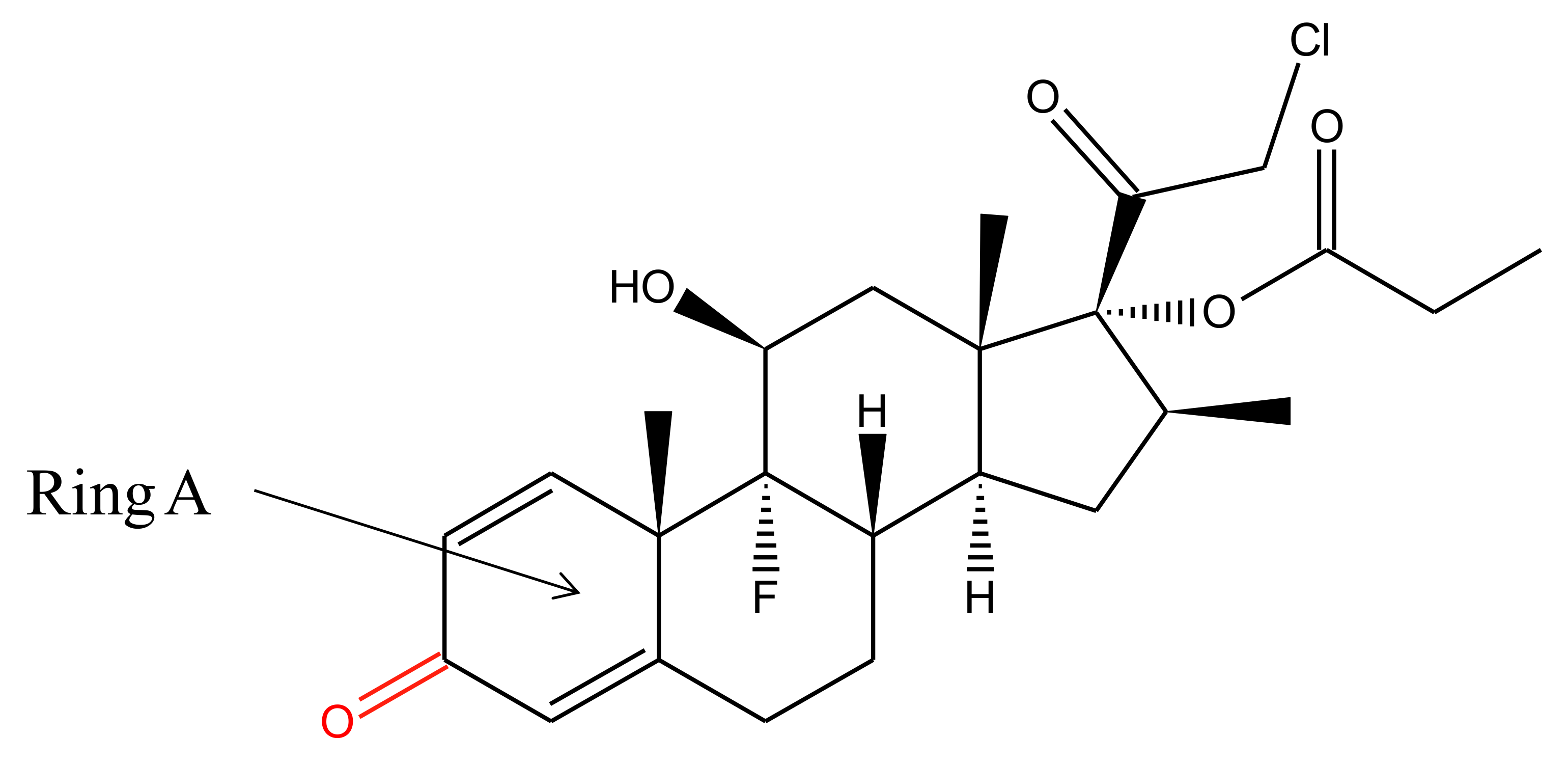
2. Physiochemical Properties and Metabolism of Clobetasol Propionate
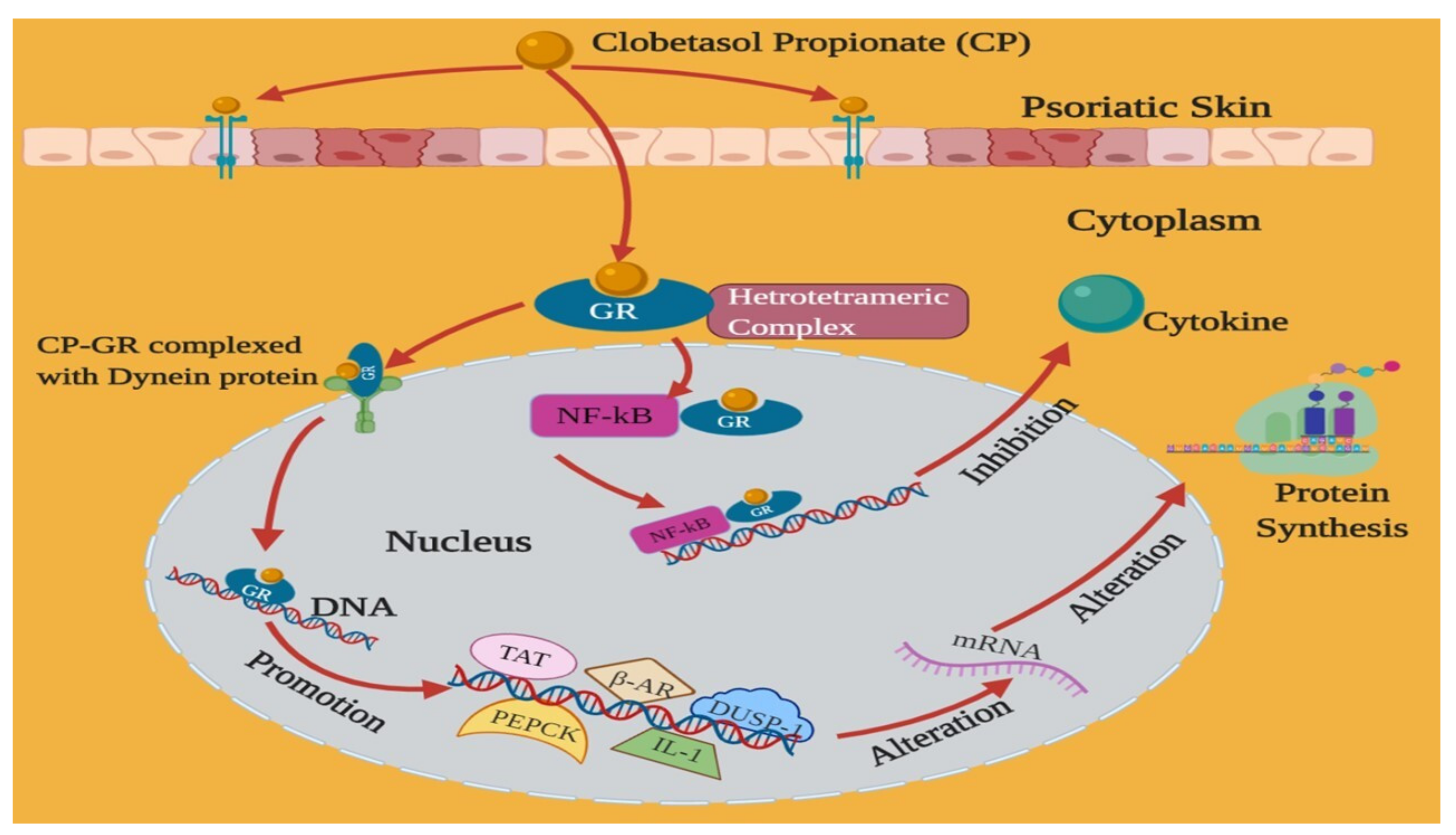
3. Mechanism of Action of Clobetasol Propionate
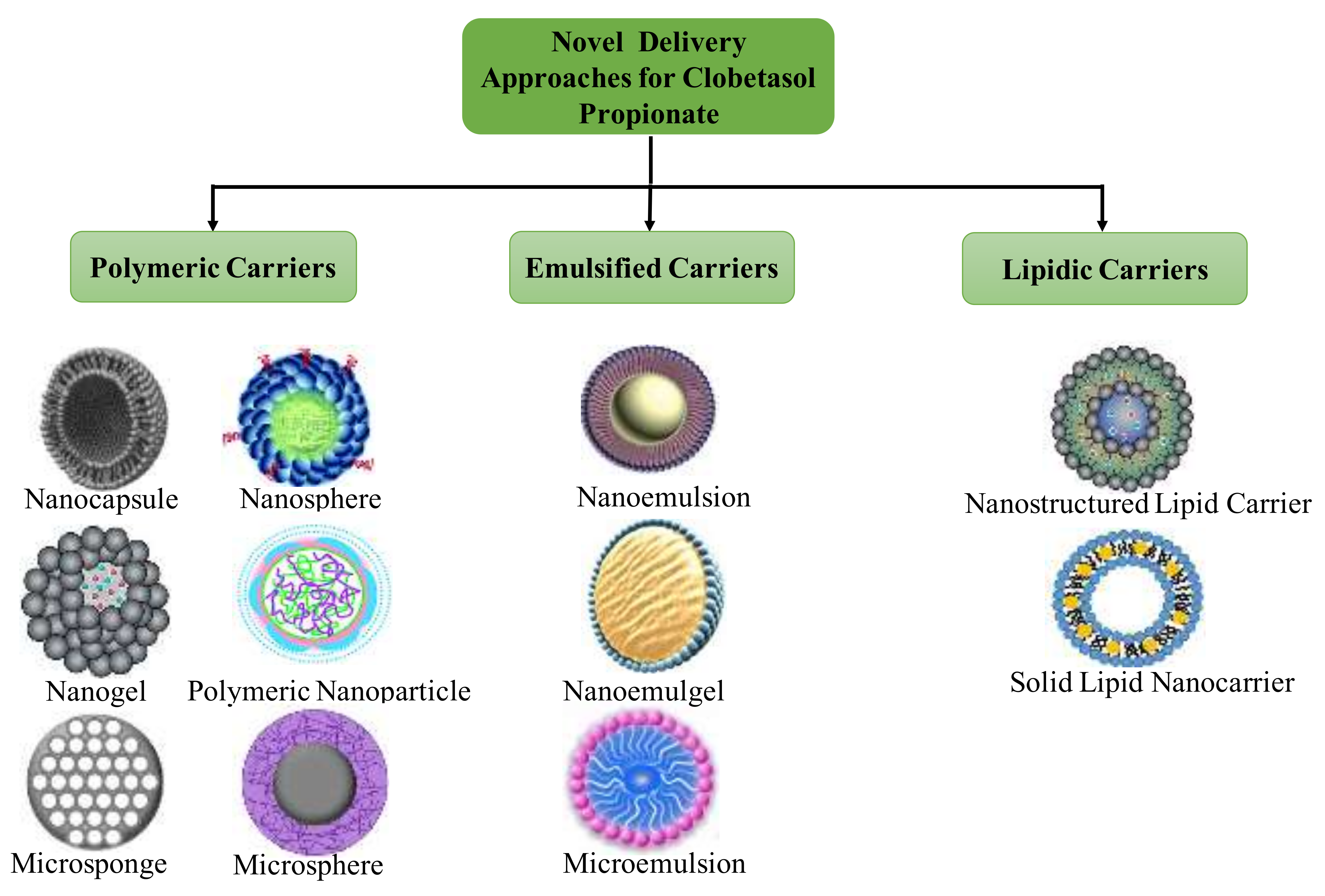
4. Novel Formulations Reported for Clobetasol Propionate
4.1. Nanoemulsions
4.2. Chitin Nanogel
4.3. Solid Lipid Nanoparticles
4.4. Nanostructured Lipid Carriers
4.5. Nanocapsules
4.6. Nanoparticles
4.7. Lecithin/Chitosan Nanoparticles
4.8. Miscellaneous Cargos
4.9. Other Novel Formulations Reported
4.9.1. Poly (d, l-lactic-co-glycolic Acid) Microspheres
4.9.2. Microemulsions
4.9.3. Microsponges
5. Cell Line Studies of Novel Clobetasol Propionate Formulations
6. In Vivo Studies of Novel Clobetasol Propionate Formulations
7. Stability Concerns of Clobetasol Propionate
8. Safety, Tolerability, and Toxicity Concerns of Clobetasol Propionate
9. Conclusions and Future Prospectus
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | cyclooxygenase |
| CP | clobetasol propionate |
| DUSP | dual-specificity protein phosphate |
| DUSP-1 | dual-specificity protein phosphatase 1 |
| GR | glucocorticoid receptors |
| GREs | glucocorticoid-responsive elements |
| Hsp90 | heat-shock protein 90 |
| ICH | International Conference on Harmonization |
| IL-1 | interleukin 1 |
| IκBa | inhibitory nuclear factor-κBa |
| LCNC | loaded lipid core nanocapsule |
| LOX | lipoxygenase |
| mRNA | messenger RNA |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide |
| NF-κb | nuclear factor kappa-light-chain-enhancer |
| NLCs | nanostructured lipid carriers |
| NTPDase | nucleoside triphosphate phosphohydrolase |
| PdI | polydispersity index |
| PEPCK | phosphoenolpyruvate carboxykinase |
| PLA | poly(DL-lactide) |
| PLGA | poly(lactic-co-glycolic acid) |
| SLNs | solid lipid nanoparticles |
| TAT | tyrosine aminotransferase |
| US-FDA | United States-Food and Drug Administration |
| UV-Vis | ultraviolet-visible |
References
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.J.; Spencer, L.; Hu, J.; Balkrishnan, R.; Fleischer, A.B., Jr.; Feldman, S.R. Class I topical corticosteroid use by psoriasis patients in an academic practice. J. Dermatol. Treat. 2004, 15, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Yentzer, B.A. Topical clobetasol propionate in the treatment of psoriasis: A review of newer formulations. Am. J. Clin. Dermatol. 2009, 10, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Kumar, S.; Prasad, M.; Rao, R. Eudragit RS100 based microsponges for dermal delivery of clobetasol propionate in psoriasis management. J. Drug Deliv. Sci. Technol. 2020, 55, 101347. [Google Scholar] [CrossRef]
- Schafer-Korting, M.; Kleuser, B.; Ahmed, M.; Holtje, H.D.; Korting, H.C. Glucocorticoids for human skin: New aspects of the mechanism of action. Skin Pharmacol. Physiol. 2005, 18, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Senyiğit, T.; Sonvico, F.; Barbieri, S.; Ozer, O.; Santi, P.; Colombo, P. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J. Control. Release Off. J. Control. Release Soc. 2010, 142, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Angelo, T.; El-Sayed, N.; Jurisic, M.; Koenneke, A.; Gelfuso, G.M.; Cunha-Filho, M.; Taveira, S.F.; Lemor, R.; Schneider, M.; Gratieri, T. Effect of physical stimuli on hair follicle deposition of clobetasol-loaded Lipid Nanocarriers. Sci. Rep. 2020, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.J.; Lehman, P.A.; Feldman, S.R.; Spellman, M.C. Bioavailability of clobetasol propionate in different vehicles. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.C.; Kimball, A.B. Clobetasol propionate foam in the treatment of psoriasis. Expert Opin. Pharmacother. 2005, 6, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Anroop, B.; Ghosh, B.; Parcha, V.; Khanam, J. Transdermal delivery of atenolol: Effect of prodrugs and iontophoresis. Curr. Drug Deliv. 2009, 6, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Reddy, C.; Jacob, S. Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin (ISBS) Int. Soc. Digit. Imaging Skin (ISDIS) Int. Soc. Skin Imaging (ISSI) 2009, 15, 187–194. [Google Scholar] [CrossRef]
- Anroop, B.; Ghosh, B.; Parcha, V.; Kumar, A.; Khanam, J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J. Drug Deliv. Sci. Technol. 2005, 15, 187–190. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, M.; Rao, R. Topical delivery of clobetasol propionate loaded nanosponge hydrogel for effective treatment of psoriasis: Formulation, physicochemical characterization, antipsoriatic potential and biochemical estimation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111605. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Jacob, S.; Bharadia, P.; Haroun, M. Proniosomal vesicles as an effective strategy to optimize naproxen transdermal delivery. J. Drug Deliv. Sci. Technol. 2021, 63. [Google Scholar] [CrossRef]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: Ex vivo permeation and skin irritation studies. Colloids Surf. B Biointerfaces 2013, 102, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Cornell, R.C. Topical clobetasol-17-propionate: Review of its clinical efficacy and safety. J. Am. Acad. Dermatol. 1986, 15, 246–255. [Google Scholar] [CrossRef]
- Iqbal, J.; Gupta, A.; Husain, A. Photochemistry of clobetasol propionate, a steroidal anti-inflammatory drug. Arkivoc 2006, 11, 91–98. [Google Scholar] [CrossRef]
- Van der Laan, S.; Meijer, O.C. Pharmacology of glucocorticoids: Beyond receptors. Eur. J. Pharmacol. 2008, 585, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.A. Mechanisms of action of topical therapies and the rationale for combination therapy. J. Am. Acad. Dermatol. 2005, 53, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ali, M.S.; Alam, N.; Siddiqui, M.R.; Shamim, M.; Safhi, M. In vivo study of clobetasol propionate loaded nanoemulsion for topical application in psoriasis and atopic dermatitis. Drug Invent. Today 2013, 5, 8–12. [Google Scholar] [CrossRef]
- Sarfaraz Alam, M.; Ali, M.S.; Zakir, F.; Alam, N.; Intakhab Alam, M.; Ahmad, F.; Siddiqui, M.R.; Ali, M.D.; Ansari, M.S.; Ahmad, S.; et al. Enhancement of Anti-Dermatitis Potential of Clobetasol Propionate by DHA [Docosahexaenoic Acid] Rich Algal Oil Nanoemulsion Gel. Iran. J. Pharm. Res. IJPR 2016, 15, 35–52. [Google Scholar]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1473–1482. [Google Scholar] [CrossRef]
- Fontana, M.C.; Rezer, J.F.; Coradini, K.; Leal, D.B.; Beck, R.C. Improved efficacy in the treatment of contact dermatitis in rats by a dermatological nanomedicine containing clobetasol propionate. Eur. J. Pharm. Biopharm. 2011, 79, 241–249. [Google Scholar] [CrossRef]
- Reddy, R.; Satyanarayana, S.; Reddy, V. Development and evaluation of clobetasol–loaded solid lipid nanoparticles for topical treatment of psoriasis. Int. J. Appl. Pharm. 2019, 11, 143–150. [Google Scholar] [CrossRef]
- Silva, L.A.D.; Taveira, S.F.; Lima, E.M.; Marreto, R.N. In vitro skin penetration of clobetasol from lipid nanoparticles: Drug extraction and quantitation in different skin layers. Braz. J. Pharm. Sci. 2012, 48, 811–817. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: Design and in vivo characterization. Drug Deliv. Transl. Res. 2016, 6, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Andrade, L.M.; de Sá, F.A.; Marreto, R.N.; Lima, E.M.; Gratieri, T.; Taveira, S.F. Clobetasol-loaded nanostructured lipid carriers for epidermal targeting. J. Pharm. Pharmacol. 2016, 68, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.C.; Coradini, K.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C. Nanocapsules prepared from amorphous polyesters: Effect on the physicochemical characteristics, drug release, and photostability. J. Nanosci. Nanotechnol. 2010, 10, 3091–3099. [Google Scholar] [CrossRef] [PubMed]
- Jaques, J.A.; Rezer, J.F.; Ruchel, J.B.; Souza Vdo, C.; Pinheiro Kde, V.; Schlemmer, K.B.; Schlemmer, J.B.; Bertoldo, T.M.; Martins, N.M.; Bertoncheli Cde, M.; et al. An experimental model of contact dermatitis: Evaluation of the oxidative profile of Wistar rats treated with free and nanoencapsulated clobetasol. Redox Rep. Commun. Free Radic. Res. 2012, 17, 206–213. [Google Scholar] [CrossRef]
- Mathes, C.; Melero, A.; Conrad, P.; Vogt, T.; Rigo, L.; Selzer, D.; Prado, W.A.; De Rossi, C.; Garrigues, T.M.; Hansen, S.; et al. Nanocarriers for optimizing the balance between interfollicular permeation and follicular uptake of topically applied clobetasol to minimize adverse effects. J. Control. Release Off. J. Control. Release Soc. 2016, 223, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M.; Silva, L.A.D.; Krawczyk-Santos, A.P.; Amorim, I.; Rocha, P.; Lima, E.M.; Anjos, J.L.V.; Alonso, A.; Marreto, R.N.; Taveira, S.F. Improved tacrolimus skin permeation by co-encapsulation with clobetasol in lipid nanoparticles: Study of drug effects in lipid matrix by electron paramagnetic resonance. Eur. J. Pharm. Biopharm. 2017, 119, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Pukale, S.S.; Sharma, S.; Dalela, M.; Singh, A.K.; Mohanty, S.; Mittal, A.; Chitkara, D. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020, 115, 393–409. [Google Scholar] [CrossRef]
- Şenyiğit, T.; Sonvico, F.; Rossi, A.; Tekmen, I.; Santi, P.; Colombo, P.; Nicoli, S.; Özer, Ö. In vivo Assessment of Clobetasol Propionate-Loaded Lecithin-Chitosan Nanoparticles for Skin Delivery. Int. J. Mol. Sci. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Ghalayani Esfahani, A.; Altomare, L.; Varoni, E.M.; Bertoldi, S.; Farè, S.; De Nardo, L. Electrophoretic bottom up design of chitosan patches for topical drug delivery. J. Mater. Sci. Mater. Med. 2019, 30, 40. [Google Scholar] [CrossRef]
- Dadwal, A.; Mishra, N.; Rawal, R.K.; Narang, R.K. Development and characterisation of clobetasol propionate loaded Squarticles as a lipid nanocarrier for treatment of plaque psoriasis. J. Microencapsul. 2020, 37, 341–354. [Google Scholar] [CrossRef]
- Badıllı, U.; Sen, T.; Tarımcı, N. Microparticulate based topical delivery system of clobetasol propionate. AAPS PharmSciTech 2011, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo--part II: Rheological characterization and in vivo assessment through dermatopharmacokinetic and pilot clinical studies. Colloids Surf. B Biointerfaces 2014, 119, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Langasco, R.; Tanrıverdi, S.T.; Özer, Ö.; Roldo, M.; Cossu, M.; Rassu, G.; Giunchedi, P.; Gavini, E. Prolonged skin retention of clobetasol propionate by bio-based microemulsions: A potential tool for scalp psoriasis treatment. Drug Dev. Ind. Pharm. 2018, 44, 398–406. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, D.F.; Fontana, M.C.; Pohlmann, A.R.; Guterres, S.S.; Carlos, R.; Beck, R. Nanoencapsulation of Clobetasol Propionate Decreases Its Penetration to Skin Layers Without Changing Its Relative Skin Distribution. J. Nanosci. Nanotechnol. 2015, 15, 875–879. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Jacob, S.; Patel, S.S.; Venugopala, K.N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin solid lipid nanoparticles for topical ocular therapy: Optimization, evaluation, and in vivo studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Yuan, H.; Zhang, H.H.; Fang, M. Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int. J. Pharm. 2002, 239, 121–128. [Google Scholar] [CrossRef]
- Kalariya, M.; Padhi, B.K.; Chougule, M.; Misra, A. Clobetasol propionate solid lipid nanoparticles cream for effective treatment of eczema: Formulation and clinical implications. Indian J. Exp. Biol. 2005, 43, 233–240. [Google Scholar] [PubMed]
- Prasad, M.; Ranjan, K.; Brar, B.; Shah, I.; Lalmbe, U.; Manimegalai, J.; Vashisht, B.; Gaury, M.; Kumar, P.; Khurana, S.K.; et al. Virus-Host Interactions: New Insights and Advances in Drug Development Against Viral Pathogens. Curr. Drug Metab. 2017, 18, 942–970. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Bortot, B.; Ruozi, B.; Dolcetta, D.; Vandelli, M.A.; Forni, F.; Severini, G.M. Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. Curr. Med. Chem. 2013, 20, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.C.; Coradini, K.; Guterres, S.S.; Pohlmann, A.R.; Beck, R.C. Nanoencapsulation as a way to control the release and to increase the photostability of clobetasol propionate: Influence of the nanostructured system. J. Biomed. Nanotechnol. 2009, 5, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.E.; Said, Z.; Santocildes-Romero, M.E.; Baker, S.R.; D’Apice, K.; Hansen, J.; Madsen, L.S.; Thornhill, M.H.; Hatton, P.V.; Murdoch, C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018, 178, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.E.; Shehata, T.M.; Mohamed, M.E.; Younis, N.S.; Elsewedy, H.S. Enhancement of Curcumin Anti-Inflammatory Effect via Formulation into Myrrh Oil-Based Nanoemulgel. Polymers 2021, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, T.; Cunha-Filho, M.S.; Gelfuso, G.M.; Gratieri, T. Chromatographic method for clobetasol propionate determination in hair follicles and in different skin layers. Biomed. Chromatogr. BMC 2017, 31, e3804. [Google Scholar] [CrossRef] [PubMed]
- Pels, R.; Sterry, W.; Lademann, J. Clobetasol propionate—Where, when, why? Drugs Today 2008, 44, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Kommanaboyina, B.; Rhodes, C.T. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev. Ind. Pharm. 1999, 25, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Fauzee, A.F.; Walker, R.B. Forced degradation studies of clobetasol 17-propionate in methanol, propylene glycol, as bulk drug and cream formulations by RP-HPLC. J. Sep. Sci. 2013, 36, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, N.; Rajpoot, K.; Tekade, M.; Kalyane, D.; Nair, A.B.; Venugopala, K.N.; Tekade, R.K. Development of metronidazole loaded chitosan nanoparticles using QBD approach—a novel and potential antibacterial formulation. Pharmaceutics 2020, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Alam, M.S.; Alam, N.; Anwer, T.; Safhi, M.M. Accelerated Stability Testing of a Clobetasol Propionate-Loaded Nanoemulsion as per ICH Guidelines. Sci. Pharm. 2013, 81, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Onuki, Y.; Fukami, T.; Koide, T. Comparison of various pharmaceutical properties of clobetasol propionate cream formulations - considering stability of mixture with moisturizer. J. Pharm. Health Care Sci. 2020, 6, 1. [Google Scholar] [CrossRef]
- Alam, S.; Sharma, P. Stability study of clobetasol propionate loaded tea tree oil nanoemulsion as per ICH guidelines. Res. J. Pharm. Technol. 2016, 9, 1999–2004. [Google Scholar] [CrossRef]
- Horn, E.J.; Domm, S.; Katz, H.I.; Lebwohl, M.; Mrowietz, U.; Kragballe, K. Topical corticosteroids in psoriasis: Strategies for improving safety. J. Eur. Acad. Dermatol. Venereol. JEADV 2010, 24, 119–124. [Google Scholar] [CrossRef]
- Laws, P.M.; Young, H.S. Topical treatment of psoriasis. Expert Opin. Pharmacother. 2010, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Ohman, E.M.; Rogers, S.; Meenan, F.O.; McKenna, T.J. Adrenal suppression following low-dose topical clobetasol propionate. J. R. Soc. Med. 1987, 80, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Staughton, R.C.; August, P.J. Cushing’s syndrome and pituitary-adrenal suppression due to clobetasol propionate. Br. Med. J. 1975, 2, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Hosking, G.P.; Elliston, H. Benign intracranial hypertension in a child with eczema treated with topical steroids. Br. Med. J. 1978, 1, 550–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siklar, Z.; Bostanci, I.; Atli, O.; Dallar, Y. An infantile Cushing syndrome due to misuse of topical steroid. Pediatr. Dermatol. 2004, 21, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.L. The role of clobetasol propionate emollient 0.05% in the treatment of patients with dry, scaly, corticosteroid-responsive dermatoses. Clin. Ther. 1998, 20, 26–39. [Google Scholar] [CrossRef]
| Carrier Systems | Fabrication Methods | Evaluation | References |
|---|---|---|---|
| Nanoemulsions | Aqueous phase titration method | In vivo anticontact dermatitis, anti-inflammatory, and irritation studies using Wistar rats | [21] |
| Rich algal oil nanoemulsion gel | Aqueous phase titration method | In vivo skin irritation, anti-inflammatory studies, and Nucleoside triphosphate phosphohydrolase activity of lymphocytes | [22] |
| Nanoemulsion-loaded gels | Spontaneous emulsification method | Codelivery of CP and calcipotriol for the management of psoriasis with dermatokinetics and skin distribution | [23] |
| Lipid-core nanocapsules, nanoemulsions | Interfacial deposition of the polymers, spontaneous emulsification | In vivo efficacy against contact dermatitis and Nucleoside triphosphate phosphohydrolase activity of lymphocytes using female Wistar rats | [24] |
| Chitin nanogels | Controlled regeneration method | In vitro and in vivo antipsoriatic studies with oxidative stress markers | [7] |
| SLNs | Emulsification–homogenization method | Ex vivo diffusion study | [25] |
| Nanostructured lipid carriers | Microemulsion technique | CP accumulation in stratum corneum in porcine ear skin | [26] |
| Nanostructured lipid carrier gels | The hot high-pressure homogenization method | In vitro release; in vivo anti-inflammatory assay using Wistar albino rats | [27] |
| Nanostructured lipid carriers | Microemulsion technique | Epidermal targeting and permeation studies using porcine ear skin | [28] |
| Nanocapsules | Interfacial deposition of the polymers | In vitro drug release and photo stability | [29] |
| Nanocapsules | Interfacial deposition | In vivo induction and treatment of contact dermatitis in female Wistar rats and oxidative stress assessment in liver tissue | [30] |
| Nanospheres, nanocapsules, lipid-core nanocapsules | Nanoprecipitation-solvent evaporation technique | Optimization between interfollicular permeation and follicular uptake balance to minimize adverse effects | [31] |
| Lipid nanoparticles | Microemulsion technique | Co-delivery with tacrolimus | [32] |
| Hybrid nanoparticles | Monowave assisted ring-opening polymerization | In vivo antipsoriatic activity | [33] |
| Lecithin/chitosan nanoparticles | Ionic interaction | Evaluation of skin barrier function and damage | [34] |
| Chitosan patches | Electrophoretic deposition | For fast drug delivery in oral mucosa disease | [35] |
| Squarticles (nanoemulgels) | Homogenization method | Enhancing the better permeation, increasing skin retention | [36] |
| PLGA microspheres | Oil/water emulsion-solvent evaporation method | In vitro drug release studies with sustained release | [37] |
| Microemulsion based gels | Homogenization method | Ex vivo skin permeation on male Wistar albino rat skin and in vivo skin irritation studies on Albino rabbits | [16] |
| Microemulsion based gels | Homogenization method | In vivo dermatokinetics and pilot clinical studies for vitiligo treatment | [38] |
| Microemulsions | Homogenization method | Drug distribution through microscopy, ex vivo skin permeation studies | [39] |
| Eudragit microsponge gels | Quasi emulsion solvent diffusion method | Therapeutic efficacy of the drug for psoriasis | [4] |
| Lipid nanocarriers | Microemulsion technique | In vitro cutaneous permeation, in vivo hair follicle targeting with physical stimuli (IR, US, mechanical message) | [8] |
| Lipid-core nanocapsule gels | Interfacial deposition of preformed polymers | In vitro skin permeation and penetration in abdominal porcine skin | [40] |
| Cyclodextrin based nanosponge hydrogel | Melt method | In vivo antipsoriatic activity | [14] |
| Carrier Systems | Cell Lines | Assays | References |
|---|---|---|---|
| Nanoemulsion-loaded gels | HaCaT | Ex vivo efficacy study (MTT assay) | [23] |
| Chitin nanogels | L929, HaCaT, and THP1 | Cyto-compatibility, Cell uptake study, COX and LOX activity | [7] |
| Mucoadhesive patches | Immortalized oral keratinocytes FNB6-TERT | Cytotoxicity studies | [56] |
| Hybrid nanoparticles | HaCaT | Cellular uptake studies, in vitro cytotoxicity assay, apoptosis assay, and Cell-cycle analysis | [33] |
| Nanostructured lipid carriers | HaCaT | Cell viability study | [8] |
| Nanosponge hydrogels | THP1 | Cytocompatibility studies | [14] |
| Delivery Systems | Animals Used | Activity/Bioassay | Remarks | References |
|---|---|---|---|---|
| Lipid-core nanocapsules, nanoemulsions | Female Wistar rats | 5% Nickle sulfate-induced dermatitis, NTPDase activity of lymphocytes | Enhanced NTPDase activity using lipid core nanocapsule-loaded hydrogels | [24] |
| Nanocapsule loaded hydrogels | Female Wistar rats | Nickle sulfate-induced dermatitis, biochemical assays of liver | Enhanced protective action against the oxidative damage using CP-loaded nanocapsules | [30] |
| Nanoemulsions | Wistar rats | Anti-inflammatory activity (Hind paw edema method) | Maximum inhibition of edema observed with prepared formulation | [21] |
| Nanoemulsions | Wistar rats | Skin irritation test | The formulation showed low irritation potential | [21] |
| Microemulsion based gels | Albino rabbits | Skin irritation test | Microemulsion-based gel found to be less irritant than marketed formulation | [16] |
| Microemulsion based gels | Albino Wistar rats | Dermatopharmacokinetic study | Enhanced therapeutic activity at the site of action and improvement in bioavailability | [38] |
| Nanoemulsions | Wistar rats of either sex | Anti-inflammatory activity (Hind paw edema method) | Hydrogel-thickened nanogel formulation has better anti-inflammatory activity than plain gel | [22] |
| Nanoemulsions | Wistar rats of either sex | Skin irritation test | Nanoemulsion showed more irritation potential than placebo formulation but was found safe for human use | [22] |
| NLCs | Male Wistar rats | Anti-inflammatory activity (paw edema method) | Decreased inflammation for a longer period was demonstrated by using NLCs | [27] |
| Nanoemulsion-loaded gels | Balb C mice | Antipsoriatic activity | Nanoemulsion-loaded gel displayed maximum antipsoriatic activity in comparison to plain gel and marketed formulation | [23] |
| Nanoemulsion-loaded gels | Balb C mice | Skin irritation test | Nanoemulsion-loaded gel showed very low irritation potential as compared to plain gel and marketed formulation | [23] |
| Nanogels | Balb C mice | Imiquimod induced psoriasis model | Nanogel presented better antipsoriatic activity than marketed formulation | [7] |
| Nanogels | Balb C mice | Skin irritation test | Nanogel was not found to induce any noticeable changes on the mice back skin | [7] |
| Nanoparticles | Male albino Wistar rats | Anti-inflammatory activity (carrageenan-induced hind paw edema model | Nanoparticles demonstrated significantly higher anti-inflammatory activity when compared to a sodium deoxycholate gel and commercial cream (Dermovate) containing the same drug. | [34] |
| Microsponge based gels | Swiss albino mice | Antipsoriatic activity (mouse tail model) | Microsponges displayed a higher efficacy than plain gel | [4] |
| Hybrid nanoparticles | Swiss albino mice | Antipsoriatic activity (imiquimod induced psoriasis-like inflammation) | Enhanced antipsoriatic potential | [33] |
| Squarticles (nanoemulgels) | Wistar rats | Ultraviolet B exposure; Skin irritation study and pharmacokinetic study | Enhanced antipsoriatic activity compared to marketed formulation, no sign of skin irritation, least penetration of the CP in the blood, and high CP deposition in pilosebaceous glands was observed | [36] |
| Nanosponge hydrogels | Swiss mice | Antipsoriatic activity (mouse tail model) | Enhanced antipsoriatic potential compared to plain CP gel | [14] |
| Carrier Systems | Storage Conditions | Evaluation | References |
|---|---|---|---|
| Nanocapsules, nanospheres, and nanoemulsions | Kept in dark at room temperature (25 ± 2 °C) for 9 months | Drug content, pH, encapsulation efficiency, particle size, PdI, and zeta potential | [55] |
| Nanocapsules | Stored in dark at room temperature for 3 months | Particle size, PdI, and zeta potential | [29] |
| Lecithin/chitosan nanoparticles and their gels | 25 °C and 60% RH for 3 months | Particle size, PdI, and zeta potential for nanoparticles; pH, viscosity, and drug content for nanoparticle-based gels | [6] |
| Nanoemulsions | 40 °C ± 2 °C/75% ± 5% RH; 30 °C ± 2 °C/65% RH ± 5% RH | Accelerated stability studies; Shelf life of nanoemulsions | [67] |
| Microemulsions and microemulsion based gels | 2–8 °C and 40 ± 2 °C/75 ± 5% RH for three months | Globule size and PdI for microemulsions, appearance for microemulsion-based gel | [16] |
| Tea tree oil nanoemulsion | As per ICH guidelines for 3 months | Accelerated stability studies, Phase separation, Ostwald ripening, coalescence, and creaming | [69] |
| Nanoemulsion gel | Centrifugation (5000 rpm) for 30 min, heating and cooling cycles, and Freeze-thaw cycles | Physical stability studies | [22] |
| Nanocarriers | Room temperature (25 ± 2 °C) for 3 months | Particle size, PdI, pH, and zeta potential | [31] |
| Nanostructured lipid gel | 5 ± 1, 25 ± 2, 40 ± 2, 60 ± 2 °C, and 75 ± 5% RH for 6 months | Shelf life of the prepared formulation | [27] |
| NLCs | Room temperature (25 ± 2 °C) 4 °C for 7 days | Colloidal stability assessment using Turbiscan Lab apparatus for 90 min | [28] |
| Chitin nanogels | 2–8 °C, 25 ± 5 °C and 40 °C with 65% RH for 3 months | Appearance, physical state, odor, color, and particle size | [7] |
| Bio-based microemulsions | Centrifugation (13,000 rpm) for 30 min; Also, at 2–8 °C and room temperature (25 ± 2 °C) | Physical stability studies | [39] |
| Microsponge gel | 5 ± 2 °C, 25 ± 2 °C and 40 ± 2 °C for 40 days | Appearance, pH, drug content, and in vitro release pattern | [4] |
| NLCs | 5, 25 and 40 °C for 30 days | Hydrodynamic diameter, PdI, zeta potential, pH, and entrapment efficiency | [8] |
| Squarticles based gel | 4 ± 2, 25 ± 2 and 45 ± 2 °C for 6 months | Entrapment efficiency, PdI, particle size, and drug content at periodical intervals | [36] |
| Nanosponges | 25 °C for 3 months | Particle size, zeta potential, PdI, and drug content | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Kumar, S.; Dalal, P.; Nagpal, C.; Dalal, S.; Rao, R.; Sreeharsha, N.; Jacob, S. Novel Dermal Delivery Cargos of Clobetasol Propionate: An Update. Pharmaceutics 2022, 14, 383. https://doi.org/10.3390/pharmaceutics14020383
Nair AB, Kumar S, Dalal P, Nagpal C, Dalal S, Rao R, Sreeharsha N, Jacob S. Novel Dermal Delivery Cargos of Clobetasol Propionate: An Update. Pharmaceutics. 2022; 14(2):383. https://doi.org/10.3390/pharmaceutics14020383
Chicago/Turabian StyleNair, Anroop B., Sunil Kumar, Pooja Dalal, Chahat Nagpal, Sweta Dalal, Rekha Rao, Nagaraja Sreeharsha, and Shery Jacob. 2022. "Novel Dermal Delivery Cargos of Clobetasol Propionate: An Update" Pharmaceutics 14, no. 2: 383. https://doi.org/10.3390/pharmaceutics14020383
APA StyleNair, A. B., Kumar, S., Dalal, P., Nagpal, C., Dalal, S., Rao, R., Sreeharsha, N., & Jacob, S. (2022). Novel Dermal Delivery Cargos of Clobetasol Propionate: An Update. Pharmaceutics, 14(2), 383. https://doi.org/10.3390/pharmaceutics14020383








