Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications
Abstract
1. Introduction
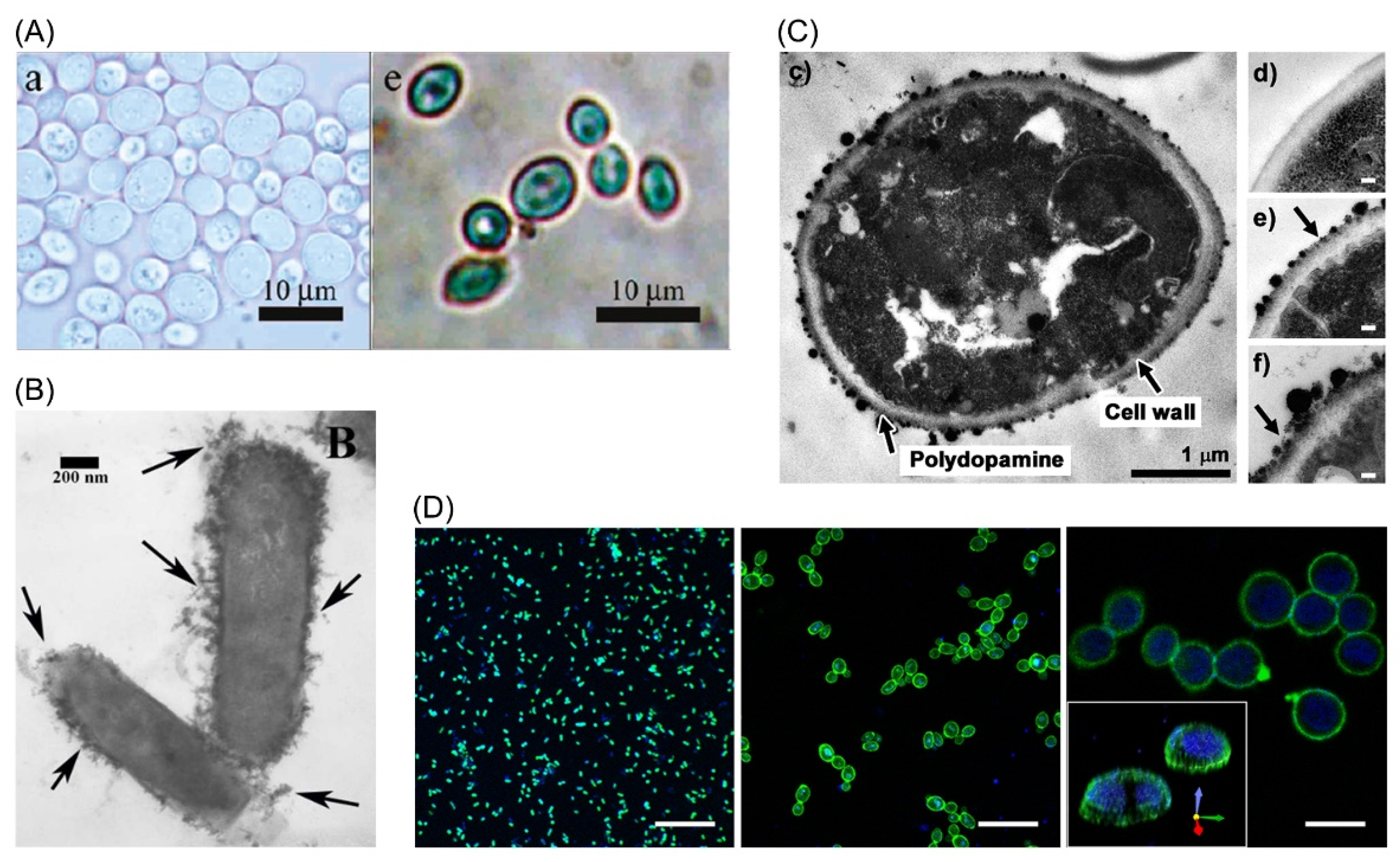
2. History of LbL Single-Cell Encapsulation
3. Biomaterials Employed in LbL Cell Encapsulation
3.1. Polycations Utilized for Non-Mammalian Cell Coating
3.1.1. Polyethylenimine (PEI)
3.1.2. PAH
3.1.3. PDDA
3.2. Polycations Utilized for Mammalian Cell Coating
3.2.1. Gelatin
3.2.2. Cationic Cellulose
3.2.3. Polyamidoamine
3.2.4. Chitosan
3.2.5. Poly-L-lysine (PLL)

3.3. Polyanions Utilized for Mammalian Cell Coating
3.3.1. Alginate
3.3.2. HA
3.3.3. PSS
3.4. Other Materials
| Materials | Cell Types | Applications | Reference | |
|---|---|---|---|---|
| Polycations (for non-mammalian cells) | PEI | Yeast cells | Function maintenance | [38] |
| PAH | Yeast cells | Cell immobilization | [40] | |
| PDDA | Allochromatium vinosum | Physical protection | [43] | |
| Polycations (for mammalian cells) | Gelatin | PC12 cells; NSCs | Protection and payload delivery | [48,49] |
| Cationic cellulose | Islet cells | Function maintenance | [57] | |
| Polyamidoamine | Islet cells | Immunocamouflage | [63] | |
| Chitosan | MSCs; Islet cells | Directed differentiation; Fibration prevention | [75,76] | |
| PLL | MSCs | Function maintenance | [87] | |
| Polyanions | Alginate | Fibroblasts, endothelial cells | Oxidative stress reaction control | [101] |
| HA | MSCs; NSCs | Directed differentiation | [110,111] | |
| PSS | Islet cells | Immunocamouflage | [115] | |
| Others | Metallic nanoparticles | S. cerevisiae | Function maintenance | [116] |
| Carbon nanotubes | S. cerevisiae | Toxicity evaluation | [117] | |
| Graphite oxide | S. cerevisiae | Antimicrobial | [118] | |
| Magnetic nanoparticles | Alcanivorax borkumensis; Yeast cells; HeLa cells | Cell functionalization; Spatial locomotion | [119,120,122] | |
| Mussel-inspired polydopamine | Yeast cells | Cell protection | [124] | |
| DNA molecules | Escherichia coli, Yeast cells, MCF-7 cells | Function maintenance | [126] | |
4. Interactions of LbL Multilayers with Interfaces
4.1. Properties of Materials Adsorption
4.1.1. Interactions on Planar Interfaces
4.1.2. Interactions on Colloidal Particles
4.2. Properties of Polyelectrolyte Multilayers
4.2.1. Amount of Adsorbed Materials
4.2.2. Morphology of Polyelectrolyte Multilayers
4.3. Charge Balance of LbL Multilayers
4.3.1. Charge Reversal
4.3.2. Charges Distribution in the Multilayer Structure
5. Functional Regulation of Cells by LbL Encapsulation
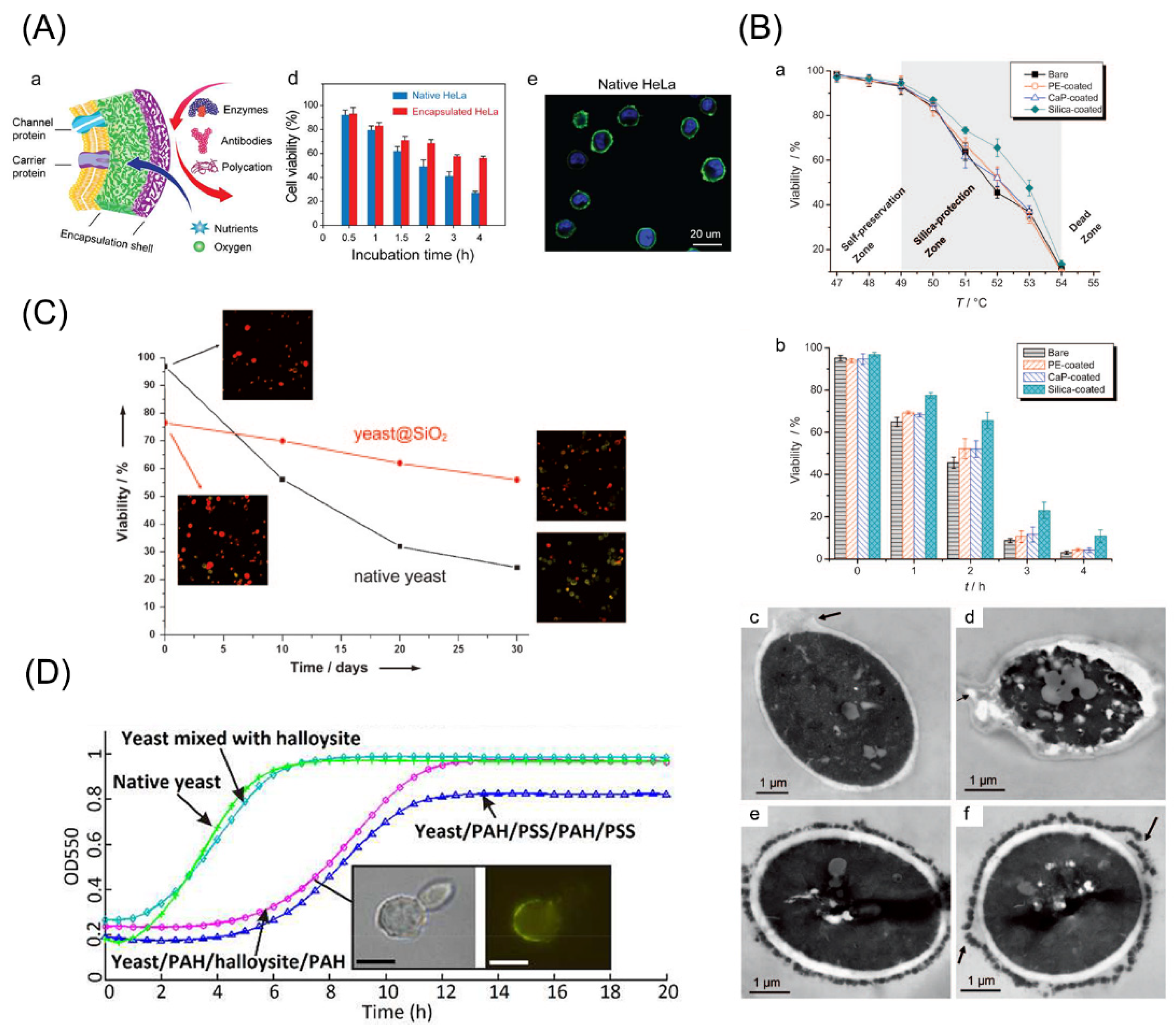
6. Applications of Drug Delivery via LbL Cell Encapsulation
6.1. Environment-Adjustable Release
6.2. ROS-Controlled Release
6.3. Biology Induced Release
6.4. Drug Delivery via Cell Encapsulation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guerzoni, L.P.B.; Tsukamoto, Y.; Gehlen, D.B.; Rommel, D.; Haraszti, T.; Akashi, M.; De Laporte, L. A Layer-by-Layer Single-Cell Coating Technique To Produce Injectable Beating Mini Heart Tissues via Microfluidics. Biomacromolecules 2019, 20, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Wang, Z.; Pan, H.; Lin, Y.; Chang, D. Sensitive detection of prostate-specific antigen based on dual signal amplification of Fc@MgAl-LDH and NH(2)-MIL-101(Fe). Biosens. Bioelectron. 2021, 190, 113437. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Rodríguez, S.; Molins-Legua, C.; Campíns-Falcó, P.; Giroud, F.; Gross, A.J.; Cosnier, S. Monofunctional pyrenes at carbon nanotube electrodes for direct electron transfer H(2)O(2) reduction with HRP and HRP-bacterial nanocellulose. Biosens. Bioelectron. 2021, 187, 113304. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Hatami, J.; Mano, J.F. Coating Strategies Using Layer-by-layer Deposition for Cell Encapsulation. Chem. Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Healthc. Mater. 2019, 8, e1800939. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210, 831–835. [Google Scholar] [CrossRef]
- Hou, S.; Li, W.; Watzele, S.; Kluge, R.M.; Xue, S.; Yin, S.; Jiang, X.; Döblinger, M.; Welle, A.; Garlyyev, B.; et al. Metamorphosis of Heterostructured Surface-Mounted Metal-Organic Frameworks Yielding Record Oxygen Evolution Mass Activities. Adv. Mater. 2021, 33, e2103218. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Tong, Z.; Gao, B.; Gao, N.; Shen, T.; Wan, Y.; Yu, Z.; Liu, L.; Ma, X.; et al. Self-Assembly of Hydrophobic and Self-Healing Bionanocomposite-Coated Controlled-Release Fertilizers. ACS Appl. Mater. Interfaces 2020, 12, 27598–27606. [Google Scholar] [CrossRef]
- Barberio, A.E.; Smith, S.G.; Correa, S.; Nguyen, C.; Nhan, B.; Melo, M.; Tokatlian, T.; Suh, H.; Irvine, D.J.; Hammond, P.T. Cancer Cell Coating Nanoparticles for Optimal Tumor-Specific Cytokine Delivery. ACS Nano 2020, 14, 11238–11253. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S.; Voigt, A.; Mitlöhner, R.; Sukhorukov, G.; Donath, E.; Möhwald, H. Scanning force microscopy investigation of polyelectrolyte nano-and microcapsule wall texture. Langmuir 2000, 16, 4059–4063. [Google Scholar] [CrossRef]
- Neu, B.; Voigt, A.; Mitlohner, R.; Leporatti, S.; Gao, C.Y.; Donath, E.; Kiesewetter, H.; Mohwald, H.; Meiselman, H.J.; Baumler, H. Biological cells as templates for hollow microcapsules. J. Microencapsul. 2001, 18, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sugunan, A.; Melin, P.; Schnürer, J.; Hilborn, J.G.; Dutta, J. Nutrition-Driven Assembly of Colloidal Nanoparticles: Growing Fungi Assemble Gold Nanoparticles as Microwires. Adv. Mater. 2007, 19, 77–81. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, S.; Zhang, Y.; Wang, Q.; Ren, T. Biotemplate-directed fabrication of size-controlled monodisperse magnetic silica microspheres. Colloids Surf. B 2015, 131, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Almodóvar, J.; Boudou, T.; Picart, C. Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D. Adv. Healthc. Mater. 2015, 4, 811–830. [Google Scholar] [CrossRef]
- Li, M.; Ai, H.; Mills, D.K.; Lvov, Y.M.; McShane, M.J.; Gale, B.K. Using microfabrication and electrostatic layer-by-layer (LbL) sel-assembly technologies to improve the growth and alignment of smooth muscle cells. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Madison, WI, USA, 2–4 May 2002. [Google Scholar] [CrossRef]
- Hiraoka, R.; Funasaki, Y.; Ishii, J.; Maruyama, T. Rational design of a degradable polyanion for layer-by-layer assembly for encapsulation and release of cationic functional biomolecules. Chem. Commun. 2015, 51, 17447–17450. [Google Scholar] [CrossRef]
- Diaspro, A.; Silvano, D.; Krol, S.; Cavalleri, O.; Gliozzi, A. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir 2002, 18, 5047–5050. [Google Scholar] [CrossRef]
- Mansouri, S.; Fatisson, J.; Miao, Z.; Merhi, Y.; Winnik, F.o.M.; Tabrizian, M. Silencing Red Blood Cell Recognition toward Anti-A Antibody by Means of Polyelectrolyte Layer-by-Layer Assembly in a Two-Dimensional Model System. Langmuir 2009, 25, 14071–14078. [Google Scholar] [CrossRef]
- Balkundi, S.S.; Veerabadran, N.G.; Eby, D.M.; Johnson, G.R.; Lvov, Y.M. Encapsulation of Bacterial Spores in Nanoorganized Polyelectrolyte Shells. Langmuir 2009, 25, 14011–14016. [Google Scholar] [CrossRef]
- Li, W.; Guan, T.; Zhang, X.; Wang, Z.; Wang, M.; Zhong, W.; Feng, H.; Xing, M.; Kong, J. The Effect of Layer-by-Layer Assembly Coating on the Proliferation and Differentiation of Neural Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-j.; Wang, J.; Miao, Y.; Liu, Y.-q.; Jiang, W.; Fan, Z.-x.; Darabi, M.-A.; Hu, Z.-q.; Xing, M. Cytokine loaded layer-by-layer ultrathin matrices to deliver single dermal papilla cells for spot-by-spot hair follicle regeneration. J. Mater. Chem. B 2016, 4, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Skaat, H.; Kennedy, S.M.; Hu, J.; Darnell, M.; Raimondo, T.M.; Mooney, D.J. Switchable release of entrapped nanoparticles from alginate hydrogels. Adv. Healthc. Mater. 2015, 4, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Swaminathan, V.; Malkovskiy, A.; Santhanam, S.; McConnell, K.; George, P.M. Single-Cell Encapsulation via Click-Chemistry Alters Production of Paracrine Factors from Neural Progenitor Cells. Adv. Sci. 2020, 7, 1902573. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, R.M.; Burke, N.A.; Zhou, C.; Stöver, H.D. Synthetic polycations with controlled charge density and molecular weight as building blocks for biomaterials. J. Biomater. Sci. Polym. Ed. 2016, 27, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Ivashkov, O.V.; Sybachin, A.V.; Efimova, A.A.; Pergushov, D.V.; Orlov, V.N.; Schmalz, H.; Yaroslavov, A.A. The Influence of the Chain Length of Polycations on their Complexation with Anionic Liposomes. Chemphyschem 2015, 16, 2849–2853. [Google Scholar] [CrossRef]
- Kulikov, S.; Khairullin, R.; Varlamov, V. Influence of polycations on antibacterial activity of lysostaphin. Appl. Biochem. Microbiol. 2015, 51, 683–687. [Google Scholar] [CrossRef]
- Qiu, F.; Becker, K.W.; Knight, F.C.; Baljon, J.J.; Sevimli, S.; Shae, D.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials 2018, 182, 82–91. [Google Scholar] [CrossRef]
- Kryger, M.B.; Pedersen, S.L.; Wohl, B.M.; Zelikin, A.N. Tools of gene transfer applied to the intracellular delivery of non-nucleic acid polyanionic drugs. Chem. Commun. 2016, 52, 889–891. [Google Scholar] [CrossRef]
- La, C.C.; Takeuchi, L.E.; Abbina, S.; Vappala, S.; Abbasi, U.; Kizhakkedathu, J.N. Targeting Biological Polyanions in Blood: Strategies toward the Design of Therapeutics. Biomacromolecules 2020, 21, 2595–2621. [Google Scholar] [CrossRef]
- Wang, X.; Niu, D.; Hu, C.; Li, P. Polyethyleneimine-based nanocarriers for gene delivery. Curr. Pharm. Des. 2015, 21, 6140–6156. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Hu, Y.; Ren, Y.; Nam, H.; Santos, J.L.; Ng, S.; Gong, L.; Brummet, M.; Carrington, C.A.; Ullman, C.G.; et al. Scalable Purification of Plasmid DNA Nanoparticles by Tangential Flow Filtration for Systemic Delivery. ACS Appl. Mater. Interfaces 2021, 13, 30326–30336. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Shou, C.; He, M.; Xu, J.; Cheng, Y.; Yuan, Z.; Lan, M.; Zhao, Y.; Yang, Y.; Chen, X.; et al. A combination of LightOn gene expression system and tumor microenvironment-responsive nanoparticle delivery system for targeted breast cancer therapy. Acta Pharm. Sin. B 2020, 10, 1741–1753. [Google Scholar] [CrossRef]
- Sedlacek, O.; Janouskova, O.; Verbraeken, B.; Hoogenboom, R. Straightforward Route to Superhydrophilic Poly(2-oxazoline)s via Acylation of Well-Defined Polyethylenimine. Biomacromolecules 2019, 20, 222–230. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, W.; Teo, P.Y.; Engler, A.C.; Coady, D.J.; Hedrick, J.L.; Yang, Y.Y. Mitigated Cytotoxicity and Tremendously Enhanced Gene Transfection Efficiency of PEI through Facile One-Step Carbamate Modification. Adv. Healthc. Mater. 2013, 2, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zheng, J.; Xiao, L.; Shang, T.; Cai, Y.; Li, Y.; Xu, Y.; Chen, X.; Liu, Y.; Yang, B. Construction of a Phenylboronic Acid-Functionalized Nano-Prodrug for pH-Responsive Emodin Delivery and Antibacterial Activity. ACS omega 2021, 6, 8672–8679. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Harbaugh, S.; Drachuk, I.; Shchepelina, O.; Kelley-Loughnane, N.; Stone, M.; Tsukruk, V.V. Hydrogen-bonded LbL shells for living cell surface engineering. Soft Matter 2011, 7, 2364–2372. [Google Scholar] [CrossRef]
- Dalchand, N.; Doğangün, M.; Ohno, P.E.; Ma, E.; Martinson, A.B.F.; Geiger, F.M. Perturbation of Hydrogen-Bonding Networks over Supported Lipid Bilayers by Poly(allylamine hydrochloride). J. Phys. Chem. B 2019, 123, 4251–4257. [Google Scholar] [CrossRef]
- Krol, S.; Nolte, M.; Diaspro, A.; Mazza, D.; Magrassi, R.; Gliozzi, A.; Fery, A. Encapsulated living cells on microstructured surfaces. Langmuir 2005, 21, 705–709. [Google Scholar] [CrossRef]
- Konnova, S.A.; Sharipova, I.R.; Demina, T.A.; Osin, Y.N.; Yarullina, D.R.; Ilinskaya, O.N.; Lvov, Y.M.; Fakhrullin, R.F. Biomimetic cell-mediated three-dimensional assembly of halloysite nanotubes. Chem. Commun. 2013, 49, 4208–4210. [Google Scholar] [CrossRef]
- Wandrey, C.; Hernandez-Barajas, J.; Hunkeler, D. Diallyldimethylammonium chloride and its polymers. In Radical Polymerisation Polyelectrolytes; Springer: Berlin/Heidelberg, Germany, 1999; pp. 123–183. [Google Scholar]
- Franz, B.; Balkundi, S.S.; Dahl, C.; Lvov, Y.M.; Prange, A. Layer-by-Layer Nano-Encapsulation of Microbes: Controlled Cell Surface Modification and Investigation of Substrate Uptake in Bacteria. Macromol. Biosci. 2010, 10, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Han, L.; Zhong, Y. Layer-by-layer films assembled from natural polymers for sustained release of neurotrophin. Biomed. Mater. 2015, 10, 055006. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, N.; Rheem, H.B.; Kim, B.J.; Park, J.H.; Choi, I.S. A Decade of Advances in Single-Cell Nanocoating for Mammalian Cells. Adv. Healthc. Mater. 2021, 10, e2100347. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Echave, M.C.; Hernáez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Guan, T.; Zhang, X.; Khosrozadeh, A.; Xing, M.; Kong, J. Manipulable Permeability of Nanogel Encapsulation on Cells Exerts Protective Effect against TNF-α-Induced Apoptosis. ACS Biomater. Sci. Eng. 2018, 4, 2825–2835. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Liu, S.; Li, L. Three-Dimensional Bioprinting of Oppositely Charged Hydrogels with Super Strong Interface Bonding. ACS Appl. Mater. Interfaces 2018, 10, 11164–11174. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Yu, Y.; Tyrikos-Ergas, T.; Zhu, Y.; Fittolani, G.; Bordoni, V.; Singhal, A.; Fair, R.J.; Grafmüller, A.; Seeberger, P.H.; Delbianco, M. Systematic Hydrogen-Bond Manipulations To Establish Polysaccharide Structure-Property Correlations. Angew. Chem. Int. Ed. Engl. 2019, 58, 13127–13132. [Google Scholar] [CrossRef]
- Kaldéus, T.; Träger, A.; Berglund, L.A.; Malmström, E.; Lo Re, G. Molecular Engineering of the Cellulose-Poly(Caprolactone) Bio-Nanocomposite Interface by Reactive Amphiphilic Copolymer Nanoparticles. ACS Nano 2019, 13, 6409–6420. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Zhou, C.; Wan, W.; Yu, S.; Qian, K.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. Simultaneous surface functionalization and drug loading: A novel method for fabrication of cellulose nanocrystals-based pH responsive drug delivery system. Int. J. Biol. Macromol. 2021, 182, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Fall, A.; Chaduc, I.; Wågberg, L.; Charleux, B.; Malmström, E.; D’Agosto, F.; Lansalot, M.; Carlmark, A. Modification of cellulose model surfaces by cationic polymer latexes prepared by RAFT-mediated surfactant-free emulsion polymerization. Polym. Chem. 2014, 5, 6076–6086. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Mallorquí, F.; Rodríguez-Comas, J.; Ramón-Azcón, J. Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication 2021, 13, 035044. [Google Scholar] [CrossRef] [PubMed]
- Prochorov, A.; Tretjak, S.; Goranov, V.; Glinnik, A.; Goltsev, M. Treatment of insulin dependent diabetes mellitus with intravascular transplantation of pancreatic islet cells without immunosuppressive therapy. Adv. Med. Sci. 2008, 53, 240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moradian, C.; Rahbarizadeh, F. PE38-based gene therapy of HER2-positive breast cancer stem cells via VHH-redirected polyamidoamine dendrimers. Sci. Rep. 2021, 11, 15517. [Google Scholar] [CrossRef]
- Qi, X.; Qin, J.; Fan, Y.; Qin, X.; Jiang, Y.; Wu, Z. Carboxymethyl Chitosan-Modified Polyamidoamine Dendrimer Enables Progressive Drug Targeting of Tumors via pH-Sensitive Charge Inversion. J. Biomed. Nanotechnol. 2016, 12, 667–678. [Google Scholar] [CrossRef]
- Urbán, P.; Ranucci, E.; Fernàndez-Busquets, X. Polyamidoamine nanoparticles as nanocarriers for the drug delivery to malaria parasite stages in the mosquito vector. Nanomedicine 2015, 10, 3401–3414. [Google Scholar] [CrossRef]
- Frost, R.; Coué, G.; Engbersen, J.F.; Zäch, M.; Kasemo, B.; Svedhem, S. Bioreducible insulin-loaded nanoparticles and their interaction with model lipid membranes. J. Colloid Interface Sci. 2011, 362, 575–583. [Google Scholar] [CrossRef]
- Gattás-Asfura, K.M.; Stabler, C.L. Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers. ACS Appl. Mater. Interfaces 2013, 5, 9964–9974. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent advances in chitosan-based layer-by-layer biomaterials and their biomedical applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Hirt-Burri, N.; Jeannerat, A.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Peptide-decorated chitosan derivatives enhance fibroblast adhesion and proliferation in wound healing. Carbohydr. Polym. 2016, 142, 114–123. [Google Scholar] [CrossRef]
- Li, L.; Zhao, F.; Zhao, B.; Zhang, J.; Li, C.; Qiao, R. Chitosan Grafted with Phosphorylcholine and Macrocyclic Polyamine as an Effective Gene Delivery Vector: Preparation, Characterization and In Vitro Transfection. Macromol. Biosci. 2015, 15, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Cai, Z.; Shang, S.; Song, Z. Synthesis and antibacterial activity of alkylated chitosan under basic ionic liquid conditions. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Facchi, S.P.; Scariot, D.B.; Bueno, P.V.; Souza, P.R.; Figueiredo, L.C.; Follmann, H.D.; Nunes, C.S.; Monteiro, J.P.; Bonafé, E.G.; Nakamura, C.V. Preparation and cytotoxicity of N-modified chitosan nanoparticles applied in curcumin delivery. Int. J. Biol. Macromol. 2016, 87, 237–245. [Google Scholar] [CrossRef]
- Torabi, S.; Mahdavian, A.R.; Sanei, M.; Abdollahi, A. Chitosan and functionalized acrylic nanoparticles as the precursor of new generation of bio-based antibacterial films. Mater. Sci. Eng. C 2016, 59, 1–9. [Google Scholar] [CrossRef]
- Beidokhti, H.R.N.; Ghaffarzadegan, R.; Mirzakhanlouei, S.; Ghazizadeh, L.; Dorkoosh, F.A. Preparation, Characterization, and Optimization of Folic Acid-Chitosan-Methotrexate Core-Shell Nanoparticles by Box-Behnken Design for Tumor-Targeted Drug Delivery. AAPS PharmSciTech 2017, 18, 115–129. [Google Scholar] [CrossRef]
- Liang, H.; Li, J.; He, Y.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Engineering multifunctional films based on metal-phenolic networks for rational pH-responsive delivery and cell imaging. ACS Biomater. Sci. Eng. 2016, 2, 317–325. [Google Scholar] [CrossRef]
- Mekhail, M.; Jahan, K.; Tabrizian, M. Genipin-crosslinked chitosan/poly-L-lysine gels promote fibroblast adhesion and proliferation. Carbohydr. Polym. 2014, 108, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Sura, H.S.; Magnay, J.; Green, D.; Oreffo, R.O.; Dobson, J.P.; El Haj, A.J. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. Part A 2010, 16, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Karabıyık Acar, Ö.; Bedir, S.; Kayitmazer, A.B.; Kose, G.T. Chondro-inductive hyaluronic acid/chitosan coacervate-based scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2021, 188, 300–312. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.S.; Kim, J.W.; Lee, E.Y.; Rhee, M.; You, Y.H.; Khang, G.; Park, C.G.; Yoon, K.H. Suppression of Fibrotic Reactions of Chitosan-Alginate Microcapsules Containing Porcine Islets by Dexamethasone Surface Coating. Endocrinol. Metab. 2021, 36, 146–156. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Wang, X.-W.; Ng, S.; Quek, C.-H.; Ho, H.-T.; Lao, X.-J.; Yu, H. Encapsulating live cells with water-soluble chitosan in physiological conditions. J. Biotechnol. 2005, 117, 355–365. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zou, S.; Pei, Y.; Cheng, D.; Ai, H.; Shuai, X. Polyethylenimine-grafted copolymer of poly(l-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials 2011, 32, 1694–1705. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Ding, S.; You, Y. Fluorinated PEG-Polypeptide Polyplex Micelles Have Good Serum-Resistance and Low Cytotoxicity for Gene Delivery. Macromol. Biosci. 2017, 17, 1700114. [Google Scholar] [CrossRef]
- Jing, H.; Cheng, W.; Zhang, J.-W.; Han, X.; Shao, H.; Sun, Y.-X. Galactosylated poly-L-lysine targeted microbubbles for ultrasound mediated antisense c-myc gene transfection in hepatocellular carcinoma cells. Arch. Med. Sci. AMS 2015, 11, 292. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, J.; Sun, Q.; Murdoch, W.J.; Shen, Y. A multifunctional PEG–PLL drug conjugate forming redox-responsive nanoparticles for intracellular drug delivery. J. Mater. Chem. B 2015, 3, 7594–7603. [Google Scholar] [CrossRef] [PubMed]
- Franiak-Pietryga, I.; Maciejewski, H.; Ostrowska, K.; Appelhans, D.; Voit, B.; Misiewicz, M.; Kowalczyk, P.; Bryszewska, M.; Borowiec, M. Dendrimer-based nanoparticles for potential personalized therapy in chronic lymphocytic leukemia: Targeting the BCR-Signaling Pathway. Int. J. Biol. Macromol. 2016, 88, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.H.; Hsu, H.J.; Bugno, J.; Tam, K.A.; Hong, S. Chemical Structure and Surface Modification of Dendritic Nanomaterials Tailored for Therapeutic and Diagnostic Applications. Curr. Top. Med. Chem. 2017, 17, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Clark, E.C.; Fong, E.L.; Lee, E.J.; Lu, S.; Tabata, Y.; Mikos, A.G. Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly (l-Lysine) for applications in cartilage tissue engineering. Biomaterials 2016, 83, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Veerabadran, N.G.; Goli, P.L.; Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 2007, 7, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Zhang, Y.Z.; Chen, X.L. Diversity of Three-Dimensional Structures and Catalytic Mechanisms of Alginate Lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Virumbrales-Muñoz, M.; Paz-Artigas, L.; Ciriza, J.; Alcaine, C.; Espona-Noguera, A.; Doblaré, M.; Sáenz Del Burgo, L.; Ziani, K.; Pedraz, J.L.; Fernández, L.; et al. Force Spectroscopy Imaging and Constriction Assays Reveal the Effects of Graphene Oxide on the Mechanical Properties of Alginate Microcapsules. ACS Biomater. Sci. Eng. 2021, 7, 242–253. [Google Scholar] [CrossRef]
- Vos, P.d.; Andersson, A.; Tam, S.; Faas, M.; Halle, J. Advances and barriers in mammalian cell encapsulation for treatment of diabetes. Immunol. Endocr. Metab. Agents Med. Chem. 2006, 6, 139–153. [Google Scholar] [CrossRef][Green Version]
- Kong, D.; Xu, H.; Chen, M.; Yu, Y.; Qian, Y.; Qin, T.; Tong, Y.; Xia, Q.; Hang, H. Co-encapsulation of HNF4α overexpressing UMSCs and human primary hepatocytes ameliorates mouse acute liver failure. Stem Cell. Res. Ther. 2020, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Ørning, P.; Hoem, K.S.; Coron, A.E.; Skjåk-Bræk, G.; Mollnes, T.E.; Brekke, O.L.; Espevik, T.; Rokstad, A.M. Alginate microsphere compositions dictate different mechanisms of complement activation with consequences for cytokine release and leukocyte activation. J. Control. Release 2016, 229, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Spasojevic, M.; Bhujbal, S.; Paredes, G.; Haan, B.J.; Schouten, A.J.; Vos, P. Considerations in binding diblock copolymers on hydrophilic alginate beads for providing an immunoprotective membrane. J. Biomed. Mater. Res. Part A 2014, 102, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Charbonier, F.; Indana, D.; Chaudhuri, O. Tuning Viscoelasticity in Alginate Hydrogels for 3D Cell Culture Studies. Curr. Protoc. 2021, 1, e124. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Zhu, S. Selective biosorption mechanism of methylene blue by a novel and reusable sugar beet pulp cellulose/sodium alginate/iron hydroxide composite hydrogel. Int. J. Biol. Macromol. 2021, 188, 993–1002. [Google Scholar] [CrossRef]
- Kendall, W.F., Jr.; Opara, E.C. Polymeric Materials for Perm-Selective Coating of Alginate Microbeads. Methods Mol. Biol. 2017, 1479, 95–109. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Monier, M.; Alatawi, R.A. Synthesis and characterization of photo-crosslinkable 4-styryl-pyridine modified alginate. Carbohydr. Polym. 2016, 145, 121–131. [Google Scholar] [CrossRef]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.N.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418–431. [Google Scholar] [CrossRef]
- Genç, H.; Hazur, J.; Karakaya, E.; Dietel, B.; Bider, F.; Groll, J.; Alexiou, C.; Boccaccini, A.R.; Detsch, R.; Cicha, I. Differential Responses to Bioink-Induced Oxidative Stress in Endothelial Cells and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 2358. [Google Scholar] [CrossRef]
- Sondermeijer, H.P.; Witkowski, P.; Woodland, D.; Seki, T.; Aangenendt, F.J.; van der Laarse, A.; Itescu, S.; Hardy, M.A. Optimization of alginate purification using polyvinylidene difluoride membrane filtration: Effects on immunogenicity and biocompatibility of three-dimensional alginate scaffolds. J. Biomater. Appl. 2016, 31, 510–520. [Google Scholar] [CrossRef]
- Kumar, S.; Ingle, H.; Prasad, D.V.R.; Kumar, H. Recognition of bacterial infection by innate immune sensors. Crit. Rev. Microbiol. 2013, 39, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, J.; Deng, F.; Guo, C.; Yang, Q.; Wu, H.; Ni, Y.; Huang, L.; Chen, L.; Ding, C. Dual-functionalized hyaluronic acid as a facile modifier to prepare polyanionic collagen. Carbohydr. Polym. 2019, 215, 358–365. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Grewal, M.G.; Gray, V.P.; Letteri, R.A.; Highley, C.B. User-defined, temporal presentation of bioactive molecules on hydrogel substrates using supramolecular coiled coil complexes. Biomater. Sci. 2021, 9, 4374–4387. [Google Scholar] [CrossRef]
- Khoshakhlagh, P.; Moore, M.J. Photoreactive interpenetrating network of hyaluronic acid and Puramatrix as a selectively tunable scaffold for neurite growth. Acta Biomater. 2015, 16, 23–34. [Google Scholar] [CrossRef]
- Kim, M.; Erickson, I.E.; Huang, A.H.; Garrity, S.T.; Mauck, R.L.; Steinberg, D.R. Donor Variation and Optimization of Human Mesenchymal Stem Cell Chondrogenesis in Hyaluronic Acid. Tissue Eng. Part A 2018, 24, 1693–1703. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, Y.H.; Huang, A.P.; Hsu, S.H. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120. [Google Scholar] [CrossRef]
- Harel, Z.; Harel, S.; Shah, P.S.; Wald, R.; Perl, J.; Bell, C.M. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: A systematic review. Am. J. Med. 2013, 126, 9–24. [Google Scholar] [CrossRef]
- Choi, W.S.; Choi, I.S.; Lee, J.K.; Yoon, K.R. Preparation of fluorescein-functionalized electrospun fibers coated with TiO2 and gold nanoparticles for visible-light-induced photocatalysis. Mater. Chem. Phys. 2015, 163, 213–218. [Google Scholar] [CrossRef]
- Åkerfeldt, M.; Nilsson, E.; Gillgard, P.; Walkenström, P. Textile piezoelectric sensors–melt spun bi-component poly (vinylidene fluoride) fibres with conductive cores and poly (3,4-ethylene dioxythiophene)-poly (styrene sulfonate) coating as the outer electrode. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Murakami, Y.; Iwata, H.; Kitano, E.; Kitamura, H.; Ikada, Y. Interaction of poly(styrene sulfonic acid) with the classical pathway of the serum complement system. J. Biomater. Sci. Polym. Ed. 2005, 16, 685–697. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Zamaleeva, A.I.; Morozov, M.V.; Tazetdinova, D.I.; Alimova, F.K.; Hilmutdinov, A.K.; Zhdanov, R.I.; Kahraman, M.; Culha, M. Living fungi cells encapsulated in polyelectrolyte shells doped with metal nanoparticles. Langmuir 2009, 25, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chouhan, R.S.; Gurbuz, Y.; Niazi, J.H.; Qureshi, A.S. cerevisiae whole-cell based capacitive biochip for the detection of toxicity of different forms of carbon nanotubes. Sens. Actuators B Chem. 2015, 218, 253–260. [Google Scholar] [CrossRef]
- Yang, X.-N.; Xue, D.-D.; Li, J.-Y.; Liu, M.; Jia, S.-R.; Chu, L.-Q.; Wahid, F.; Zhang, Y.-M.; Zhong, C. Improvement of antimicrobial activity of graphene oxide/bacterial cellulose nanocomposites through the electrostatic modification. Carbohydr. Polym. 2016, 136, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Konnova, S.A.; Lvov, Y.M.; Fakhrullin, R.F. Nanoshell Assembly for Magnet-Responsive Oil-Degrading Bacteria. Langmuir 2016, 32, 12552–12558. [Google Scholar] [CrossRef] [PubMed]
- Gorobets, S.; Gorobets, O.Y.; Demianenko, I.; Nikolaenko, R. Self-organization of magnetite nanoparticles in providing Saccharomyces cerevisiae Yeasts with magnetic properties. J. Magn. Magn. Mater. 2013, 337, 53–57. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; García-Alonso, J.; Paunov, V.N. A direct technique for preparation of magnetically functionalised living yeast cells. Soft Matter 2010, 6, 391–397. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Zamaleeva, A.I.; Ishmuchametova, D.G.; Osin, Y.N.; Kiyasov, A.P.; Nurgaliev, D.K.; Ilinskaya, O.N.; Fakhrullin, R.F. A direct technique for magnetic functionalization of living human cells. Langmuir 2011, 27, 14386–14393. [Google Scholar] [CrossRef]
- Gal, N.; Massalha, S.; Samuelly-Nafta, O.; Weihs, D. Effects of particle uptake, encapsulation, and localization in cancer cells on intracellular applications. Med. Eng. Phys. 2015, 37, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kang, S.M.; Lee, K.-B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A Mussel-Inspired Persistent ROS-Scavenging, Electroactive, and Osteoinductive Scaffold Based on Electrochemical-Driven In Situ Nanoassembly. Small 2019, 15, e1805440. [Google Scholar] [CrossRef]
- Gao, T.; Chen, T.; Feng, C.; He, X.; Mu, C.; Anzai, J.I.; Li, G. Design and fabrication of flexible DNA polymer cocoons to encapsulate live cells. Nat. Commun. 2019, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.S.; Zheng, Z.; Sarwar, M.I.; Félix, O.; Decher, G. Nanoprotective layer-by-layer coatings with epoxy components for enhancing abrasion resistance: Toward robust multimaterial nanoscale films. ACS Nano 2013, 7, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pineda, E.; Andreozzi, P.; Diamanti, E.; Anguiano, R.; Ziolo, R.F.; Moya, S.E.; José Rodríguez-Presa, M.; Gervasi, C.A. Effects of valinomycin doping on the electrical and structural properties of planar lipid bilayers supported on polyelectrolyte multilayers. Bioelectrochemistry 2021, 138, 107688. [Google Scholar] [CrossRef] [PubMed]
- Kékicheff, P.; Schneider, G.F.; Decher, G. Size-controlled polyelectrolyte complexes: Direct measurement of the balance of forces involved in the triggered collapse of layer-by-layer assembled nanocapsules. Langmuir 2013, 29, 10713–10726. [Google Scholar] [CrossRef]
- Tezel, G.B.; Arole, K.; Holta, D.E.; Radovic, M.; Green, M.J. Interparticle interactions and rheological signatures of Ti(3)C(2)T(z) MXene dispersions. J. Colloid Interface Sci. 2021, 605, 120–128. [Google Scholar] [CrossRef]
- Frank, C.; Novak, J.; Banerjee, R.; Gerlach, A.; Schreiber, F.; Vorobiev, A.; Kowarik, S. Island size evolution and molecular diffusion during growth of organic thin films followed by time-resolved specular and off-specular scattering. Phys. Rev. B 2014, 90, 045410. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, S.; Lee, M.; Kim, Q.; An, S.; Jhe, W. Probing nonlinear rheology layer-by-layer in interfacial hydration water. Proc. Natl. Acad. Sci. USA 2015, 112, 15619–15623. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Wu, K.C.-W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Bracic, M.; Kulterer, M.; Doliška, A.; Stana-Kleinschek, K.; Ribitsch, V. Adsorption of carboxymethyl cellulose on polymer surfaces: Evidence of a specific interaction with cellulose. Langmuir 2012, 28, 11440–11447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-X.; Miao, J.; Liu, B. Layer-by-layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Mazilu, D.; Mazilu, I.; Seredinski, A.; Kim, V.; Simpson, B.; Banks, W. Cooperative sequential adsorption models on a Cayley tree: Analytical results and applications. J. Stat. Mech. Theory Exp. 2012, 2012, P09002. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.-I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.; Liu, J.; Xie, Z.; Herron, J.A. Palladium-platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kuroda, K. Layer-by-layer assembly of imogolite nanotubes and polyelectrolytes into core-shell particles and their conversion to hierarchically porous spheres. Sci. Technol. Adv. Mater. 2008, 9, 025018. [Google Scholar] [CrossRef]
- Mathivanan, N.; Paramasivam, G.; Vergaelen, M.; Rajendran, J.; Hoogenboom, R.; Sundaramurthy, A. Hydrogen-Bonded Multilayer Thin Films and Capsules Based on Poly(2-n-propyl-2-oxazoline) and Tannic Acid: Investigation on Intermolecular Forces, Stability, and Permeability. Langmuir 2019, 35, 14712–14724. [Google Scholar] [CrossRef]
- Huang, J.; Ware, H.O.T.; Hai, R.; Shao, G.; Sun, C. Conformal Geometry and Multimaterial Additive Manufacturing through Freeform Transformation of Building Layers. Adv. Mater. 2021, 33, e2005672. [Google Scholar] [CrossRef]
- Katagiri, K.; Yamazaki, S.-I.; Inumaru, K.; Koumoto, K. Anti-reflective coatings prepared via layer-by-layer assembly of mesoporous silica nanoparticles and polyelectrolytes. Polym. J. 2015, 47, 190–194. [Google Scholar] [CrossRef]
- Sergeeva, Y.N.; Huang, T.; Felix, O.; Jung, L.; Tropel, P.; Viville, S.; Decher, G. What is really driving cell–surface interactions? Layer-by-layer assembled films may help to answer questions concerning cell attachment and response to biomaterials. Biointerphases 2016, 11, 019009. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A closer physico-chemical look to the Layer-by-Layer electrostatic self-assembly of polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef] [PubMed]
- Vaterrodt, A.; Thallinger, B.; Daumann, K.; Koch, D.; Guebitz, G.M.; Ulbricht, M. Antifouling and antibacterial multi-functional polyzwitterion/enzyme coating on silicone catheter material prepared by electrostatic layer-by-layer assembly. Langmuir 2016, 32, 1347–1359. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Wen, L.; Zhou, X.; Xu, J.; Yang, S. Dynamics of the layer-by-layer assembly of a poly (acrylic acid)–lanthanide complex colloid and poly (diallyldimethyl ammonium). Soft Matter 2016, 12, 867–875. [Google Scholar] [CrossRef]
- Selin, V.; Ankner, J.F.; Sukhishvili, S.A. Diffusional Response of Layer-by-Layer Assembled Polyelectrolyte Chains to Salt Annealing. Macromolecules 2015, 48, 3983–3990. [Google Scholar] [CrossRef]
- Varga, I.; Mezei, A.; Mészáros, R.; Claesson, P.M. Controlling the interaction of poly (ethylene imine) adsorption layers with oppositely charged surfactant by tuning the structure of the preadsorbed polyelectrolyte layer. Soft Matter 2011, 7, 10701–10712. [Google Scholar] [CrossRef]
- Popa, I.; Gillies, G.; Papastavrou, G.; Borkovec, M. Attractive and repulsive electrostatic forces between positively charged latex particles in the presence of anionic linear polyelectrolytes. J. Phys. Chem. B 2010, 114, 3170–3177. [Google Scholar] [CrossRef]
- Bellanger, H.; Casdorff, K.; Muff, L.F.; Ammann, R.; Burgert, I.; Michen, B. Layer-by-layer deposition on a heterogeneous surface: Effect of sorption kinetics on the growth of polyelectrolyte multilayers. J. Colloid Interface Sci. 2017, 500, 133–141. [Google Scholar] [CrossRef]
- Ciejka, J.; Grzybala, M.; Gut, A.; Szuwarzynski, M.; Pyrc, K.; Nowakowska, M.; Szczubiałka, K. Tuning the Surface Properties of Poly(Allylamine Hydrochloride)-Based Multilayer Films. Materials 2021, 14, 2361. [Google Scholar] [CrossRef]
- Popa, I.; Papastavrou, G.; Borkovec, M. Charge regulation effects on electrostatic patch-charge attraction induced by adsorbed dendrimers. Phys. Chem. Chem. Phys. 2010, 12, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Weng, G.M.; Lipton, J.; Li, C.M.; Van Tassel, P.R.; Taylor, A.D. Weak polyelectrolyte-based multilayers via layer-by-layer assembly: Approaches, properties, and applications. Adv. Colloid Interface Sci. 2020, 282, 102200. [Google Scholar] [CrossRef] [PubMed]
- Petrila, L.M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pyo, J.B.; Kim, T.S. Layer-by-Layer Assembly of Free-Standing Nanofilms by Controlled Rolling. Langmuir 2018, 34, 5831–5836. [Google Scholar] [CrossRef]
- Choi, D.; Heo, J.; Hong, J. Investigation of the Structural Mechanism and Film Growth on Cytoprotective Type I Collagen-Based Nanocoating of Individual Cellular Surfaces. Langmuir 2021, 37, 4587–4598. [Google Scholar] [CrossRef]
- Min, L.; GAO, Y.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Abstract LB-102: Layer-by-layer engineering of upconversion nanoparticle based siRNA and miRNA delivery system for cancer therapy. Cancer Res. 2015, 75, LB-102. [Google Scholar] [CrossRef]
- Batys, P.; Nosek, M.; Weroński, P. Structure analysis of layer-by-layer multilayer films of colloidal particles. Appl. Surf. Sci. 2015, 332, 318–327. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zembala, M.; Michna, A. Polyelectrolyte adsorption layers studied by streaming potential and particle deposition. J. Colloid Interface Sci. 2006, 303, 353–364. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Wang, R. Enhanced hollow fiber membrane performance via semi-dynamic layer-by-layer polyelectrolyte inner surface deposition for nanofiltration and forward osmosis applications. React. Funct. Polym. 2015, 86, 154–160. [Google Scholar] [CrossRef]
- Bucur, C.B.; Lita, A.; Osada, N.; Muldoon, J. A soft, multilayered lithium–electrolyte interface. Energy Environ. Sci. 2016, 9, 112–116. [Google Scholar] [CrossRef]
- Fuller, M.; Köper, I. Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability. Polymers 2018, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, I.; Rosická, D.; Hierrezuelo, J.; Borkovec, M. Charging and stability of anionic latex particles in the presence of linear poly (ethylene imine). J. Colloid Interface Sci. 2011, 360, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; Freitag, J.S.; Smith, D.M.; Stöver, H.D.H. Charge-Shifting Polycations Based on N,N-(dimethylamino)ethyl Acrylate for Improving Cytocompatibility During DNA Delivery. ACS Omega 2020, 5, 9114–9122. [Google Scholar] [CrossRef]
- Cai, C.; Mao, S.; Kissel, T. O-013-Layer-by-layer nanostructured protein loaded nanoparticles: A feasibility study using lysozyme as model protein and chitosan as coating material. Asian J. Pharm. Sci. 2016, 1, 64–65. [Google Scholar] [CrossRef][Green Version]
- Richardson, J.J.; Tardy, B.L.; Ejima, H.; Guo, J.; Cui, J.; Liang, K.; Choi, G.H.; Yoo, P.J.; De Geest, B.G.; Caruso, F. Thermally Induced Charge Reversal of Layer-by-Layer Assembled Single-Component Polymer Films. ACS Appl. Mater. Interfaces 2016, 8, 7449–7455. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Lee, S.H.; Na, W.; Lim, J.W.; Park, G.; Park, C.; Lee, H.; Kang, A.; Haam, S.; Choi, I.; et al. Cell-mimic polymersome-shielded islets for long-term immune protection of neonatal porcine islet-like cell clusters. J. Mater. Chem. B 2020, 8, 2476–2482. [Google Scholar] [CrossRef]
- Carroll, L.; Mridha, A.R.; Tuch, B.E. Encapsulation and Transplantation of Pancreatic Progenitor Cells. Methods Mol. Biol. 2019, 2029, 93–102. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Guyot, S.; Lherminier, J.; Wache, Y.; Saurel, R.; Husson, F. Protection of living yeast cells by micro-organized shells of natural polyelectrolytes. Process Biochem. 2015, 50, 1528–1536. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Li, X.; Wang, X.; Yang, Y.; Kawazoe, N.; Chen, G. Nanoencapsulation of individual mammalian cells with cytoprotective polymer shell. Biomaterials 2017, 133, 253–262. [Google Scholar] [CrossRef]
- González-Ferrero, C.; Irache, J.M.; Marín-Calvo, B.; Ortiz-Romero, L.; Virto-Resano, R.; González-Navarro, C.J. Encapsulation of probiotics in soybean protein-based microparticles preserves viable cell concentration in foods all along the production and storage processes. J. Microencapsul. 2020, 37, 242–253. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Liu, P.; Yan, Y.; Xu, X.; Tang, R. Extracellular silica nanocoat confers thermotolerance on individual cells: A case study of material-based functionalization of living cells. ChemBioChem 2010, 11, 2368–2373. [Google Scholar] [CrossRef]
- Yang, S.H.; Lee, K.B.; Kong, B.; Kim, J.H.; Kim, H.S.; Choi, I.S. Biomimetic encapsulation of individual cells with silica. Angew. Chem. Int. Ed. 2009, 48, 9160–9163. [Google Scholar] [CrossRef] [PubMed]
- Kempaiah, R.; Salgado, S.; Chung, W.L.; Maheshwari, V. Graphene as membrane for encapsulation of yeast cells: Protective and electrically conducting. Chem. Commun. 2011, 47, 11480–11482. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Georgiou, A.I.; MacFarlane, R.J.; Klontzas, M.E.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Fibronectin stimulates the osteogenic differentiation of murine embryonic stem cells. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Konno, T.; Ishihara, K. Phospholipid polymer hydrogel microsphere modulates the cell cycle profile of encapsulated cells. Soft Matter 2013, 9, 4628–4634. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Vishwapathi, V.K.; Quarshie, A.; Moinuddin, Z.; Page, J.; Kendrekar, P.; Mashele, S.S. Self-Assembled Lipid Cubic Phase and Cubosomes for the Delivery of Aspirin as a Model Drug. Langmuir 2017, 33, 9907–9915. [Google Scholar] [CrossRef]
- Nilsson, C.; Edwards, K.; Eriksson, J.; Larsen, S.W.; Østergaard, J.; Larsen, C.; Urtti, A.; Yaghmur, A. Characterization of oil-free and oil-loaded liquid-crystalline particles stabilized by negatively charged stabilizer citrem. Langmuir 2012, 28, 11755–11766. [Google Scholar] [CrossRef]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Alinejad, Y.; Bitar, C.M.E.; Martinez Villegas, K.; Perignon, S.; Hoesli, C.A.; Lerouge, S. Chitosan Microbeads Produced by One-Step Scalable Stirred Emulsification: A Promising Process for Cell Therapy Applications. ACS Biomater. Sci. Eng. 2020, 6, 288–297. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. View 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Selin, V.; Ankner, J.F.; Sukhishvili, S.A. Ionically Paired Layer-by-Layer Hydrogels: Water and Polyelectrolyte Uptake Controlled by Deposition Time. Gels 2018, 4, 7. [Google Scholar] [CrossRef]
- Hsu, B.B.; Park, M.H.; Hagerman, S.R.; Hammond, P.T. Multimonth controlled small molecule release from biodegradable thin films. Proc. Natl. Acad. Sci. USA 2014, 111, 12175–12180. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anes, A.; Gargouri, M.; Laure, W.; Van Den Berghe, H.; Courcot, E.; Sobocinski, J.; Tabary, N.; Chai, F.; Blach, J.F.; Addad, A.; et al. Bioinspired Titanium Drug Eluting Platforms Based on a Poly-β-cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections. ACS Appl. Mater. Interfaces 2015, 7, 12882–12893. [Google Scholar] [CrossRef]
- Guo, X.; Carter, M.C.D.; Appadoo, V.; Lynn, D.M. Tunable and Selective Degradation of Amine-Reactive Multilayers in Acidic Media. Biomacromolecules 2019, 20, 3464–3474. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Wang, Z.; Shi, S.; Nan, K.; Xu, Q.; Ye, Z.; Chen, H. A self-defensive antibacterial coating acting through the bacteria-triggered release of a hydrophobic antibiotic from layer-by-layer films. J. Mater. Chem. B 2017, 5, 1498–1506. [Google Scholar] [CrossRef]
- Tao, B.; Deng, Y.; Song, L.; Ma, W.; Qian, Y.; Lin, C.; Yuan, Z.; Lu, L.; Chen, M.; Yang, X.; et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surf. B Biointerfaces 2019, 177, 242–252. [Google Scholar] [CrossRef]
- Nam, K.; Kim, T.; Kim, Y.M.; Yang, K.; Choe, D.; Mensah, L.B.; Choi, K.Y.; Roh, Y.H. Size-controlled synthesis of polymerized DNA nanoparticles for targeted anticancer drug delivery. Chem. Commun. 2019, 55, 4905–4908. [Google Scholar] [CrossRef]
- Choi, K.Y.; Correa, S.; Min, J.; Li, J.; Roy, S.; Laccetti, K.H.; Dreaden, E.; Kong, S.; Heo, R.; Roh, Y.H.; et al. Binary Targeting of siRNA to Hematologic Cancer Cells In Vivo using Layer-by-Layer Nanoparticles. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Yuan, W.; Li, C.M. Exponentially growing layer-by-layer assembly to fabricate pH-responsive hierarchical nanoporous polymeric film and its superior controlled release performance. Chem. Commun. 2010, 46, 9161–9163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, P. pH-sensitive fluorescent hepatocyte-targeting multilayer polyelectrolyte hollow microspheres as a smart drug delivery system. Mol. Pharm. 2014, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xing, M.; Li, B. Capsule-Integrated Polypeptide Multilayer Films for Effective pH-Responsive Multiple Drug Co-Delivery. ACS Appl. Mater. Interfaces 2018, 10, 44267–44278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, S.; Liu, H.; Xing, M.; Ding, B.; Li, B. Pinecone-Inspired Nanoarchitectured Smart Microcages Enable Nano/Microparticle Drug Delivery. Adv. Funct. Mater. 2020, 30, 2002434. [Google Scholar] [CrossRef]
- Zhang, S.; Vaida, J.; Parenti, J.; Lindsey, B.A.; Xing, M.; Li, B. Programmed Multidrug Delivery Based on Bio-Inspired Capsule-Integrated Nanocoatings for Infected Bone Defect Treatment. ACS Appl. Mater. Interfaces 2021, 13, 12454–12462. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Swelling and Smoothing of Polyelectrolyte Multilayers by Salt. Langmuir 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- Pethes, I.; Bakó, I.; Pusztai, L. Chloride ions as integral parts of hydrogen bonded networks in aqueous salt solutions: The appearance of solvent separated anion pairs. Phys. Chem. Chem. Phys. 2020, 22, 11038–11044. [Google Scholar] [CrossRef]
- Lvov, Y.; Caruso, F. Biocolloids with ordered urease multilayer shells as enzymatic reactors. Anal. Chem. 2001, 73, 4212–4217. [Google Scholar] [CrossRef]
- Lvov, Y.; Antipov, A.A.; Mamedov, A.; Möhwald, H.; Sukhorukov, G.B. Urease Encapsulation in Nanoorganized Microshells. Nano Lett. 2001, 1, 125–128. [Google Scholar] [CrossRef]
- Woo, J.; Na, Y.; Choi, W.I.; Kim, S.; Kim, J.; Hong, J.; Sung, D. Functional ferrocene polymer multilayer coatings for implantable medical devices: Biocompatible, antifouling, and ROS-sensitive controlled release of therapeutic drugs. Acta Biomater. 2021, 125, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Johnston, A.P.; Dodds, S.J.; Kamphuis, M.M.; Ferguson, C.; Parton, R.G.; Nice, E.C.; Heath, J.K.; Caruso, F. Uptake and intracellular fate of disulfide-bonded polymer hydrogel capsules for Doxorubicin delivery to colorectal cancer cells. ACS Nano 2010, 4, 2928–2936. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Yao, P.; Dai, Q.; Xia, P.; Zhang, D.; et al. Folic acid-modified ROS-responsive nanoparticles encapsulating luteolin for targeted breast cancer treatment. Drug Deliv. 2021, 28, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Seras-Franzoso, J.; Sánchez-Chardi, A.; Garcia-Fruitós, E.; Vázquez, E.; Villaverde, A. Cellular uptake and intracellular fate of protein releasing bacterial amyloids in mammalian cells. Soft Matter 2016, 12, 3451–3460. [Google Scholar] [CrossRef]
- Zelikin, A.N. Drug releasing polymer thin films: New era of surface-mediated drug delivery. ACS Nano 2010, 4, 2494–2509. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.M.; Bilodeau, L.; Tabrizian, M. Delivery platform for hydrophobic drugs: Prodrug approach combined with self-assembled multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar] [CrossRef] [PubMed]
- Orozco, V.H.; Kozlovskaya, V.; Kharlampieva, E.; López, B.L.; Tsukruk, V.V. Biodegradable self-reporting nanocomposite films of poly(lactic acid) nanoparticles engineered by layer-by-layer assembly. Polymer 2010, 51, 4127–4139. [Google Scholar] [CrossRef]
- Boi, S.; Dellacasa, E.; Bianchini, P.; Petrini, P.; Pastorino, L.; Monticelli, O. Encapsulated functionalized stereocomplex PLA particles: An effective system to support mucolytic enzymes. Colloids Surf. B Biointerfaces 2019, 179, 190–198. [Google Scholar] [CrossRef]
- Ham, T.R.; Farrag, M.; Leipzig, N.D. Covalent growth factor tethering to direct neural stem cell differentiation and self-organization. Acta Biomater. 2017, 53, 140–151. [Google Scholar] [CrossRef]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Thanos, C.G.; Skinner, S.J.; Geaney, M.; Emerich, D.F. Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington’s disease. Cell Transplant. 2007, 16, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Orive, G.; Thanos, C.; Tornoe, J.; Wahlberg, L.U. Encapsulated cell therapy for neurodegenerative diseases: From promise to product. Adv. Drug Deliv. Rev. 2014, 67, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B. Two-Year Intraocular Delivery of Ciliary Neurotrophic Factor by Encapsulated Cell Technology Implants in Patients with Chronic Retinal Degenerative DiseasesIntraocular Delivery of CNTF via ECT. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter-Jönhagen, M.; Linderoth, B.; Lind, G.; Aladellie, L.; Almkvist, O.; Andreasen, N.; Blennow, K.; Bogdanovic, N.; Jelic, V.; Kadir, A. Encapsulated cell biodelivery of nerve growth factor to the basal forebrain in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2012, 33, 18–28. [Google Scholar] [CrossRef]
- Perez-Bouza, A.; Di Santo, S.; Seiler, S.; Meyer, M.; Andereggen, L.; Huber, A.; Guzman, R.; Widmer, H.R. Simultaneous Transplantation of Fetal Ventral Mesencephalic Tissue and Encapsulated Genetically Modified Cells Releasing GDNF in a Hemi-Parkinsonian Rat Model of Parkinson’s Disease. Cell Transplant. 2017, 26, 1572–1581. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, 297. https://doi.org/10.3390/pharmaceutics14020297
Li W, Lei X, Feng H, Li B, Kong J, Xing M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics. 2022; 14(2):297. https://doi.org/10.3390/pharmaceutics14020297
Chicago/Turabian StyleLi, Wenyan, Xuejiao Lei, Hua Feng, Bingyun Li, Jiming Kong, and Malcolm Xing. 2022. "Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications" Pharmaceutics 14, no. 2: 297. https://doi.org/10.3390/pharmaceutics14020297
APA StyleLi, W., Lei, X., Feng, H., Li, B., Kong, J., & Xing, M. (2022). Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics, 14(2), 297. https://doi.org/10.3390/pharmaceutics14020297







