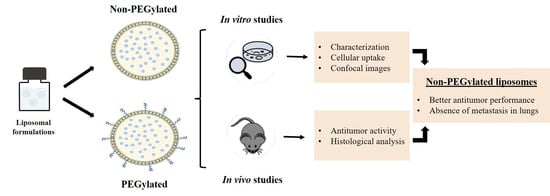
PEGylated versus Non-PEGylated pH-Sensitive Liposomes: New Insights from a Comparative Antitumor Activity Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Liposome Preparation
2.3. Determination of Size Distribution, Zeta Potential, and Encapsulation Efficiency
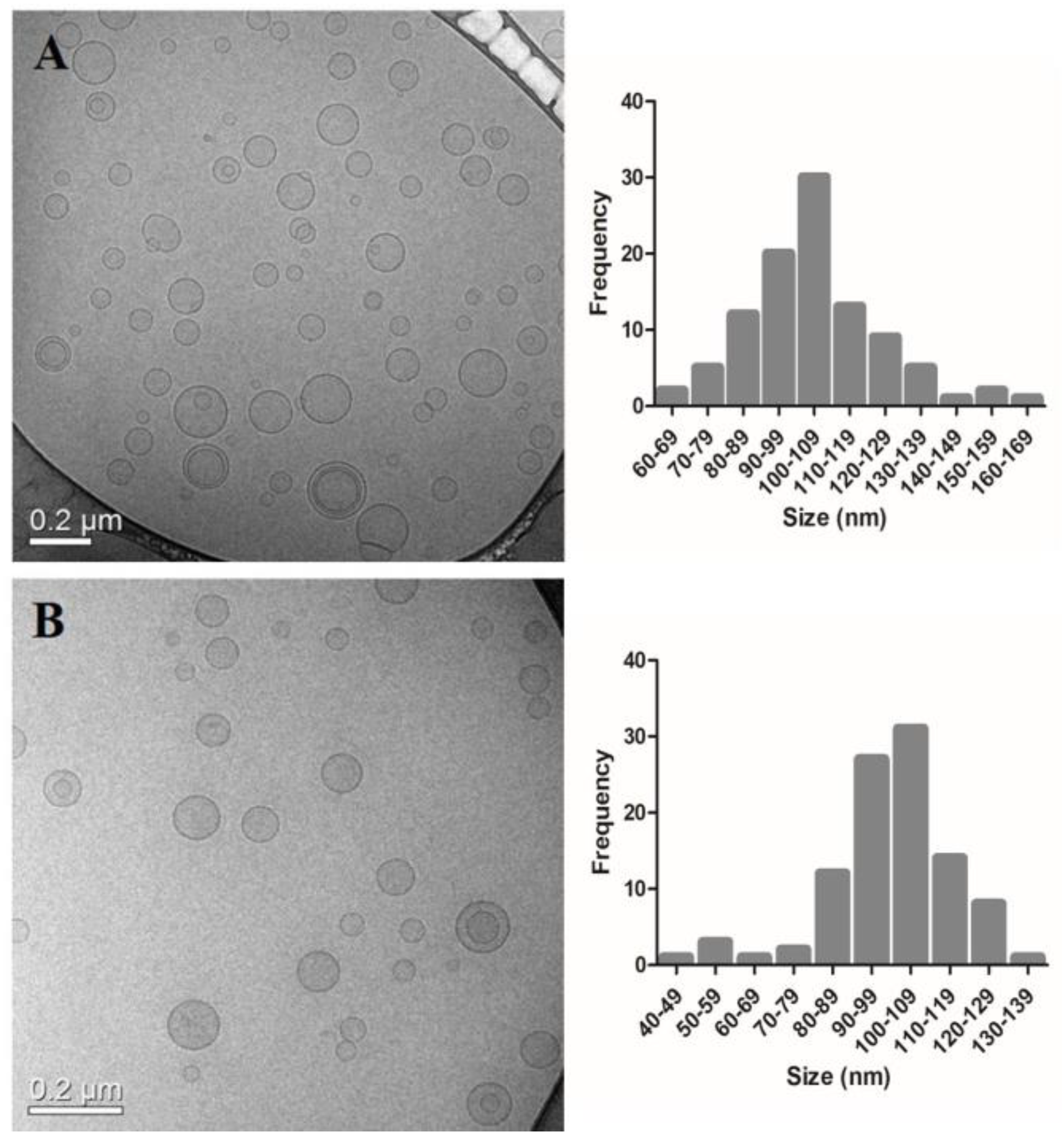
2.4. Cryogenic Transmission Electron Microscopy
2.5. Cell Culture
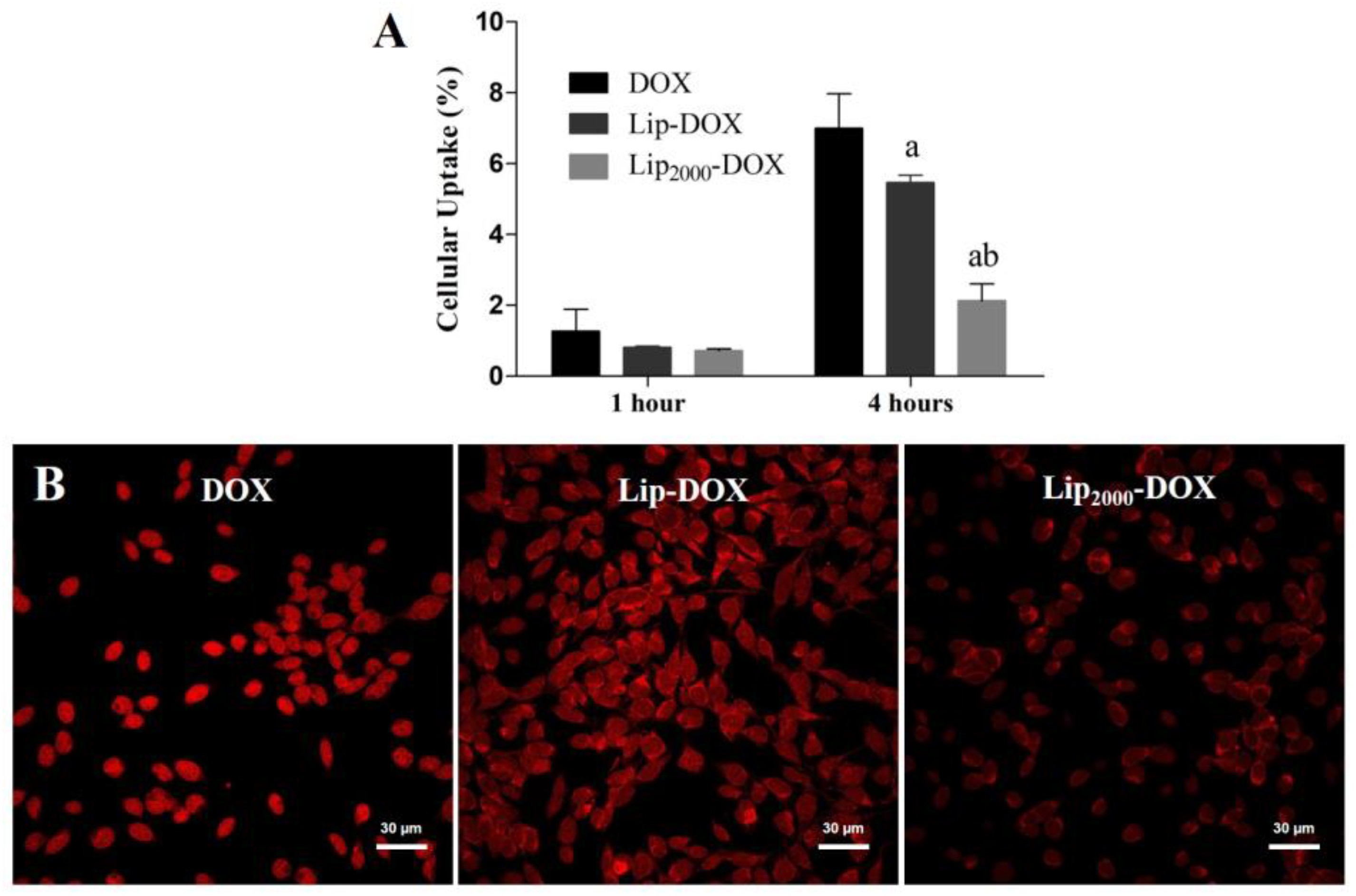
2.6. Cellular Uptake
2.7. In Vivo Antitumor Activity
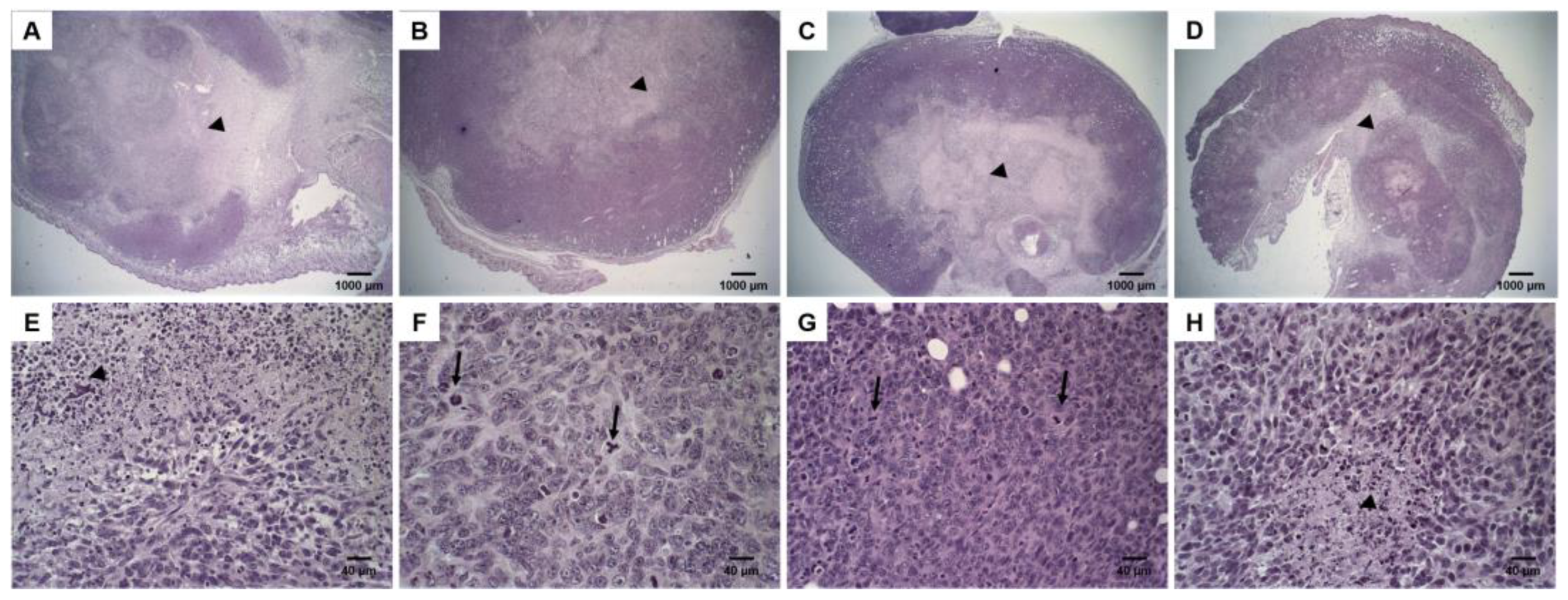
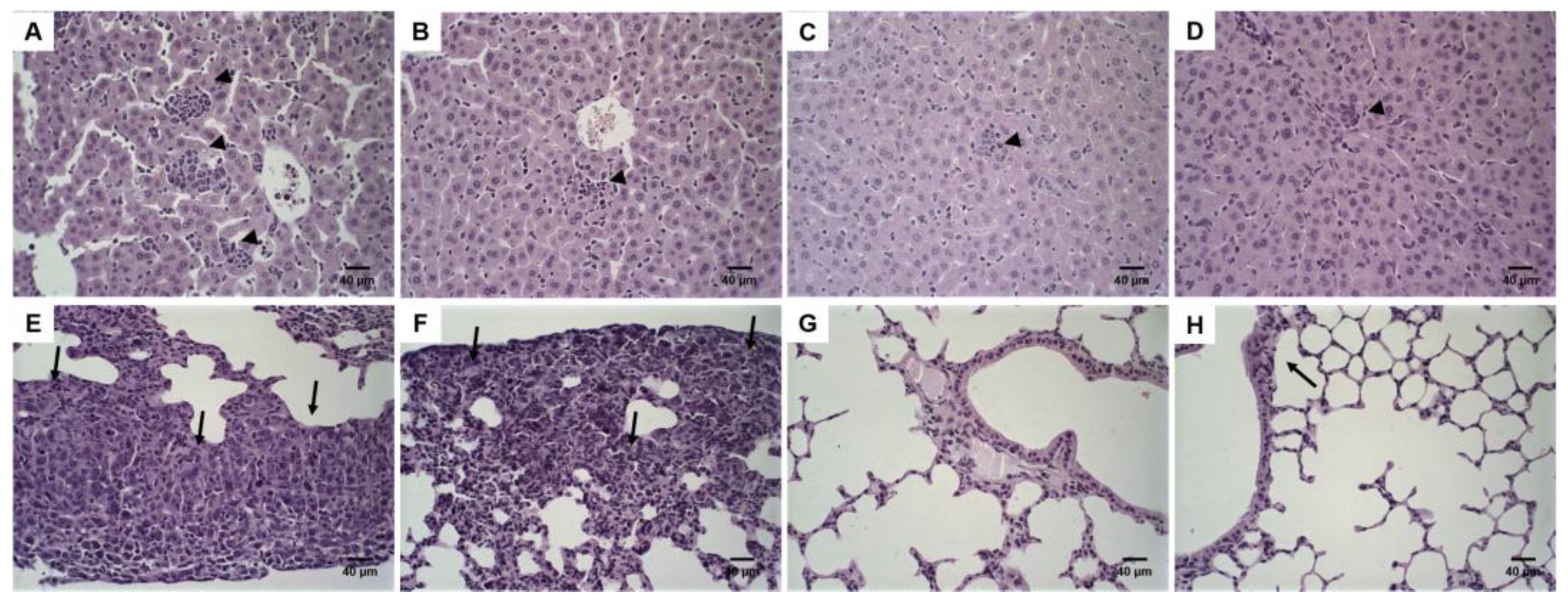
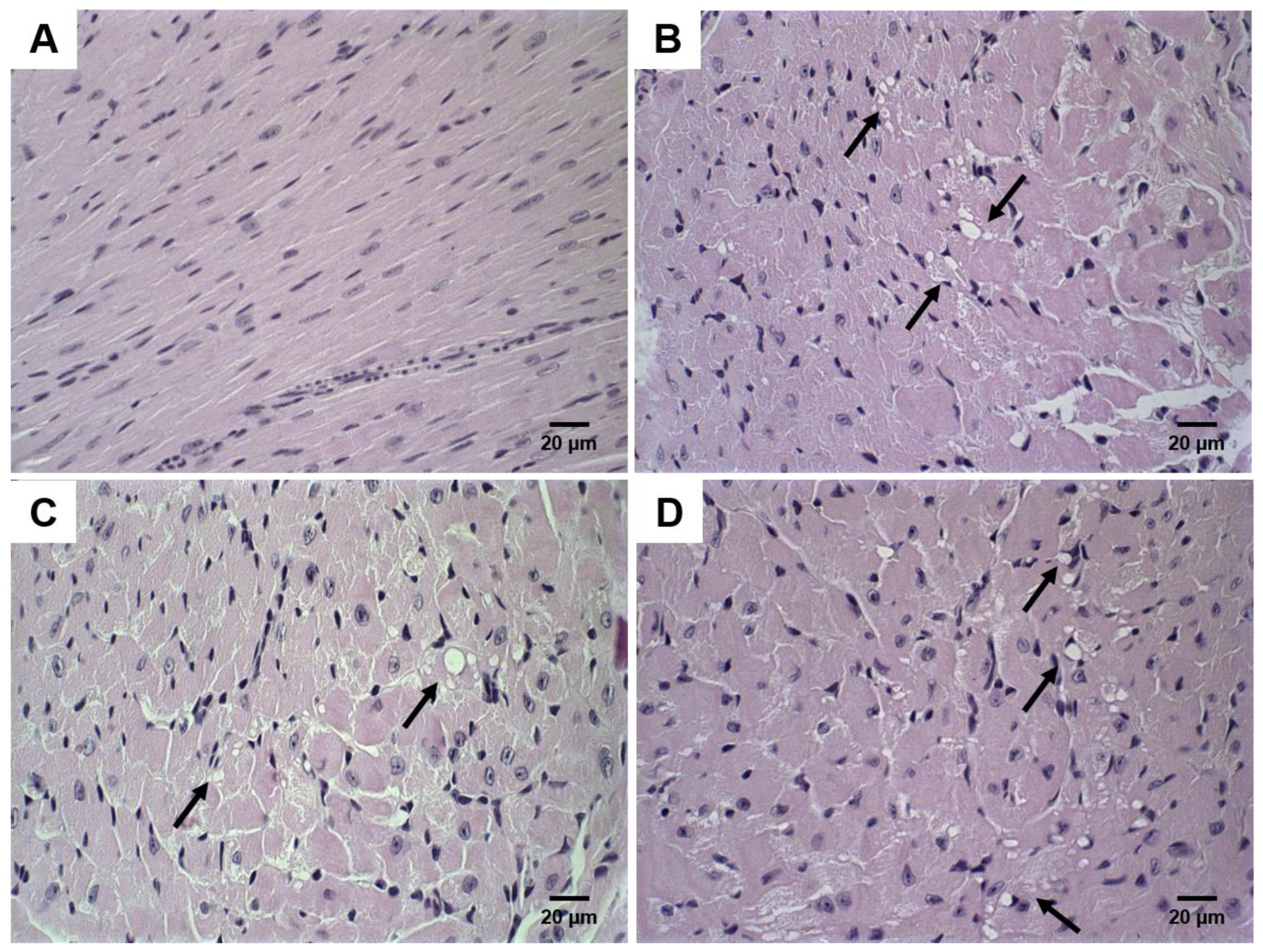
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
3.1. Liposomes Physicochemical Characterization
3.2. Cryogenic Transmission Electron Microscopy
3.3. Cellular Uptake
3.4. Antitumor Activity
3.5. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.; Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Huwyler, J.; Drewe, J.; Krähenbühl, S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008, 3, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Reshetov, V.; Lassalle, H.-P.; François, A.; Dumas, D.; Hupont, S.; Gräfe, S.; Filipe, V.; Jiskoot, W.; Guillemin, F.; Zorin, V.; et al. Photodynamic therapy with conventional and PEGylated liposomal formulations of mTHPC (temoporfin): Comparison of treatment efficacy and distribution characteristics in vivo. Int. J. Nanomed. 2013, 8, 3817–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the Use of PEGylation for Drug Delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Charrois, G.J.R.; Allen, T.M. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim. Biophys. Acta Biomembr. 2004, 1663, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Parr, M.J.; Masin, D.; Cullis, P.R.; Bally, M.B. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: The lack of beneficial effects by coating liposomes with poly(ethylene glycol). J. Pharmacol. Exp. Ther. 1997, 280, 1319–1327. [Google Scholar] [PubMed]
- Mayer, L.D.; Cullis, P.R.; Bally, M.B. Designing therapeutically optimized liposomal anticancer delivery systems: Lessons from conventional liposomes. In Medical Applications of Liposomes; Elsevier: Amsterdam, The Netherlands, 1998; pp. 231–257. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; César, I.D.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C.; et al. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Devine, D.V.; Marjan, J.M. The role of immunoproteins in the survival of liposomes in the circulation. Crit. Rev. Ther. Drug Carr. Syst. 1997, 14, 105–131. [Google Scholar] [CrossRef]
- Yan, X.; Scherphof, G.L.; Kamps, J.A.A.M. Liposome Opsonization. J. Liposome Res. 2005, 15, 109–139. [Google Scholar] [CrossRef] [PubMed]
- de Barros, A.L.B.; Mota, L.D.G.; Soares, D.C.F.; Coelho, M.M.A.; Oliveira, M.C.; Cardoso, V.N. Tumor bombesin analog loaded long-circulating and pH-sensitive liposomes as tool for tumor identification. Bioorg. Med. Chem. Lett. 2011, 21, 7373–7375. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Silva, J.O.; Monteiro, L.O.F.; Leite, E.A.; Cassali, G.D.; Rubello, D.; Cardoso, V.N.; Ferreira, L.A.M.; Oliveira, M.C.; de Barros, A.L.B. Doxorubicin-loaded nanocarriers: A comparative study of liposome and nanostructured lipid carrier as alternatives for cancer therapy. Biomed. Pharmacother. 2016, 84, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tardi, P.G.; Boman, N.L.; Cullis, P.R. Liposomal doxorubicin. J. Drug Target. 1996, 4, 129–140. [Google Scholar] [CrossRef]
- de Souza, C.M.; de Carvalho, L.F.; Vieira, T.D.; Silva, A.C.A.E.; Lopes, M.T.P.; Ferreira, M.A.N.D.; Andrade, S.P.; Cassali, G.D. Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed. Pharmacother. 2012, 66, 491–498. [Google Scholar] [CrossRef] [PubMed]
- duPre’, S.A.; Hunter, K.W. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp. Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Nakade, A.; Hirama, R.; Miyagishima, A.; Nozawa, Y.; Hirota, S.; Sonobe, T. Effects of mixed polyethyleneglycol modification on fixed aqueous layer thickness and antitumor activity of doxorubicin containing liposome. Int. J. Pharm. 2002, 238, 171–180. [Google Scholar] [CrossRef]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Berk, D.A.; Leunig, M.; Fukumura, D.; Torchilin, V.P.; Dellian, M.; Yuan, F.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Harasym, T.O.; Tardi, P.; Longman, S.A.; Ansell, S.M.; Bally, M.B.; Cullis, P.R.; Choi, L.S.L. Poly(ethylene glycol)-Modified Phospholipids Prevent Aggregation during Covalent Conjugation of Proteins to Liposomes. Bioconjug. Chem. 2005, 6, 187–194. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Su, Y.; Tian, Q.; Li, B.; Quan, J.; Deng, Y. Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. Drug Deliv. 2016, 23, 1092–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Allen, T.M.; Austin, G.A.; Chonn, A.; Lin, L.; Lee, K.C. Uptake of liposomes by cultured mouse bone marrow macrophages: Influence of liposome composition and size. BBA Biomembr. 1991, 1061, 56–64. [Google Scholar] [CrossRef]
- Molino, N.M.; Bilotkach, K.; Fraser, D.A.; Ren, D.; Wang, S.W. Complement activation and cell uptake responses toward polymer- functionalized protein nanocapsules. Biomacromolecules 2012, 13, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2006, 88, 11460–11464. [Google Scholar] [CrossRef] [Green Version]
- Chin, D.J.; Straubinger, R.M.; Acton, S.; Nathke, I.; Brodsky, F.M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2006, 86, 9289–9293. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.D.; Fernandes, R.S.; Oda, C.M.R.; Ferreira, T.H.; Botelho, A.F.M.; Melo, M.M.; de Miranda, M.C.; Gomes, D.A.; Cassali, G.D.; Townsend, D.M.; et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef]
- Khan, D.R.; Webb, M.N.; Cadotte, T.H.; Gavette, M.N. Use of Targeted Liposome-based Chemotherapeutics to Treat Breast Cancer. Breast Cancer Basic Clin. Res. 2015, 9, BCBCR-S29421. [Google Scholar] [CrossRef] [Green Version]

| Formulation | Mean Diameter (nm) | PDI | Zeta Potential (mV) | EP |
|---|---|---|---|---|
| Lip | 136.3 ± 3.9 | 0.12 ± 0.09 | −9.7 ± 1.2 | - |
| Lip2000 | 137.0 ± 2.1 | 0.13 ± 0.07 | −4.1 ± 0.6 a | - |
| Lip-DOX | 139.4 ± 3.8 | 0.11 ± 0.07 | −8.9 ± 0.8 | 93.1 ± 1.2 |
| Lip2000-DOX | 140.2 ± 3.1 | 0.12 ± 0.08 | −4.0 ± 1.2 b | 92.3 ± 0.9 |
| Treatment | Best-Fit Model | Correlation Coefficient (r2) | IR (%) |
|---|---|---|---|
| Lip | y = 72.2x − 419.7 | 0.9723 | - |
| DOX | y = −4.9x2 − 176.5x − 917.3 | 0.9675 | 36.1 |
| Lip2000-DOX | y = −1.9x2 − 93.3x − 450.7 | 0.9238 | 40.3 |
| Lip-DOX | y = 0.4x3 − 24.8x2 − 426.7x − 1886 | 0.9916 | 60.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, S.S.; de Oliveira Silva, J.; Fernandes, R.S.; Miranda, S.E.M.; Leite, E.A.; de Farias, M.A.; Portugal, R.V.; Cassali, G.D.; Townsend, D.M.; Oliveira, M.C.; et al. PEGylated versus Non-PEGylated pH-Sensitive Liposomes: New Insights from a Comparative Antitumor Activity Study. Pharmaceutics 2022, 14, 272. https://doi.org/10.3390/pharmaceutics14020272
Nunes SS, de Oliveira Silva J, Fernandes RS, Miranda SEM, Leite EA, de Farias MA, Portugal RV, Cassali GD, Townsend DM, Oliveira MC, et al. PEGylated versus Non-PEGylated pH-Sensitive Liposomes: New Insights from a Comparative Antitumor Activity Study. Pharmaceutics. 2022; 14(2):272. https://doi.org/10.3390/pharmaceutics14020272
Chicago/Turabian StyleNunes, Shirleide Santos, Juliana de Oliveira Silva, Renata Salgado Fernandes, Sued Eustaquio Mendes Miranda, Elaine Amaral Leite, Marcelo Alexandre de Farias, Rodrigo Villares Portugal, Geovanni Dantas Cassali, Danyelle M. Townsend, Mônica Cristina Oliveira, and et al. 2022. "PEGylated versus Non-PEGylated pH-Sensitive Liposomes: New Insights from a Comparative Antitumor Activity Study" Pharmaceutics 14, no. 2: 272. https://doi.org/10.3390/pharmaceutics14020272
APA StyleNunes, S. S., de Oliveira Silva, J., Fernandes, R. S., Miranda, S. E. M., Leite, E. A., de Farias, M. A., Portugal, R. V., Cassali, G. D., Townsend, D. M., Oliveira, M. C., & de Barros, A. L. B. (2022). PEGylated versus Non-PEGylated pH-Sensitive Liposomes: New Insights from a Comparative Antitumor Activity Study. Pharmaceutics, 14(2), 272. https://doi.org/10.3390/pharmaceutics14020272