Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SPIONs Characterization
2.3. Cytotoxicity
2.4. Immunocytochemistry
2.5. Cresyl Violet Staining (Staining of Nissl Bodies)
2.6. Neurite Outgrowth Analysis
2.7. Statistical Analysis
3. Results
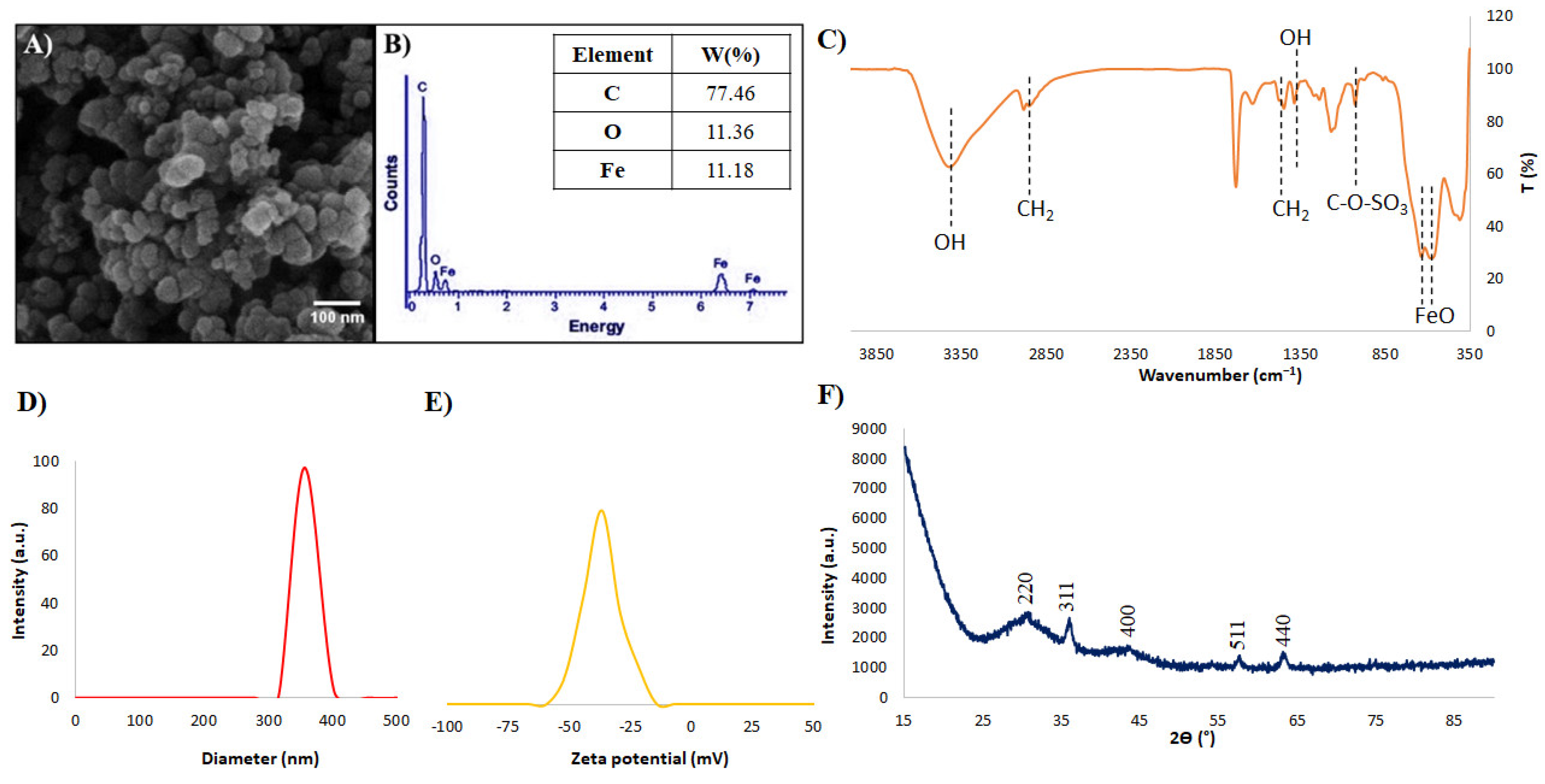
3.1. SPIONs Characterization
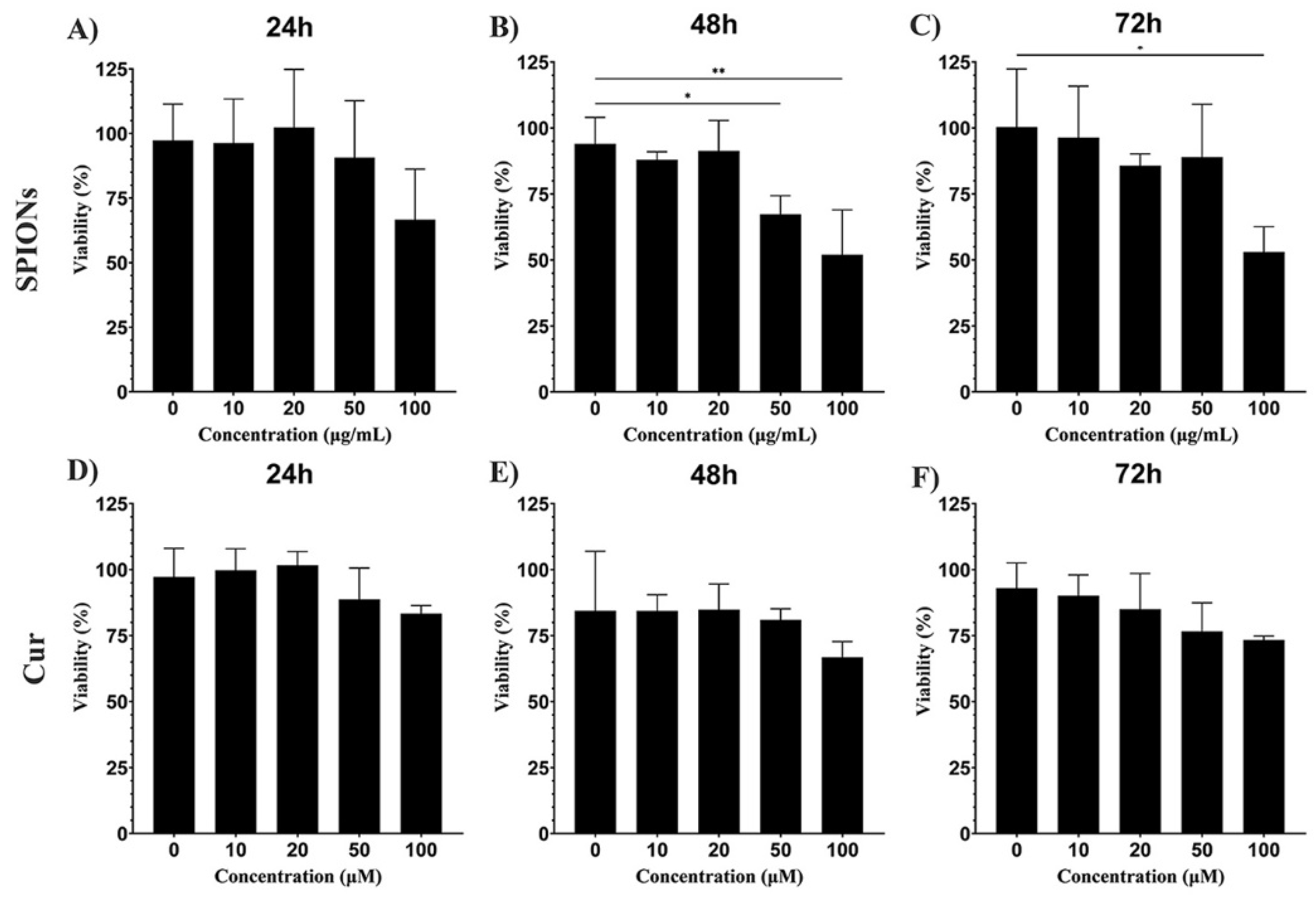
3.2. Cytotoxicity
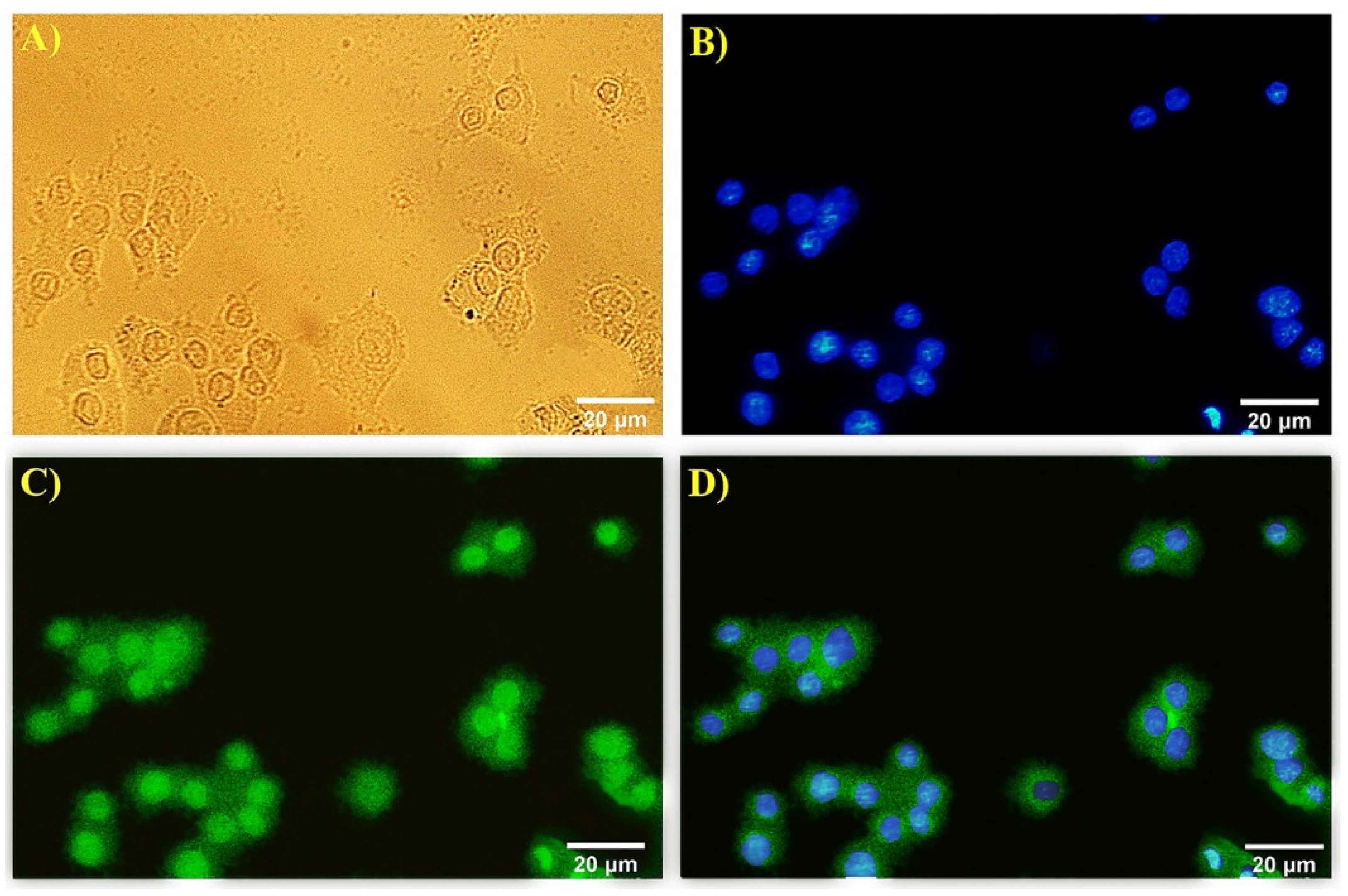
3.3. Immunocytochemistry
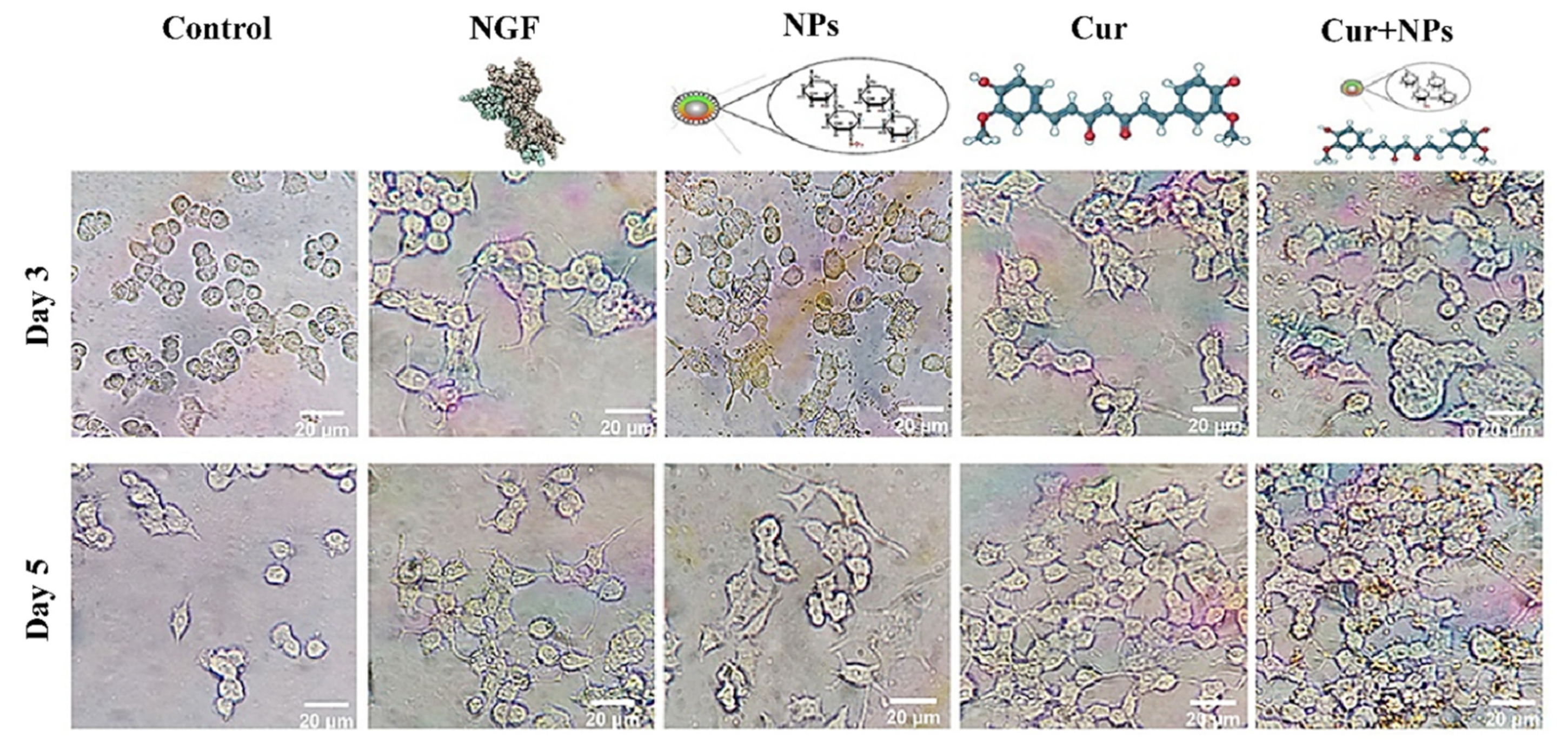
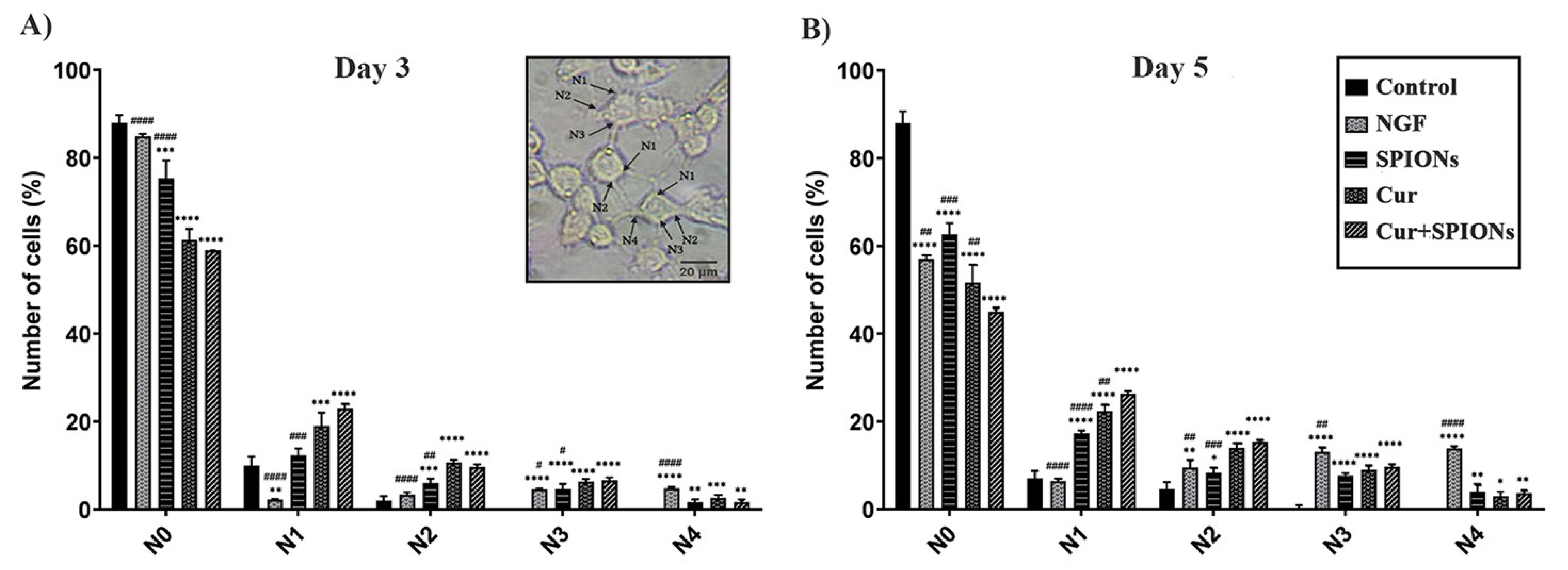
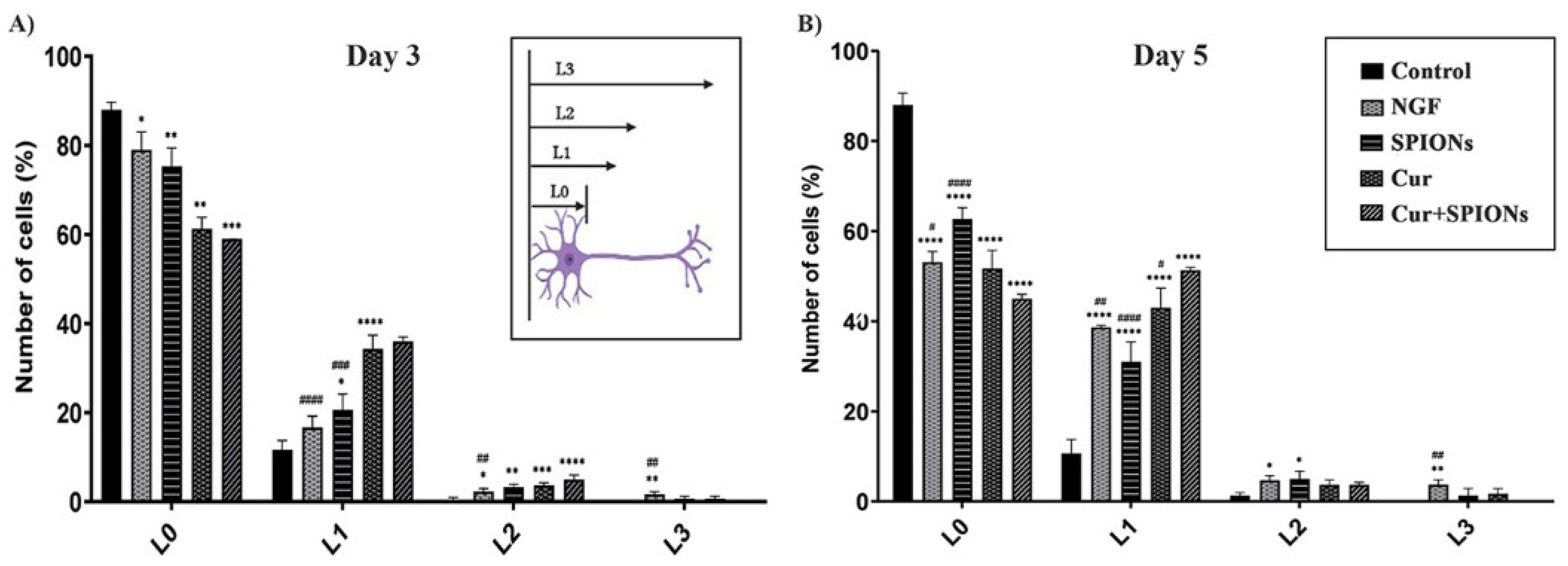
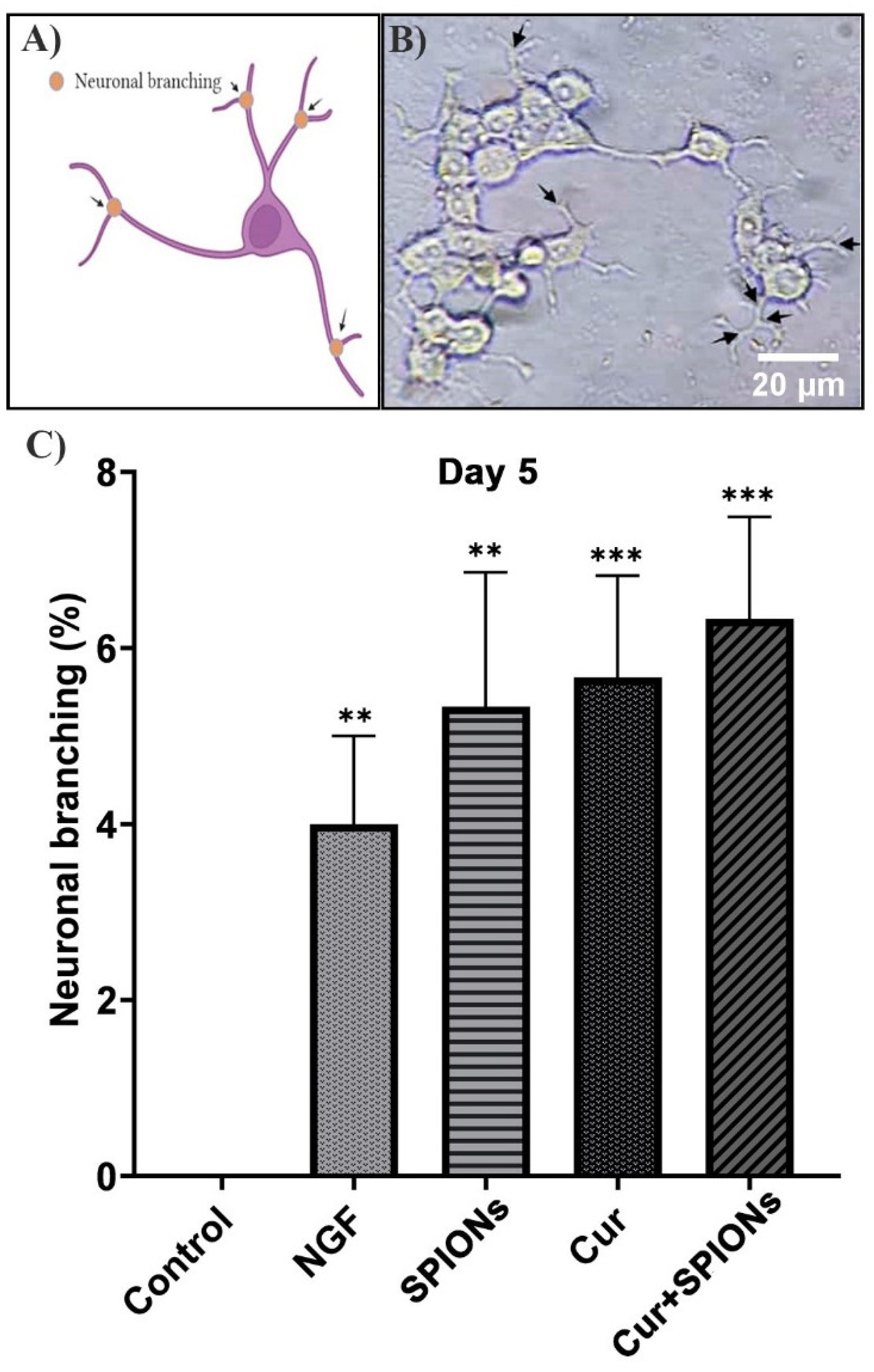
3.4. Neurite Outgrowth Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.A.; Lee, N.; Kim, B.H.; Rhee, W.J.; Yoon, S.; Hyeon, T.; Park, T.H. Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials 2011, 32, 2871–2877. [Google Scholar] [CrossRef]
- Tondo, G.; De Marchi, F. From Biomarkers to Precision Medicine in Neurodegenerative Diseases: Where Are We? MDPI: Basel, Switzerland, 2022; Volume 11, p. 4515. [Google Scholar]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Giridharan, V.V.; De Quevedo, C.E.B.; Petronilho, F. Microbiota-gut-brain axis in the Alzheimer’s disease pathology-an overview. Neurosci. Res. 2022, 181, 17–21. [Google Scholar] [CrossRef]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 2021, 231, 107989. [Google Scholar] [CrossRef]
- Neri, S.; Mastroianni, G.; Gardella, E.; Aguglia, U.; Rubboli, G. Epilepsy in neurodegenerative diseases. Epileptic Disord. 2022, 24, 249–273. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef]
- Kanu, L.N.; Ciolino, J.B. Nerve growth factor as an ocular therapy: Applications, challenges, and future directions. In Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2021; Volume 36, pp. 224–231. [Google Scholar]
- Dikmen, M.; Kaya-Tilki, E.; Engur, S.; Ozturk, Y. Neuritogenic activity of epigallocatechin gallate and curcumin combination on rat adrenal pheochromocytoma cells. Fresenius Env. Bull 2017, 26, 4726–4733. [Google Scholar]
- Phan, C.W.; Sabaratnam, V.; Bovicelli, P.; Righi, G.; Saso, L. Negletein as a neuroprotectant enhances the action of nerve growth factor and induces neurite outgrowth in PC12 cells. Biofactors 2016, 42, 591–599. [Google Scholar] [CrossRef]
- Xu, D.; Wu, D.; Qin, M.; Nih, L.R.; Liu, C.; Cao, Z.; Ren, J.; Chen, X.; He, Z.; Yu, W. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv. Mater. 2019, 31, 1900727. [Google Scholar] [CrossRef]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, B.; Wang, A.; Hao, D.; Zhang, Q.; Zhao, J.; Liu, C.; Zhang, L.; Zhang, R.; Yang, H. Modulatory Effects of natural products on neuronal differentiation. Neuropsychiatry 2018, 8, 1593–1611. [Google Scholar] [CrossRef]
- Liao, K.-K.; Wu, M.-J.; Chen, P.-Y.; Huang, S.-W.; Chiu, S.-J.; Ho, C.-T.; Yen, J.-H. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK-and PKC-dependent pathways. J. Agric. Food Chem. 2012, 60, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 16953–16965. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Dikmen, M. Comparison of the effects of curcumin and RG108 on NGF-induced PC-12 Adh cell differentiation and neurite outgrowth. J. Med. Food 2017, 20, 376–384. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; pp. 197–212. [Google Scholar]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.-D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef]
- Nocito, M.C.; De Luca, A.; Prestia, F.; Avena, P.; La Padula, D.; Zavaglia, L.; Sirianni, R.; Casaburi, I.; Puoci, F.; Chimento, A. Antitumoral Activities of Curcumin and Recent Advances to ImProve Its Oral Bioavailability. Biomedicines 2021, 9, 1476. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of functional biocompatible nanomaterials to improve curcumin bioavailability. Front. Chem. 2020, 8, 589957. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging trends in nanomaterials for antibacterial applications. Int. J. Nanomed. 2021, 16, 5831. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin nanoformulations with metal oxide nanomaterials for biomedical applications. Nanomaterials 2021, 11, 460. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Salehi, B.; Del Prado-Audelo, M.L.; Cortés, H.; Leyva-Gómez, G.; Stojanović-Radić, Z.; Singh, Y.D.; Patra, J.K.; Das, G.; Martins, N.; Martorell, M. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J. Clin. Med. 2020, 9, 746. [Google Scholar] [CrossRef]
- Pandit, R.S.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Douziech-Eyrolles, L.; Marchais, H.; Hervé, K.; Munnier, E.; Souce, M.; Linassier, C.; Dubois, P.; Chourpa, I. Nanovectors for anticancer agents based on superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2007, 2, 541. [Google Scholar]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011, 2, 510. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Magnetic Nanoparticles as Delivery Systems to Penetrate the Blood-Brain Barrier. In Nanomedicines for Brain Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 173–208. [Google Scholar]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wang, Y.-X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ma, C.; Zhu, M.-Q.; Ju, W.-N.; Yang, Y.; Wang, X. Application of iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer’s disease. Front. Cell. Neurosci. 2020, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Katebi, S.; Esmaeili, A.; Ghaedi, K.; Zarrabi, A. Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int. J. Nanomed. 2019, 14, 2157. [Google Scholar] [CrossRef]
- Mendonça, L.M.; da Silva Machado, C.; Teixeira, C.C.C.; de Freitas, L.A.P.; Bianchi, M.d.L.P.; Antunes, L.M.G. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013, 34, 205–211. [Google Scholar] [CrossRef]
- Shahpiri, Z.; Bahramsoltani, R.; Farzaei, M.H.; Farzaei, F.; Rahimi, R. Phytochemicals as future drugs for Parkinson’s disease: A comprehensive review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef]
- Marcus, M.; Skaat, H.; Alon, N.; Margel, S.; Shefi, O. NGF-conjugated iron oxide nanoparticles promote differentiation and outgrowth of PC12 cells. Nanoscale 2015, 7, 1058–1066. [Google Scholar] [CrossRef]
- Li, N.; Zhong, Q.J.F.H. Casein core-polysaccharide shell nanocomplexes stable at pH 4.5 enabled by chelating and complexation properties of dextran sulfate. Food Hydrocoll. 2020, 103, 105723. [Google Scholar] [CrossRef]
- Mostaghasi, E.; Zarepour, A.; Zarrabi, A. Folic acid armed Fe3O4-HPG nanoparticles as a safe nano vehicle for biomedical theranostics. J. Taiwan Inst. Chem. Eng. 2018, 82, 33–41. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.-K.; Lin, J.; Fuchs, J.R.; Marcucci, G. Curcumin is a potent DNA hypomethylation agent. Bioorganic Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Szymusiak, M.; Hu, X.; Plata, P.A.L.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef]
- Fan, C.-D.; Li, Y.; Fu, X.-T.; Wu, Q.-J.; Hou, Y.-J.; Yang, M.-F.; Sun, J.-Y.; Fu, X.-Y.; Zheng, Z.-C.; Sun, B.-L. Reversal of beta-amyloid-induced neurotoxicity in PC12 cells by curcumin, the important role of ROS-mediated signaling and ERK pathway. Cell. Mol. Neurobiol. 2017, 37, 211–222. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xu, K.; Gu, J.; Huang, L.; Zhang, L.; Liu, N.; Kong, J.; Xing, M.; Zhang, L. Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum. Toxicol. Lett. 2018, 292, 151–161. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, R.; Mir, R.H.; Wani, T.U.; Shah, A.J.; Mohi-Ud-Din, I.; Dar, M.A.; Pottoo, F.H. Novel drug delivery system for curcumin: Implementation to improve therapeutic efficacy against neurological disorders. Comb. Chem. High Throughput Screen. 2022, 25, 607–615. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Barreto, G.E.; Bland, A.R.; Sahebkar, A. Curcumin: A small molecule with big functionality against amyloid aggregation in neurodegenerative diseases and type 2 diabetes. Biofactors 2021, 47, 570–586. [Google Scholar] [CrossRef]
- Doytchinova, I.; Atanasova, M.; Salamanova, E.; Ivanov, S.; Dimitrov, I. Curcumin inhibits the primary nucleation of amyloid-beta peptide: A molecular dynamics study. Biomolecules 2020, 10, 1323. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742. [Google Scholar]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef]
- Shahbaz, S.K.; Koushki, K.; Sathyapalan, T.; Majeed, M.; Sahebkar, A. PLGA-Based Curcumin Delivery System: An Interesting Therapeutic Approach in the Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2022, 2, 309–323. [Google Scholar] [CrossRef]
- Khan, F.A.; Almohazey, D.; Alomari, M.; Almofty, S.A. Impact of nanoparticles on neuron biology: Current research trends. Int. J. Nanomed. 2018, 13, 2767. [Google Scholar] [CrossRef]
- Abbas, E.; Hassan, M.A.; Sokpor, G.; Kiszka, K.; Pham, L.; Kerimoglu, C.; Fischer, A.; Nguyen, H.P.; Staiger, J.F.; Tuoc, T. Conditional loss of BAF (mSWI/SNF) scaffolding subunits affects specification and proliferation of oligodendrocyte precursors in developing mouse forebrain. Front. Cell Dev. Biol. 2021, 9, 619538. [Google Scholar] [CrossRef]
- Lein, P.; Gallagher, P.J.; Amodeo, J.; Howie, H.; Roth, J.A. Manganese induces neurite outgrowth in PC12 cells via upregulation of αv integrins. Brain Res. 2000, 885, 220–230. [Google Scholar] [CrossRef]
- Roth, J.A.; Horbinski, C.; Higgins, D.; Lein, P.; Garrick, M.D. Mechanisms of manganese-induced rat pheochromocytoma (PC12) cell death and cell differentiation. Neurotoxicology 2002, 23, 147–157. [Google Scholar] [CrossRef]
- Hong, J.-H.; Noh, K.-M.; Yoo, Y.-E.; Choi, S.-Y.; Park, S.-Y.; Kim, Y.-H.; Chung, J.-M. Iron promotes the survival and neurite extension of serum-starved PC12 cells in the presence of NGF by enhancing cell attachment. Mol. Cells 2003, 15, 10–19. [Google Scholar]
- Lin, W.; Higgins, D.; Pacheco, M.; Aletta, J.; Perini, S.; Marcucci, K.; Roth, J. Manganese induces spreading and process outgrowth in rat pheochromocytoma (PC12) cells. J. Neurosci. Res. 1993, 34, 546–561. [Google Scholar] [CrossRef]
- Dai, R.; Hang, Y.; Liu, Q.; Zhang, S.; Wang, L.; Pan, Y.; Chen, H. Improved neural differentiation of stem cells mediated by magnetic nanoparticle-based biophysical stimulation. J. Mater. Chem. B 2019, 7, 4161–4168. [Google Scholar] [CrossRef]
- Mohammadalizadeh, M.; Dabirian, S.; Akrami, M.; Hesari, Z.J.N. SPION based magnetic PLGA nanofibers for neural differentiation of mesenchymal stem cells. Nanotechnology 2022, 33, 375101. [Google Scholar] [CrossRef]
- Oravecz, K.; Kalka, D.; Jeney, F.; Cantz, M.; Nagy, I.Z. Hydroxyl free radicals induce cell differentiation in SK-N-MC neuroblastoma cells. Tissue Cell 2002, 34, 33–38. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part. Fibre Toxicol. 2009, 6, 1. [Google Scholar] [CrossRef]
- Higuchi, M.; Onishi, K.; Masuyama, N.; Gotoh, Y. The phosphatidylinositol-3 kinase (PI3K)-Akt pathway suppresses neurite branch formation in NGF-treated PC12 cells. Genes Cells 2003, 8, 657–669. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Hafez, A.A.; Abdorahim, M.; Abdollahifar, M.A.; Shabani, R.; Peirovi, H.; Simchi, A.; Ashtari, K.J.B. Pharmacotherapy Curcumin loading potentiates the neuroprotective efficacy of Fe3O4 magnetic nanoparticles in cerebellum cells of schizophrenic rats. Biomed. Pharmacother. 2018, 108, 1244–1252. [Google Scholar] [CrossRef]
- De Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Dames, P.; Gleich, B.; Flemmer, A.; Hajek, K.; Seidl, N.; Wiekhorst, F.; Eberbeck, D.; Bittmann, I.; Bergemann, C.; Weyh, T. Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2007, 2, 495–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, M.; Esmaeili, A.; Zarrabi, A.; Zarepour, A. Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics 2022, 14, 2692. https://doi.org/10.3390/pharmaceutics14122692
Zarei M, Esmaeili A, Zarrabi A, Zarepour A. Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics. 2022; 14(12):2692. https://doi.org/10.3390/pharmaceutics14122692
Chicago/Turabian StyleZarei, Mahshid, Abolghasem Esmaeili, Ali Zarrabi, and Atefeh Zarepour. 2022. "Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells" Pharmaceutics 14, no. 12: 2692. https://doi.org/10.3390/pharmaceutics14122692
APA StyleZarei, M., Esmaeili, A., Zarrabi, A., & Zarepour, A. (2022). Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics, 14(12), 2692. https://doi.org/10.3390/pharmaceutics14122692







