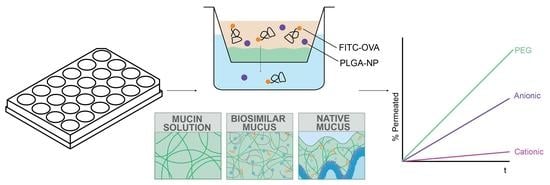
Optimisation of a High-Throughput Model for Mucus Permeation and Nanoparticle Discrimination Using Biosimilar Mucus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLGA Nanoparticles with Varying Surface Properties
2.2.1. PEGylation of Anionic PLGA Nanoparticle Surface via PLUF127 Adsorption
2.2.2. Quantification of Adsorbed PLUF127 Mass
2.3. Transwell Experiments
2.3.1. Preparation of Mucus Mimics
- A 5% mucin solution was prepared via solvation of Type III mucin in MES buffer.
- BSM was prepared via a previously published method [14,26]. Briefly, 5% mucin and 0.9% PAA were dissolved in pH 6.5 MES buffer, with the addition of NaOH to rectify pH. Next, 3.1% BSA, 0.15% Tween 80, 0.36% cholesterol 0.18% (w/v) PC were added to the mixture and stirred until homogeneous. The prepared BSM was stored overnight at 4 °C prior to use.
- Native porcine intestinal mucus (PIM) was harvested from the intestine of deceased pigs obtained from a local slaughterhouse. Mucus collection was conducted within 30 min from slaughter and kept on ice until snap freezing with liquid nitrogen within 2 h from collection. Permission was gained from the slaughterhouse for the use of waste materials in scientific testing and therefore ethical approval was not necessary for this study.
2.3.2. Permeation Experiments
3. Results
3.1. Fabrication of Nanoparticles with Diverse Surface Properties
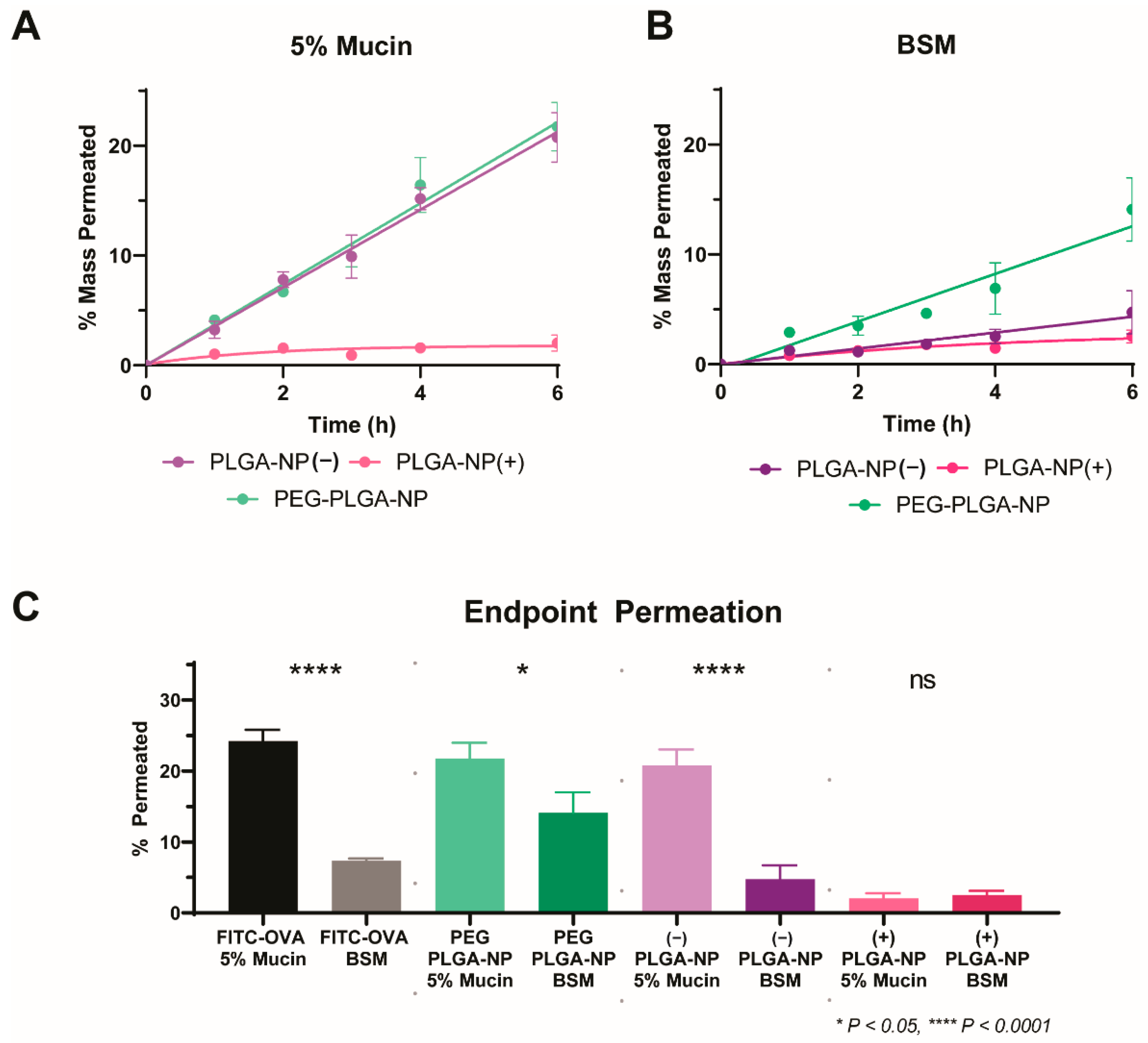
3.2. Development and Validation of a High-Throughput Biorelevant Mucus Model
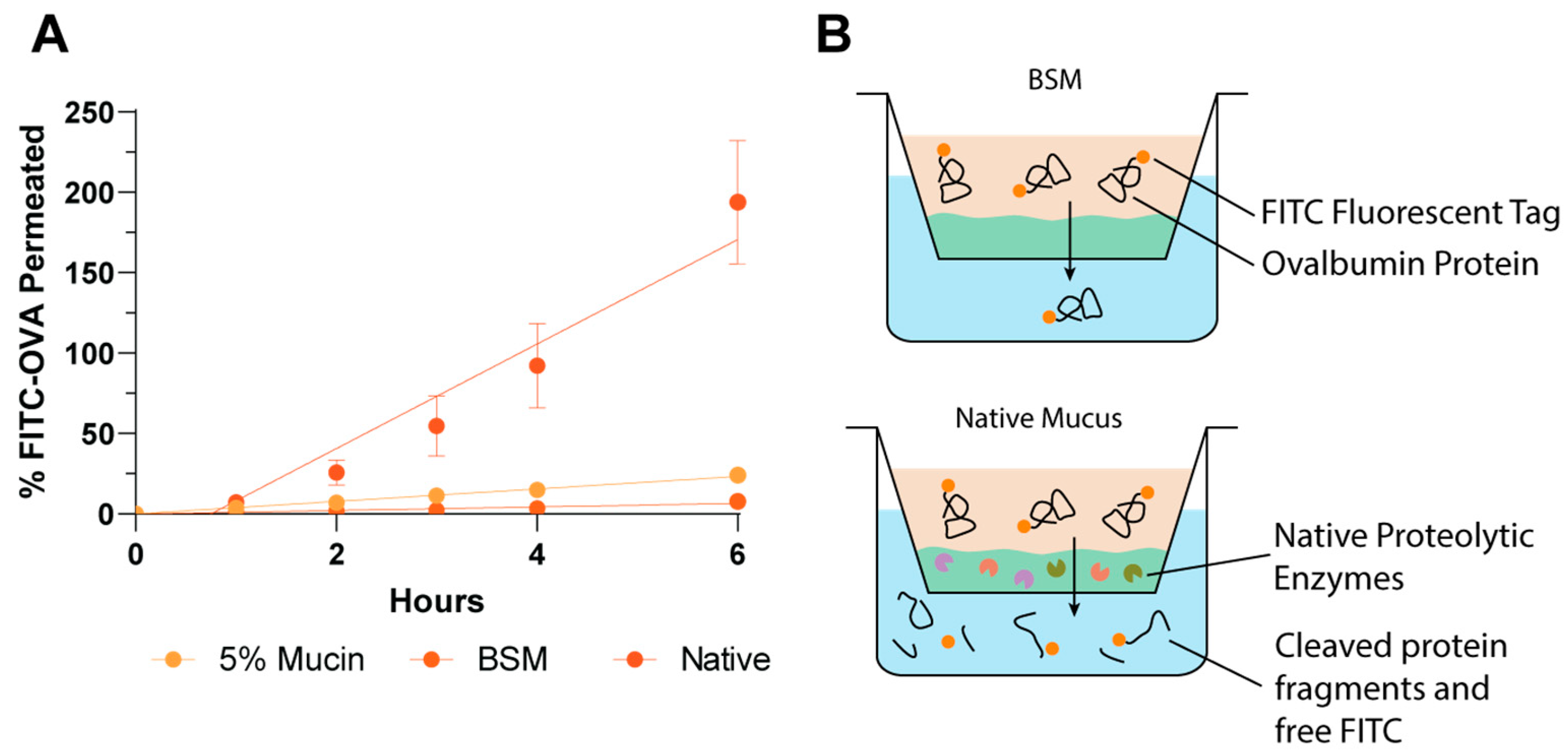
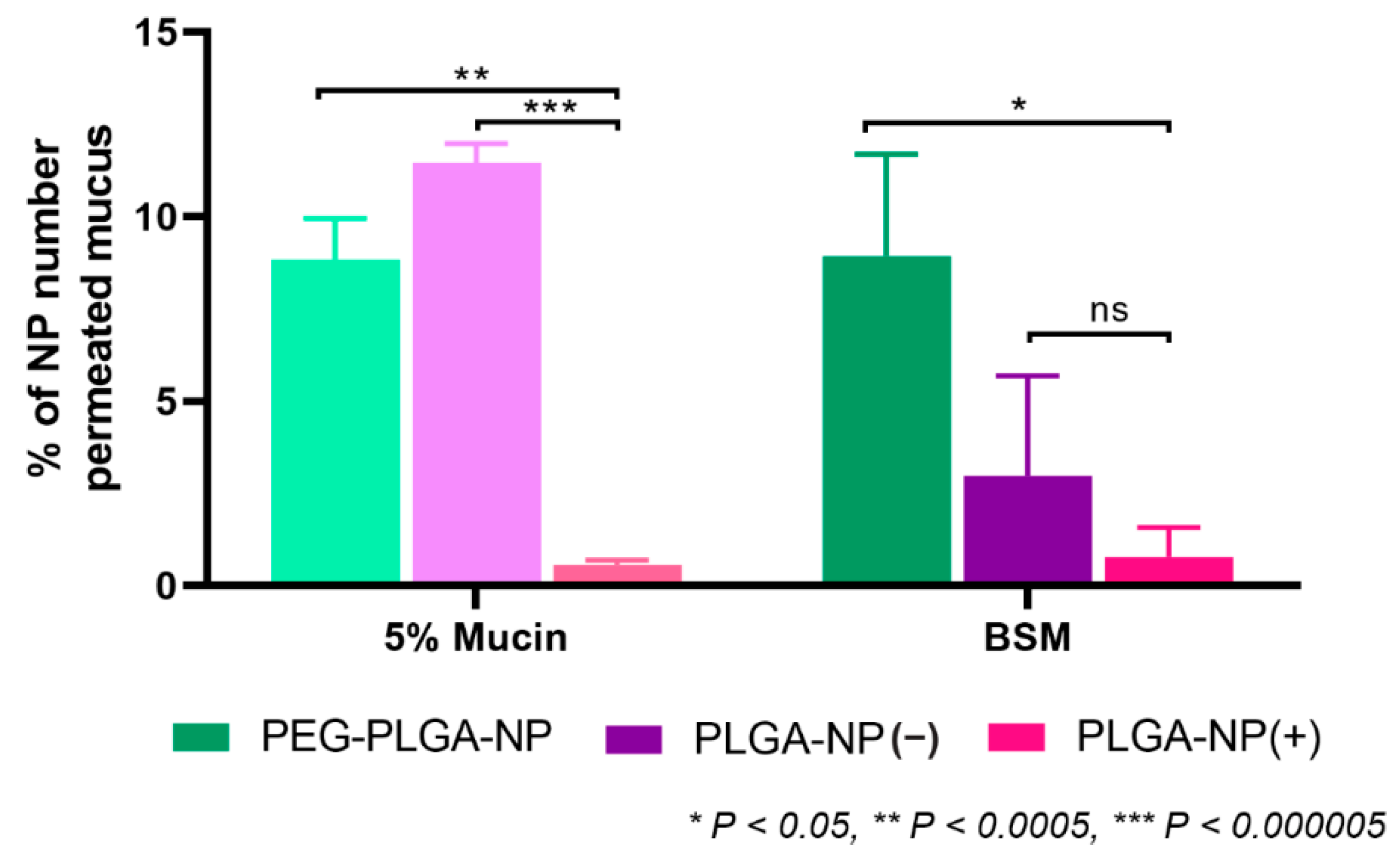
3.3. Application of a High-Throughput Mucus Model as a Screening Tool to Discriminate between Nanoparticle Mucus Permeability
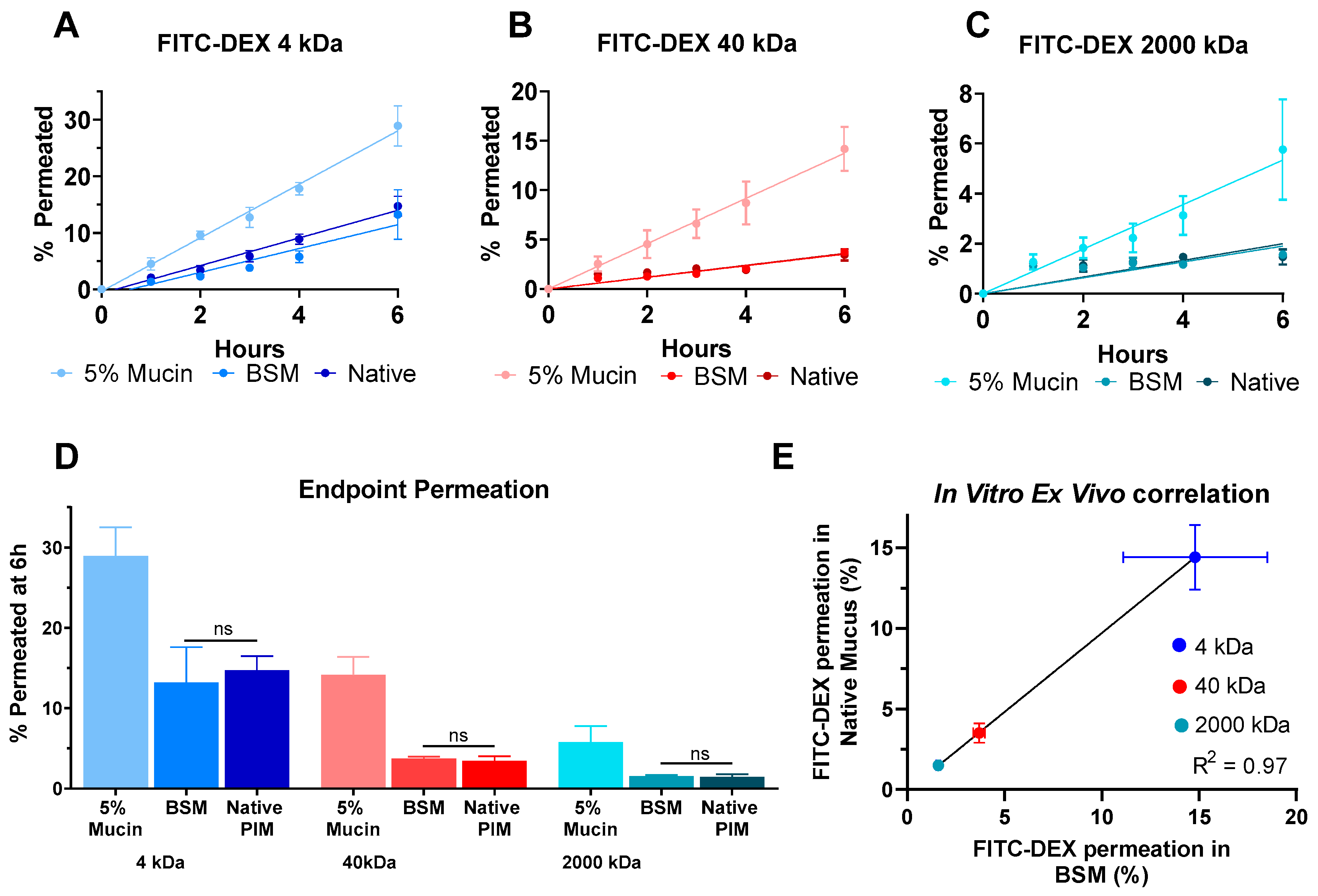
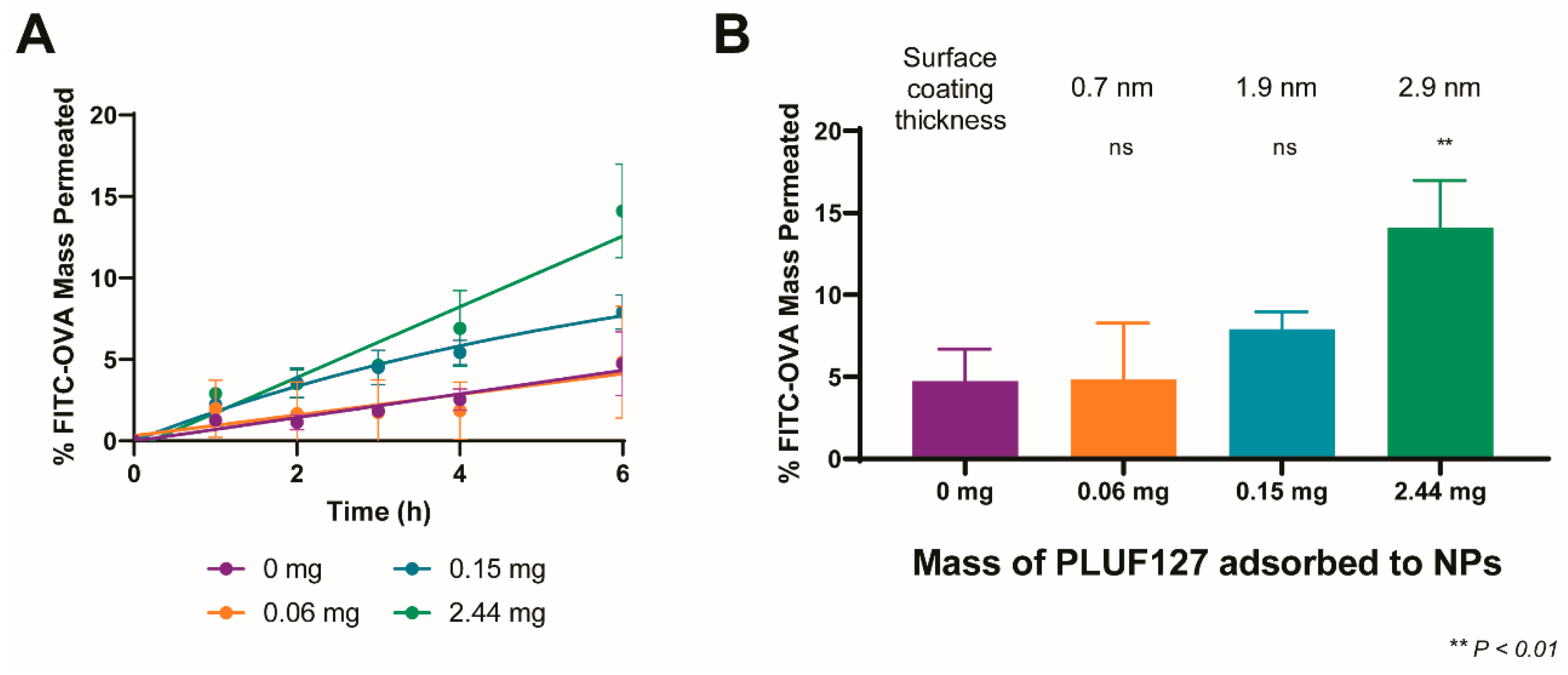
3.4. Influence of the Degree of PEGylation on Permeation in BSM
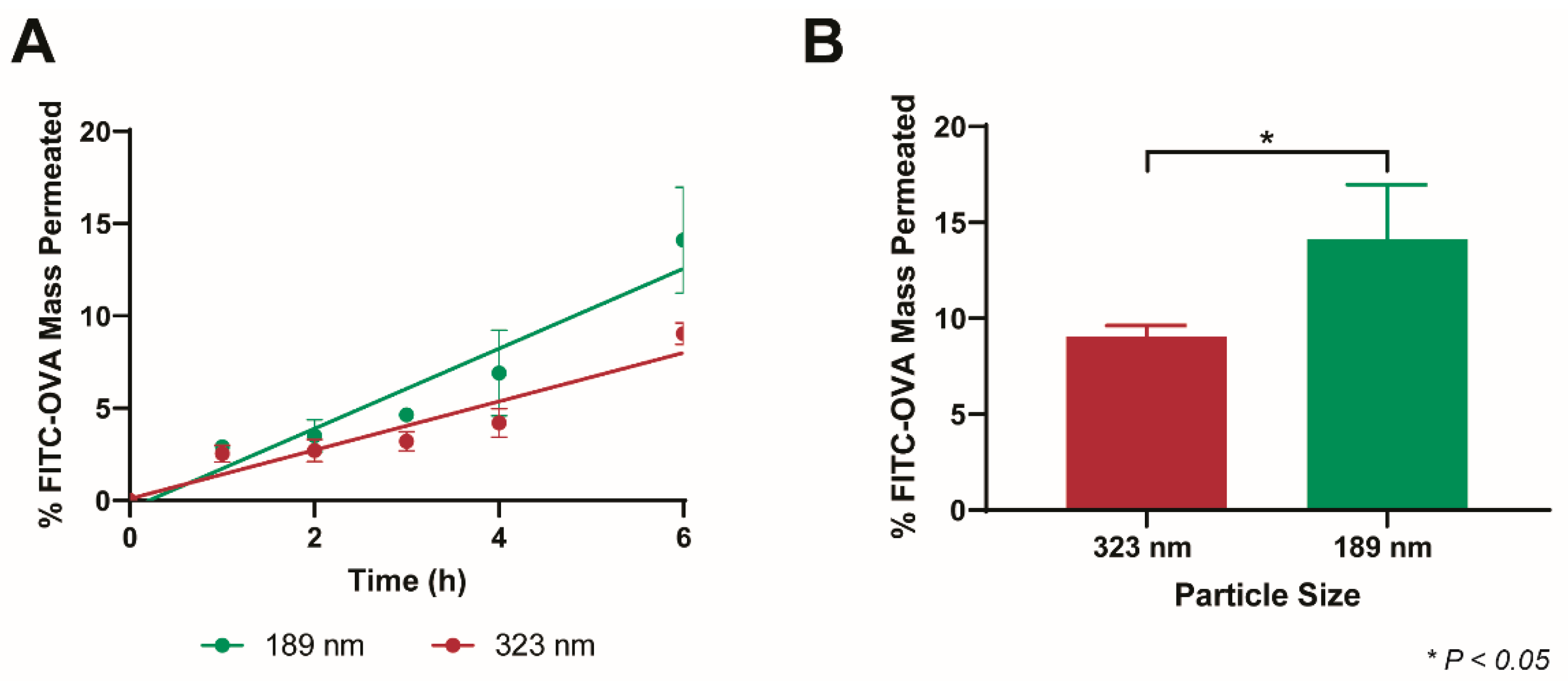
3.5. Size-Dependent Permeation of PEG-PLGA-NP in BSM
3.6. Model Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahan, A.; Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef]
- Sharma, A.; Kwak, J.-G.; Kolewe, K.W.; Schiffman, J.D.; Forbes, N.S.; Lee, J. In Vitro Reconstitution of an Intestinal Mucus Layer Shows That Cations and pH Control the Pore Structure That Regulates Its Permeability and Barrier Function. ACS Appl. Bio Mater. 2020, 3, 2897–2909. [Google Scholar] [CrossRef]
- Wright, L.; Joyce, P.; Barnes, T.J.; A Prestidge, C. Mimicking the Gastrointestinal Mucus Barrier: Laboratory-Based Approaches to Facilitate an Enhanced Understanding of Mucus Permeation. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Crouch, L.I.; Liberato, M.V.; Urbanowicz, P.A.; Baslé, A.; Lamb, C.A.; Stewart, C.J.; Cooke, K.; Doona, M.; Needham, S.; Brady, R.R.; et al. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat. Commun. 2020, 11, 4017. [Google Scholar] [CrossRef]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef]
- Joyner, K.; Song, D.; Hawkins, R.F.; Silcott, R.D.; Duncan, G.A. Designing viscoelastic mucin-based hydrogels. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2017, 124, 34–49. [Google Scholar] [CrossRef]
- Boegh, M.; Baldursdottir, S.G.; Nielsen, M.H.; Müllertz, A.; Nielsen, H.M. Development and rheological profiling of biosimilar mucus. Nord. Rheol. Society. Annu. Trans. 2013, 21, 233–240. [Google Scholar]
- Wright, L.; Wignall, A.; Jõemetsa, S.; Joyce, P.; Prestidge, C.A. A Membrane-Free Microfluidic Approach to Mucus Permeation for Efficient Differentiation of Mucoadhesive and Mucopermeating Nanoparticulate Systems. Drug Deliv. Transl. 2022, in press. [Google Scholar]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Aljayyoussi, G.; Abdulkarim, M.; Griffiths, P.; Gumbleton, M. Pharmaceutical Nanoparticles and the Mucin Biopolymer Barrier. BioImpacts 2012, 2, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; García-Díaz, M.; Müllertz, A.; Nielsen, H.M. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur. J. Pharm. Biopharm. 2015, 95, 136–143. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Sundby, A.; Bakke, A. Integrated function and control of the gut|Gut Secretion and Digestion. In Encyclopedia of Fish Physiology; Academic Press: Cambridge, MA, USA, 2011; pp. 1301–1310. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- Muralidhara, B.K.; Wong, M. Critical considerations in the formulation development of parenteral biologic drugs. Drug Discov. Today 2020, 25, 574–581. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Wright, L.; Joyce, P.; Barnes, T.J.; Lundmark, R.; Bergström, C.A.; Hubert, M.; Prestidge, C.A. A Comparison of Chitosan, Mesoporous Silica and Poly(lactic-co-glycolic) Acid Nanocarriers for Optimising Intestinal Uptake of Oral Protein Therapeutics. J. Pharm. Sci. 2020, 110, 217–227. [Google Scholar] [CrossRef]
- Yang, M.; Lai, S.K.; Wang, Y.-Y.; Zhong, W.; Happe, C.; Zhang, M.; Fu, J.; Hanes, J. Biodegradable Nanoparticles Composed Entirely of Safe Materials that Rapidly Penetrate Human Mucus. Angew. Chem. Int. Ed. 2011, 50, 2597–2600. [Google Scholar] [CrossRef]
- Fleer, G.J. Polymer Adsorption and It’s Effect on Colloidal Stability: A Theoretical and Experimental Study on the Polyvinyl Alcohol-Silver iodide System; Landbouwuniversiteit Wageningen: Wageningen, The Netherlands, 1971. [Google Scholar]
- Barnes, T.J.; Prestidge, C.A. PEO–PPO–PEO Block Copolymers at the Emulsion Droplet-Water Interface. Langmuir 2000, 16, 4116–4121. [Google Scholar] [CrossRef]
- Ahmed, F.; Alexandridis, P.; Neelamegham, S. Synthesis and Application of Fluorescein-Labeled Pluronic Block Copolymers to the Study of Polymer−Surface Interactions. Langmuir 2000, 17, 537–546. [Google Scholar] [CrossRef]
- Boegh, M.; Baldursdóttir, S.G.; Müllertz, A.; Nielsen, H.M. Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur. J. Pharm. Biopharm. 2014, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Venugopal, V.; Tsianou, M.; Alexandridis, P. Adsorption of Pluronic block copolymers on silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 155–164. [Google Scholar] [CrossRef]
- Lin, Y.; Alexandridis, P. Temperature-Dependent Adsorption of Pluronic F127 Block Copolymers onto Carbon Black Particles Dispersed in Aqueous Media. J. Phys. Chem. B 2002, 106, 10834–10844. [Google Scholar] [CrossRef]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a Barrier to Drug Delivery—Understanding and Mimicking the Barrier Properties. Basic Clin. Pharmacol. Toxicol. 2014, 116, 179–186. [Google Scholar] [CrossRef]
- Kočevar-Nared, J.; Kristl, J.; Šmid-Korbar, J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials 1997, 18, 677–681. [Google Scholar] [CrossRef]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.-Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Inchaurraga, L.; Martín-Arbella, N.; Zabaleta, V.; Quincoces, G.; Peñuelas, I.; Irache, J.M. In vivo study of the mucus-permeating properties of PEG-coated nanoparticles following oral administration. Eur. J. Pharm. Biopharm. 2015, 97, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Iwasaki, A.; Fujita, T. Effect of surface charge, particle size, and modification by polyethylene glycol of liposomes on their association with Caco-2 cells across an unstirred water layer. Die Pharm. Int. J. Pharm. Sci. 2018, 73, 3–8. [Google Scholar] [CrossRef]
- Krupa, L.; Bajka, B.; Staroń, R.; Dupont, D.; Singh, H.; Gutkowski, K.; Macierzanka, A. Comparing the permeability of human and porcine small intestinal mucus for particle transport studies. Sci. Rep. 2020, 10, 20290. [Google Scholar] [CrossRef]
- Bajka, B.H.; Rigby, N.M.; Cross, K.L.; Macierzanka, A.; Mackie, A.R. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surfaces B Biointerfaces 2015, 135, 73–80. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Poelma, F.G.; Junginger, H.E.; Tukker, J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 1991, 70, 235–240. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Varum, F.J.O.; Veiga, F.; Sousa, J.; Basit, A.W. Mucus thickness in the gastrointestinal tract of laboratory animals. J. Pharm. Pharmacol. 2011, 64, 218–227. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232. [Google Scholar] [CrossRef]
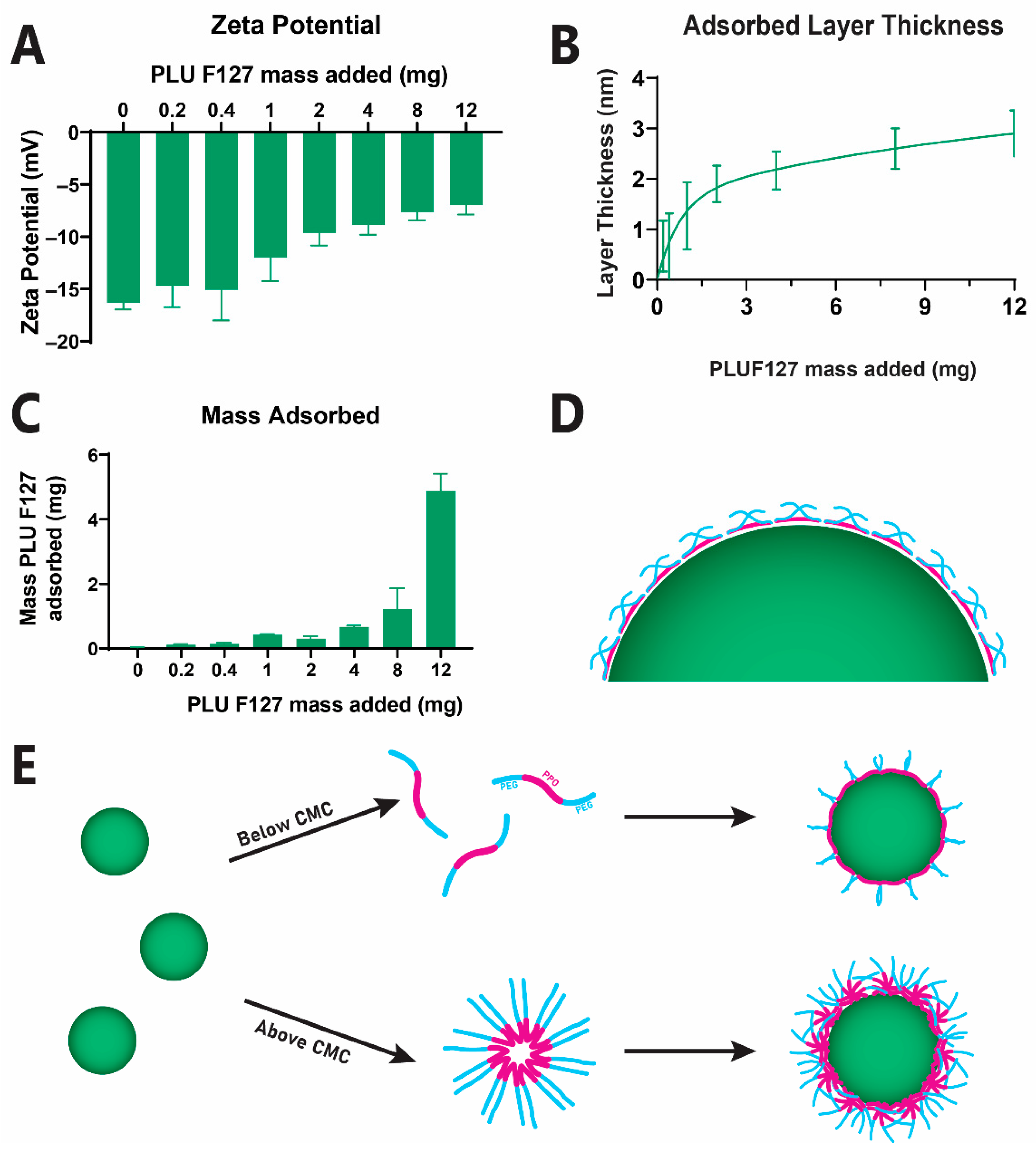
| Nanoparticle Type | Surfactant | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| PLGA-NP(−) | 1% PVA + 0.1% TPGS | 156.6 ± 2.4 | −19.0 ± 0.2 |
| PEG-PLGA-NP | 1% PVA + 0.1% TPGS Pluronic F127 | 188.8 ± 3.4 | −7.0 ± 0.9 |
| PLGA-NP(+) | 1% CTAB | 170.5 ± 5.2 | +12.1 ± 1.9 |
| Particle Size | zP (mV) | PLUF127 Coating Thickness (nm) | PLUF127 Mass Adsorbed (mg) |
|---|---|---|---|
| 188.8 ± 3.4 | −7.0 ± 0.89 | 2.9 ± 0.4 | 4.9 ± 0.5 |
| 322.7 ± 4.5 | −5.0 ± 0.08 | 4.2 ± 0.1 | 4.6 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, L.; Barnes, T.J.; Joyce, P.; Prestidge, C.A. Optimisation of a High-Throughput Model for Mucus Permeation and Nanoparticle Discrimination Using Biosimilar Mucus. Pharmaceutics 2022, 14, 2659. https://doi.org/10.3390/pharmaceutics14122659
Wright L, Barnes TJ, Joyce P, Prestidge CA. Optimisation of a High-Throughput Model for Mucus Permeation and Nanoparticle Discrimination Using Biosimilar Mucus. Pharmaceutics. 2022; 14(12):2659. https://doi.org/10.3390/pharmaceutics14122659
Chicago/Turabian StyleWright, Leah, Timothy J. Barnes, Paul Joyce, and Clive A. Prestidge. 2022. "Optimisation of a High-Throughput Model for Mucus Permeation and Nanoparticle Discrimination Using Biosimilar Mucus" Pharmaceutics 14, no. 12: 2659. https://doi.org/10.3390/pharmaceutics14122659
APA StyleWright, L., Barnes, T. J., Joyce, P., & Prestidge, C. A. (2022). Optimisation of a High-Throughput Model for Mucus Permeation and Nanoparticle Discrimination Using Biosimilar Mucus. Pharmaceutics, 14(12), 2659. https://doi.org/10.3390/pharmaceutics14122659