Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Experimental Design
2.2.2. Preparation of FLB-SPLs
2.2.3. Characterization FLB-SPLs
2.2.4. Optimization of FLB-SPLs
Characterization of the Optimized FLB-SPLs
2.2.5. In Vivo Pharmacokinetic Study
Animals
Study Design
FLB Assay
Analysis of Pharmacokinetic Parameters
3. Results and Discussion
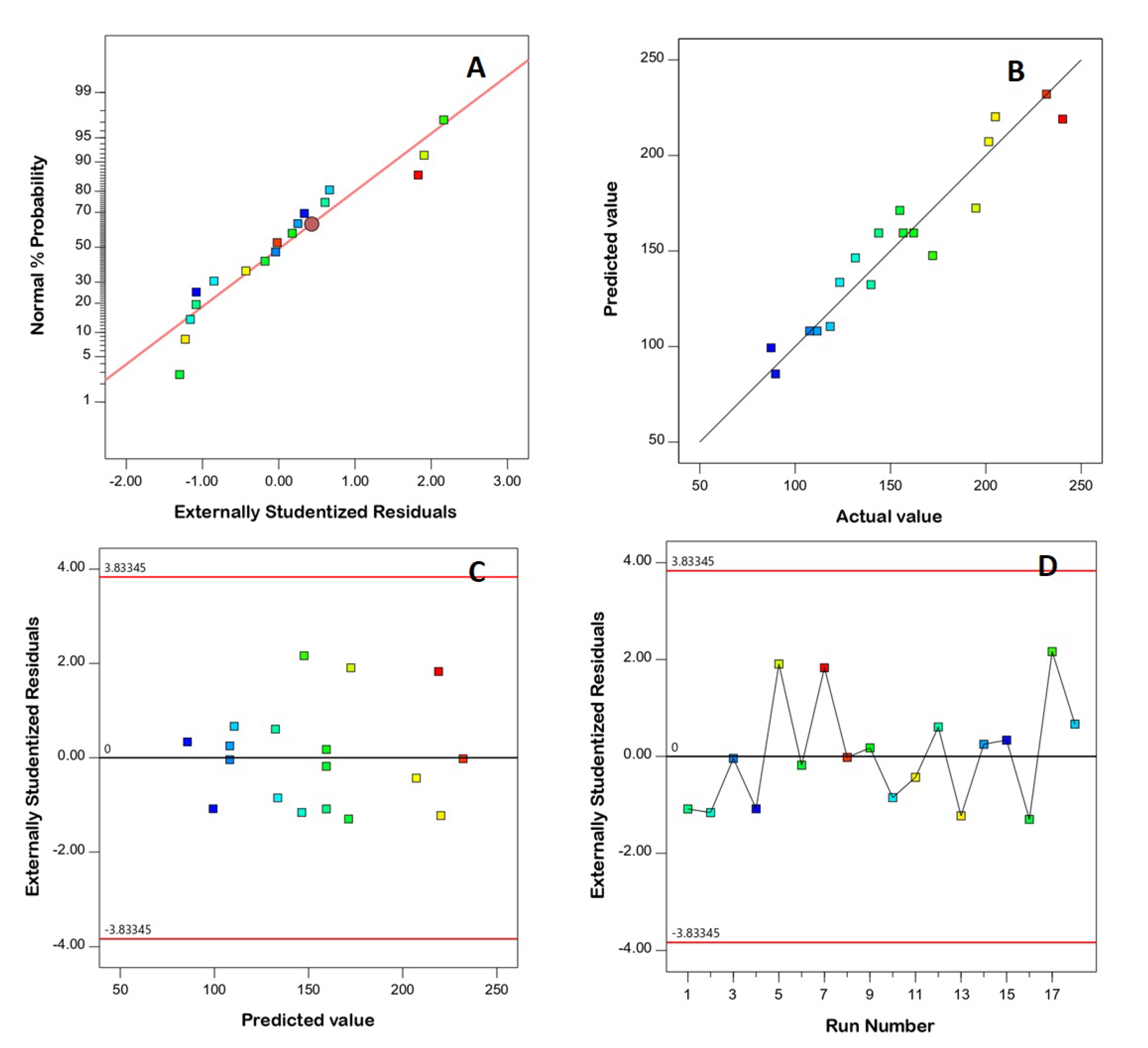
3.1. Model Fit Statistics
3.2. Diagnostic Analysis
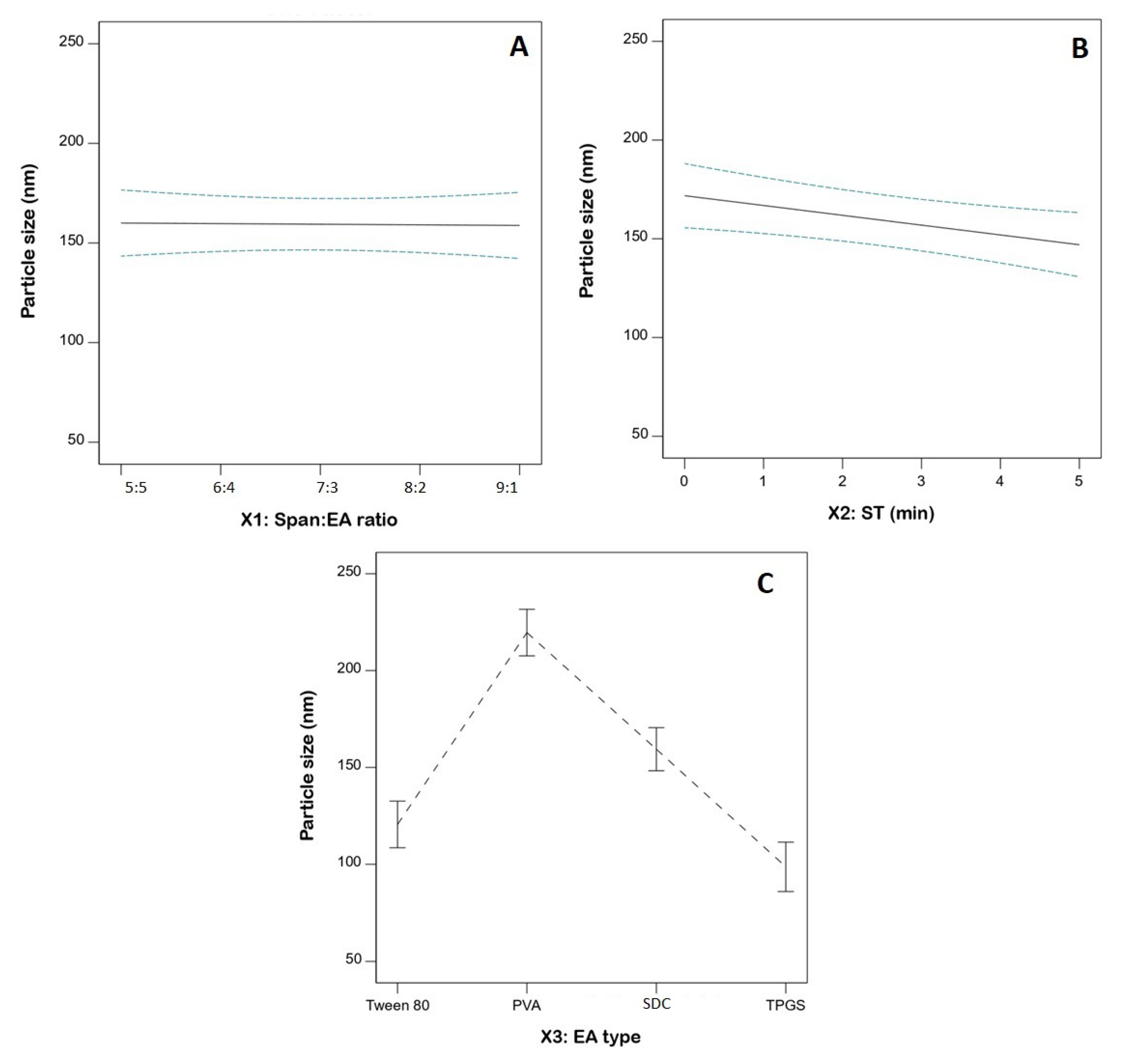
3.3. Statistical Analysis for the Influence of Variables on PS (Y1)
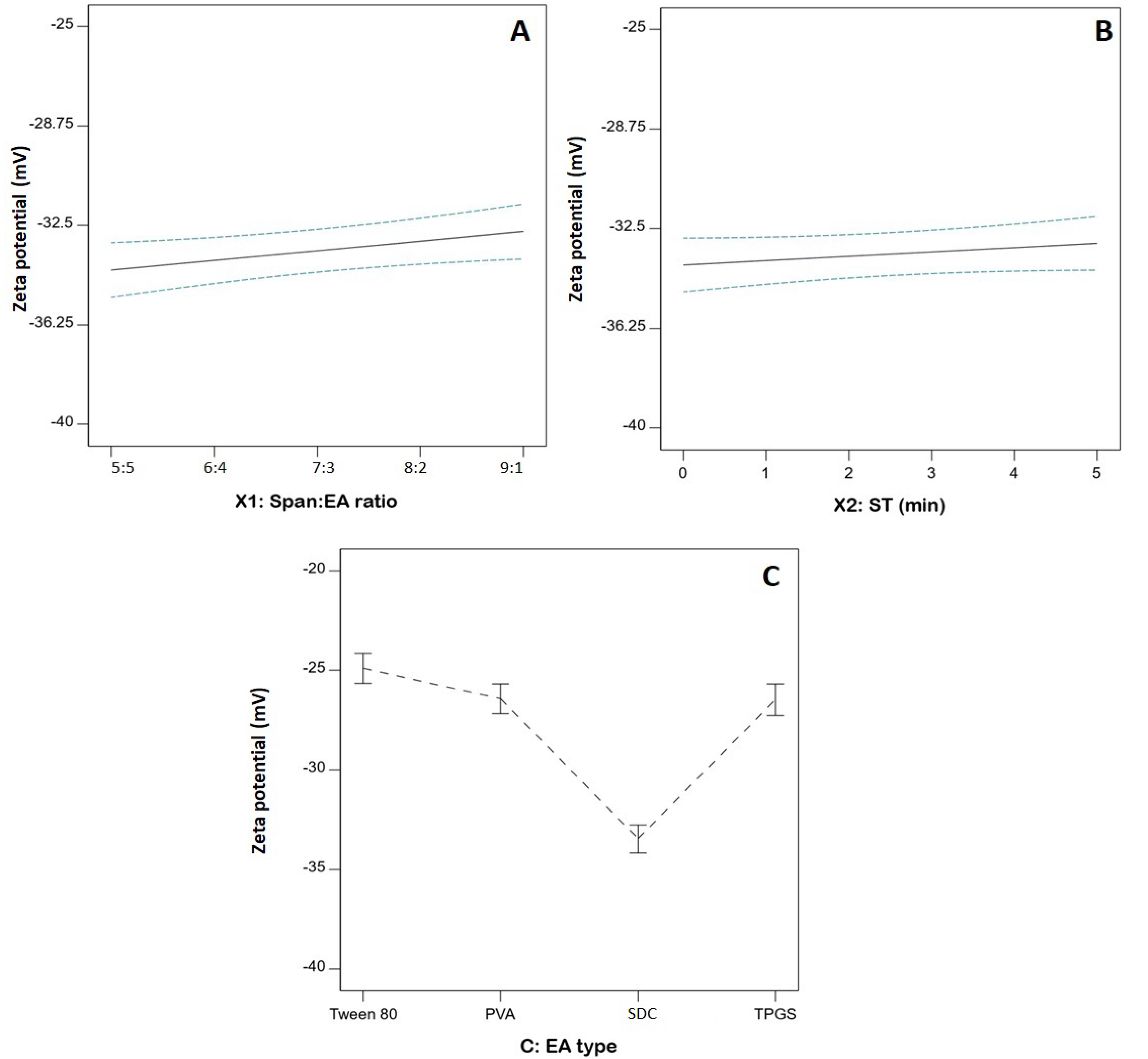
3.4. Statistical Analysis of the Influence of Variables on ZP (Y2)
3.5. Optimization of FLB-SPLs
3.6. Characterization of Optimized FLB-SPLs
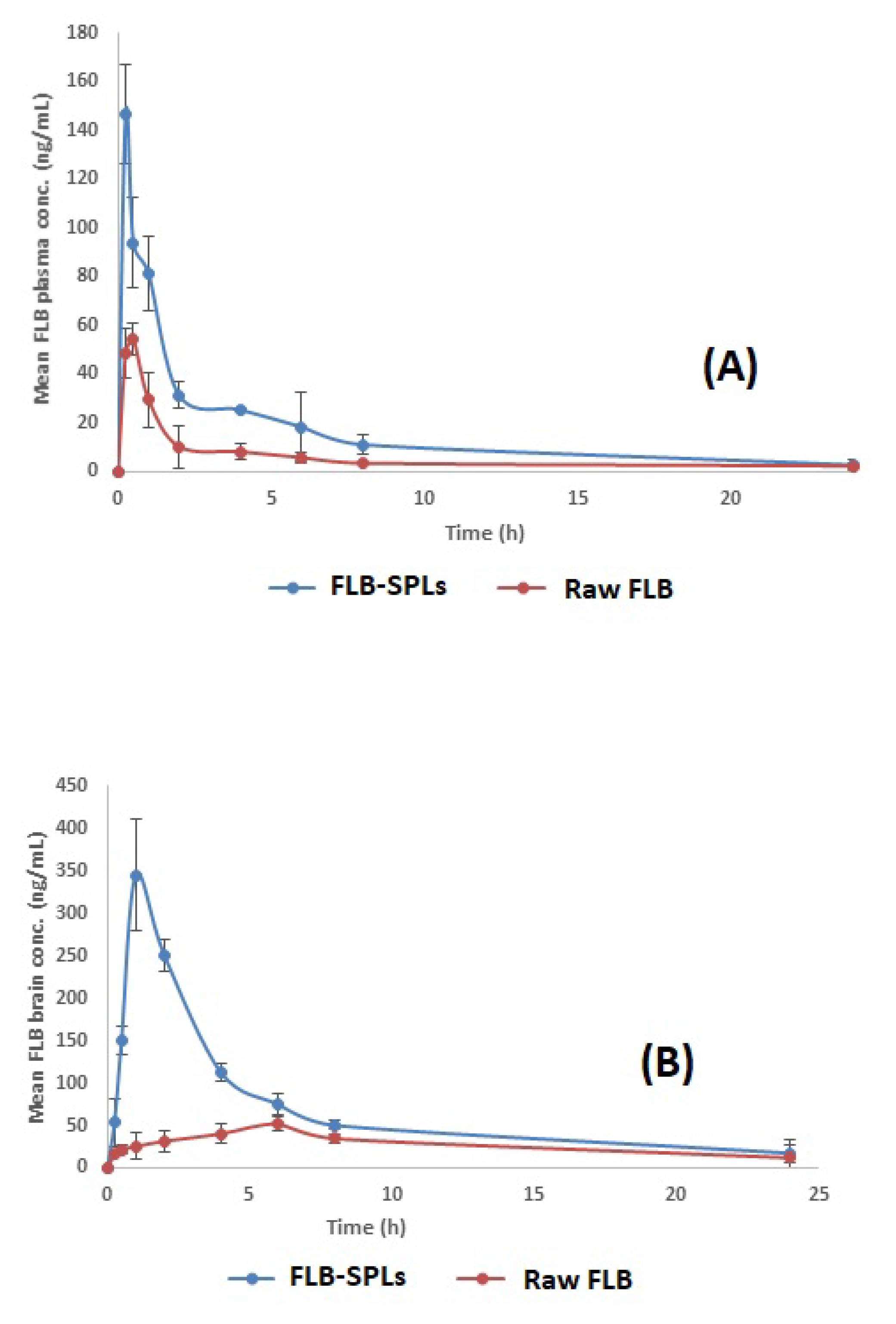
3.7. In Vivo Pharmacokinetic Assessment of Optimized FLB-SPLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelman, F.; Atrio, J. Flibanserin for Hypoactive Sexual Desire Disorder: Place in Therapy. Ther. Adv. Chronic Dis. 2017, 8, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.V.; Chang, C.; Sewell, C.; Easley, O.; Nguyen, C.; Dunn, S.; Lehrfeld, K.; Lee, L.; Kim, M.-J.; Slagle, A.F.; et al. FDA Approval of Flibanserin—Treating Hypoactive Sexual Desire Disorder. N. Engl. J. Med. 2016, 374, 101–104. [Google Scholar] [CrossRef]
- Allers, K.A.; Dremencov, E.; Ceci, A.; Flik, G.; Ferger, B.; Cremers, T.I.F.H.; Ittrich, C.; Sommer, B. Acute and Re-peated Flibanserin Administration in Female Rats Modulates Monoamines Differentially across Brain Areas: A Microdialysis Study. J. Sex. Med. 2010, 7, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.J.; Makhlouf, A.I.A. Scalable Flibanserin Nanocrystal-Based Novel Sublingual Platform for Female Hypoactive Sexual Desire Disorder: Engineering, Optimization Adopting the Desirability Function Approach and in Vivo Pharmacokinetic Study. Drug Deliv. 2021, 28, 1301–1311. [Google Scholar] [CrossRef]
- Dooley, E.M.; Miller, M.K.; Clayton, A.H. Flibanserin: From Bench to Bedside. Sex. Med. Rev. 2017, 5, 461–469. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H.M.; Tima, S.; Asfour, H.Z.; Al-Rabia, M.W.; Negm, A.A.; Sultan, M.H.; Madkhali, O.A.A.; et al. Intranasal Niosomal in Situ Gel as a Promising Approach for En-hancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/in Vivo Evaluation. Pharmaceutics 2020, 12, 485. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Helal, D.A.; Elkhoudary, M.M.; Abd Elhameed, A.G.; Yasser, M. Formulation and Optimization of Nanospanlastics for Improving the Bioavailability of Green Tea Epigallocatechin Gallate. Pharmaceuticals 2021, 14, 68. [Google Scholar] [CrossRef]
- Ansari, M.D.; Saifi, Z.; Pandit, J.; Khan, I.; Solanki, P.; Sultana, Y.; Aqil, M. Spanlastics a Novel Nanovesicular Carrier: Its Potential Application and Emerging Trends in Therapeutic Delivery. AAPS PharmSciTech 2022, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kaur, I.P. Spanlastics-A Novel Nanovesicular Carrier System for Ocular Delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Saleh, A.; Khalifa, M.; Shawky, S.; Bani-Ali, A.; Eassa, H. Zolmitriptan Intranasal Spanlastics for Enhanced Mi-graine Treatment; Formulation Parameters Optimized via Quality by Design Approach. Sci. Pharm. 2021, 89, 24. [Google Scholar] [CrossRef]
- Badria, F.; Mazyed, E. Formulation of Nanospanlastics as a Promising Approach for Improving the Topical De-livery of a Natural Leukotriene Inhibitor (3-Acetyl-11-Keto-β-Boswellic Acid): Statistical Optimization, in Vitro Characterization, and Ex Vivo Permeation Study. Drug Des. Dev. Ther. 2020, 14, 3697–3721. [Google Scholar] [CrossRef] [PubMed]
- Al-mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced Non Invasive Trans-Tympanic Delivery of Ciprofloxacin through Encapsulation into Nano-Spanlastic Vesicles: Fabrication, in-Vitro Characterization, and Comparative Ex-Vivo Permeation Studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E.A. Design and Optimization of Surfactant-Based Nan-ovesicles for Ocular Delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-Ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Topical Delivery of Fenoprofen Calcium via Elas-tic Nano-Vesicular Spanlastics: Optimization Using Experimental Design and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2898–2909. [Google Scholar] [CrossRef]
- Ahmad, J.; Rizwanullah, M.; Amin, S.; Warsi, M.H.; Ahmad, M.Z.; Barkat, M.A. Nanostructured Lipid Carriers (NLCs): Nose-to-Brain Delivery and Theranostic Application. Curr. Drug Metab. 2020, 21, 1136–1143. [Google Scholar] [CrossRef]
- Abhaihaidelmonem, R.; Nabarawi, M.E.; Attia, A. Development of Novel Bioadhesive Granisetron Hydrochlo-ride Spanlastic Gel and Insert for Brain Targeting and Study Their Effects on Rats. Drug Deliv. 2018, 25, 70–77. [Google Scholar] [CrossRef]
- Yassin, G.E.; Amer, R.I.; Fayez, A.M. Carbamazepine Loaded Vesicular Structures for Enhanced Brain Targeting via Intranasal Route: Optimization, in Vitro Evaluation, and in Vivo Study. Int. J. Appl. Pharm. 2019, 11, 264–274. [Google Scholar] [CrossRef]
- Darekar, T.; Aithal, K.S.; Shirodkar, R.; Kumar, L.; Attari, Z.; Lewis, S. Characterization and in Vivo Evaluation of Lacidipine Inclusion Complexes with β-Cyclodextrin and Its Derivatives. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 225–235. [Google Scholar] [CrossRef]
- Naguib, M.J.; Elsayed, I.; Teaima, M.H. Simultaneous Optimization of Oral and Transdermal Nanovesicles for Bioavailability Enhancement of Ivabradine Hydrochloride. Int. J. Nanomed. 2021, 16, 2917–2931. [Google Scholar] [CrossRef]
- Hassan, D.H.; Shohdy, J.N.; El-Setouhy, D.A.; El-Nabarawi, M.; Naguib, M.J. Compritol-Based Nanostrucutured Lipid Carriers (NLCs) for Augmentation of Zolmitriptan Bioavailability via the Transdermal Route: In Vitro Op-timization, Ex Vivo Permeation, In Vivo Pharmacokinetic Study. Pharmaceutics 2022, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.A.; Abdelmalak, N.S.; Naguib, M.J.; Rashed, H.M.; Ibrahim, A.B. Intranasal Brain-Targeted Clonazepam Polymeric Micelles for Immediate Control of Status Epilepticus: In Vitro Optimization, Ex Vivo Determination of Cytotoxicity, in Vivo Biodistribution and Pharmacodynamics Studies. Drug Deliv. 2016, 23, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Joseph Naguib, M.; Moustafa Kamel, A.; Thabet Negmeldin, A.; Elshafeey, A.H.; Elsayed, I. Molecular Docking and Statistical Optimization of Taurocholate-Stabilized Galactose Anchored Bilosomes for the Enhancement of Sofosbuvir Absorption and Hepatic Relative Targeting Efficiency. Drug Deliv. 2020, 27, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, H.M.; Badr-Eldin, S.M.; Assiri, N.Y.; Alhakamy, N.A.; Privitera, A.; Caraci, F.; Caruso, G. Sur-face-Tailoring of Emulsomes for Boosting Brain Delivery of Vinpocetine via Intranasal Route: In Vitro Optimiza-tion and in Vivo Pharmacokinetic Assessment. Drug Deliv. 2022, 29, 2671. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocar-rier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M. Biodegradable Self-Assembled Nanoparticles of PEG-PLGA Amphiphilic Di-block Copolymer as a Promising Stealth System for Augmented Vinpocetine Brain Delivery. Int. J. Pharm. 2020, 588, 119778. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; El-Say, K.M.; Aljaeid, B.M.; Badr-Eldin, S.M.; Ahmed, T.A. Optimized Vinpocetine-Loaded Vita-min E D-α-Tocopherol Polyethylene Glycol 1000 Succinate-Alpha Lipoic Acid Micelles as a Potential Transder-mal Drug Delivery System: In Vitro and Ex Vivo Studies. Int. J. Nanomed. 2018, 14, 33–43. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal Optimized Solid Lipid Nanoparticles Loaded in Situ Gel for Enhancing Trans-Mucosal Delivery of Simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ghanbarzadeh, S.; Mohammadhassani, Z.; Hamishehkar, H. Formulation of Gammaoryza-nol-Loaded Nanoparticles for Potential Application in Fortifying Food Products. Adv. Pharm. Bull. 2014, 4, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Alhakamy, N.A.; Fahmy, U.A.; Ahmed, O.A.A.; Neamatallah, T.; Tima, S.; Almaghrabi, R.H.; Alkudsi, F.M.; Alamoudi, A.A.; et al. Merging Experimental Design and Nanotechnology for the Development of Optimized Simvastatin Spanlastics: A Promising Combined Strategy for Augmenting the Suppression of Various Human Cancer Cells. Pharmaceutics 2022, 14, 1024. [Google Scholar] [CrossRef]
- El-Helw, A.R.M.; Fahmy, U.A. Improvement of Fluvastatin Bioavailability by Loading on Nanostructured Lipid Carriers. Int. J. Nanomed. 2015, 10, 5797–5804. [Google Scholar] [CrossRef]
- Xu, Q.; Crossley, A.; Czernuszka, J. Preparation and Characterization of Negatively Charged Poly(Lactic-Co-Glycolic Acid) Microspheres. J. Pharm. Sci. 2009, 98, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, S.; Feng, S.S. Vitamin E TPGS as a Molecular Biomaterial for Drug Delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Wongtrakul, P.; Manosroi, J.; Sakai, H.; Sugawara, F.; Yuasa, M.; Abe, M. Characterization of Vesi-cles Prepared with Various Non-Ionic Surfactants Mixed with Cholesterol. Colloids Surf. B Biointerfaces 2003, 30, 129–138. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z. Preparation and Performance Evaluation of Emulsomes as a Drug Delivery System for Silybin. Arch. Pharm. Res. 2015, 38, 2193–2200. [Google Scholar] [CrossRef]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Elshafeey, A.H.; Mohamed, M.I. Response Surface Optimization, Ex Vivo and In Vivo Investigation of Nasal Spanlastics for Bioavailability Enhancement and Brain Targeting of Risperidone. Int. J. Pharm. 2017, 530, 1–11. [Google Scholar] [CrossRef]
- Haroon, H.B.; Mukherjee, D.; Anbu, J.; Teja, B.V. Thiolated Chitosan-Centella Asiatica Nanocomposite: A Poten-tial Brain Targeting Strategy Through Nasal Route. AAPS PharmSciTech 2021, 22, 251. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Maeng, H.J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Tirucherai, G.S.; Yang, C.; Mitra, A.K. Prodrugs in Nasal Drug Delivery. Expert Opin. Biol. Ther. 2001, 1, 49–66. [Google Scholar] [CrossRef] [PubMed]
- McMartin, C.; Hutchinson, L.E.F.; Hyde, R.; Peters, G.E. Analysis of Structural Requirements for the Absorption of Drugs and Macromolecules from the Nasal Cavity. J. Pharm. Sci. 1987, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.-I.; Kubo, H.; Natsume, H.; Sugibayashi, K.; Morimoto, Y.; Yamashita, S. The Structural Barrier of Ab-sorptive Mucosae: Site Difference of the Permeability of Fluorescein Isothiocyanate-Labelled Dextran in Rabbits. Biopharm. Drug Dispos. 1993, 14, 685–695. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Thakur, R.; Meidan, V.M.; Michniak, B. Mucosal Drug Delivery: Membranes, Methodologies, and Applications. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 195–256. [Google Scholar] [CrossRef] [PubMed]
- Haschke, M.; Suter, K.; Hofmann, S.; Witschi, R.; Fröhlich, J.; Imanidis, G.; Drewe, J.; Briellmann, T.A.; Dussy, F.E.; Krähenbühl, S.; et al. Pharmacokinetics and Pharmacodynamics of Nasally Delivered Midazolam. Br. J. Clin. Pharmacol. 2010, 69, 607–616. [Google Scholar] [CrossRef] [PubMed]



| Independent Variables | Levels | |||
|---|---|---|---|---|
| X1: Span: edge activator ratio (w/w) | 5:5 | 7:3 | 9:1 | |
| X2: Sonication time (min) | 0.0 | 2.5 | 5.0 | |
| X3: Edge activator type | Tween 80 | PVA | SDC | TPGS |
| Responses | Desirability constraint | |||
| Y1: Particle size (nm) | Minimize | |||
| Y2: Absolute zeta potential (mV) | Maximize | |||
| Run No. | Independent Variables | Average Responses ± SD * | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| 1 | 7:3 | 2.5 | SDC | 143.8 ± 4.9 | −32.9 ± 1.1 |
| 2 | 9:1 | 5.0 | SDC | 131.6 ± 2.7 | −31.4 ± 1.7 |
| 3 | 7:3 | 5.0 | Tween 80 | 107.6 ± 2.1 | −24.9 ± 0.7 |
| 4 | 5:5 | 2.5 | TPGS | 87.4 ± 1.9 | −26.4 ± 0.9 |
| 5 | 5:5 | 0.0 | SDC | 194.8 ± 3.3 | −36.4 ± 1.2 |
| 6 | 7:3 | 2.5 | SDC | 156.7 ± 3.7 | −33.1 ± 1.4 |
| 7 | 9:1 | 2.5 | PVA | 240.3 ± 6.9 | −26.1 ± 0.9 |
| 8 | 7:3 | 0.0 | PVA | 231.8 ± 7.1 | −26.9 ± 1.1 |
| 9 | 7:3 | 2.5 | SDC | 162.1 ± 5.3 | −34.2 ± 1.6 |
| 10 | 5:5 | 0.0 | Tween 80 | 123.4 ± 4.2 | −25.7 ± 0.6 |
| 11 | 7:3 | 5.0 | PVA | 201.5 ± 5.8 | −27.1± 0.5 |
| 12 | 9:1 | 0.0 | Tween 80 | 139.8 ± 3.8 | −24.8 ± 0.5 |
| 13 | 5:5 | 2.5 | PVA | 205.0 ± 5.9 | −25.6 ± 0.9 |
| 14 | 7:3 | 5.0 | Tween 80 | 111.5 ± 2.4 | −24.2 ± 0.4 |
| 15 | 9:1 | 5.0 | TPGS | 89.7 ± 2.1 | −25.8 ± 0.6 |
| 16 | 9:1 | 0.0 | SDC | 154.9 ± 3.9 | −32.1 ± 0.9 |
| 17 | 5:5 | 5.0 | SDC | 172.1 ± 4.1 | −34.1 ± 1.3 |
| 18 | 9:1 | 0.0 | TPGS | 118.4 ± 3.5 | −26.5 ± 0.8 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 33,012.17 | 5 | 6602.43 | 26.84 | <0.0001 |
| X1: Span: EA | 3.96 | 1 | 3.96 | 0.0161 | 0.9011 |
| X2: ST (min) | 1852.57 | 1 | 1852.57 | 7.53 | 0.0178 |
| X3: EA type | 30,808.12 | 3 | 10,269.37 | 41.75 | <0.0001 |
| Residual | 2951.98 | 12 | 246.00 | ||
| Lack of fit | 2767.56 | 9 | 307.51 | 5.00 | 0.1062 |
| Pure error | 184.42 | 3 | 61.47 | ||
| Cor total | 35,964.15 | 17 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 262.59 | 5 | 52.52 | 55.17 | <0.0001 |
| X1: Span: EA | 5.61 | 1 | 5.61 | 5.89 | 0.0319 |
| X2: ST (min) | 2.00 | 1 | 2.00 | 2.10 | 0.1728 |
| X3: EA type | 250.92 | 3 | 83.64 | 87.86 | <0.0001 |
| Residual | 11.42 | 12 | 0.9520 | ||
| Lack of fit | 10.20 | 9 | 1.13 | 2.78 | 0.2168 |
| Pure error | 1.23 | 3 | 0.4083 | ||
| Cor total | 274.02 | 17 |
| Pharmacokinetic Parameter | Plasma Data | Brain Data | ||
|---|---|---|---|---|
| Raw FLB | FLB-SPL | Raw FLB | FLB-SPL | |
| Cmax (ng/mL) | 54.37 ± 6.56 | 147.08 ± 24.04 | 51.91 ± 8.31 | 345.02 ± 65.15 |
| Tmax (h) | 0.5 | 0.25 | 6 | 1 |
| AUC0–24 (ng·h/mL) | 145.04 ± 23.82 | 387.16 ± 38.90 | 671.46 ± 36.17 | 1673.2 ± 125.28 |
| AUC0–∞ (ng·h/mL) | 195.39 ± 25.64 | 413.29 ± 40.12 | 849.95 ± 40.27 | 1901.22 ± 130.16 |
| Relative bioavailability | --- | 211.52% | --- | 223.68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, W.S.; Hareeri, R.H.; Bazuhair, M.; Alfaleh, M.A.; Alhakamy, N.A.; Fahmy, U.A.; Alamoudi, A.A.; Badr-Eldin, S.M.; Ahmed, O.A.; AlGhamdi, S.A.; et al. Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment. Pharmaceutics 2022, 14, 2627. https://doi.org/10.3390/pharmaceutics14122627
Alharbi WS, Hareeri RH, Bazuhair M, Alfaleh MA, Alhakamy NA, Fahmy UA, Alamoudi AA, Badr-Eldin SM, Ahmed OA, AlGhamdi SA, et al. Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment. Pharmaceutics. 2022; 14(12):2627. https://doi.org/10.3390/pharmaceutics14122627
Chicago/Turabian StyleAlharbi, Waleed S., Rawan H. Hareeri, Mohammed Bazuhair, Mohamed A. Alfaleh, Nabil A. Alhakamy, Usama A. Fahmy, Abdullah A. Alamoudi, Shaimaa M. Badr-Eldin, Osama A. Ahmed, Shareefa A. AlGhamdi, and et al. 2022. "Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment" Pharmaceutics 14, no. 12: 2627. https://doi.org/10.3390/pharmaceutics14122627
APA StyleAlharbi, W. S., Hareeri, R. H., Bazuhair, M., Alfaleh, M. A., Alhakamy, N. A., Fahmy, U. A., Alamoudi, A. A., Badr-Eldin, S. M., Ahmed, O. A., AlGhamdi, S. A., & Naguib, M. J. (2022). Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment. Pharmaceutics, 14(12), 2627. https://doi.org/10.3390/pharmaceutics14122627








