Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nano Spray-Drying Experiments
2.3. Characterization of Spray-Dried Samples
2.4. Preparation of Oleogels
2.5. Ex Vivo Raman Microscopic Investigations
2.6. Animals
2.7. In Vivo Tests
3. Results and Discussion
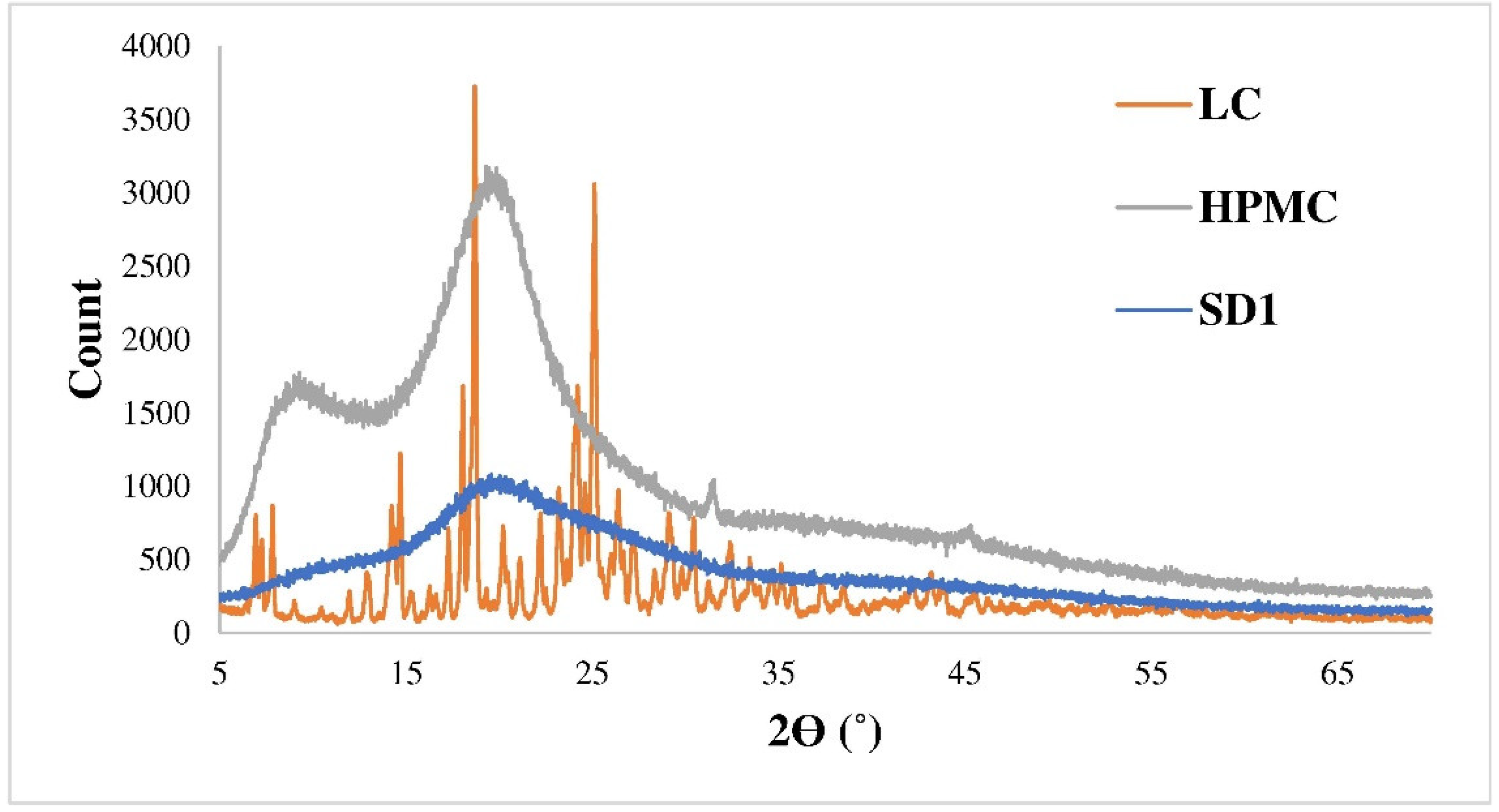
3.1. Characteristics of the Spray-Dried Samples
3.2. Size of the Oleogels
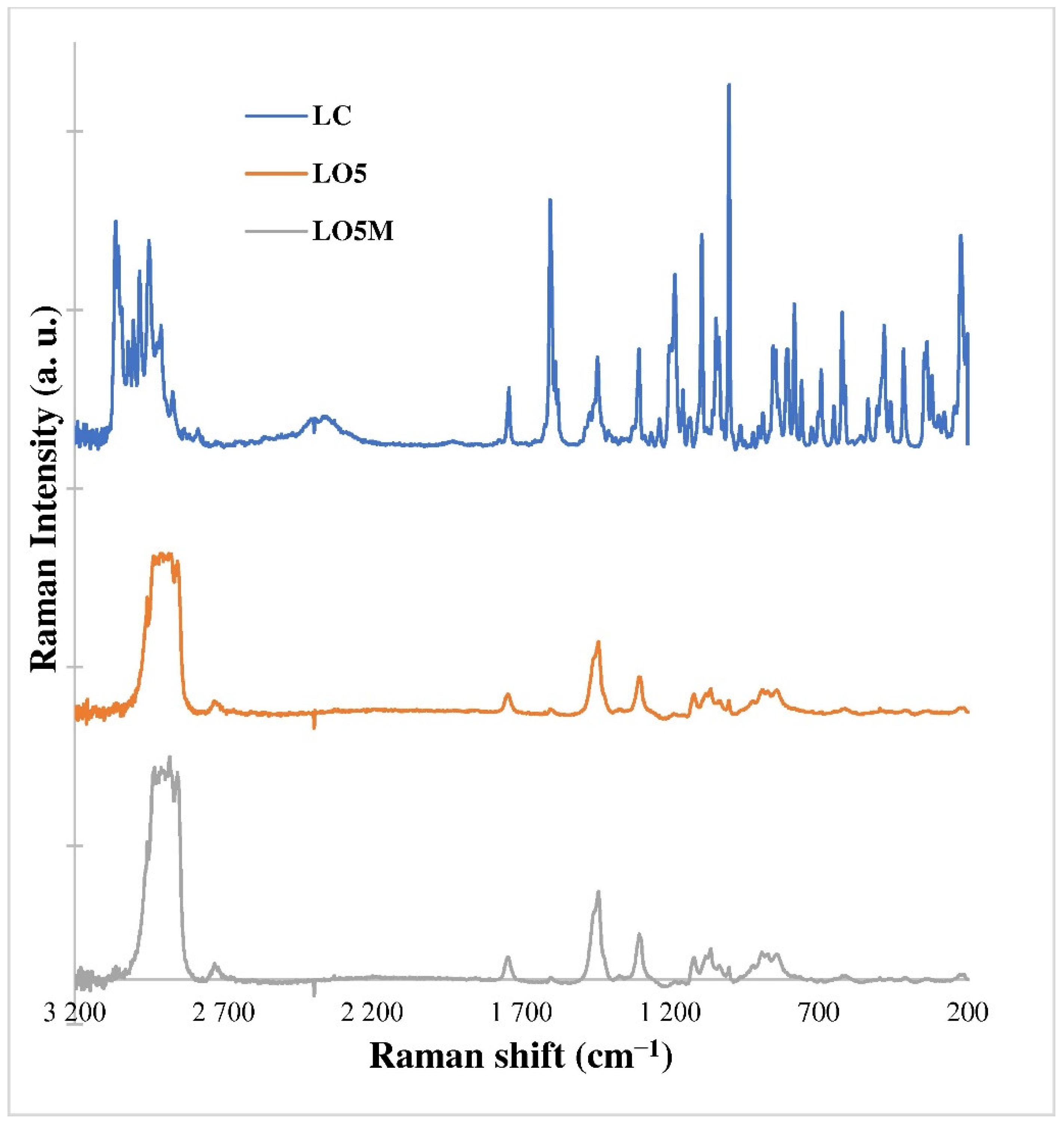
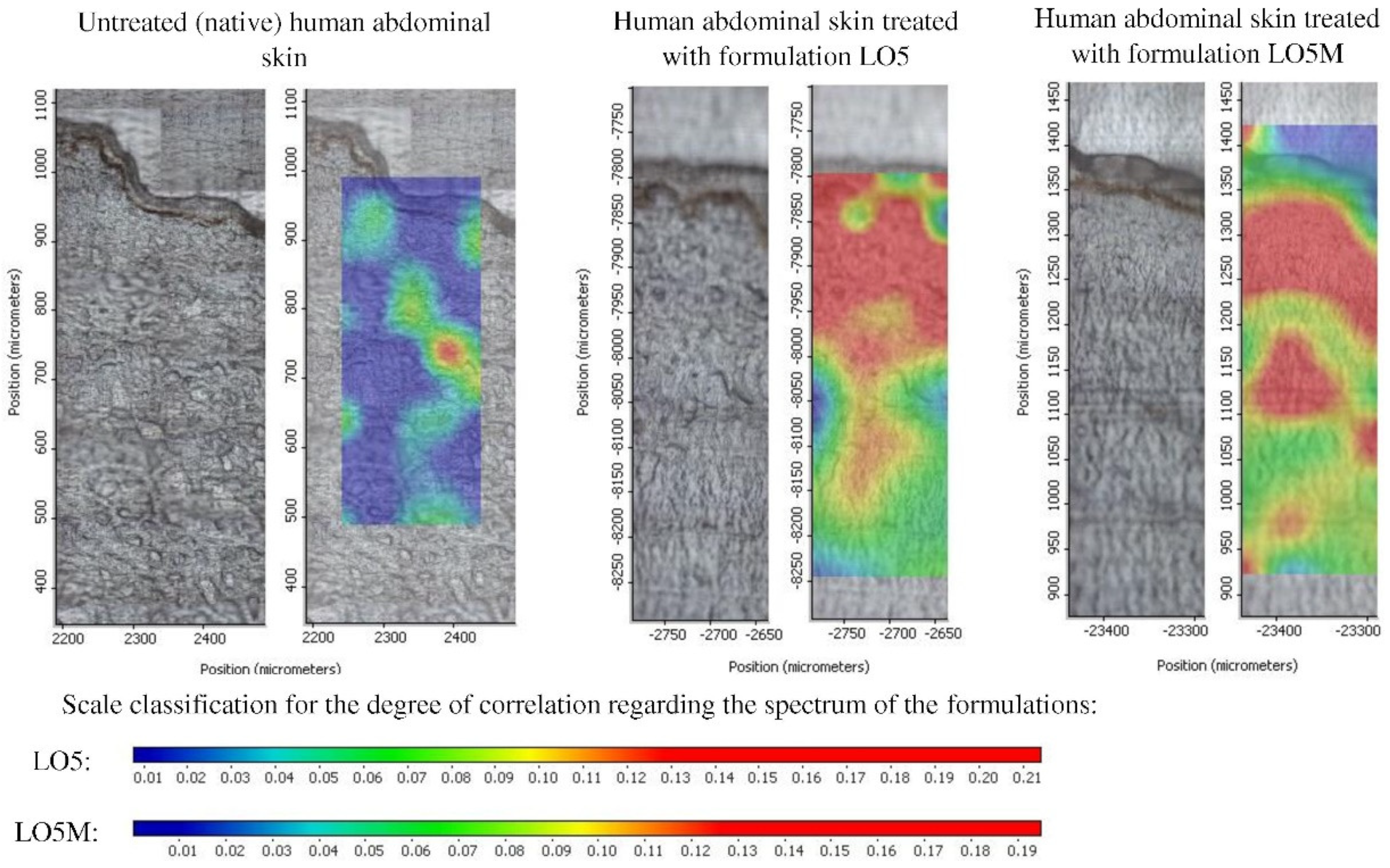
3.3. Raman Microscopic Investigations
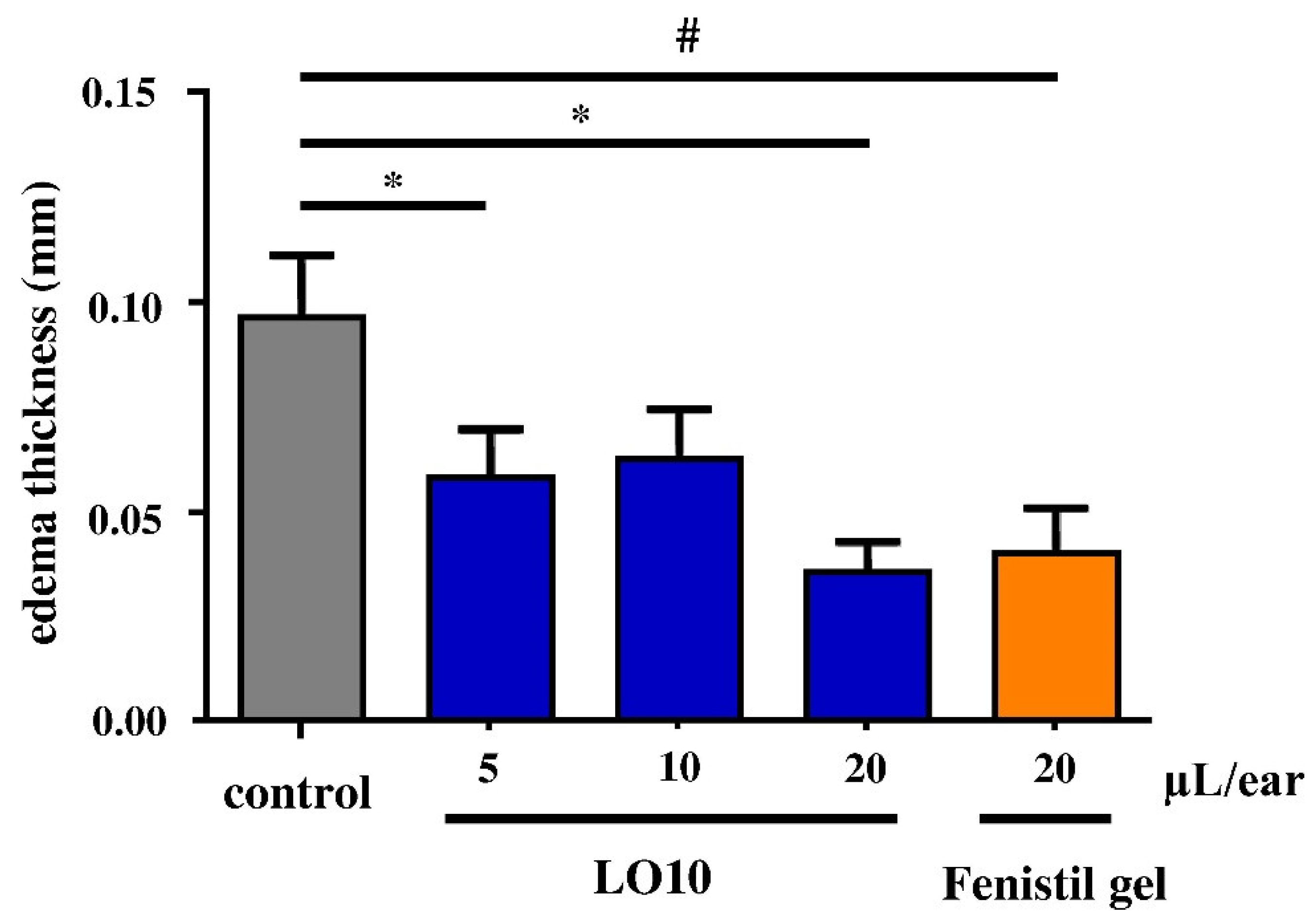
3.4. In Vivo Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanani, A.; Schellenberg, R.; Warrington, R. Urticaria and angioedema. AACI 2011, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Criado, P.R.; Criado, R.F.J.; Valente, N.Y.S.; Queiroz, L.B.; Martins, J.E.C.; Vasconcellos, C. The inflammatory response in drug-induced acute urticaria: Ultrastructural study of the dermal microvascular unit. JEADV 2005, 20, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.W.; Zechnich, A.D.; Haxby, D.G. Second-Generation Antihistamines. Drugs 1999, 57, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Physicochemical, Pharmacological and Pharmacokinetic Properties of the Zwitterionic Antihistamines Cetirizine and Levocetirizine. Curr. Med. Chem. 2008, 15, 2173–2191. [Google Scholar] [CrossRef] [PubMed]
- Hair, P.I.; Scott, L.J. A Review of its Use in the Management of Allergic Rhinitis and Skin Allergies. Drugs 2006, 66, 973–996. [Google Scholar] [CrossRef]
- Kazsoki, A.; Palcsó, B.; Omer, S.M.; Kovacs, Z.; Zelkó, R. Formulation of Levocetirizine-Loaded Core–Shell Type Nanofibrous Orally Dissolving Webs as a Potential Alternative for Immediate Release Dosage Forms. Pharmaceutics 2022, 14, 1442. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Randall, K.L.; Hawkins, C.A. Antihistamines and allergy. Aust. Prescr. 2018, 41, 42–45. [Google Scholar] [CrossRef]
- Patil, P.B.; Datir, S.K.; Saudagar, R.B. A Review on Topical Gels as Drug Delivery System. JDDT 2019, 9, 989–994. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Sughir, A.A.; Damodar, G. Oleogel: A promising base for transdermal formulations. Asian J. Pharm. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Valoppi, F.; Pal, K. Oleogels and Organogels: A Promising Tool for New Functionalities. Gels 2022, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Walch, H. Topical Application of Cetirizine and Loratadine. U.S. Patent 6,790,847, 14 September 2004. [Google Scholar]
- Ciurlizza, C.; Fernández, F.; Calpena, A.C.; Lázaro, R.; Parra, A.; Clares, B. Semisolid formulations containing cetirizine: Human skin permeation and topical antihistaminic evaluation in a rabbit model. Arch. Dermatol. Res. 2014, 306, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Rajkapoor, B.; Vijayaraghavan, C. Design and physico-chemical evaluation of cetirizine dihydrochloride transderml patches. Int. J. Res. Pharm. Sci. 2011, 2, 518–520. [Google Scholar]
- Majumder, J.; Deb, J.; Husain, A.; Jana, S.S.; Dastidar, P. Cetirizine derived supramolecular topical gel in action: Rational design, characterization and in vivo self-delivery application in treating skin allergy in mice. J. Mater. Chem. B 2015, 3, 6634–6644. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Cun, D.; Tong, H.H.Y.; Zheng, Y. Application of Nano- and Micro-Particles on the Topical Therapy of Skin-Related Immune Disorders. Curr. Pharm. Des. 2015, 21, 2643–2667. [Google Scholar] [CrossRef]
- Kircik, L.H. Microsphere Technology: Hype or Help? J. Clin. Aesthet. Dermatol. 2011, 4, 27–31. [Google Scholar]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef]
- Jain, M.S.; Lohare, G.B.; Bari, M.; Chavan, R.; Barhate, S.; Shah, C.B. Spray Drying in Pharmaceutical Industry: A Review. Res. J. Pharm. Dos. Technol. 2011, 4, 74–79. [Google Scholar]
- Amelian, A.; Szekalska, M.; Ciosek, P.; Basa, A.; Winnicka, K. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying. Acta Pharm. 2017, 67, 113–124. [Google Scholar] [CrossRef]
- ZYRTEC® Liquid Gels. Available online: https://www.zyrtecprofessional.com/products/zyrtec-liquid-gels (accessed on 20 November 2022).
- Cal, K. Skin Disposition of Menthol After its Application in the Presence of Drug Substances. Biopharm. Drug Dispos. 2008, 29, 449–454. [Google Scholar] [CrossRef]
- Rowe, R.C. Handbook of Pharmaceutical Excipients, 6th ed.; Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK; American Pharmacists Association: Washington, DC, USA, 2009; p. 433. [Google Scholar]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 346–401. [Google Scholar]
- Malvern Instruments Ltd. Mastersizer 2000 User Manual issue 2.0 (English); Malvern Instruments Ltd.: Malvern, UK, 1999. [Google Scholar]
- Caspers, P.J.; Nico, C.; Schut, T.C.B.; de Sterke, J.; Pudney, P.D.A.; Curto, P.R.; Illand, A.; Puppels, G.J. Method to quantify the in vivo skin penetration of topically applied materials based on confocal Raman spectroscopy. TBIO 2019, 1, e201900004. [Google Scholar] [CrossRef]
- Bakonyi, M.; Gácsi, A.; Kovács, A.; Budai Szűcs, M.; Berkó, S.; CsányI, E. Following-up skin penetration of lidocaine from different vehicles by Raman spectroscopic mapping. J. Pharm. Biomed. Anal. 2018, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]

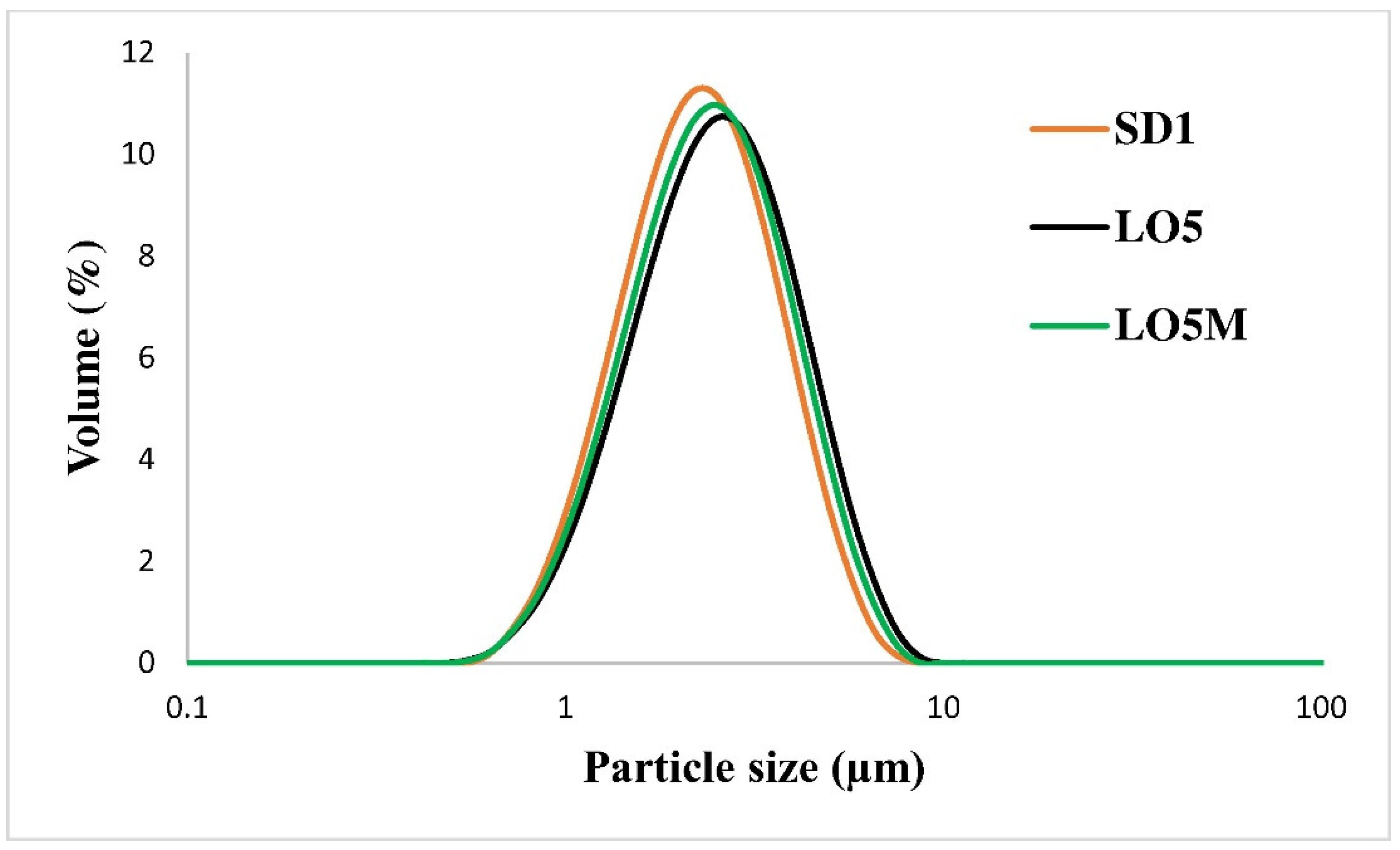

| Sample | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | D(4,3) (µm) | Span |
|---|---|---|---|---|---|
| LC (sonicated) | 1.44 | 6.07 | 19.78 | 10.68 | 3.02 |
| SD1 | 1.31 | 2.43 | 4.39 | 2.67 | 1.27 |
| SD2 | 1.31 | 2.39 | 4.14 | 2.59 | 1.18 |
| LO5 * | 1.39 | 2.71 | 4.99 | 2.99 | 1.33 |
| LO5M * | 1.35 | 2.58 | 4.72 | 2.85 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirankó, M.; Tóth, J.; Fodor-Kardos, A.; Móricz, K.; Szenes-Nagy, A.B.; Gácsi, A.; Spaits, T.; Gyenis, J.; Feczkó, T. Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema. Pharmaceutics 2022, 14, 2577. https://doi.org/10.3390/pharmaceutics14122577
Mirankó M, Tóth J, Fodor-Kardos A, Móricz K, Szenes-Nagy AB, Gácsi A, Spaits T, Gyenis J, Feczkó T. Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema. Pharmaceutics. 2022; 14(12):2577. https://doi.org/10.3390/pharmaceutics14122577
Chicago/Turabian StyleMirankó, Mirella, Judit Tóth, Andrea Fodor-Kardos, Krisztina Móricz, Antal Balázs Szenes-Nagy, Attila Gácsi, Tamás Spaits, János Gyenis, and Tivadar Feczkó. 2022. "Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema" Pharmaceutics 14, no. 12: 2577. https://doi.org/10.3390/pharmaceutics14122577
APA StyleMirankó, M., Tóth, J., Fodor-Kardos, A., Móricz, K., Szenes-Nagy, A. B., Gácsi, A., Spaits, T., Gyenis, J., & Feczkó, T. (2022). Topical Formulation of Nano Spray-Dried Levocetirizine Dihydrochloride against Allergic Edema. Pharmaceutics, 14(12), 2577. https://doi.org/10.3390/pharmaceutics14122577








