Machine-Learning Exploration of Exposure-Effect Relationships of Cisplatin in Head and Neck Cancer Patients
Abstract
1. Introduction
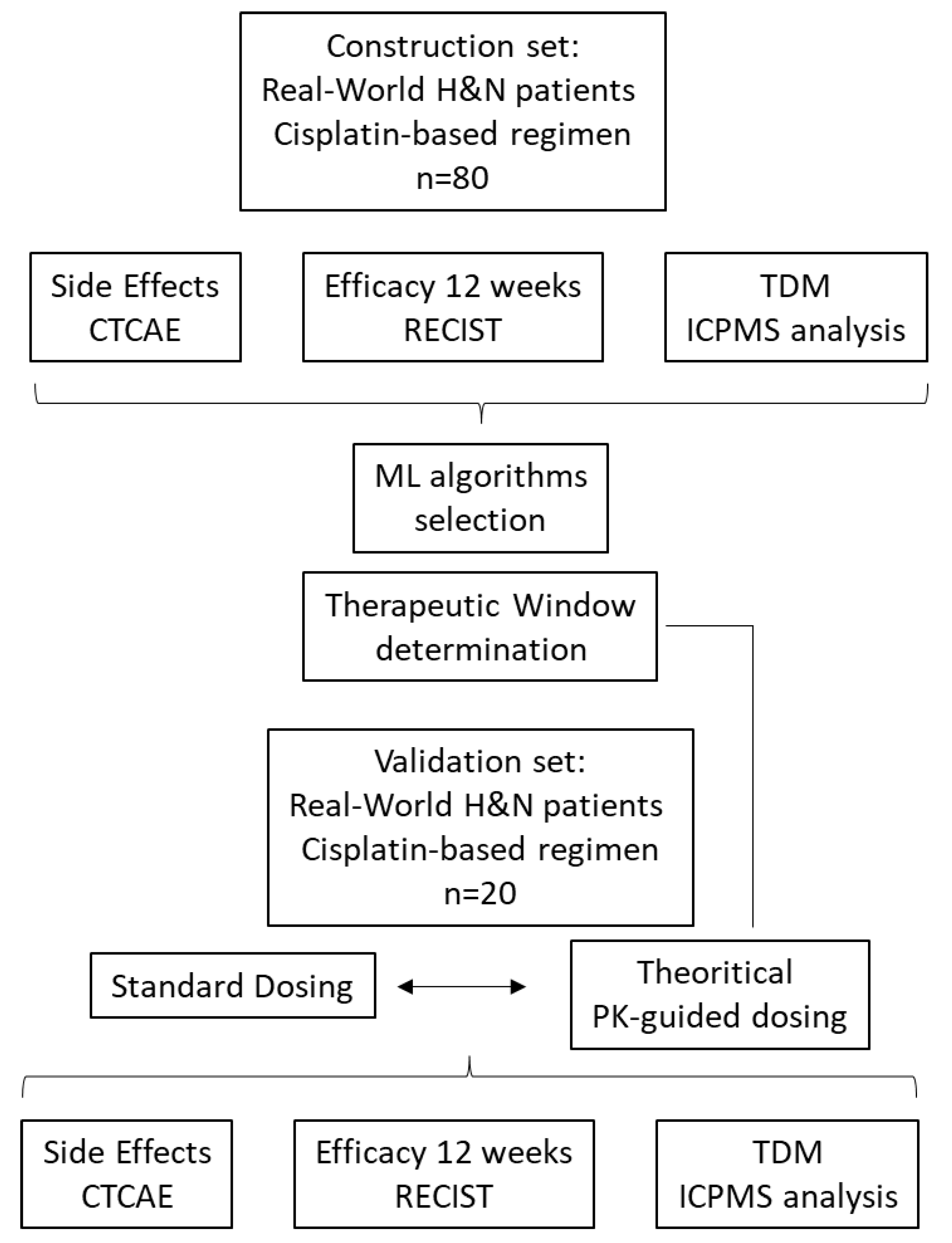
2. Materials and Methods
2.1. Patients
2.2. Data Collection
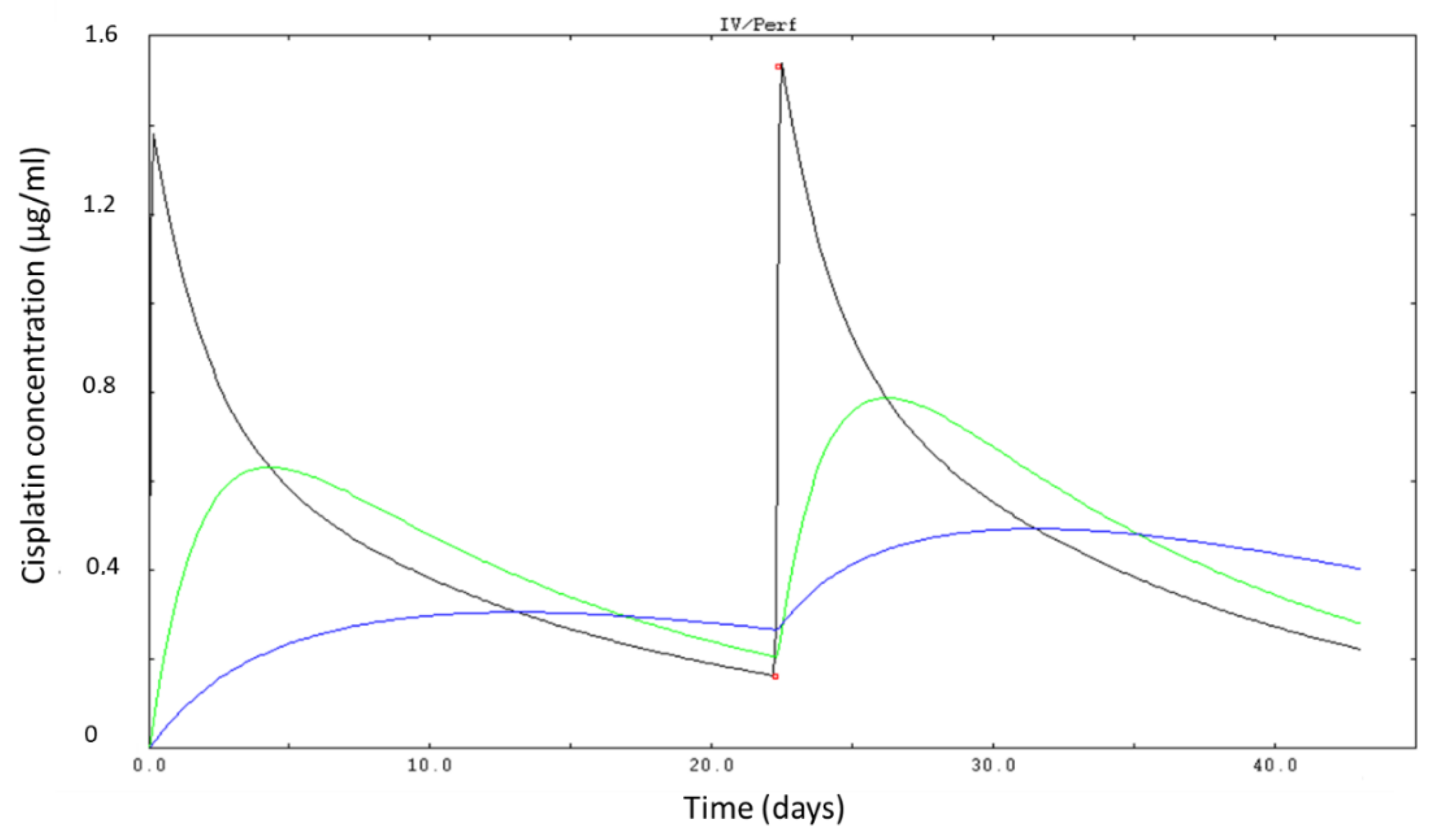
2.3. Cisplatin PK Parameters Identification
2.4. Exposure Parameters
2.5. Machine Learning
2.6. Exploration of Exposure-Effect Relationships
2.7. Validation Set for Adaptive Dosage
3. Results
3.1. Exposure and PK Parameters
3.2. Clinical Data
3.3. Exposure-Effect Relationships
3.4. Determination of the Cisplatin Therapeutic Range
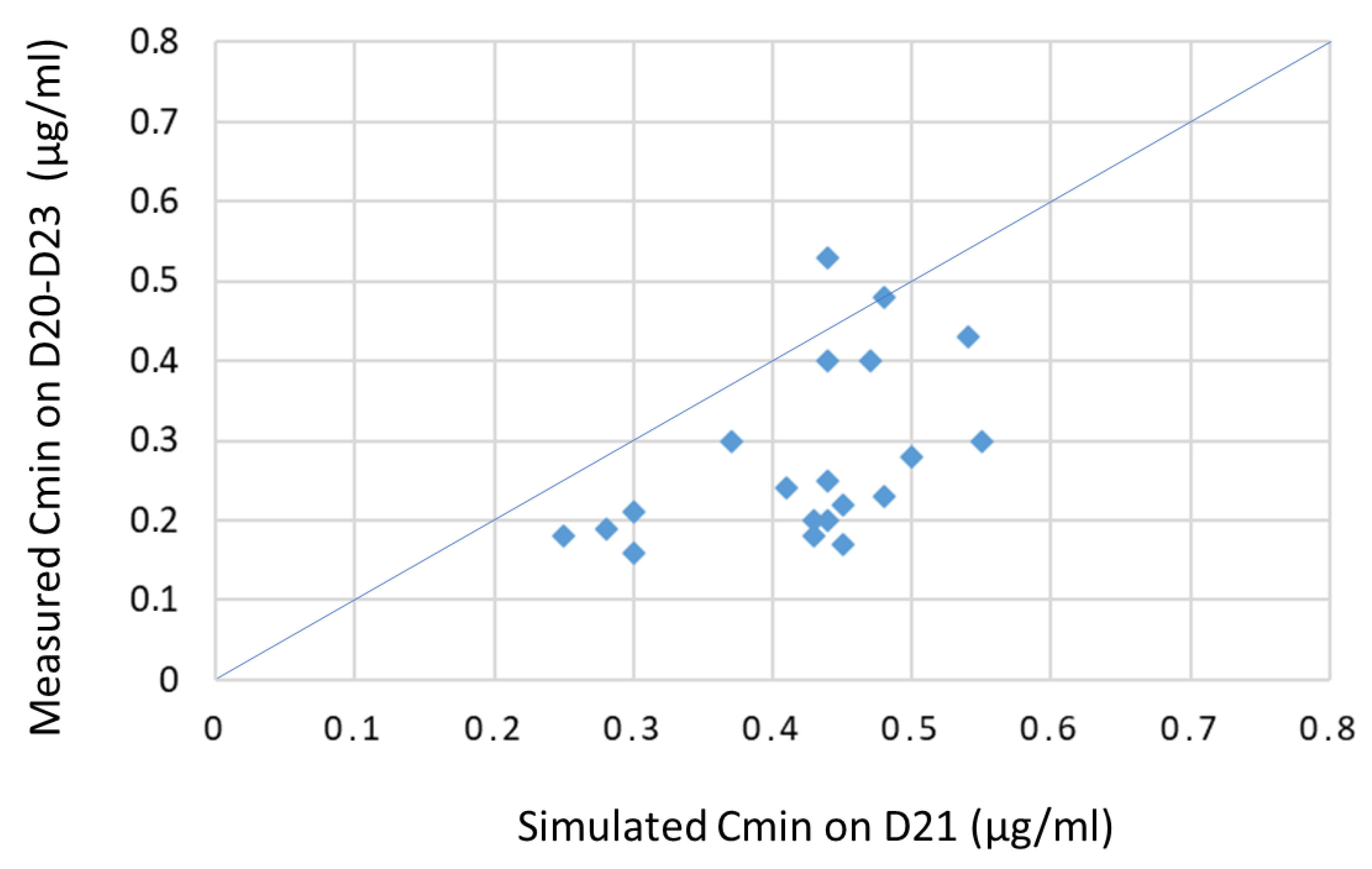
3.5. Validation Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Fernández Hidalgo, O.; González, F.; Gil, A.; Campbell, W.; Barrajón, E.; Lacave, A.J. 120 hours simultaneous infusion of Cisplatin and fluorouracil in metastatic breast cancer. Am. J. Clin. Oncol. 1989, 12, 397–401. [Google Scholar] [CrossRef]
- Boni, C.; Moretti, G.; Savoldi, L.; Armaroli, L.; Barbieri, W.; Bisagni, G.; Caroggio, A.; Iotti, C.; Pedroni, C.; Manenti, A.L.; et al. Neoadjuvant chemotherapy with continuous infusion of Cisplatin and fluorouracil in stage II–IV, M0 squamous cell carcinoma of the head and neck. Tumori 1996, 82, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Monjanel-Mouterde, S.; Ciccolini, J.; Bagarry, D.; Zonta-David, M.; Duffaud, F.; Favre, R.; Durand, A. Population pharmacokinetics of Cisplatin after 120-h infusion: Application to routine adaptive control with feedback. J. Clin. Pharm. Ther. 2003, 28, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Mercier, C.; Ciccolini, J.; Pourroy, B.; Fanciullino, R.; Tranchand, B.; Monjanel-Mouterde, S.; Baciuchka-Palmaro, M.; Dupuis, C.; Yang, C.; et al. Therapeutic drug monitoring for dose individualization of Cisplatin in testicular cancer patients based upon total platinum measurement in plasma. Ther. Drug Monit. 2006, 28, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, T.; Adelstein, D.J. In squamous cell head and neck cancer: Which platinum, how much and how often? Expert Rev. Anticancer Ther. 2014, 14, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.J.; Vokes, E.E. Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives. Oncologist 2021, 26, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Pajak, T.F.; Gwede, C.K.; Paulus, R.; Cooper, J.; Forastiere, A. TAME: Development of a new method for summarising adverse events of cancer treatment by the radiation therapy oncology group. Lancet Oncol. 2007, 8, 613–624. [Google Scholar] [CrossRef]
- Szturz, P.; Cristina, V.; Herrera Gómez, R.G.; Bourhis, J.; Simon, C.; Vermorken, J.B. Cisplatin Eligibility Issues and Alternative Regimens in Locoregionally Advanced Head and Neck Cancer: Recommendations for Clinical Practice. Front. Oncol. 2019, 9, 464. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 1 June 2022).
- Available online: https://www.legifrance.gouv.fr/jorf/article_jo/JORFARTI000037187516 (accessed on 1 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Aus-tria: R Foundation for Statistical Computing. Available online: https://www.R-project.org (accessed on 2 January 2022).
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T. KNIME—The Konstanz Infor-mation Miner: Version 2.0 and Beyond. SIGKDD Explor. Newsl. 2009, 11, 26–31. [Google Scholar] [CrossRef]
- Marin, C.; Krache, A.; Palmaro, C.; Lucas, M.; Hilaire, V.; Ugdonne, R.; De Victor, B.; Quaranta, S.; Solas, C.; Lacarelle, B.; et al. A Simple and Rapid UPLC-UV Method for Detecting DPD Deficiency in Patients With Cancer. Clin. Transl. Sci. 2020, 13, 761–768. [Google Scholar] [CrossRef]
- Manhar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.K.; Lopez-Perez, L.; Martinez, R.X.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. A Simple and Rapid UPLC-UV Method for Detecting DPD Deficiency in Patients With Cancer. Global burden of ototoxic hearing loss associated with platinum-based cancer treatment: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 79, 102203. [Google Scholar] [CrossRef]
- Aguiar Zdovc, J.; Vaupotič, M.; Marolt, G.; Knez, L.; Režonja Kukec, R.; Čufer, T.; Vovk, T.; Grabnar, I. Population pharmacokinetics of cisplatin in small cell lung cancer patients guided with informative priors. Cancer Chemother. Pharmacol. 2022, 90, 301–313. [Google Scholar] [CrossRef]
- Morita-Ogawa, T.; Sugita, H.; Minami, H.; Yamaguchi, T.; Hanada, K. Population pharmacokinetics and renal toxicity of cisplatin in cancer patients with renal dysfunction. Cancer Chemother. Pharmacol. 2020, 86, 559–566. [Google Scholar] [CrossRef]


| Characteristics | Mean ± SD |
|---|---|
| Number of patients | 80 |
| Gender (Female/Male) | 23/57 |
| Age (years) | 58 ± 11 |
| Body weight (kg) | 65.5 ± 13 |
| BSA (m2) | 1.76 ± 0.19 |
| Dose (mg) | 164 ± 27 |
| Cl (L/h) | 4.2 ± 1.3 |
| AUC (µg/mL/h) | 42.0 ± 14.4 |
| Co (µg/mL) | 0.21 ± 0.17 |
| Cmax (µg/mL) | 3.3 ± 1.0 |
| Cpredicted (µg/mL) | 0.41 ± 0.15 |
| Creatinine (µMol/L) | 72.3 ± 18.7 |
| Model | Accuracy | Recall | Precision |
|---|---|---|---|
| Generalized Linear Model | 0.71 | 0.55 | 0.75 |
| Naive Bayes | 0.67 | 0.27 | 1 |
| Random Forest | 0.67 | 0.27 | 1 |
| Neural Network | 0.63 | 0.55 | 0.63 |
| Decision Tree | 0.63 | 0.55 | 0.6 |
| Gradient Boosted Trees | 0.58 | 0.36 | 0.57 |
| Logistic Regression | 0.58 | 0.36 | 0.57 |
| XGBoost Trees | 0.5 | 0.18 | 0.4 |
| Within Therapeutic Range (n = 44) | Outside Therapeutic Range (n = 36) | ||
|---|---|---|---|
| Nephrotoxicity | yes | 11 (25%) | 6 (17%) |
| no | 33 (75%) | 30 (83%) | |
| Ototoxicity | yes | 4 (9%) | 8 (22%) |
| no | 40 (91%) | 28 (78%) | |
| Efficacy | yes | 39 (89%) | 26 (72%) |
| no | 5 (11%) | 10 (28%) | |
| Clinical Benefit | yes | 28 (64%) | 15 (42%) |
| no | 16 (36%) | 21 (58%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauvin, C.; Bourguignon, L.; Carriat, L.; Mence, A.; Ghipponi, P.; Salas, S.; Ciccolini, J. Machine-Learning Exploration of Exposure-Effect Relationships of Cisplatin in Head and Neck Cancer Patients. Pharmaceutics 2022, 14, 2509. https://doi.org/10.3390/pharmaceutics14112509
Cauvin C, Bourguignon L, Carriat L, Mence A, Ghipponi P, Salas S, Ciccolini J. Machine-Learning Exploration of Exposure-Effect Relationships of Cisplatin in Head and Neck Cancer Patients. Pharmaceutics. 2022; 14(11):2509. https://doi.org/10.3390/pharmaceutics14112509
Chicago/Turabian StyleCauvin, Céleste, Laurent Bourguignon, Laure Carriat, Abel Mence, Pauline Ghipponi, Sébastien Salas, and Joseph Ciccolini. 2022. "Machine-Learning Exploration of Exposure-Effect Relationships of Cisplatin in Head and Neck Cancer Patients" Pharmaceutics 14, no. 11: 2509. https://doi.org/10.3390/pharmaceutics14112509
APA StyleCauvin, C., Bourguignon, L., Carriat, L., Mence, A., Ghipponi, P., Salas, S., & Ciccolini, J. (2022). Machine-Learning Exploration of Exposure-Effect Relationships of Cisplatin in Head and Neck Cancer Patients. Pharmaceutics, 14(11), 2509. https://doi.org/10.3390/pharmaceutics14112509









