Polyurethane/Liquid Crystal Microfibers with pDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cells
2.2. Preparation of pDNA Loaded Fibers
2.3. Morphology of the pDNA Polymer Fibers
2.4. Characteristics of pDNA Release in Electrospun Fibers
2.5. Growth of Cells on pDNA-Loaded Fibers
2.6. Efficiency of Fiberboard Cells
3. Results and Comments
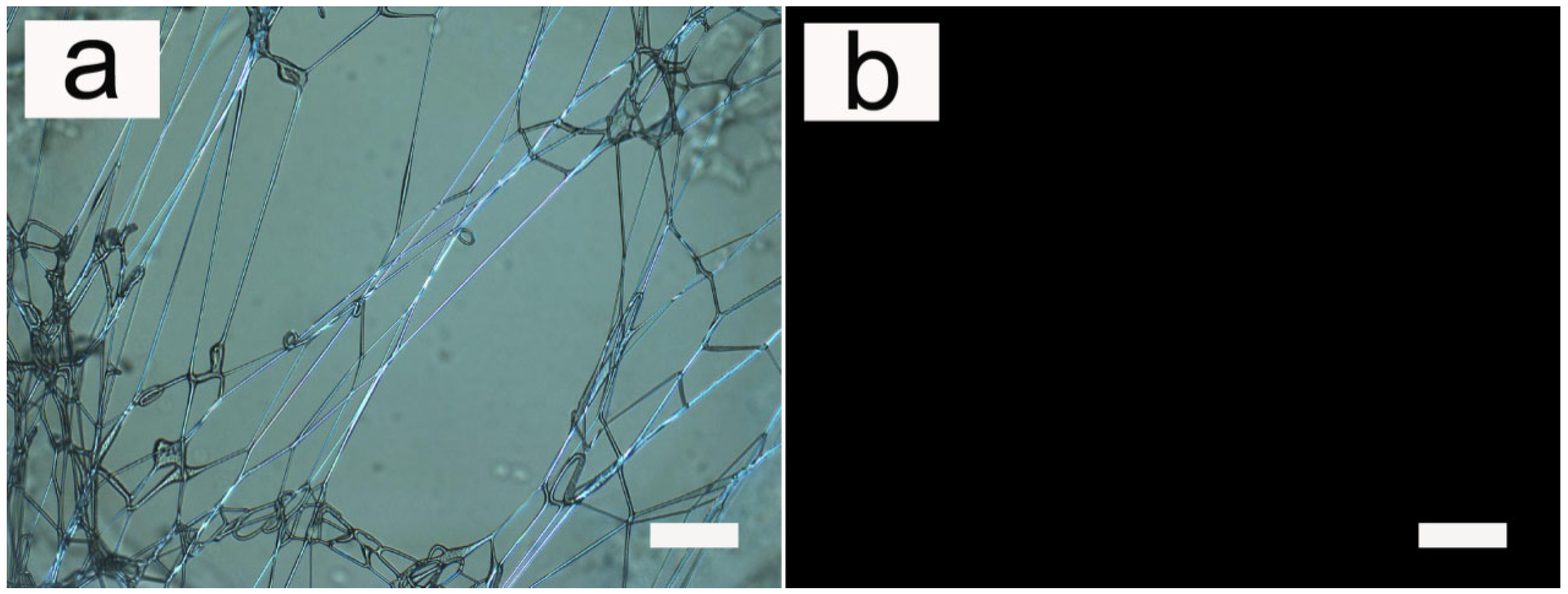
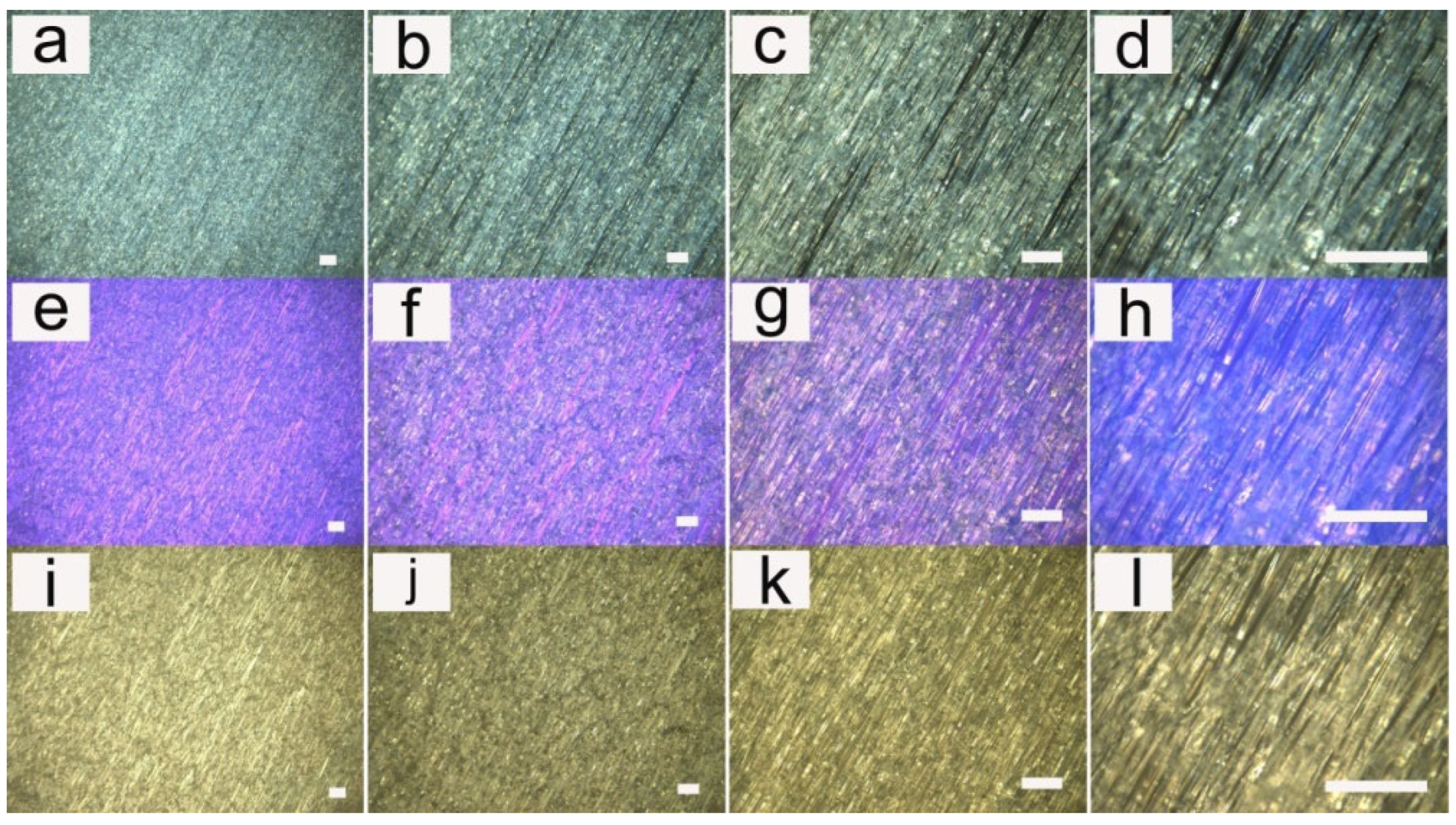
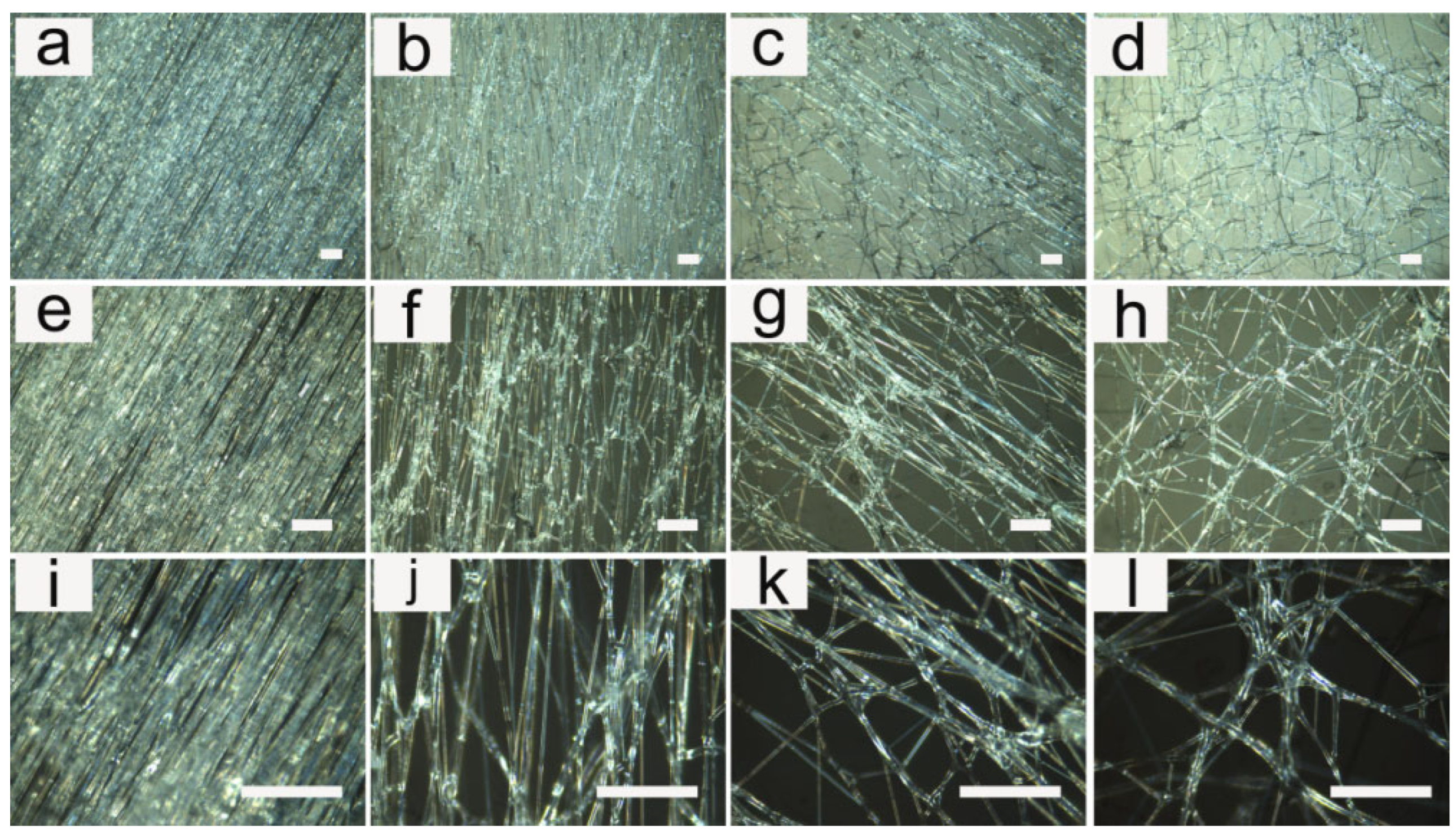
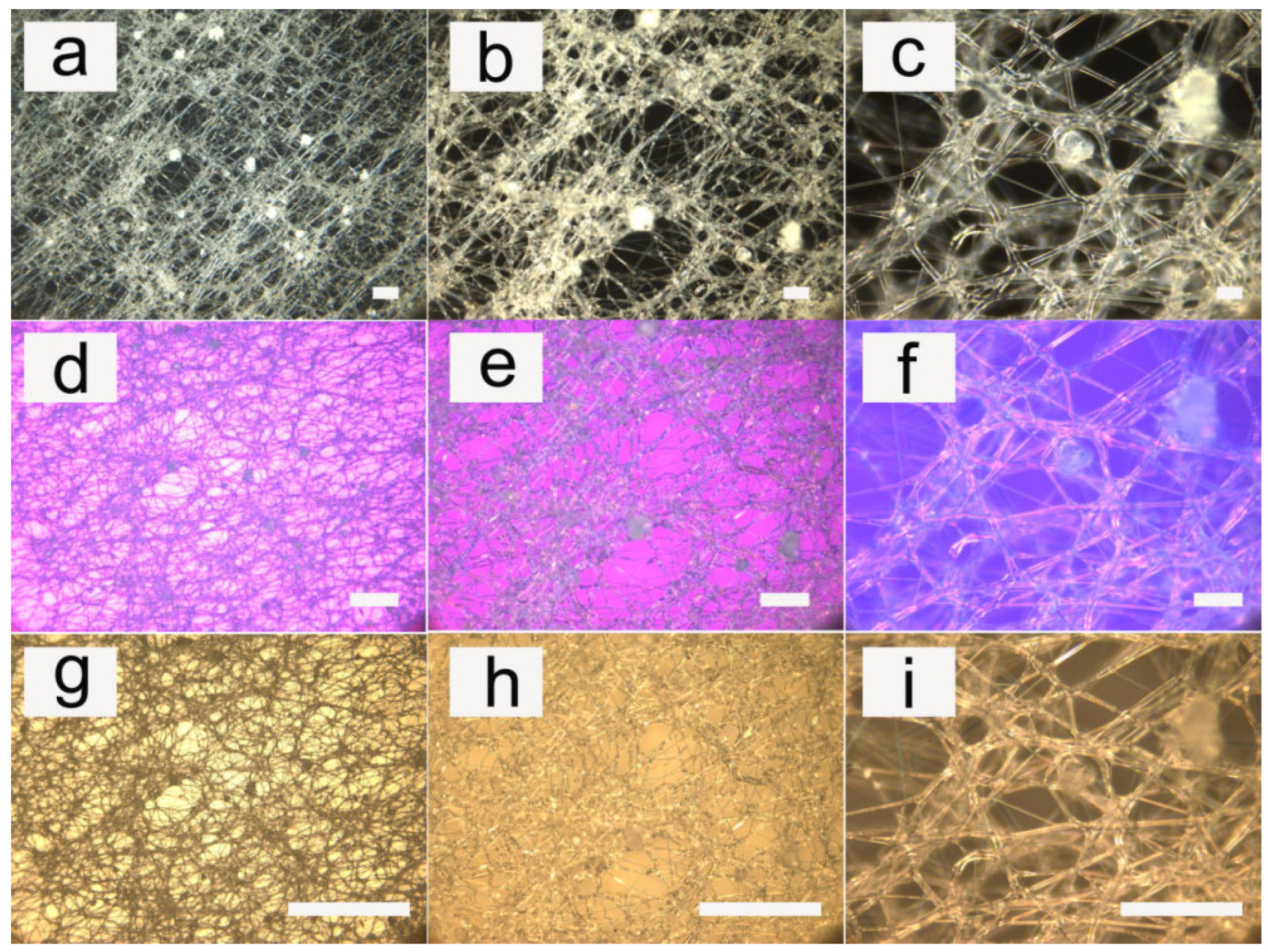
3.1. Fiber Morphology
3.2. Influence of Applied Voltage
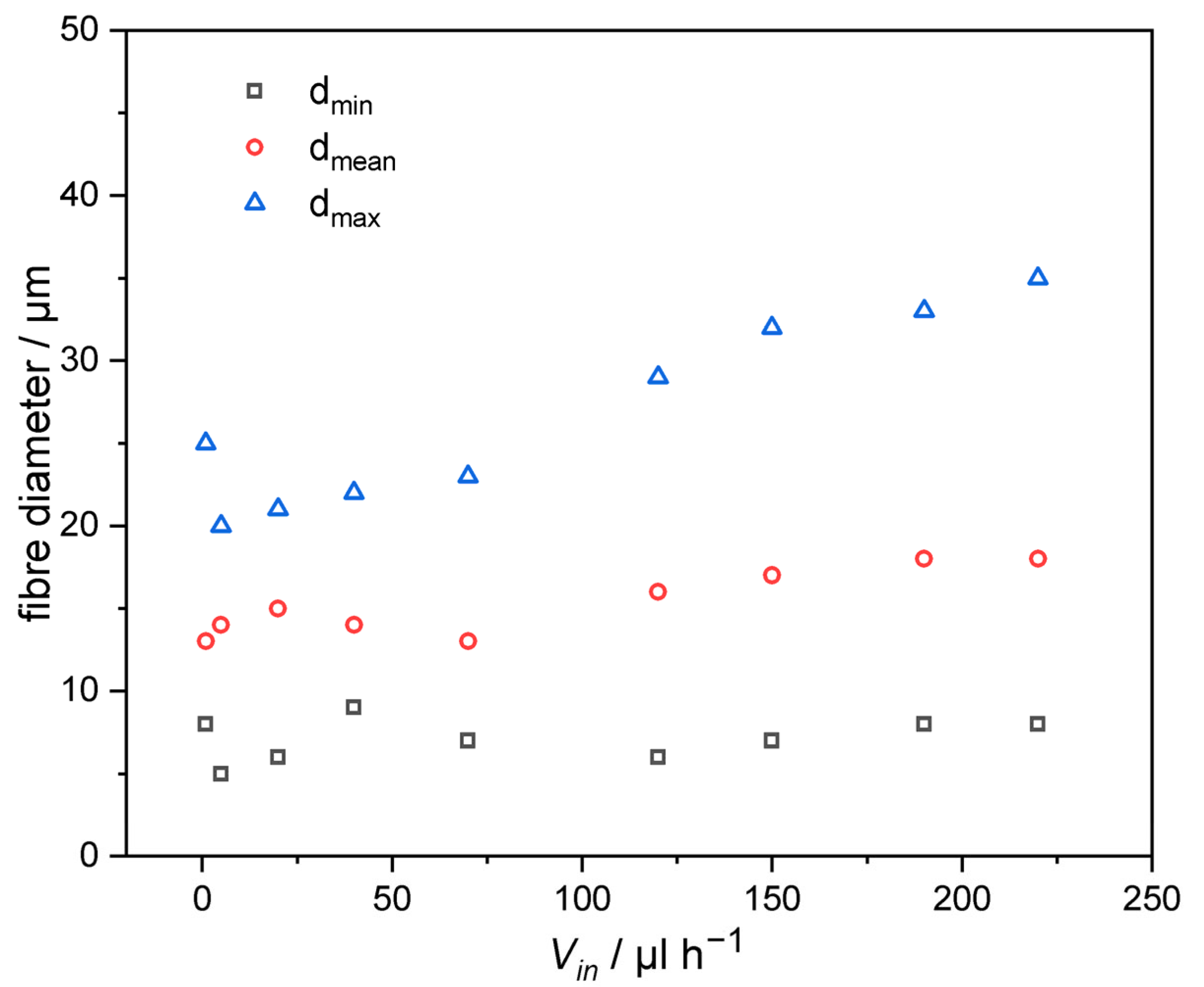
3.3. Influence of Flow Rate
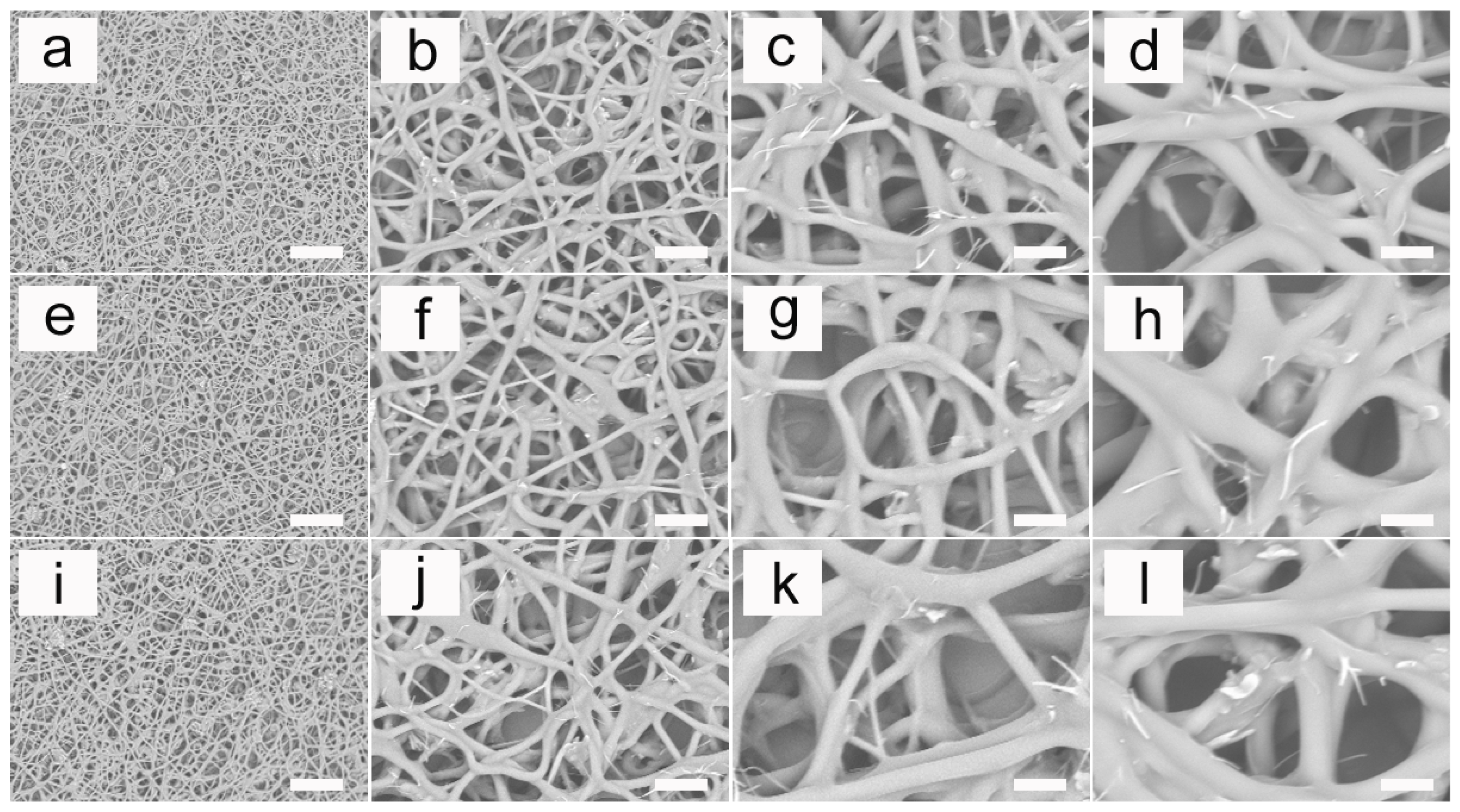
3.4. SEM Micrographs
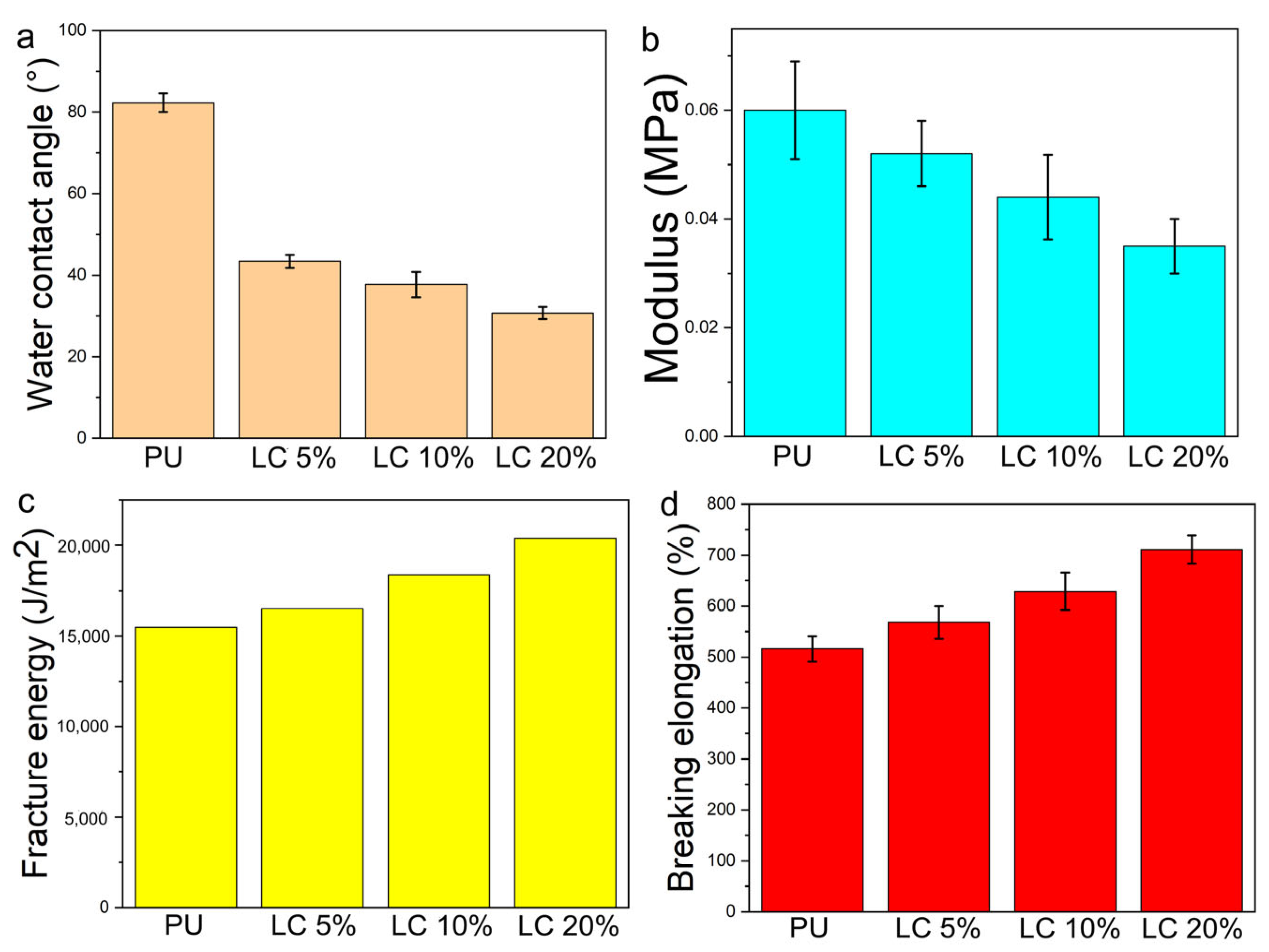
3.5. Contact Angle and Mechanical Properties of Composite Fibers
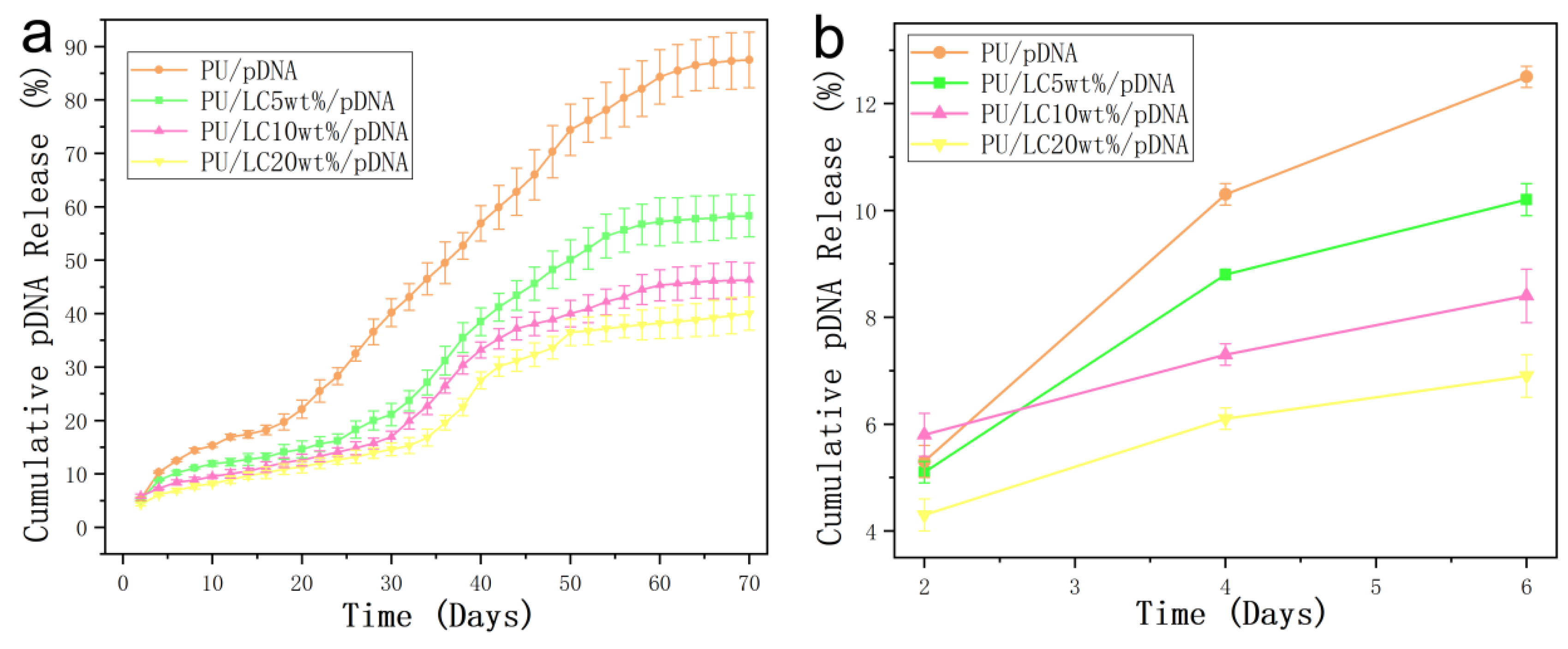
3.6. In Vitro Emission Characteristics of the pDNA-Loaded Fibers
The release rate of the pDNA on the pure PU fibers and PU/LCmicrofibers was uniform in both cases. In addition, the cumulative release amount reached about 40% after 70 days for the samples containing LC, which was observably lower than that of the PU microfibers. The fibers and LC could prolong the pDNA activity, and were beneficial in potential application in medical fields.
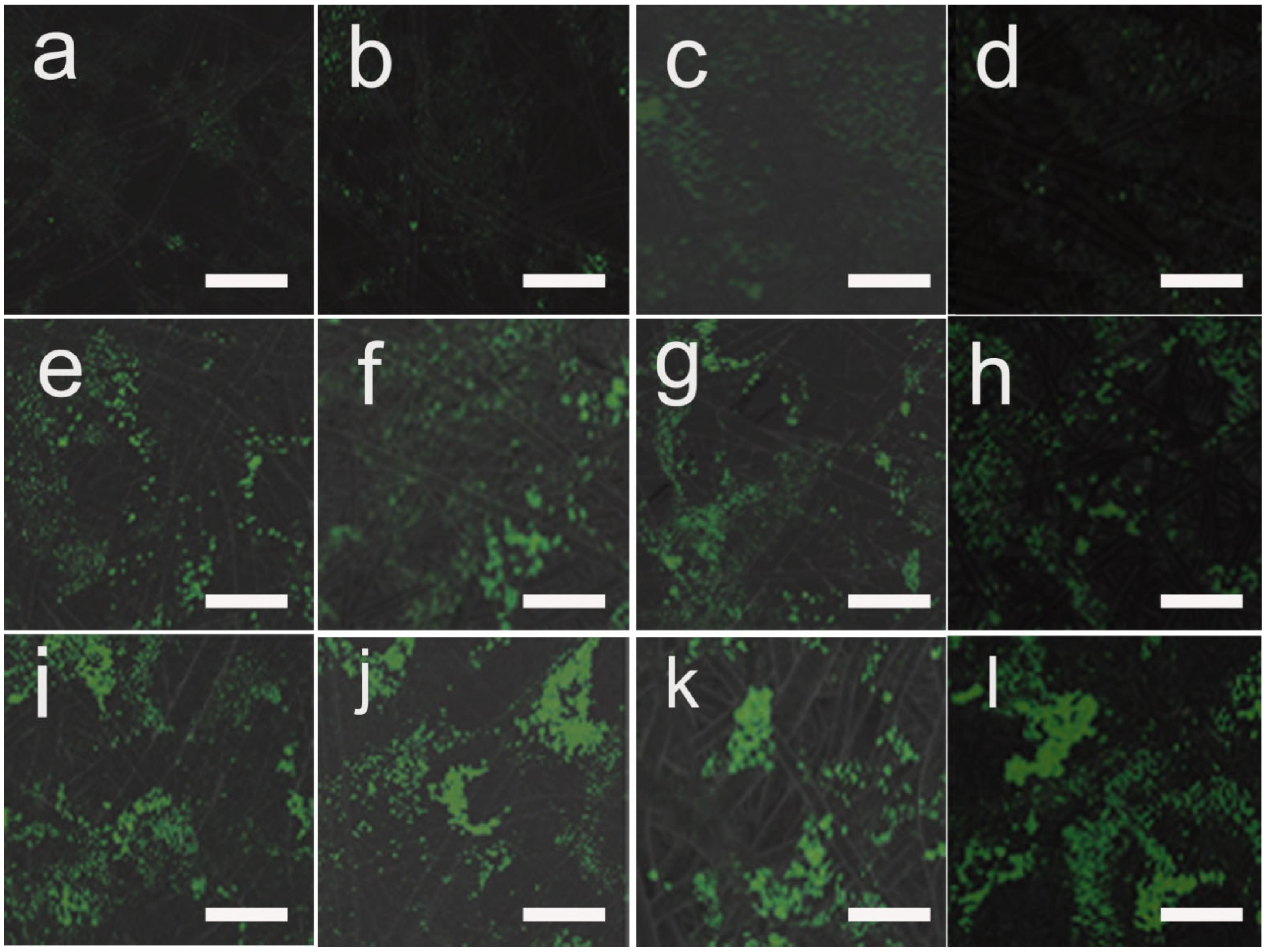
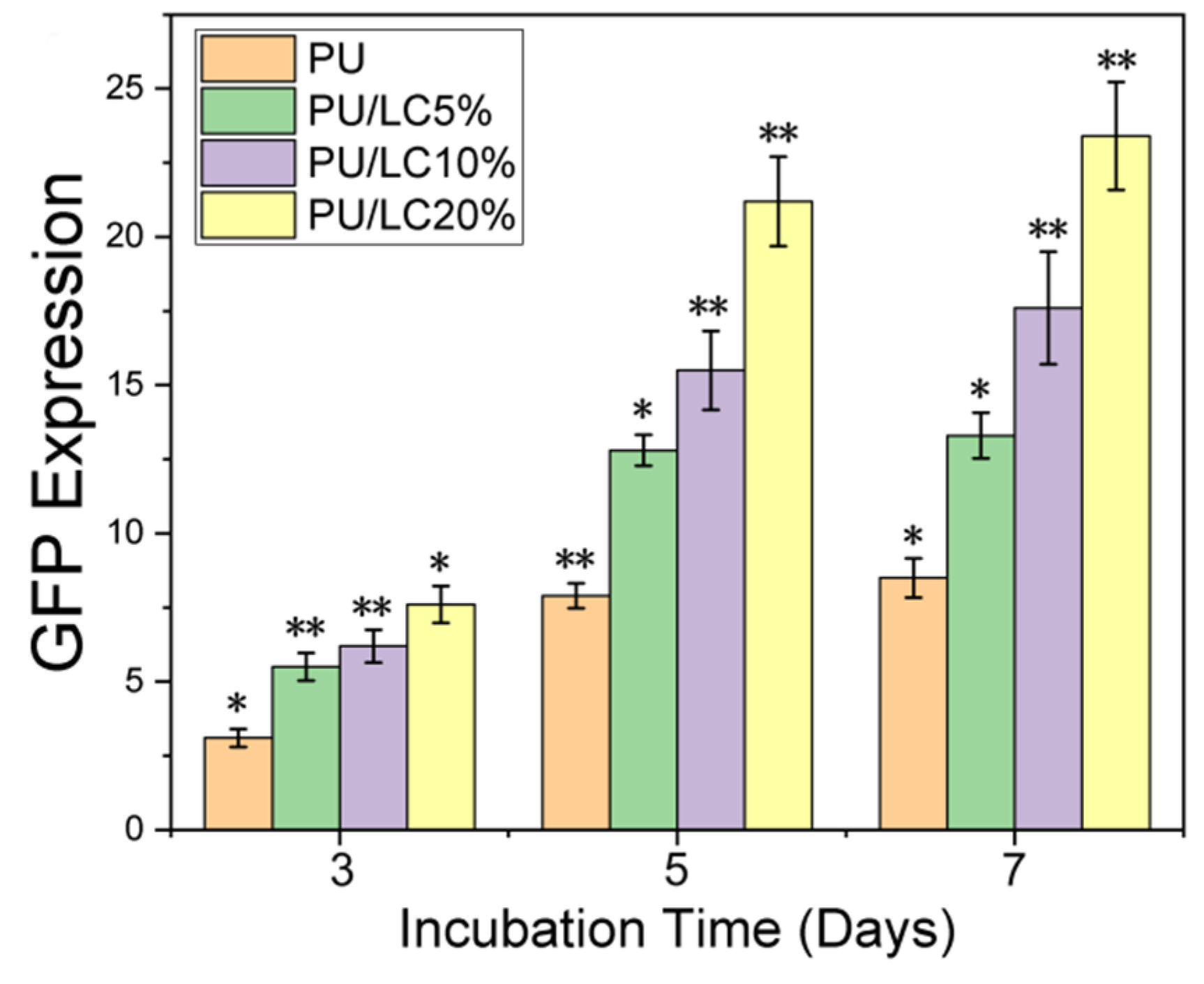
3.7. Transfection Efficiency of Cells on pDNA-Loaded Fibrous Mats
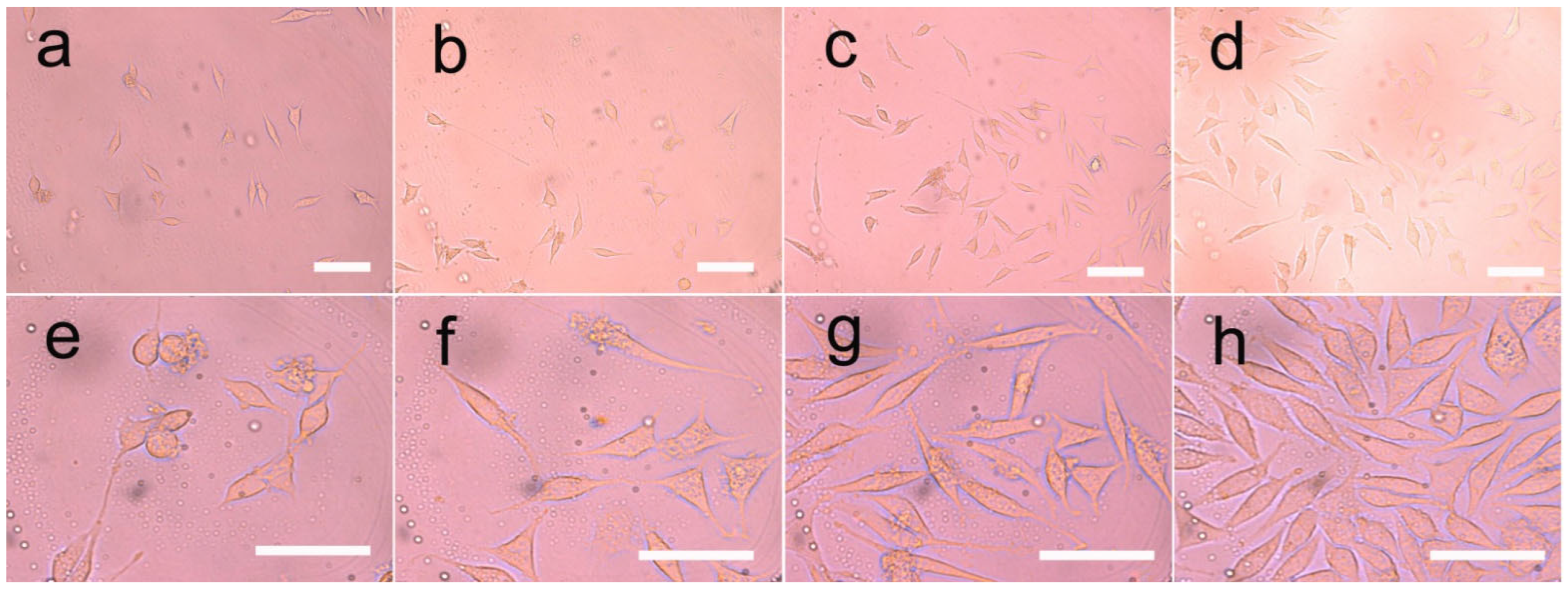
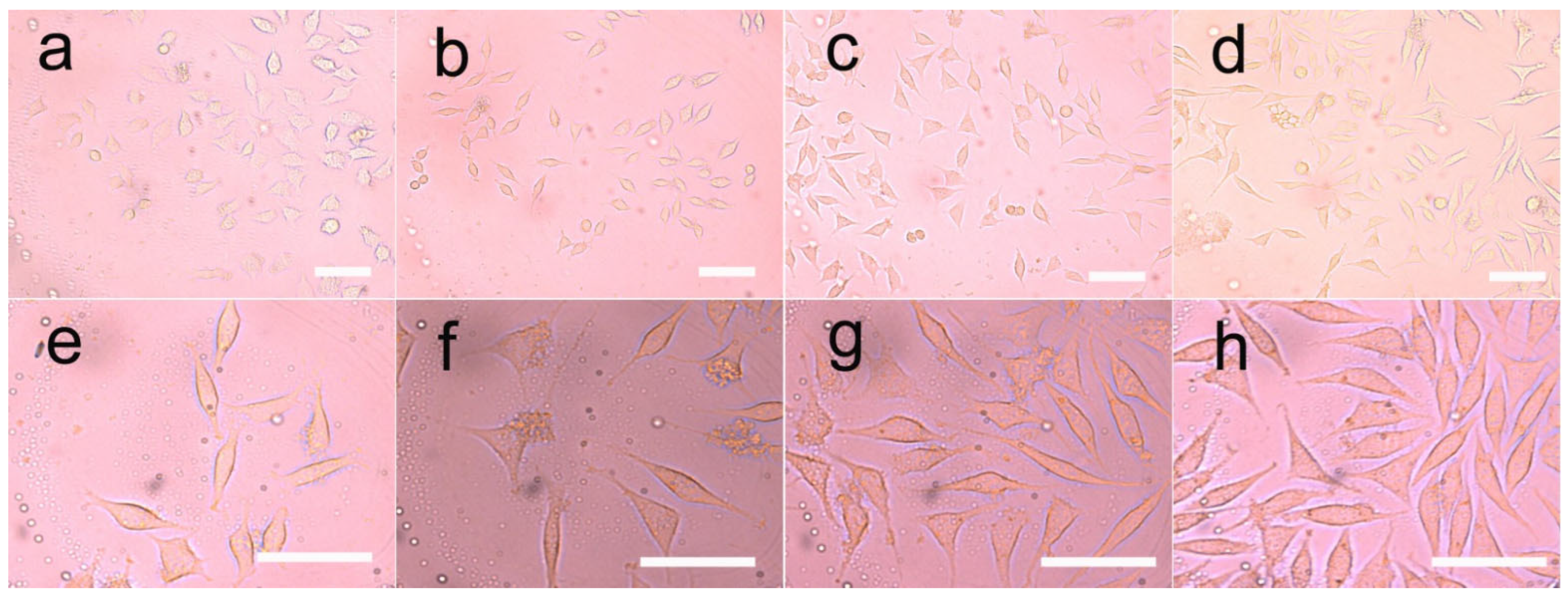
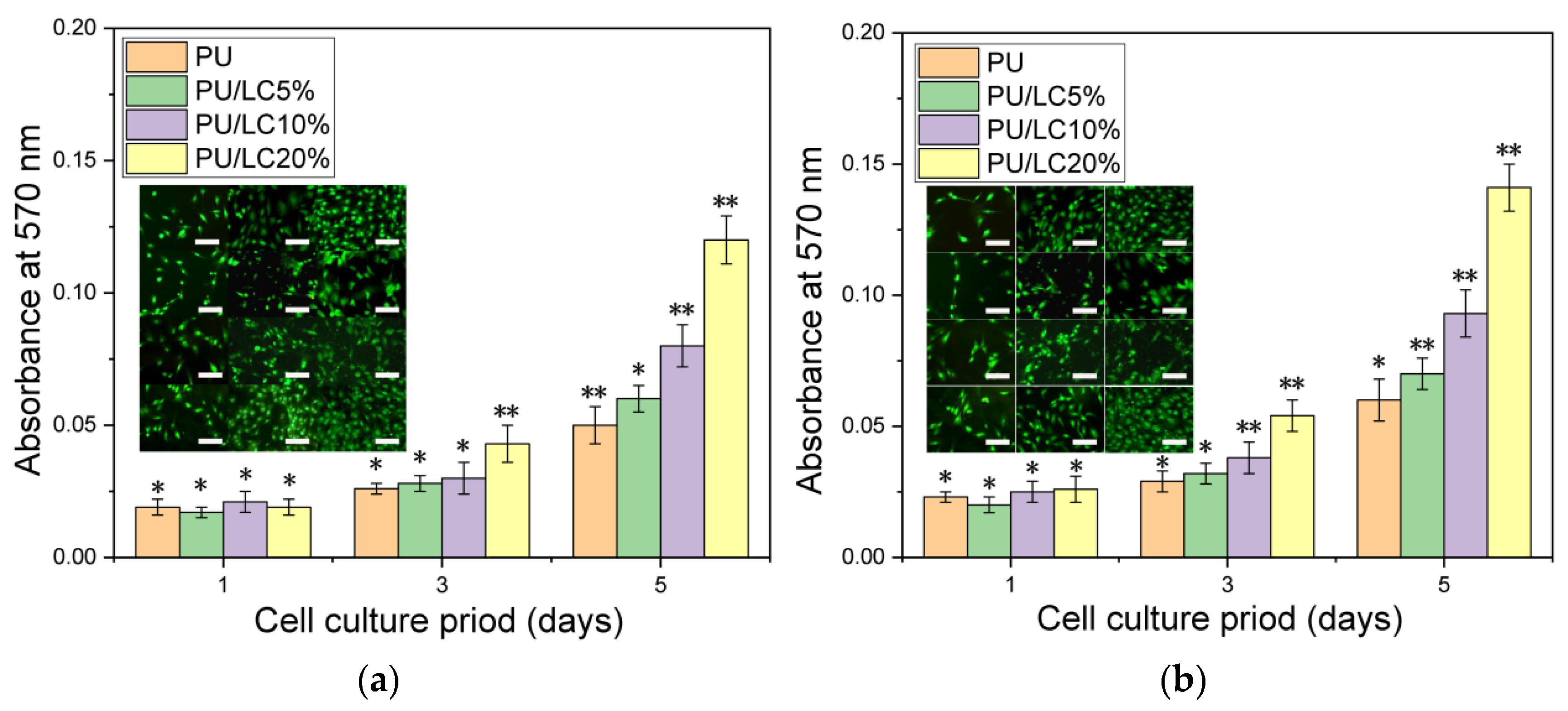
3.8. Cell Growth on the Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moghimi, E.; Chubak, I.; Statt, A.; Howard, M.P.; Founta, D.; Polymeropoulos, G.; Ntetsikas, K.; Hadjichristidis, N.; Panagiotopoulos, A.Z.; Likos, C.N.; et al. Self-organization and flow of low-functionality telechelic star polymers with varying attraction. ACS Macro Lett. 2019, 8, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Nizamova, C.I.; Kolochkova, A.E. Identification, Justification and Concretization of the Basic Skills of Self-Organization. ARPHA Proc. 2020, 3, 1789. [Google Scholar]
- Zhou, H.; Huang, H.; Bahri, M.; Browning, N.; Smith, J.; Graham, M.; Shchukin, D. Communicating assemblies of biomimetic nanocapsules. Nanoscale 2021, 13, 11343–11348. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, Y. Stimuli-responsive optical nanomaterials. Adv. Mater. 2019, 31, 1807061. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Huang, Y.; Yang, X.; Zhu, H.; Heng, L.; Xia, F. External Stimuli Responsive Liquid-Infused Surfaces Switching between Slippery and Nonslippery States: Fabrications and Applications. Adv. Funct. Mater. 2020, 30, 1901130. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Pang, X.; Lv, J.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable Azobenzene-Containing Liquid Crystal Polymers and Soft Actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef]
- Wang, L.; Urbas, A.M.; Li, Q. Nature-Inspired Emerging Chiral Liquid Crystal Nanostructures: From Molecular Self-Assembly to DNA Mesophase and Nanocolloids. Adv. Mater. 2020, 32, 1801335. [Google Scholar] [CrossRef]
- Xie, M.; Hisano, K.; Zhu, M.; Toyoshi, T.; Pan, M.; Okada, S.; Tsutsumi, O.; Kawamura, S.; Bowen, S. Flexible multifunctional sensors for wearable and robotic applications. Adv. Mater. Technol. 2019, 4, 1800626. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Chen, J.; Lee, P.S. Functional fibers and fabrics for soft robotics, wearables, and human–robot interface. Adv. Mater. 2021, 33, 2002640. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Li, J.-T.; Wei, W.-Y.; Wei, J.; Guo, J.-B. A luminescent dicyanodistyrylbenzene-based liquid crystal polymer network for photochemically patterned photonic composite film. Chin. J. Polym. Sci. 2018, 36, 776–782. [Google Scholar] [CrossRef]
- Zhao, D.-X.; Jiang, Q.; Wang, J.; Qiu, Y.; Liao, Y.-G.; Xie, X.-L. Visible Light and Temperature Regulated Reflection Colors in Self-supporting Cholesteric Liquid Crystal Physical Gels. Chin. J. Polym. Sci. 2021, 39, 1617–1625. [Google Scholar] [CrossRef]
- Mann, I.; Yu, X.; Zhang, W.-B.; Van Horn, R.M.; Jason, J.G.; Graham, M.J.; Harris, F.W.; Cheng, S.Z.D. What are the differences of polymer surface relaxation from the bulk? Chin. J. Polym. Sci. 2011, 29, 81–86. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Tan, J.; Liu, Z.; Lu, L.; Zhou, C. A gene-activating skin substitute comprising PLLA/POSS nanofibers and plasmid DNA encoding ANG and bFGF promotes in vivo revascularization and epidermalization. J. Mater. Chem. 2018, 6, 6977–6992. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafariet, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Mailley, D.; Hebraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2021, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.M.; Moreira, A.; Weber, A.; Williams, G.R.; Costa, P.F. Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics 2021, 13, 983. [Google Scholar] [CrossRef]
- Nosoudi, N.; Hart, C.; McKnight, I.; Esmaeilpour, M.; Ghomian, T.; Zadeh, A.; Raines, R.; Vick, J.E.R. Differentiation of adipose-derived stem cells to chondrocytes using electrospraying. Sci. Rep. 2021, 11, 24301. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, C.; Kim, J.-J.; Lee, J.-H.; Jin, G.-Z.; Knowles, J.C.; Kim, H.-W. Differential chondro-and osteo-stimulation in three-dimensional porous scaffolds with different topological surfaces provides a design strategy for biphasic osteochondral engineering. J. Tissue Eng. 2019, 10, 2041731419826433. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.Z.; Zhou, Y. Electrospinning of biomimetic fibrous scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 947–960. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, D.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers derived from trees as sustainable multifunctional materials: A review. Adv. Mater. 2020, 33, 2001654. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-H.; Xu, X.-H.; Huang, Y.-F.; Zhao, X.-L.; Zhao, B.; Cai, S.-Y.; Xie, M.-J.; Wang, M.-Z.; Yuan, T.-J.; He, Y.; et al. Self-Adaptive All-In-One Delivery Chip for Rapid Skin Nerves Regeneration by Endogenous Mesenchymal Stem Cells. Adv. Funct. Mater. 2020, 30, 2001751. [Google Scholar] [CrossRef]
- Syahidah, D.; Elliman, J.; Constantinoiu, C.; Owens, L. Vero cell lines expressing nuclear location signals of Penaeus merguiensis hepandensovirus: An early study. Asian J. Adv. Res. 2018, 1, 1–10. [Google Scholar]
- Zupančič, Š. Core-shell nanofibers as drug-delivery systems. Acta Pharm. 2019, 69, 131–153. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yang, T.; Mu, X.; Mai, Z.; Li, H.; Wang, Y.; Zhou, G. Smart Supramolecular Self-Assembled Nanosystem: Stimulus-Responsive Hydrogen-Bonded Liquid Crystals. Nanomaterials 2021, 11, 448. [Google Scholar] [CrossRef]
- Nayak, S.; Ola, M. Liquid Crystalline System: Novel approach in Drug Delivery. J. Pharm. Adv. Res. 2018, 1, 66–75. [Google Scholar]
- Pandey, M.; Kumari, N.; Nagamatsu, S.; Pandey, S.S. Recent advances in the orientation of conjugated polymers for organic field-effect transistors. J. Mater. Chem. 2019, 7, 13323–13351. [Google Scholar] [CrossRef]
- Stewart, G.T. Liquid crystals in biology II. Origins and processes of life. Liq. Cryst. 2004, 31, 443–471. [Google Scholar] [CrossRef]
- Hiltner, L.; Carme Calderer, M.; Arsuaga, J.; Vázquez, M. Chromonic liquid crystals and packing configurations of bacteriophage viruses. Philos. Trans. R. Soc. A 2021, 379, 20200111. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, N.; Leonhardt, B.; Jolowsky, C.; Hao, A.; Park, J.G.; Liang, R. Carbon-Nanotube-Based Electrical Conductors: Fabrication, Optimization, and Applications. Adv. Electron. Mater. 2019, 5, 1800811. [Google Scholar] [CrossRef]
- Xie, W.; Ouyang, R.; Wang, H.; Zhou, C. Construction and Biocompatibility of Three-Dimensional Composite Polyurethane Scaffolds in Liquid Crystal State. ACS Biomater. Sci. Eng. 2020, 6, 2312–2322. [Google Scholar] [CrossRef]
- Xie, W.; Ouyang, R.; Wang, H.; Li, N.; Zhou, C. Synthesis and cytotoxicity of novel elastomers based on cholesteric liquid crystals. Liq. Cryst. 2020, 47, 449–464. [Google Scholar] [CrossRef]
- Imani, F.; Karimi-Soflou, R.; Shabani, I.; Karkhaneh, A. PLA electrospun nanofibers modified with polypyrrole-grafted gelatin as bioactive electroconductive scaffold. Polymer 2021, 218, 123487. [Google Scholar] [CrossRef]
- Oztemur, J.; Yalcin-Enis, I. Development of biodegradable webs of PLA/PCL blends prepared via electrospinning: Morphological, chemical, and thermal characterization. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2021, 109, 1844–1856. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Dandurand, J.; Pepe, A.; Bochicchio, B. Thermal and dynamic mechanical behavior of poly (lactic acid)(PLA)-based electrospun scaffolds for tissue engineering. J. Appl. Polym. Sci. 2021, 138, 51313. [Google Scholar] [CrossRef]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferronit, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-based vascular grafts: In vitro tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef]
- Budurova, D.; Ublekov, F.; Penchev, H. The use of formic acid as a common solvent for electrospinning of hybrid PHB/Soy protein fibers. Mater. Lett. 2021, 301, 130313. [Google Scholar] [CrossRef]
- Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 529. [Google Scholar] [CrossRef]
- Kolte, A.; Patil, S.; Lesimple, P.; Hanrahan, J.W.; Misra, A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 2017, 524, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ebrahimian, M.; Hashemi, M. Current strategies in the modification of PLGA-based gene delivery system. Curr. Med. Chem. 2017, 24, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Ramgopal, A.; Segal, J.; Mukhtar, S.; Yu, J.; Picarsic, J.; Tersak, J.M.; Allen, S.W. A chronic eyelid lesion in a child: Multi-disciplinary approach to diagnosis, treatment and management of a highly atypical histiocytic lesion. Pediatric Hematol. Oncol. 2022, 39, 180–186. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Lu, L.; Ouyang, R.; Zhou, C. Polyurethane/Liquid Crystal Microfibers with pDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation. Pharmaceutics 2022, 14, 2489. https://doi.org/10.3390/pharmaceutics14112489
Zhang C, Lu L, Ouyang R, Zhou C. Polyurethane/Liquid Crystal Microfibers with pDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation. Pharmaceutics. 2022; 14(11):2489. https://doi.org/10.3390/pharmaceutics14112489
Chicago/Turabian StyleZhang, Chaowen, Lu Lu, Ruoran Ouyang, and Changren Zhou. 2022. "Polyurethane/Liquid Crystal Microfibers with pDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation" Pharmaceutics 14, no. 11: 2489. https://doi.org/10.3390/pharmaceutics14112489
APA StyleZhang, C., Lu, L., Ouyang, R., & Zhou, C. (2022). Polyurethane/Liquid Crystal Microfibers with pDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation. Pharmaceutics, 14(11), 2489. https://doi.org/10.3390/pharmaceutics14112489




