Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of Blends
2.2.2. Production of Granules
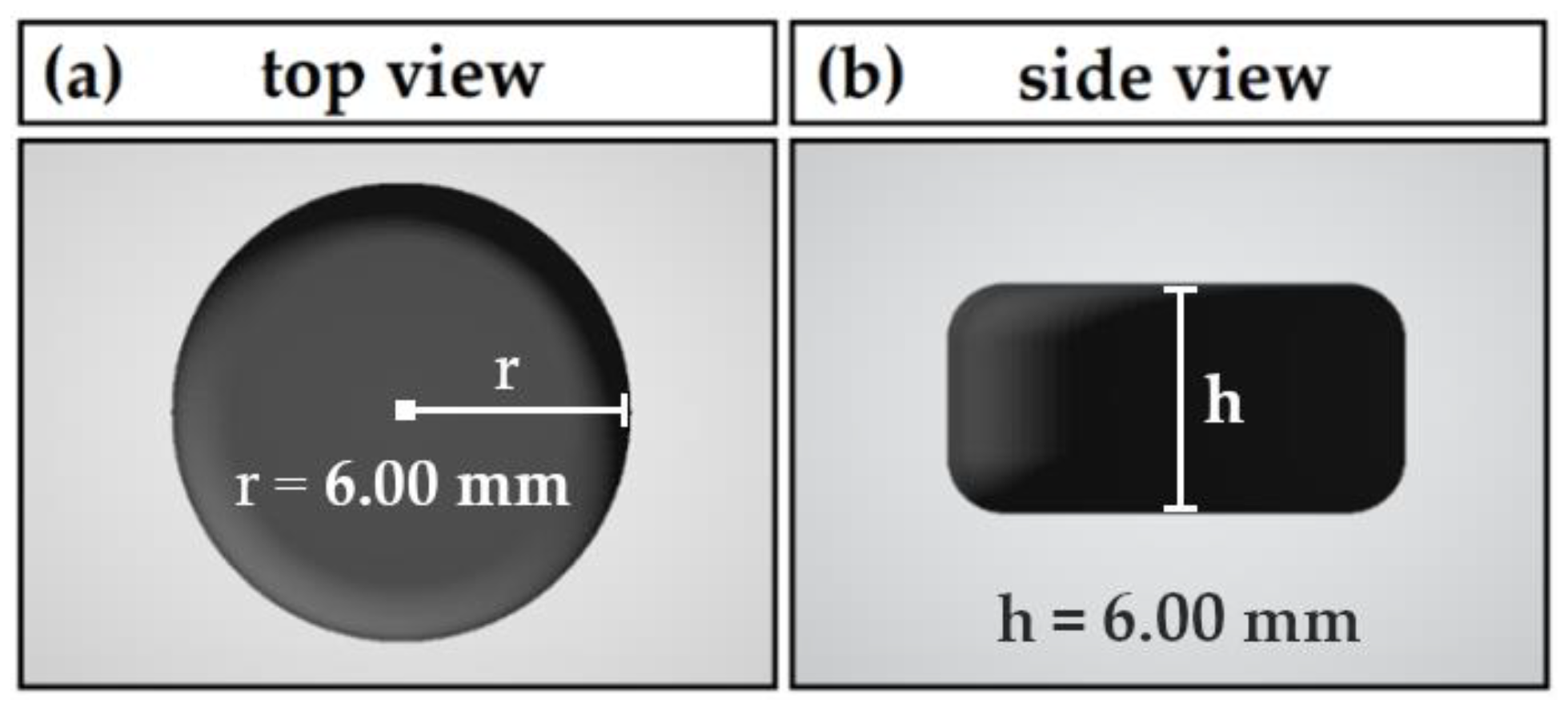
2.2.3. Printlet Design and 3D Printing Process
2.2.4. Printability Runs
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Thermogravimetric Analysis (TGA)
2.2.7. Rheology: Small Amplitude Oscillatory Shear (SAOS)
2.2.8. Uniformity of Mass of Single-Dose Dosage Forms
2.2.9. Evaluation of Content Uniformity
2.2.10. In Vitro Dissolution
3. Results
3.1. Thermogravimetric Analysis
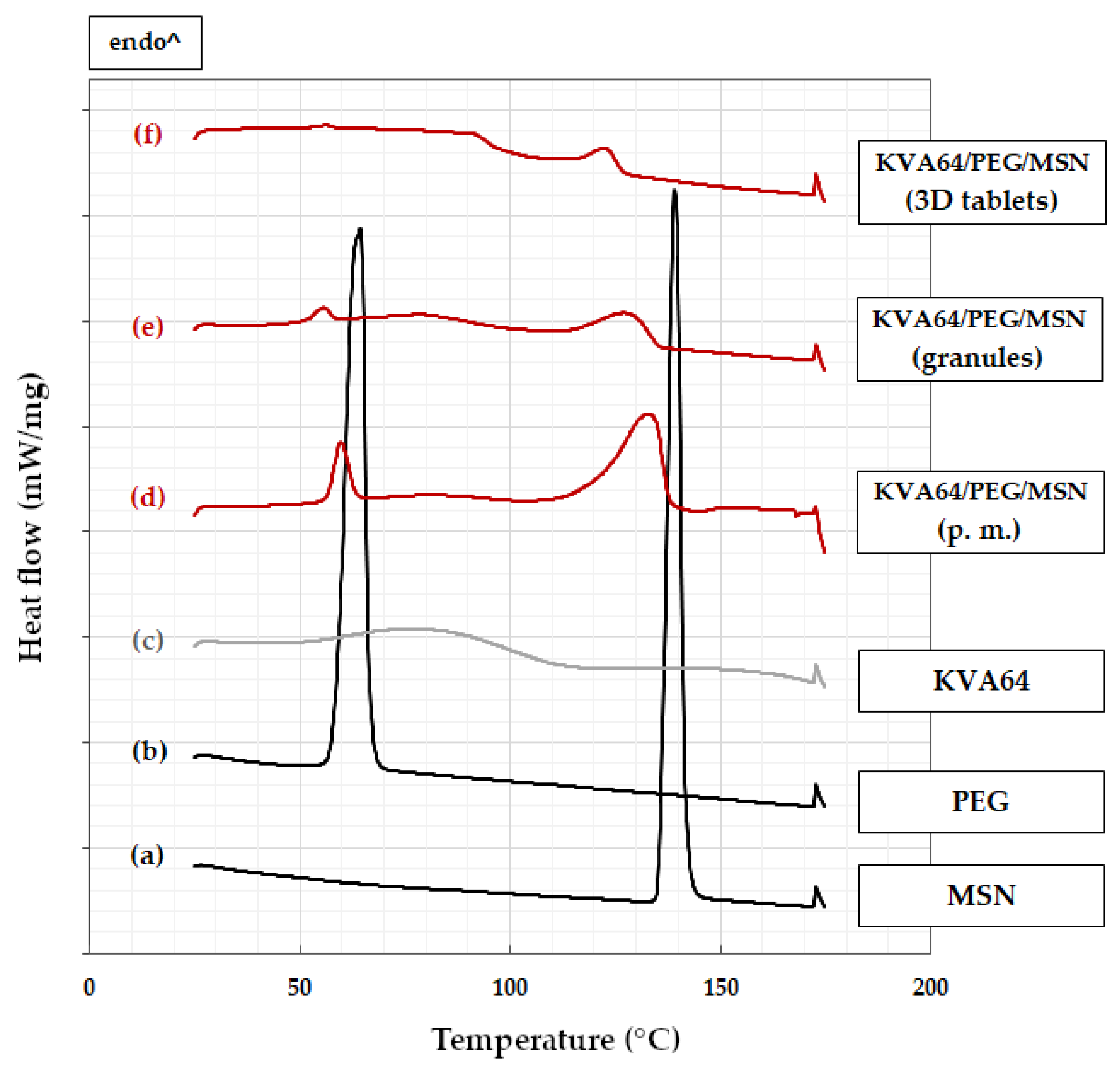
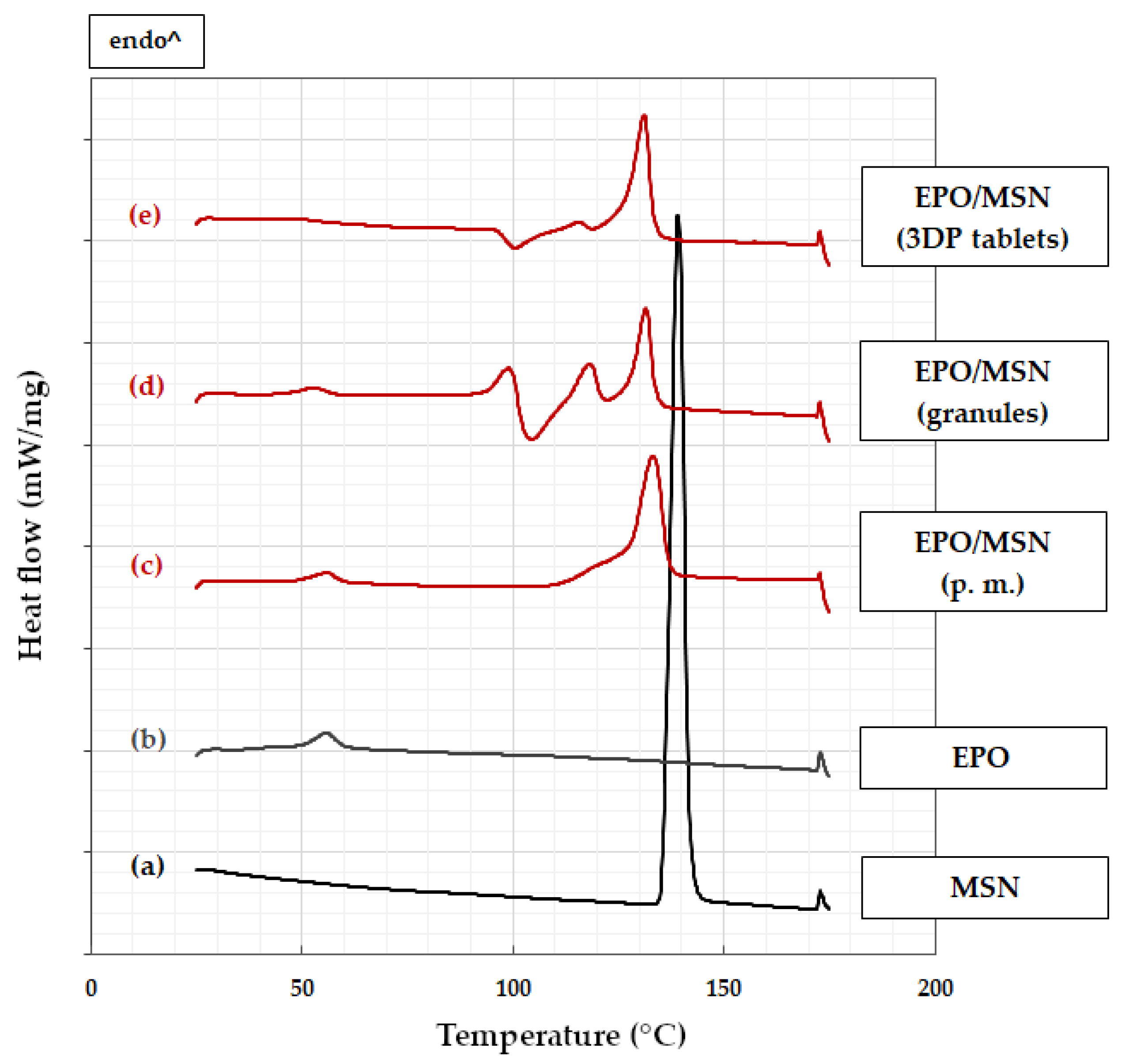
3.2. Differential Scanning Calorimetry
3.2.1. KVA64-Based Formulation
3.2.2. EPO-Based Formulation
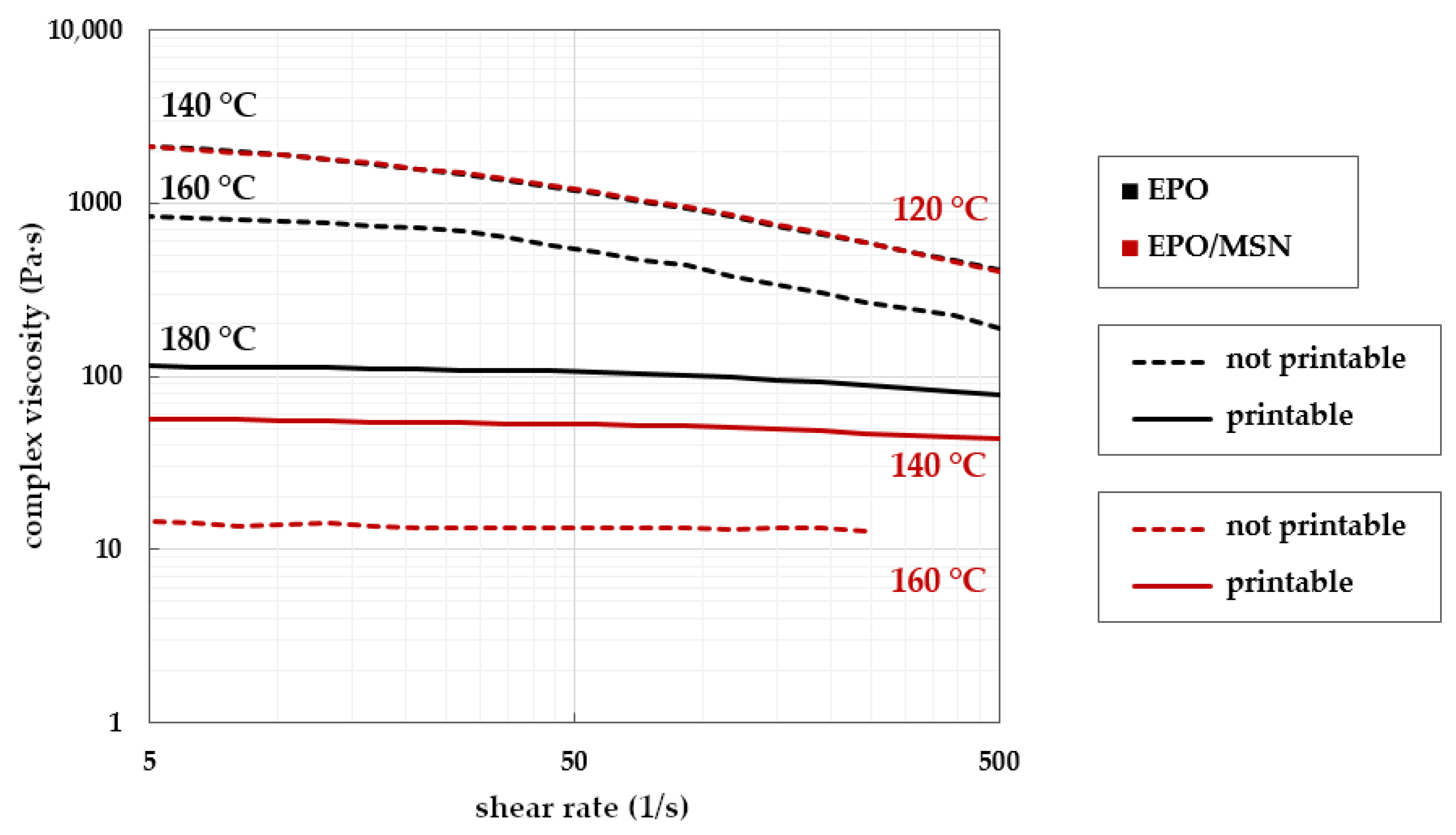
3.3. Small Amplitude Oscillatory Shear Rheology
3.3.1. Technical Challenges Regarding Melt Viscosity
3.3.2. Establishing Target Rheological Properties with Placebos
3.3.3. Transfer to Drug Loaded Formulations
3.4. Uniformity of Mass
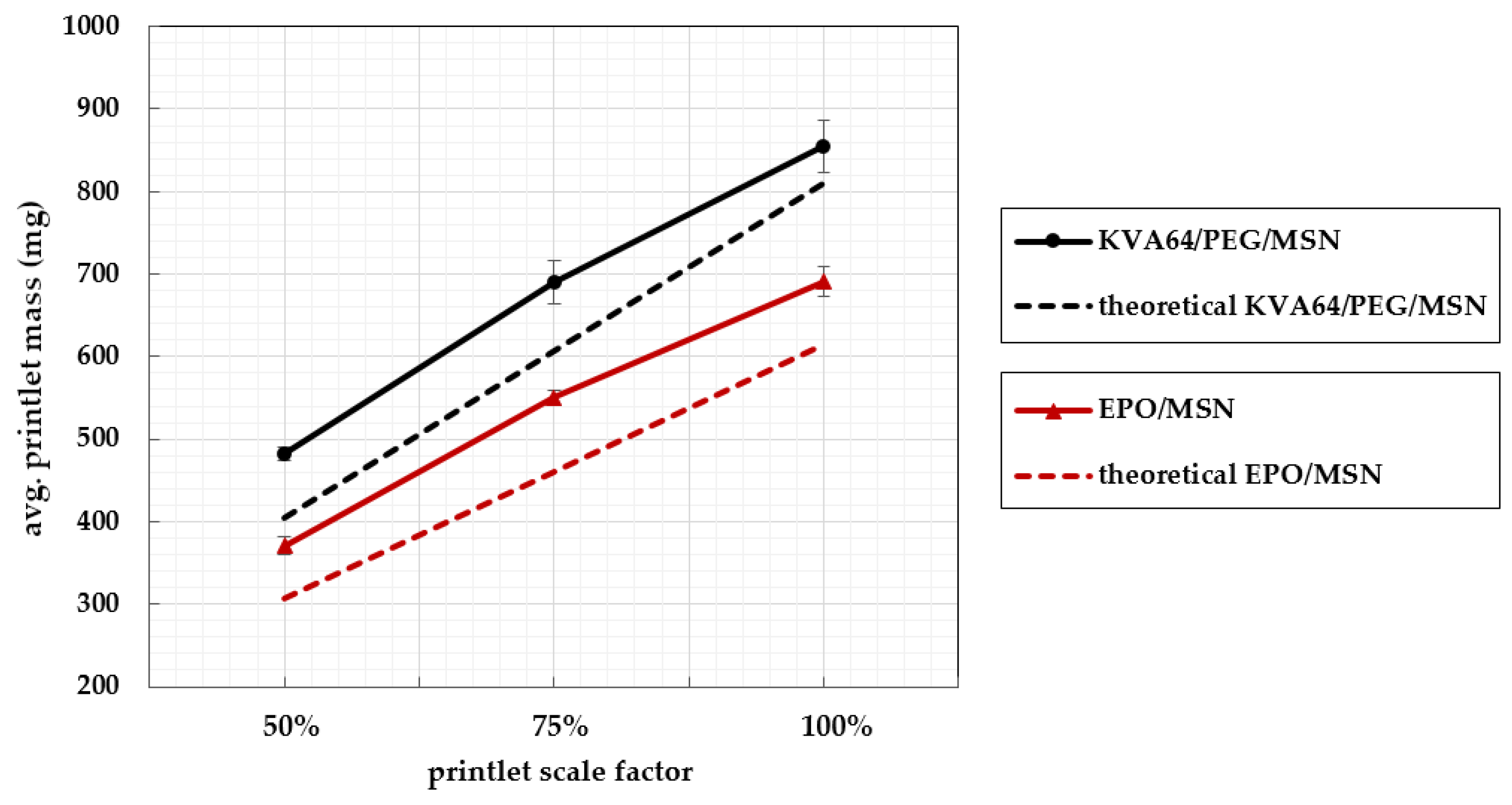
3.5. Printlet Volume–Mass Correlation
3.6. Uniformity of Dosage Units
3.7. In Vitro Dissolution of Solid Oral Dosage Forms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huber, G.; Dachtler, M.; Edinger, D. Digitalisierung in der Pharmaindustrie. In Digitale Transformation von Dienstleistungen im Gesundheitswesen II; Springer: Berlin/Heidelberg, Germany, 2017; pp. 241–255. [Google Scholar]
- Pravin, S.; Sudhir, A. Integration of 3D printing with dosage forms: A new perspective for modern healthcare. Biomed. Pharmacother. 2018, 107, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Dachtler, M.; Eggenreich, K.; Pflieger, T. Digital Health-Digital 2D/3D Printing of Personalized Medication. In Proceedings of the 4rd International Symposium on Pharmaceutical Engineering Research (SPhERe), Online, 15–17 September 2021. [Google Scholar]
- Dachtler, M.; Huber, G.; Pries, T. 2D & 3D-Print-Technologien in der Pharmazeutischen Industrie. In Digitale Transformation von Dienstleistungen im Gesundheitswesen VII; Springer: Berlin/Heidelberg, Germany, 2020; pp. 53–66. [Google Scholar]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.; Luo, Z.; Leroux, J.-C. 3D printing of a controlled fluoride delivery device for the prevention and treatment of tooth decay. J. Control. Release 2022, 348, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Menegatou, I.-M.; Papakyriakopoulou, P.; Rekkas, D.M.; Dallas, P.; Valsami, G. Design of a Personalized Nasal Device (Matrix-Piston Nasal Device, MPD) for Drug Delivery: A 3D-Printing Application. AAPS PharmSciTech 2022, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Teoh, X.Y.; Yan, J.; Gleadall, A.; Belton, P.; Bibb, R.; Qi, S. Development of combi-pills using the coupling of semi-solid syringe extrusion 3D printing with fused deposition modelling. Int. J. Pharm. 2022, 625, 122140. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, P.; Chung, S.; Bandari, S.; Repka, M.A. Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing. Int. J. Pharm. 2022, 624, 121972. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Briatico-Vangosa, F.; Moutaharrik, S.; Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Gazzaniga, A. The Chronotopic™ System for Pulsatile and Colonic Delivery of Active Molecules in the Era of Precision Medicine: Feasibility by 3D Printing via Fused Deposition Modeling (FDM). Pharmaceutics 2021, 13, 759. [Google Scholar] [CrossRef]
- Rahman, Z.; Ali, S.F.B.; Ozkan, T.; Charoo, N.A.; Reddy, I.K.; Khan, M.A. Additive manufacturing with 3D printing: Progress from bench to bedside. AAPS J. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical applications of 3D printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Reiner, G.; Pierce, S.L.; Flynn, J. Wrong drug and wrong dose dispensing errors identified in pharmacist professional liability claims. J. Am. Pharm. Assoc. 2020, 60, e50–e56. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. A guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Staessen, J.A.; Wang, J.; Bianchi, G.; Birkenhäger, W.H. Essential hypertension. Lancet 2003, 361, 1629–1641. [Google Scholar] [CrossRef]
- Fahey, T.; Schroeder, K.; Ebrahim, S.; Glynn, L. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst. Rev. 2006, 4, CD005182. [Google Scholar]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in pharmaceutical additive manufacturing: A balancing act between printability and product performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef]
- Parulski, C.; Jennotte, O.; Lechanteur, A.; Evrard, B. Challenges of fused deposition modeling 3D printing in pharmaceutical applications: Where are we now? Adv. Drug Deliv. Rev. 2021, 175, 113810. [Google Scholar] [CrossRef]
- Krueger, L.; Miles, J.A.; Popat, A. 3D printing hybrid materials using fused deposition modelling for solid oral dosage forms. J. Control. Release 2022, 351, 444–455. [Google Scholar] [CrossRef]
- Liu, X.; Chi, B.; Jiao, Z.; Tan, J.; Liu, F.; Yang, W. A large-scale double-stage-screw 3D printer for fused deposition of plastic pellets. J. Appl. Polym. Sci. 2017, 134, 45147. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Muller, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Simplification of fused deposition modeling 3D-printing paradigm: Feasibility of 1-step direct powder printing for immediate release dosage form production. Int. J. Pharm. 2020, 578, 119124. [Google Scholar] [CrossRef]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D printing of solid oral dosage forms: Numerous challenges with unique opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Uniformity of Mass of Single-Dose Preparations. In European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2019; Volume 1, pp. 335–336.
- Uniformity of Dosage Units. In European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2019; Volume 1, pp. 357–359.
- Safety Data Sheet: Metoprolol Succinate; Hangzhou-Longshine: Hangzhou, China, 2019.
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Gratson, G.M. Direct writing in three dimensions. Mater. Today 2004, 7, 32–39. [Google Scholar] [CrossRef]
- Jaluria, Y. Heat and Mass Transfer in the Extrusion of Non-Newtonian Materials. In Advances in Heat Transfer; Elsevier: Amsterdam, The Netherlands, 1996; Volume 28, pp. 145–230. [Google Scholar]
- Chiruvella, R.V.; Jaluria, Y.; Sernas, V.; Esseghir, M. Extrusion of non-Newtonian fluids in a single-screw extruder with pressure back flow. Polym. Eng. Sci. 1996, 36, 358–367. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.-J.; Schimmel, K.J. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?—A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Saydam, M.; Takka, S. Improving the dissolution of a water-insoluble orphan drug through a fused deposition modelling 3-dimensional printing technology approach. Eur. J. Pharm. Sci. 2020, 152, 105426. [Google Scholar] [CrossRef]
- Buanz, A.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Loflin, W.A.; English, J.D.; Borders, C.; Harris, L.M.; Moon, A.; Holland, J.N.; Kasper, F.K. Effect of print layer height on the assessment of 3D-printed models. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 283–289. [Google Scholar] [CrossRef]
- Ferretti, P.; Leon-Cardenas, C.; Santi, G.M.; Sali, M.; Ciotti, E.; Frizziero, L.; Donnici, G.; Liverani, A. Relationship between FDM 3D printing parameters study: Parameter optimization for lower defects. Polymers 2021, 13, 2190. [Google Scholar] [CrossRef]
- Shaqour, B.; Abuabiah, M.; Abdel-Fattah, S.; Juaidi, A.; Abdallah, R.; Abuzaina, W.; Qarout, M.; Verleije, B.; Cos, P. Gaining a better understanding of the extrusion process in fused filament fabrication 3D printing: A review. Int. J. Adv. Manuf. Technol. 2021, 114, 1279–1291. [Google Scholar] [CrossRef]
- Shaik, Y.P.; Schuster, J.; Shaik, A. A Scientific Review on Various Pellet Extruders Used In 3D Printing FDM Processes. Open Access Libr. J. 2021, 8, 1–19. [Google Scholar] [CrossRef]
- Kumar, L.J.; Pandey, P.M.; Wimpenny, D.I. 3D Printing and Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2019; Volume 311. [Google Scholar]
- Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances-Guidance for Industry; US Department of Health and Human Services-Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Anand, O.; Yu, L.X.; Conner, D.P.; Davit, B.M. Dissolution testing for generic drugs: An FDA perspective. AAPS J. 2011, 13, 328–335. [Google Scholar] [CrossRef]
- Recommendation on Dissolution Testing. In European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2019; Volume 1, pp. 727–729.
- Budavari, S.; O’Neil, M.; Smith, A.; Heckelman, P.; Kinneary, J. The Merk Index. An Encyclopedia of Chemicals, Drugs and Biologicals, 13th ed.; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 2001; Volume 1097, p. 1096. [Google Scholar]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]



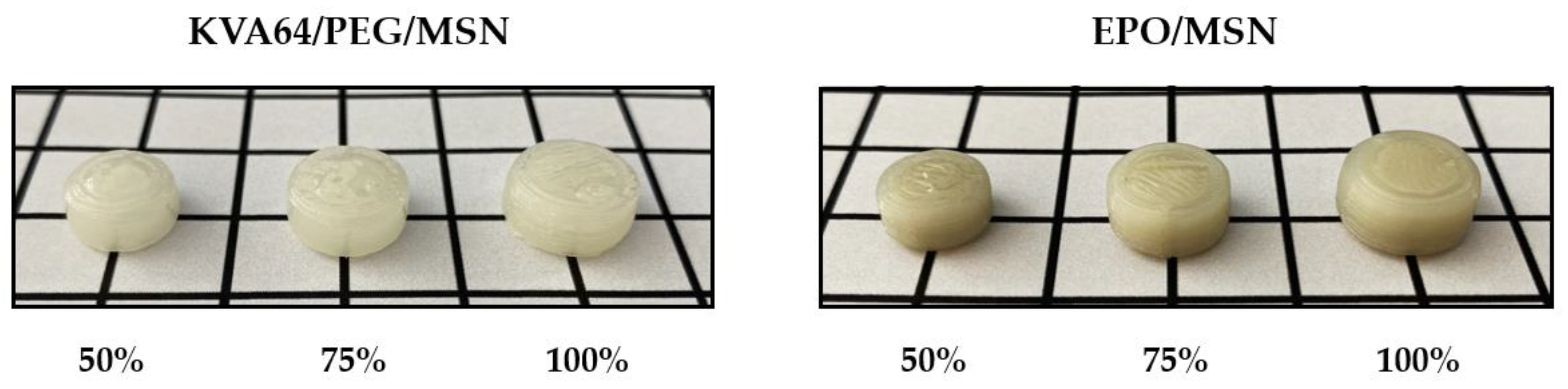

| Formulation | MSN (% w/w) | KVA64 (% w/w) | EPO (% w/w) | PEG (% w/w) |
|---|---|---|---|---|
| KVA64/PEG | - | 70 | - | 30 |
| KVA64/PEG/MSN | 25 | 65 | - | 5 |
| EPO | - | - | 100 | - |
| EPO/MSN | 25 | - | 75 | - |
| KVA64/PEG | KVA64/PEG/MSN | EPO | EPO/MSN | |
|---|---|---|---|---|
| Extrusion T. (°C) | 100 | 100 | 140 | 140 |
| Torque (N·m) | 2.0 | 2.2 | 2.4 | 2.1 |
| Screw speed (rpm) | 100 | 100 | 100 | 100 |
| 3DP T. (°C) | 140 | 140 | 180 | 140 |
| Formulation | Printlet Scale Factor 1 | Mean Mass [mg] | ±SD | First Ph. Eur. Limit 2 | Second Ph. Eur. Limit 3 |
|---|---|---|---|---|---|
| KVA64/PEG/MSN | 50% | 482 | 1.75% | 10/10 | 10/10 |
| 75% | 691 | 3.73% | 9/10 | 10/10 | |
| 100% | 854 | 4.34% | 9/10 | 10/10 | |
| EPO/MSN | 50% | 371 | 2.92% | 9/10 | 10/10 |
| 75% | 550 | 1.65% | 10/10 | 10/10 | |
| 100% | 691 | 2.62% | 10/10 | 10/10 |
| KVA64/PEG/MSN | EPO/MSN | |||
|---|---|---|---|---|
| Granules | 3DP Tablets | Granules | 3DP Tablets | |
| Mean API content (%) 1 | 96.9 ± 1.5 | 96.1 ± 1.7 | 99.7 ± 1.8 | 98.3 ± 1.3 |
| Acceptance value | 5.2 | 6.5 | 4.2 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pflieger, T.; Venkatesh, R.; Dachtler, M.; Eggenreich, K.; Laufer, S.; Lunter, D. Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics. Pharmaceutics 2022, 14, 2488. https://doi.org/10.3390/pharmaceutics14112488
Pflieger T, Venkatesh R, Dachtler M, Eggenreich K, Laufer S, Lunter D. Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics. Pharmaceutics. 2022; 14(11):2488. https://doi.org/10.3390/pharmaceutics14112488
Chicago/Turabian StylePflieger, Thomas, Rakesh Venkatesh, Markus Dachtler, Karin Eggenreich, Stefan Laufer, and Dominique Lunter. 2022. "Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics" Pharmaceutics 14, no. 11: 2488. https://doi.org/10.3390/pharmaceutics14112488
APA StylePflieger, T., Venkatesh, R., Dachtler, M., Eggenreich, K., Laufer, S., & Lunter, D. (2022). Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics. Pharmaceutics, 14(11), 2488. https://doi.org/10.3390/pharmaceutics14112488







