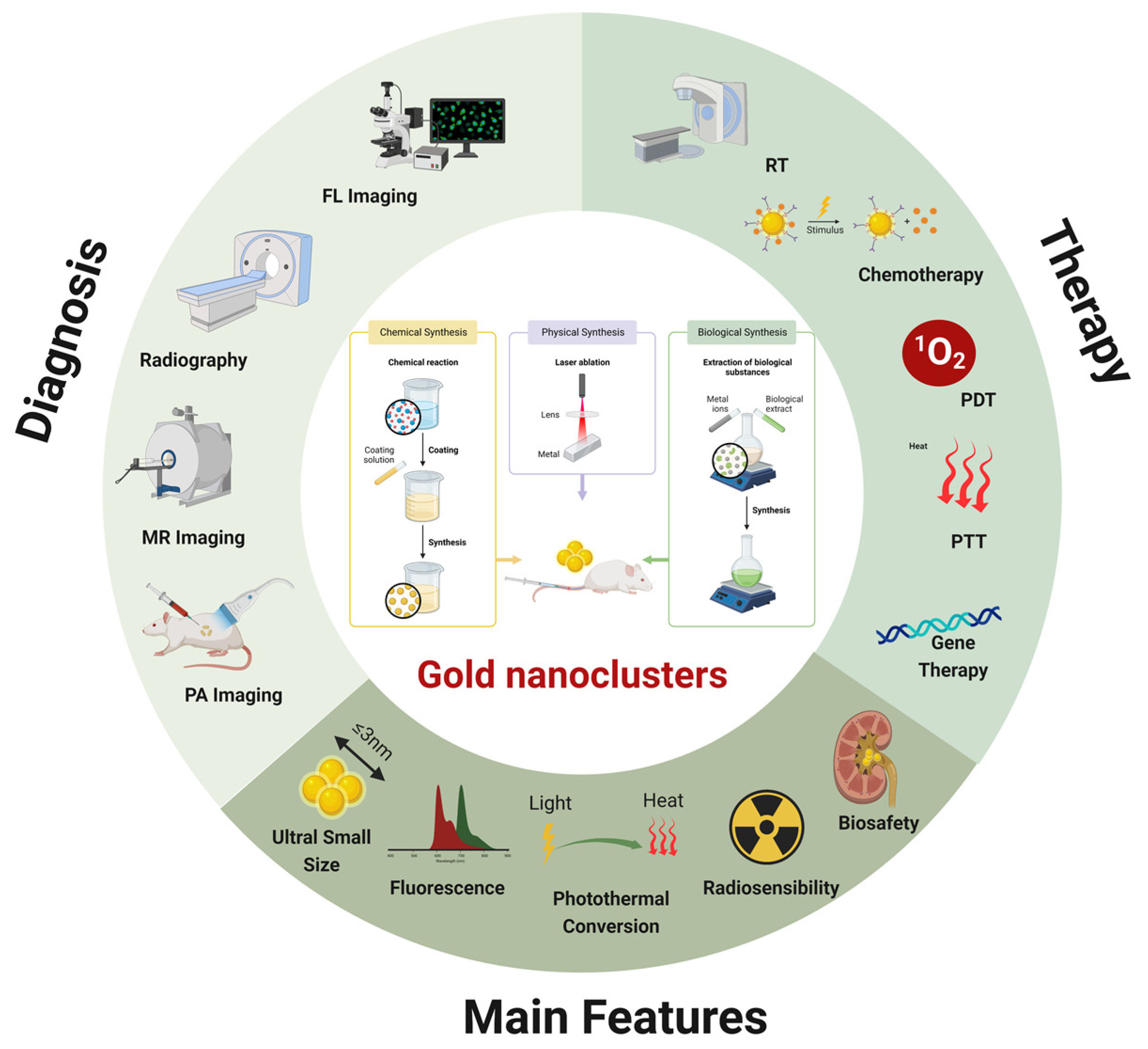
The Recent Development of Multifunctional Gold Nanoclusters in Tumor Theranostic and Combination Therapy
Abstract
1. Introduction
2. AuNCs as Imaging Agents in Tumor Theranostic
2.1. Fluorescence Imaging
2.2. Radiography
2.3. Multi-Modal Imaging
3. AuNCs as Transport Agents in Combined Therapy
3.1. Tumor Microenvironment Response
3.2. Photoactivation
3.3. Nuclear Targeting
4. AuNCs as Therapeutic Agents in Combined Therapy
4.1. Radiosensitization
4.2. Photothermal Conversion
5. Conclusions
| Multifunctional Nanoplatform | Role of AuNCs | Therapeutic Agent | Size (nm) | Imaging Mode | Cancer Types | Therapy Method | Activity | Ref |
|---|---|---|---|---|---|---|---|---|
| AuNCs-Ag@Keratin-Gd | Imaging | NM | 5 | FL, MRI | Breast cancer | Chemotherapy | In vivo and in vitro | [37] |
| CDGM NPs | Imaging, drug delivery | CAD, Ce6 | 2 | FL | Lung cancer | PDT | In vivo and in vitro | [54] |
| AuS-U11 | PTT-carrier | U11 peptide, cyanine dye Cy5.5, 5-ALA | 10 | FL | Pancreatic carcinoma | PTT, PDT | In vivo and in vitro | [55] |
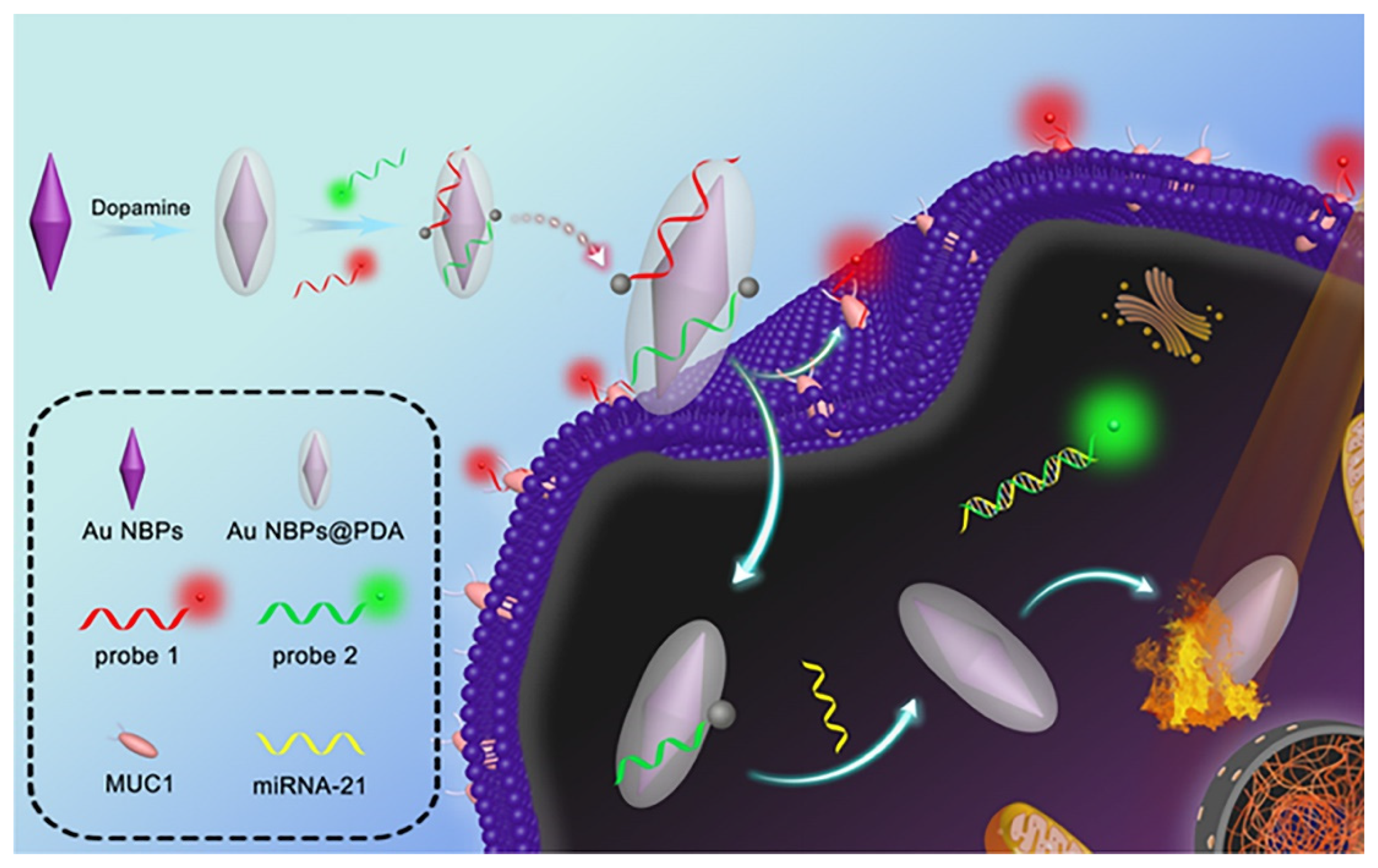
| Au NBPs@PDA/AuNCs | Imaging | Au NBPs@PDA | 2.1, 3.3 | FL | Breast cancer, hepatocarcinoma | PTT | In vitro | [56] |
| Dox@HG-CAHs | Imaging | HA-ALD, Dox | 2.8 | FL, CT | Osteosarcoma | PTT, chemotherapy | In vivo and in vitro | [61] |
| AuNCs–LHRHa | Imaging, PTT | LHRH analogues | 2.4 | FL, CT | Prostatic cancer | PTT | In vitro | [67] |
| GTSL-CYC-HER2 | Changed the zeta potential of liposomes, superior photothermal effect | HER2-modified thermosensitive liposome, cyclopamine | NA | CT, PTI | Breast cancer | Chemotherapy, PTT | In vivo and in vitro | [68] |
| Ce6&AuNCs/Gd-LDH | Imaging | Ce6 | ~2 | MRI, FL | Hepatocarcinoma | PDT | In vivo and in vitro | [70] |
| AuNCs-ICG | Imaging, radiosensitizing effects | ICG | ~1 | FL, PAI, CT | Breast cancer | PDT, RT | In vivo and in vitro | [72] |
| Qu-GNCs | Imaging | Qu | 1–3 | FL | Lung cancer | Chemotherapy | In vitro | [74] |
| Fe3O4@PAA/AuNCs/ZIF-8 NPs | Imaging | DOX | NA | MRI, CT, FL | Hepatocarcinoma | Chemotherapy | In vivo and in vitro | [75] |
| AuNCs@GTMS-FA | Imaging, phototherapeutic agents | FA | 2.8 | FL | Breast cancer | PTT, PDT | In vitro | [80] |
| AuNCs/Dzs-Dox | NSET effect, shelter therapeutic cargos | Dzs-Dox | ~1.76 | FL | Breast cancer | Gene therapy, chemotherapy | In vivo and in vitro | [87] |
| HG-GNCs/GO-5FU | Bioimaging, phototherapeutic | HA, 5FU | 2 | FL | Lung cancer, breast cancer | Chemotherapy, PDT, PTT | In vitro | [93] |
| AuNCs@mSiO2@MnO2 | Photosensitizer | MnO2 nanozyme | NA | MRI | Breast cancer | PDT | In vivo and in vitro | [94] |
| Au8NC | Radiosensitizing effects | Levonorgestrel | ~2 | FL | Esophagus cancer | RT | In vivo and in vitro | [105] |
| Au4-IO NP-cRGD | Imaging, radiosensitizing effects | IO nanocluster | 2 | FL, MRI | Breast cancer | RT, chemotherapy | In vivo and in vitro | [113] |
| PML-MF nanocarrier | Imaging | IO@AuNPs | NA | FL | Cervical cancer | PPTT, chemotherapy | In vitro | [117] |
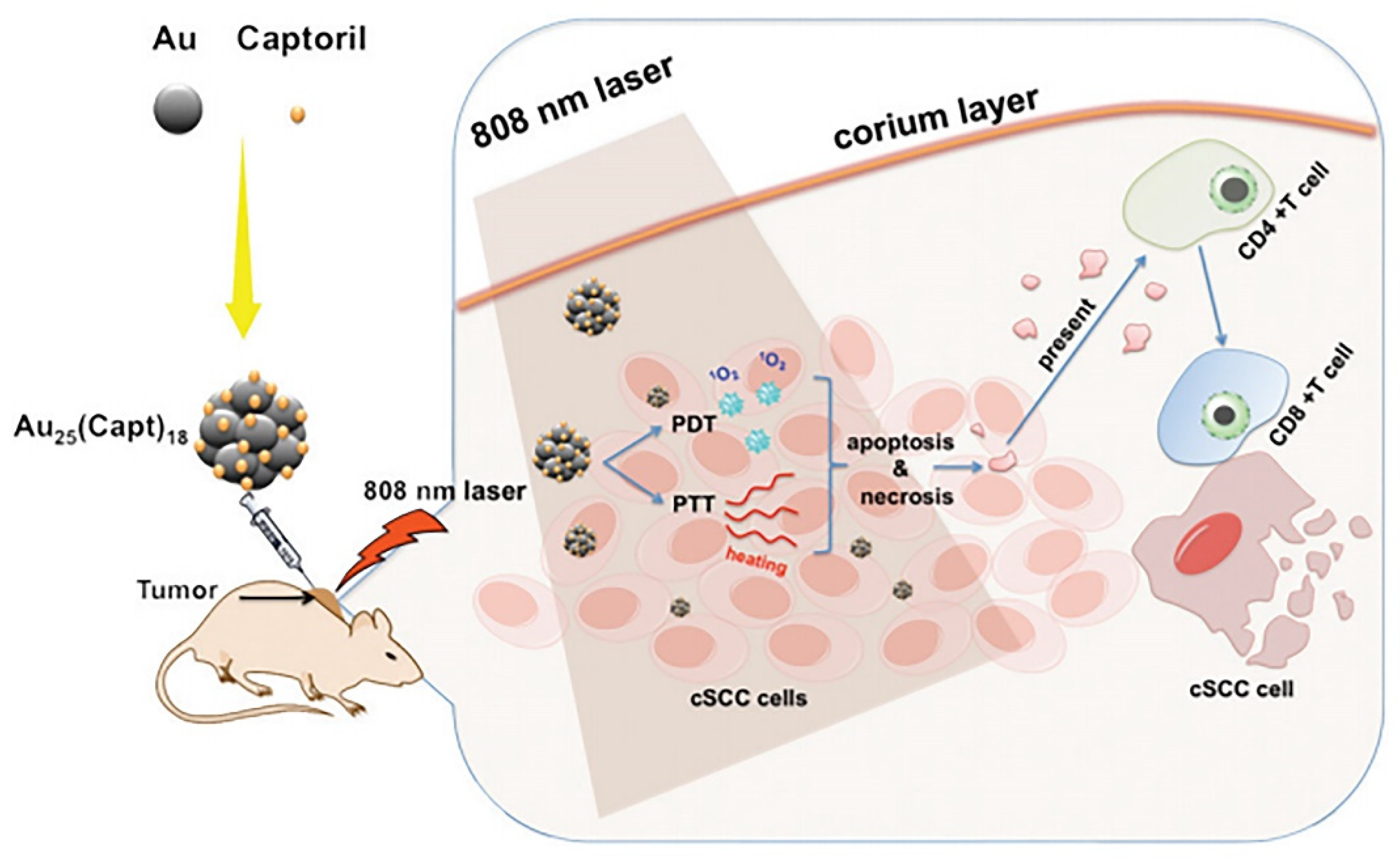
| WLPD-Au25 | Photosensitizer, drug delivery | WS2 nanoparticles, Dex, Captopril | 2.5 | CT | Breast cancer | PTT, PDT | In vivo | [118] |
| AuNCs/Cas9–gRNA | Imaging, drug delivery | Cas9–sgRNA plasmid | ~1.56 | FL | Osteosarcoma | Gene therapy | In vitro | [119] |
| K-AuNCs | Imaging, drug delivery | K | 1–3 | FL | Lung cancer | Chemotherapy | In vitro | [120] |
| EA-AB | Imaging | EB | NM | FL, MSOT Imaging | Breast cancer | Chemotherapy, PTT | In vivo and in vitro | [121] |
| Ce6-GNCs-Ab-CIK | Drug delivery | Ce6, CD3 antibody | NA | FL | Gastric cancer | Chemotherapy, PDT | In vivo and in vitro | [122] |
| Au4Cu4/Au25@Lip | Photothermogenesis effect, photoluminescence performance | Au4Cu4 nanoclusters | ~2 | FL, PTI | Cervical cancer | PTT, PDT | In vivo and in vitro | [123] |
| MB-loaded Au NC-mucin NPs | Imaging | MB | 1.9 ± 0.34 | FL | Cervical cancer | PDT | In vitro | [124] |
| ISQ@BSA-AuNC@AuNR@DAC@DR5 | SERS substrate | DAC, ISQ | NA | NM | Amelanotic Melanoma | PTT, PDT | In vivo and in vitro | [125] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Huang, X.; Wang, P.; Zhang, F.; Li, W.; Chen, G.; Chen, B. Novel iodinated gold nanoclusters for precise diagnosis of thyroid cancer. Nanoscale 2017, 9, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Park, J.H.; Lee, D.S. Stimuli-responsive polymersomes for cancer therapy. Biomater. Sci. 2016, 4, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Akgonullu, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Lu, M.; Dinel, M.P.; Blain, P.; Peng, W.; Gu, H.; Masson, J.F. Hybridization conditions of oligonucleotide-capped gold nanoparticles for SPR sensing of microRNA. Biosens. Bioelectron. 2018, 109, 230–236. [Google Scholar] [CrossRef]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Zhang, Z.; Huang, X.; Zhao, H.; Wei, J.; Li, F.; Yuan, K.; Su, L.; Xiong, Y. Green photoreduction synthesis of dispersible gold nanoparticles and their direct in situ assembling in multidimensional substrates for SERS detection. Mikrochim. Acta 2022, 189, 275. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Bulin, A.L.; Porret, E.; Musnier, B.; Chovelon, B.; Ravelet, C.; Sancey, L.; Elleaume, H.; Hainaut, P.; Coll, J.L.; et al. Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement. Nanoscale 2020, 12, 6959–6963. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, Y.; Qin, Z.; Li, C.; Jiang, H.; Wang, X. Fluorescence light up detection of aluminium ion and imaging in live cells based on the aggregation-induced emission enhancement of thiolated gold nanoclusters. Talanta 2019, 204, 548–554. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The synthesis of metal nanoclusters and their applications in bio-sensing and imaging. Methods Appl. Fluoresc. 2019, 8, 012001. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, N.; Li, M.; Huang, X.; Wu, P.; Hu, Z.; Shuai, J. Induced fluorescent enhancement of protein-directed synthesized gold nanoclusters for selective and sensitive detection of flame retardants. Sci. Total Environ. 2020, 713, 136488. [Google Scholar] [CrossRef]
- Guo, T.; Li, W.; Qian, L.; Yan, X.; Cui, D.; Zhao, J.; Ni, H.; Zhao, X.; Zhang, Z.; Li, X.; et al. Highly-selective detection of EGFR mutation gene in lung cancer based on surface enhanced Raman spectroscopy and asymmetric PCR. J. Pharm. Biomed. Anal. 2020, 190, 113522. [Google Scholar] [CrossRef] [PubMed]
- Nonappa, N. Luminescent gold nanoclusters for bioimaging applications. Beilstein. J. Nanotechnol. 2020, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wu, Q.; Jiang, Y.; Hou, M.; Zhang, P.; Liu, M.; Zhang, L.; Li, B.; Zhang, C. One-pot synthesis of (68)Ga-doped ultrasmall gold nanoclusters for PET/CT imaging of tumors. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112291. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. RGD peptide-modified fluorescent gold nanoclusters as highly efficient tumor-targeted radiotherapy sensitizers. Biomaterials 2017, 144, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Guan, R.; Su, H.; Zhang, L.; Liu, B.F.; Hu, Z.; Liu, X. Carbohydrate-protein template synthesized high mannose loading gold nanoclusters: A powerful fluorescence probe for sensitive Concanavalin A detection and specific breast cancer cell imaging. Talanta 2020, 218, 121130. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Shao, Z.S.; Song, Z.; Wang, Y.B.; Wang, H.S. Development of gold nanoclusters: From preparation to applications in the field of biomedicine. J. Mater. Chem. C 2020, 8, 14312–14333. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthc. Mater. 2014, 3, 133–141. [Google Scholar] [CrossRef]
- Ramesh, B.S.; Giorgakis, E.; Lopez-Davila, V.; Dashtarzheneha, A.K.; Loizidou, M. Detection of cell surface calreticulin as a potential cancer biomarker using near-infrared emitting gold nanoclusters. Nanotechnology 2016, 27, 285101. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, J.; Hwang, Y.H.; Xu, C.; Yu, T.; Zhang, R.; Cai, H.; Kim, D.P.; Qi, L. Synthesis of ficin-protected AuNCs in a droplet-based microreactor for sensing serum ferric ions. Talanta 2019, 200, 547–552. [Google Scholar] [CrossRef]
- Purohit, R.; Singh, S. Fluorescent gold nanoclusters for efficient cancer cell targeting. Int. J. Nanomed. 2018, 13, 15–17. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Schneider, M. Advances in biomedical and pharmaceutical applications of protein-stabilized gold nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Chen, J.T.; Yan, X.P. Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe. Anal. Chem. 2013, 85, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, J.M.; Hai, X.; Shu, Y.; Chen, X.W.; Wang, J.H. Protein-Stabilized Gadolinium Oxide-Gold Nanoclusters Hybrid for Multimodal Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6941–6949. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.M.; Craciun, A.M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic acid functionalized gold nanoclusters for enabling targeted fluorescence imaging of human ovarian cancer cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Q.; Zhou, Q.; Zhang, W.; Yue, P.; Xu, C.; Qin, X.; Yu, H.; Zhu, M. Cancer cell specific fluorescent methionine protected gold nanoclusters for in-vitro cell imaging studies. Talanta 2018, 188, 259–265. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Liu, Y.; Chen, B. Fabrication of folic acid-sensitive gold nanoclusters for turn-on fluorescent imaging of overexpression of folate receptor in tumor cells. Talanta 2016, 158, 118–124. [Google Scholar] [CrossRef]
- Xie, J.; Liang, R.; Li, Q.; Wang, K.; Hussain, M.; Dong, L.; Shen, C.; Li, H.; Shen, G.; Zhu, J.; et al. Photosensitizer-loaded gold nanocages for immunogenic phototherapy of aggressive melanoma. Acta Biomater. 2022, 142, 264–273. [Google Scholar] [CrossRef]
- Peng, L.H.; Niu, J.; Zhang, C.Z.; Yu, W.; Wu, J.H.; Shan, Y.H.; Wang, X.R.; Shen, Y.Q.; Mao, Z.W.; Liang, W.Q.; et al. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials 2014, 35, 5605–5618. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, C.; Yang, Y.; Zhang, J.; Gao, H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B 2019, 9, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Z.; Huang, Z.; Deng, H.; Chen, W.; Peng, H. Split-type electrochemiluminescent gene assay platform based on gold nanocluster probe for human papillomavirus diagnosis. Biosens. Bioelectron. 2021, 178, 113044. [Google Scholar] [CrossRef]
- Peng, H.P.; Jian, M.L.; Huang, Z.N.; Wang, W.J.; Deng, H.H.; Wu, W.H.; Liu, A.L.; Xia, X.H.; Chen, W. Facile electrochemiluminescence sensing platform based on high-quantum-yield gold nanocluster probe for ultrasensitive glutathione detection. Biosens. Bioelectron. 2018, 105, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Gao, P.; Zhang, K.Y.; Tong, X.; Yang, H.; Liu, S.; Du, J.; Zhao, Q.; Huang, W. Luminescent gold nanocluster-based sensing platform for accurate H2S detection in vitro and in vivo with improved anti-interference. Light Sci. Appl. 2017, 6, e17107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Zhao, R.; Hong, P.; Peng, S.; Zhang, X.; Wang, Y. A novel and sensitive ratiometric fluorescence assay for carbendazim based on N-doped carbon quantum dots and gold nanocluster nanohybrid. J. Hazard Mater. 2020, 386, 121958. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, Y.; Wei, L.; Wang, J.; Zhang, M.; Yang, X.; Wang, W.; Yang, G. The assembly of protein-templated gold nanoclusters for enhanced fluorescence emission and multifunctional applications. Acta Biomater. 2020, 101, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Matus, M.F.; Hakkinen, H. Atomically Precise Gold Nanoclusters: Towards an Optimal Biocompatible System from a Theoretical-Experimental Strategy. Small 2021, 17, e2005499. [Google Scholar] [CrossRef]
- Pigliacelli, C.; Acocella, A.; Diez, I.; Moretti, L.; Dichiarante, V.; Demitri, N.; Jiang, H.; Maiuri, M.; Ras, R.H.A.; Bombelli, F.B.; et al. High-resolution crystal structure of a 20 kDa superfluorinated gold nanocluster. Nat. Commun. 2022, 13, 2607. [Google Scholar] [CrossRef]
- Linko, V.; Zhang, H.; Nonappa; Kostiainen, M.A.; Ikkala, O. From Precision Colloidal Hybrid Materials to Advanced Functional Assemblies. Acc. Chem. Res. 2022, 55, 1785–1795. [Google Scholar] [CrossRef]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z.; et al. Cisplatin Prodrug-Conjugated Gold Nanocluster for Fluorescence Imaging and Targeted Therapy of the Breast Cancer. Theranostics 2016, 6, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sonia; Komal; Kukreti, S.; Kaushik, M. Gold nanoclusters: An ultrasmall platform for multifaceted applications. Talanta 2021, 234, 122623. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, M.; Zhang, S.; Li, J.; Shen, G.; Tu, Y.; Zhu, J.; Tao, J. Cytotoxicity of BSA-Stabilized Gold Nanoclusters: In Vitro and In Vivo Study. Small 2015, 11, 2571–2581. [Google Scholar] [CrossRef]
- Huang, T.H.; Zhao, F.Z.; Hu, Q.L.; Liu, Q.; Wu, T.C.; Zheng, D.; Kang, T.; Gui, L.C.; Chen, J. Bisphosphine-Stabilized Gold Nanoclusters with the Crown/Birdcage-Shaped Au11 Cores: Structures and Optical Properties. Inorg. Chem. 2020, 59, 16027–16034. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.A.; Jachimska, B.; Chen, Y. Polyallylamine hydrochloride coating enhances the fluorescence emission of Human Serum Albumin encapsulated gold nanoclusters. J. Photochem. Photobiol. B 2018, 187, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lou, X.; Yu, F.; Liu, H. Cross-linking structure-induced strong blue emissive gold nanoclusters for intracellular sensing. Analyst 2019, 144, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- You, J.G.; Tseng, W.L. Peptide-induced aggregation of glutathione-capped gold nanoclusters: A new strategy for designing aggregation-induced enhanced emission probes. Anal. Chim. Acta 2019, 1078, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.H.; Peng, H.P.; Huang, K.Y.; He, S.B.; Yuan, Q.F.; Lin, Z.; Chen, R.T.; Xia, X.H.; Chen, W. Self-Referenced Ratiometric Detection of Sulfatase Activity with Dual-Emissive Urease-Encapsulated Gold Nanoclusters. ACS Sens. 2019, 4, 344–352. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Ren, T.; Tong, Y.; Yang, B.; Li, Y. Novel bimetallic gold-silver nanoclusters with “Synergy”-enhanced fluorescence for cyanide sensing, cell imaging and temperature sensing. Talanta 2017, 170, 530–539. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Qi, Q.; Shi, H.; Hsu, E.C.; Chen, W.; Yuan, W.; Wu, Y.; Lin, S.; Zeng, Y.; et al. Gold Nanoclusters for NIR-II Fluorescence Imaging of Bones. Small 2020, 16, e2003851. [Google Scholar] [CrossRef]
- Meng, X.; Pang, X.; Zhang, K.; Gong, C.; Yang, J.; Dong, H.; Zhang, X. Recent Advances in Near-Infrared-II Fluorescence Imaging for Deep-Tissue Molecular Analysis and Cancer Diagnosis. Small 2022, 18, e2202035. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.M.; Craciun, A.M.; Focsan, M.; Vulpoi, A.; Borcan, E.L.; Astilean, S. Glutathione-capped gold nanoclusters as near-infrared-emitting efficient contrast agents for confocal fluorescence imaging of tissue-mimicking phantoms. Mikrochim. Acta 2022, 189, 337. [Google Scholar] [CrossRef]
- Wu, X.; He, X.; Wang, K.; Xie, C.; Zhou, B.; Qing, Z. Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo. Nanoscale 2010, 2, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Hou, W.; Zhang, C.; Zhi, X.; Cheng, J.; de la Fuente, J.M.; Song, J.; Cui, D. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018, 68, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Deng, Y.; Zeng, M.; Tang, Y.; Zhu, W.H.; Cheng, Y. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials 2017, 139, 30–38. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Liu, P.; Xu, S.; Luo, X. Core-Shell Multifunctional Nanomaterial-Based All-in-One Nanoplatform for Simultaneous Multilayer Imaging of Dual Types of Tumor Biomarkers and Photothermal Therapy. Anal. Chem. 2020, 92, 15169–15178. [Google Scholar] [CrossRef]
- Schockel, L.; Jost, G.; Seidensticker, P.; Lengsfeld, P.; Palkowitsch, P.; Pietsch, H. Developments in X-Ray Contrast Media and the Potential Impact on Computed Tomography. Invest. Radiol. 2020, 55, 592–597. [Google Scholar] [CrossRef]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Smilowitz, N.R.; Davis, J.; Smilowitz, H.M. Small, Long Blood Half-Life Iodine Nanoparticle for Vascular and Tumor Imaging. Sci. Rep. 2018, 8, 13803. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer. Small 2019, 15, e1900968. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Zhao, Y.; Zhao, Y.; Li, X.; He, L.; Zvyagin, A.V.; Yang, B.; Lin, Q.; Ma, X. Lotus Seedpod-Inspired Crosslinking-Assembled Hydrogels Based on Gold Nanoclusters for Synergistic Osteosarcoma Multimode Imaging and Therapy. ACS Appl. Mater. Interfaces 2022, 14, 34377–34387. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, e2001806. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.R. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Park, S.; Lim, K.; Kim, S.S.; Kim, J.O.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Facile fabrication of highly photothermal-effective albumin-assisted gold nanoclusters for treating breast cancer. Int. J. Pharm. 2018, 553, 363–374. [Google Scholar] [CrossRef]
- Duan, Q.; Yang, M.; Zhang, B.; Li, Y.; Zhang, Y.; Li, X.; Wang, J.; Zhang, W.; Sang, S. Gold nanoclusters modified mesoporous silica coated gold nanorods: Enhanced photothermal properties and fluorescence imaging. J. Photochem. Photobiol. B 2021, 215, 112111. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Yang, Y.; Xie, X.; Wang, L.; Deng, K.; Xia, X.; Yang, X.; Huang, H. Prompting peroxidase-like activity of gold nanorod composites by localized surface plasmon resonance for fast colorimetric detection of prostate specific antigen. Analyst 2018, 143, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, L.; Che, S.; Xing, H.; Guan, L.; Yang, Z.; Li, X.; Zvyagin, A.V.; Lin, Q.; Qu, W. AuNCs-LHRHa nano-system for FL/CT dual-mode imaging and photothermal therapy of targeted prostate cancer. J. Mater. Chem. B 2022, 10, 5182–5190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Hu, Y.; Xia, Y.; Li, Z.; Lu, Y.; Shen, Y. “Petal-like” size-tunable gold wrapped immunoliposome to enhance tumor deep penetration for multimodal guided two-step strategy. J. Nanobiotechnol. 2021, 19, 293. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Liu, H.; Zhou, Z.; Huang, J.; Lei, S.; Cai, S.; Chen, Z.; Guo, Y.; Chen, Z.; et al. Porous gold nanocluster-decorated manganese monoxide nanocomposites for microenvironment-activatable MR/photoacoustic/CT tumor imaging. Nanoscale 2018, 10, 3631–3638. [Google Scholar] [CrossRef]
- Mei, X.; Wang, W.; Yan, L.; Hu, T.; Liang, R.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. Hydrotalcite monolayer toward high performance synergistic dual-modal imaging and cancer therapy. Biomaterials 2018, 165, 14–24. [Google Scholar] [CrossRef]
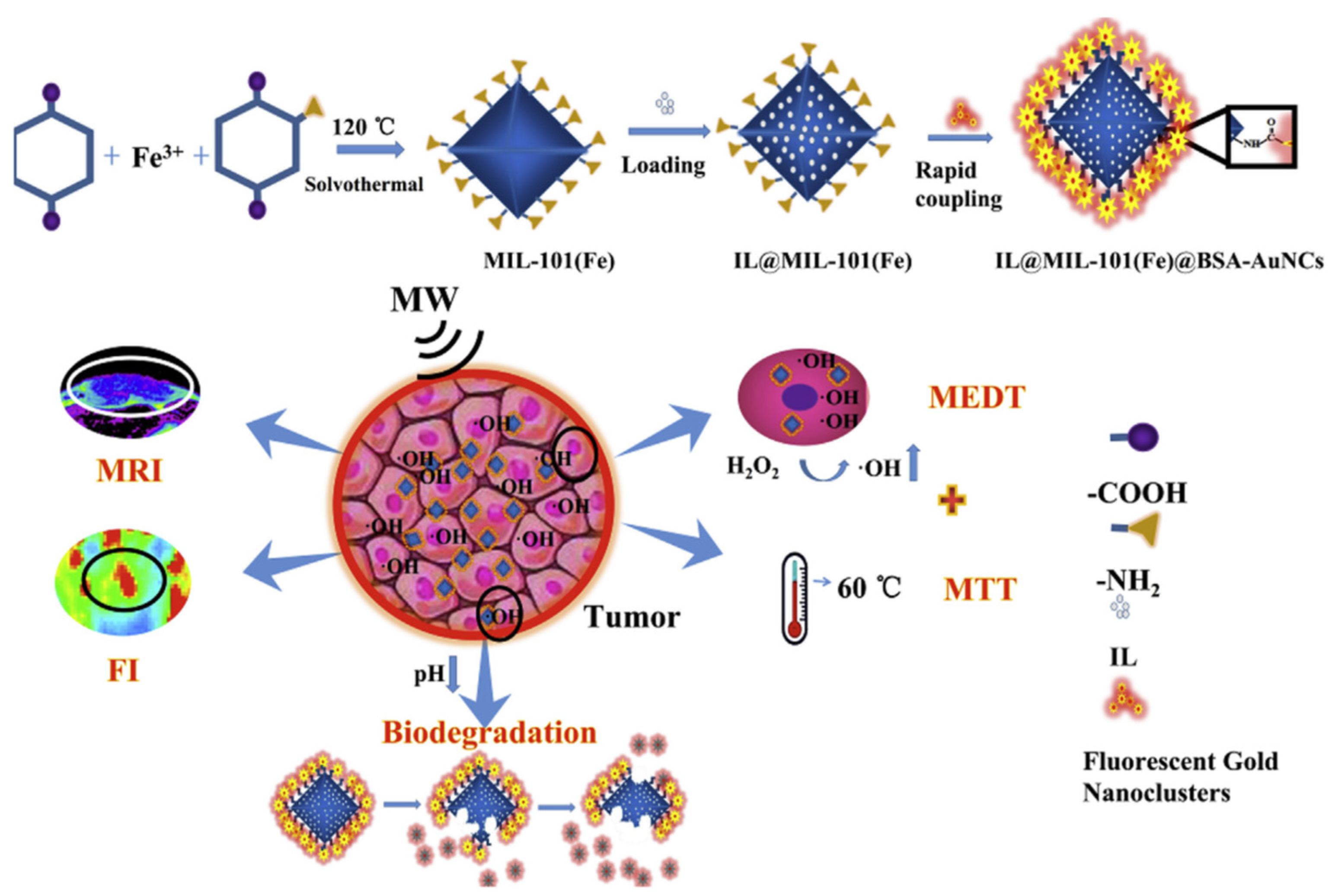
- Ma, X.; Ren, X.; Guo, X.; Fu, C.; Wu, Q.; Tan, L.; Li, H.; Zhang, W.; Chen, X.; Zhong, H.; et al. Multifunctional iron-based Metal-Organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy. Biomaterials 2019, 214, 119223. [Google Scholar] [CrossRef] [PubMed]
- Dan, Q.; Hu, D.; Ge, Y.; Zhang, S.; Li, S.; Gao, D.; Luo, W.; Ma, T.; Liu, X.; Zheng, H.; et al. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater. Sci. 2020, 8, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Wan, A. Luminescent gold nanoclusters for in vivo tumor imaging. Analyst 2020, 145, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Kim, S. Quercetin mediated gold nanoclusters explored as a dual functional nanomaterial in anticancer and bio-imaging disciplines. Colloids Surf. B Biointerfaces 2019, 178, 230–237. [Google Scholar] [CrossRef]
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal-organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278. [Google Scholar] [CrossRef]
- Jiang, M.; Lin, Y.; Fang, X.; Liu, M.; Ma, L.; Liu, J.; Chen, M.; Yang, Y.; Wang, C. Enhancement of gold-nanocluster-mediated chemotherapeutic efficiency of cisplatin in lung cancer. J. Mater. Chem. B 2021, 9, 4895–4905. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Shang, L.; Yang, C.; Kong, L.; Zhang, Z. Green tea extract-assembled nanoclusters for combinational photothermal and chemotherapy. J. Mater. Chem. B 2019, 7, 5972–5982. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Luo, F.; Wu, T.; Xu, P.; Zou, M.; Yan, J. Biosynthesis of flower-shaped Au nanoclusters with EGCG and their application for drug delivery. J. Nanobiotechnol. 2018, 16, 90. [Google Scholar] [CrossRef]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor microenvironment and nanotherapeutics. Transl. Cancer Res. 2013, 2, 309–319. [Google Scholar]
- Ovais, M.; Mukherjee, S.; Pramanik, A.; Das, D.; Mukherjee, A.; Raza, A.; Chen, C. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv. Mater. 2020, 32, e2000055. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef] [PubMed]
- Suwa, T.; Kobayashi, M.; Nam, J.-M.; Harada, H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Srinivasulu, Y.G.; Mozhi, A.; Goswami, N.; Yao, Q.; Xie, J. Traceable Nanocluster-Prodrug Conjugate for Chemo-photodynamic Combinatorial Therapy of Non-small Cell Lung Cancer. ACS Appl. Bio Mater. 2021, 4, 3232–3245. [Google Scholar] [CrossRef]
- Latorre, A.; Latorre, A.; Castellanos, M.; Rodriguez Diaz, C.; Lazaro-Carrillo, A.; Aguado, T.; Lecea, M.; Romero-Perez, S.; Calero, M.; Sanchez-Puelles, J.M.; et al. Multifunctional Albumin-Stabilized Gold Nanoclusters for the Reduction of Cancer Stem Cells. Cancers 2019, 11, 969. [Google Scholar] [CrossRef]
- Sun, H.; Ma, W.; Duan, S.; Huang, J.; Jia, R.; Cheng, H.; Chen, B.; He, X.; Wang, K. An endogenous stimulus detonated nanocluster-bomb for contrast-enhanced cancer imaging and combination therapy. Chem. Sci. 2021, 12, 12118–12129. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef]
- Song, J.; Huang, P.; Duan, H.; Chen, X. Plasmonic Vesicles of Amphiphilic Nanocrystals: Optically Active Multifunctional Platform for Cancer Diagnosis and Therapy. Acc. Chem. Res. 2015, 48, 2506–2515. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- D’Acunto, M. Detection of Intracellular Gold Nanoparticles: An Overview. Materials 2018, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Tabero, A.; Planas, O.; Gallavardin, T.; Nieves, I.; Nonell, S.; Villanueva, A. Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy. Nanomaterials 2020, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, C.; Tian, C.; Shen, Y.; Zhu, M. Engineered Targeted Hyaluronic Acid-Glutathione-Stabilized Gold Nanoclusters/Graphene Oxide-5-Fluorouracil as a Smart Theranostic Platform for Stimulus-Controlled Fluorescence Imaging-Assisted Synergetic Chemo/Phototherapy. Chem. Asian J. 2019, 14, 1418–1423. [Google Scholar] [CrossRef]
- Yin, Z.; Ji, Q.; Wu, D.; Li, Z.; Fan, M.; Zhang, H.; Zhao, X.; Wu, A.; Cheng, L.; Zeng, L. H2O2-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for “Off/On” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 14928–14937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Xu, C.; Xie, A.; Shen, Y.; Zhu, M. Improved fluorescence imaging and synergistic anticancer phototherapy of hydrosoluble gold nanoclusters assisted by a novel two-level mesoporous canal structured silica nanocarrier. Chem. Commun. 2018, 54, 2731–2734. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Chen, Y.; Li, C.; Jiang, H.; Wang, X. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Irudayaraj, J. Nuclear targeting dynamics of gold nanoclusters for enhanced therapy of HER2+ breast cancer. ACS Nano 2011, 5, 9718–9725. [Google Scholar] [CrossRef]
- Gao, P.; Wu, S.; Chang, X.; Liu, F.; Zhang, T.; Wang, B.; Zhang, K.Q. Aprotinin Encapsulated Gold Nanoclusters: A Fluorescent Bioprobe with Dynamic Nuclear Targeting and Selective Detection of Trypsin and Heavy Metal. Bioconjug. Chem. 2018, 29, 4140–4148. [Google Scholar] [CrossRef]
- Tan, H.; Liu, S.; He, Y.; Cheng, G.; Zhang, Y.; Wei, X.; Hu, L. Spider Toxin Peptide-Induced NIR Gold Nanocluster Fabrication for GSH-Responsive Cancer Cell Imaging and Nuclei Translocation. Front Bioeng. Biotechnol. 2021, 9, 780223. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Smialek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Taggart, L.E.; McMahon, S.J.; Butterworth, K.T.; Currell, F.J.; Schettino, G.; Prise, K.M. Protein disulphide isomerase as a target for nanoparticle-mediated sensitisation of cancer cells to radiation. Nanotechnology 2016, 27, 215101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Jia, T.T.; Yang, G.; Mo, S.J.; Wang, Z.Y.; Li, B.J.; Ma, W.; Guo, Y.X.; Chen, X.; Zhao, X.; Liu, J.Q.; et al. Atomically Precise Gold-Levonorgestrel Nanocluster as a Radiosensitizer for Enhanced Cancer Therapy. ACS Nano 2019, 13, 8320–8328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Jiang, Y.W.; Ma, N.; Xia, L.Y.; Cheng, X.; Jia, H.R.; Liu, P.; Gu, N.; Chen, Z.; et al. Glutathione-Depleting Gold Nanoclusters for Enhanced Cancer Radiotherapy through Synergistic External and Internal Regulations. ACS Appl. Mater. Interfaces 2018, 10, 10601–10606. [Google Scholar] [CrossRef]
- Herold, D.M.; Das, I.J.; Stobbe, C.C.; Iyer, R.V.; Chapman, J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364. [Google Scholar]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Deliv. 2022, 19, 199–212. [Google Scholar] [CrossRef]
- Samani, R.K.; Tavakoli, M.B.; Maghsoudinia, F.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Trastuzumab and folic acid functionalized gold nanoclusters as a dual-targeted radiosensitizer for megavoltage radiation therapy of human breast cancer. Eur. J. Pharm. Sci. 2020, 153, 105487. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Walker, E.; Springer, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Chemoradiotherapy of Prostate Cancer Using Gold Nanoclusters with Protease Activatable Monomethyl Auristatin E. ACS Appl. Mater. Interfaces 2022, 14, 14916–14927. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Du, X.; Jia, B.; Zhang, C.; Li, W.; Liu, T.C.; Li, Y.Q. A transformable gold nanocluster aggregate-based synergistic strategy for potentiated radiation/gene cancer therapy. J. Mater. Chem. B 2021, 9, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wang, Y.; Kang, X.; Xu, F.; Han, Z.; Zhang, C.; Wang, Z.Y.; Liu, J.Q.; Zhao, X.; Chen, X.; et al. A multifunctional AIE gold cluster-based theranostic system: Tumor-targeted imaging and Fenton reaction-assisted enhanced radiotherapy. J. Nanobiotechnol. 2021, 19, 438. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, F.; Kefayat, A.; Shahbazi-Gahrouei, D.; Motaghi, H.; Mehrgardi, M.A.; Haghjooy-Javanmard, S. AS1411 aptamer-targeted gold nanoclusters effect on the enhancement of radiation therapy efficacy in breast tumor-bearing mice. Nanomedicine 2018, 13, 2563–2578. [Google Scholar] [CrossRef]
- Chen, J.; Gong, M.; Fan, Y.; Feng, J.; Han, L.; Xin, H.L.; Cao, M.; Zhang, Q.; Zhang, D.; Lei, D.; et al. Collective Plasmon Coupling in Gold Nanoparticle Clusters for Highly Efficient Photothermal Therapy. ACS Nano 2022, 16, 910–920. [Google Scholar] [CrossRef]
- Nair, R.V.; Puthiyaparambath, M.F.; Chatanathodi, R.; Nair, L.V.; Jayasree, R.S. A nanoarchitecture of a gold cluster conjugated gold nanorod hybrid system and its application in fluorescence imaging and plasmonic photothermal therapy. Nanoscale 2022, 14, 13561–13569. [Google Scholar] [CrossRef]
- Pan, U.N.; Sanpui, P.; Paul, A.; Chattopadhyay, A. Protein-Nanoparticle Agglomerates as a Plasmonic Magneto-Luminescent Multifunctional Nanocarrier for Imaging and Combination Therapy. ACS Appl. Bio Mater. 2019, 2, 3144–3152. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Q.; Geng, S.; Lou, R.; Yin, Q.; Ye, W. Construction and evaluation of tumor nucleus-targeting nanocomposite for cancer dual-mode imaging—Guiding photodynamic therapy/photothermal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 541–551. [Google Scholar] [CrossRef]
- Tao, Y.; Yi, K.; Hu, H.; Shao, D.; Li, M. Coassembly of nucleus-targeting gold nanoclusters with CRISPR/Cas9 for simultaneous bioimaging and therapeutic genome editing. J. Mater. Chem. B 2021, 9, 94–100. [Google Scholar] [CrossRef]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, Y.; Lin, G.; Huang, S.; Zeng, F.; Wu, S. A Gold Nanocage/Cluster Hybrid Structure for Whole-Body Multispectral Optoacoustic Tomography Imaging, EGFR Inhibitor Delivery, and Photothermal Therapy. Small 2019, 15, e1900309. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Wang, X.; Liu, X.; Cheng, H.; Wang, P.; Shen, Y.; Xie, A.; Zhu, M. Self-assembled Au4Cu4/Au25 NCs@liposome tumor nanotheranostics with PT/fluorescence imaging-guided synergetic PTT/PDT. J. Mater. Chem. B 2021, 9, 6396–6405. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sailapu, S.K.; Simon, A.T.; Ghosh, S.S.; Chattopadhyay, A. Gold-Nanocluster-Embedded Mucin Nanoparticles for Photodynamic Therapy and Bioimaging. Langmuir 2019, 35, 10475–10483. [Google Scholar] [CrossRef] [PubMed]
- Sujai, P.T.; Joseph, M.M.; Karunakaran, V.; Saranya, G.; Adukkadan, R.N.; Shamjith, S.; Thomas, R.; Nair, J.B.; Swathi, R.S.; Maiti, K.K. Biogenic Cluster-Encased Gold Nanorods as a Targeted Three-in-One Theranostic Nanoenvelope for SERS-Guided Photochemotherapy against Metastatic Melanoma. ACS Appl. Bio Mater. 2019, 2, 588–600. [Google Scholar] [CrossRef]
- Zuber, G.; Weiss, E.; Chiper, M. Biocompatible gold nanoclusters: Synthetic strategies and biomedical prospects. Nanotechnology 2019, 30, 352001. [Google Scholar] [CrossRef]
- Guo, Y.; Amunyela, H.; Cheng, Y.; Xie, Y.; Yu, H.; Yao, W.; Li, H.W.; Qian, H. Natural protein-templated fluorescent gold nanoclusters: Syntheses and applications. Food Chem. 2021, 335, 127657. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Chen, B.; Zhao, G. Highly fluorescent gold nanoclusters stabilized by food proteins: From preparation to application in detection of food contaminants and bioactive nutrients. Crit. Rev. Food Sci. Nutr. 2018, 58, 689–699. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, J.; Song, Y.; He, S.; Tan, H. The Recent Development of Multifunctional Gold Nanoclusters in Tumor Theranostic and Combination Therapy. Pharmaceutics 2022, 14, 2451. https://doi.org/10.3390/pharmaceutics14112451
Liu S, Wang J, Song Y, He S, Tan H. The Recent Development of Multifunctional Gold Nanoclusters in Tumor Theranostic and Combination Therapy. Pharmaceutics. 2022; 14(11):2451. https://doi.org/10.3390/pharmaceutics14112451
Chicago/Turabian StyleLiu, Sisi, Junyao Wang, Yuxin Song, Shuya He, and Huaxin Tan. 2022. "The Recent Development of Multifunctional Gold Nanoclusters in Tumor Theranostic and Combination Therapy" Pharmaceutics 14, no. 11: 2451. https://doi.org/10.3390/pharmaceutics14112451
APA StyleLiu, S., Wang, J., Song, Y., He, S., & Tan, H. (2022). The Recent Development of Multifunctional Gold Nanoclusters in Tumor Theranostic and Combination Therapy. Pharmaceutics, 14(11), 2451. https://doi.org/10.3390/pharmaceutics14112451





