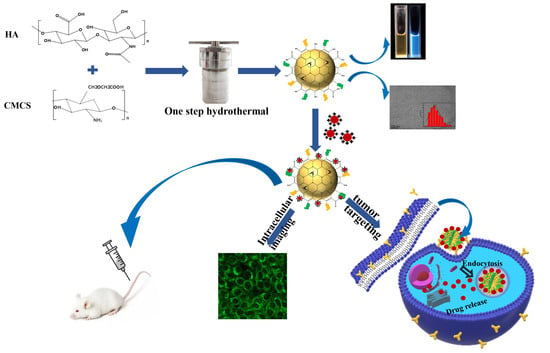
Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Carbon Dots (CDC-H)
2.2.2. Preparation of DOX–CDC-H Complexes
2.2.3. Characterization
2.2.4. Fluorescence Quantum Yields
2.2.5. Buffering Ability
2.2.6. Drug Release Studies In Vitro
2.2.7. In Vitro Cytotoxicity Assay
2.2.8. Laser Scanning Confocal Microscopy Studies
2.2.9. In Vivo Antitumor Activity
2.2.10. Statistical Analysis
3. Results
3.1. Results and Discussion
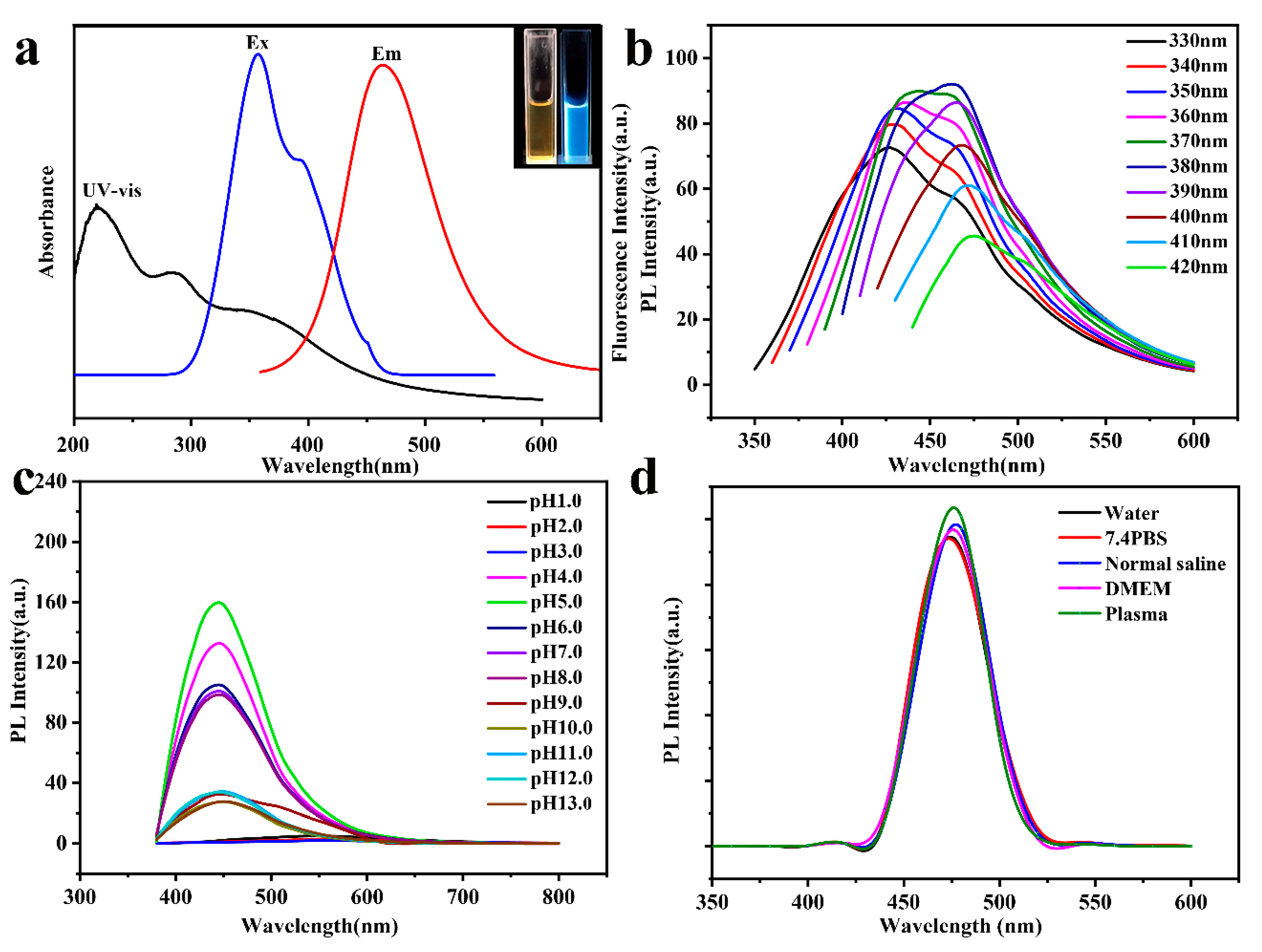
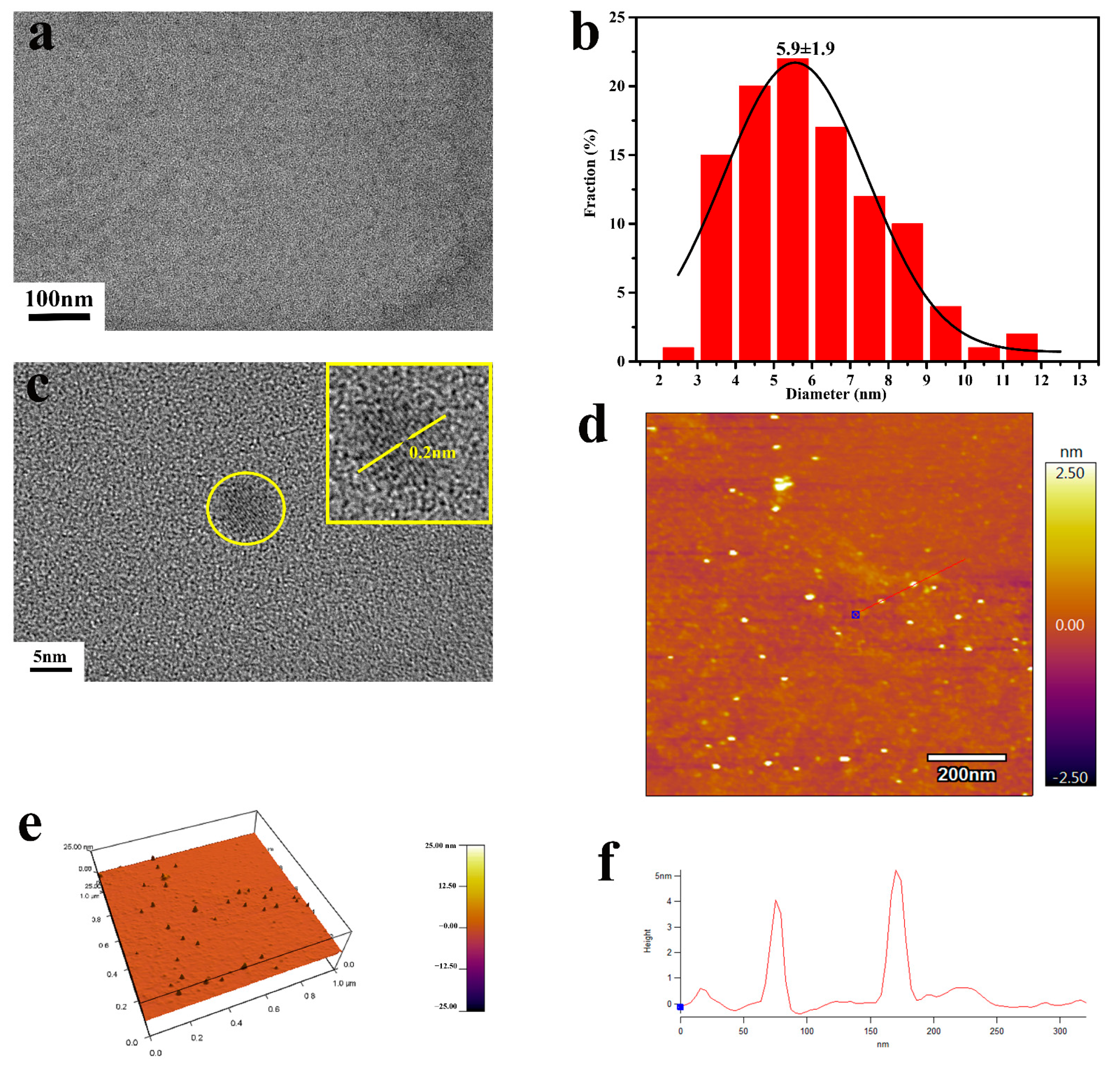
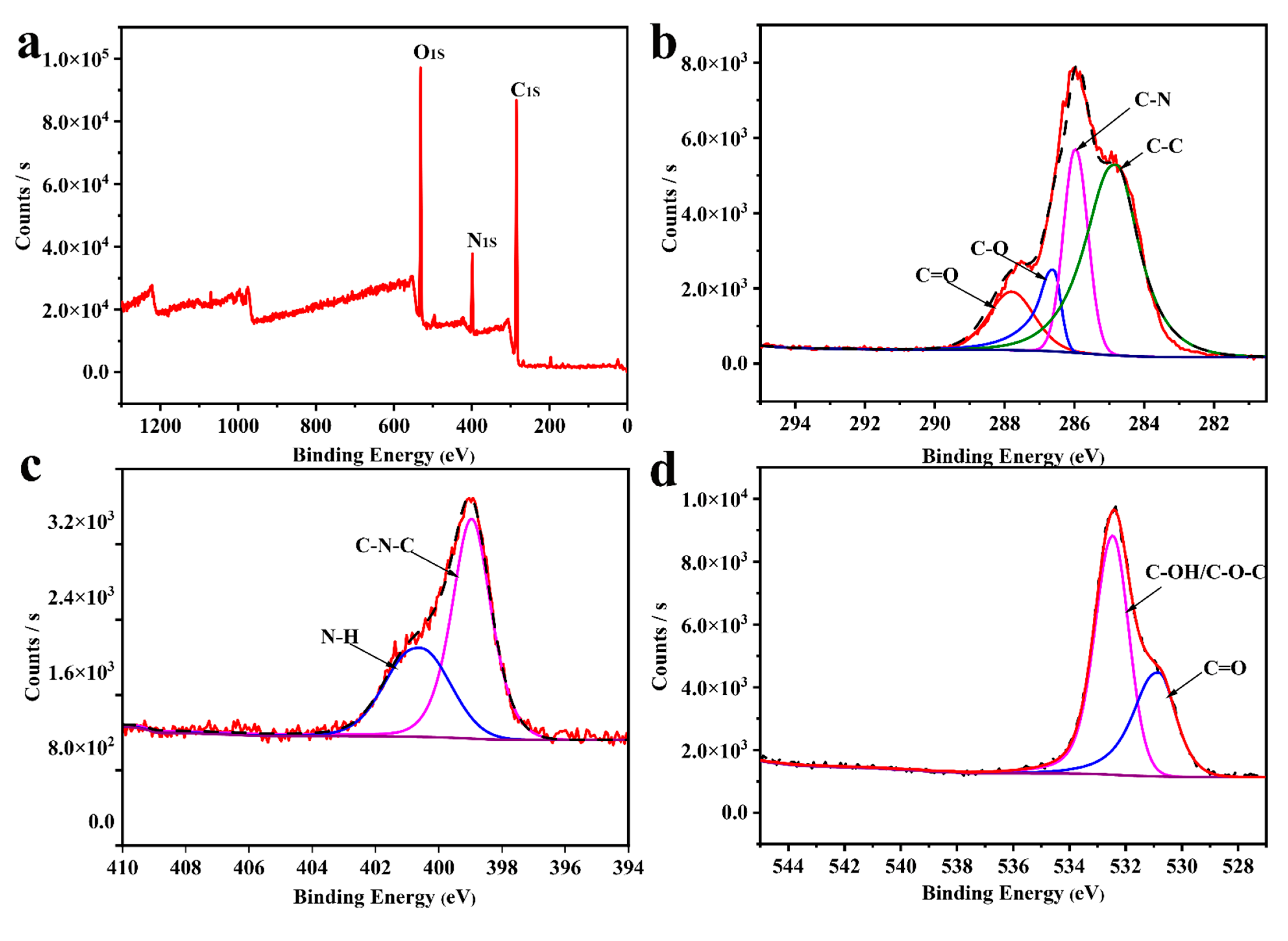
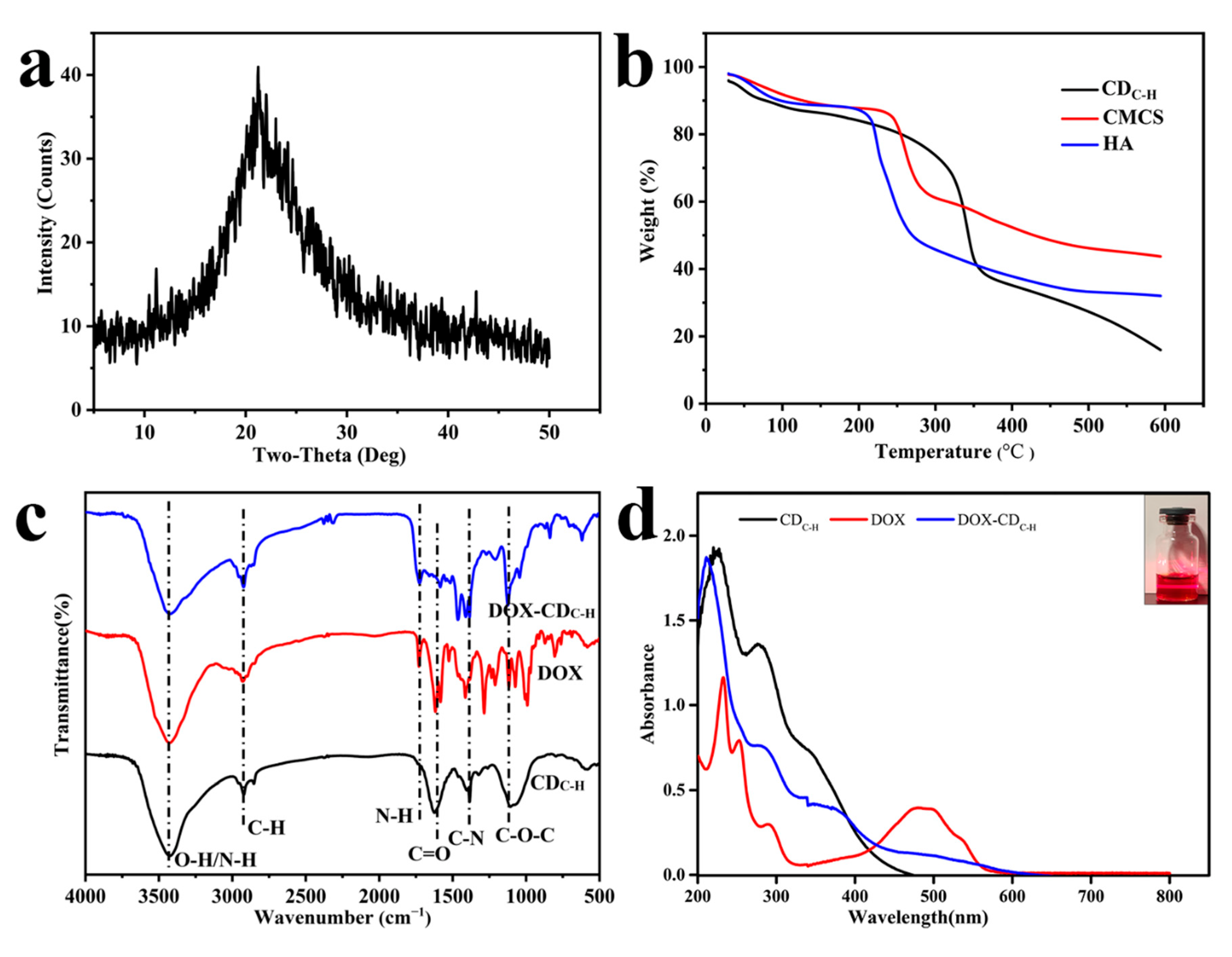
3.1.1. Preparation and Characterization of CDC-H and DOX–CDC-H
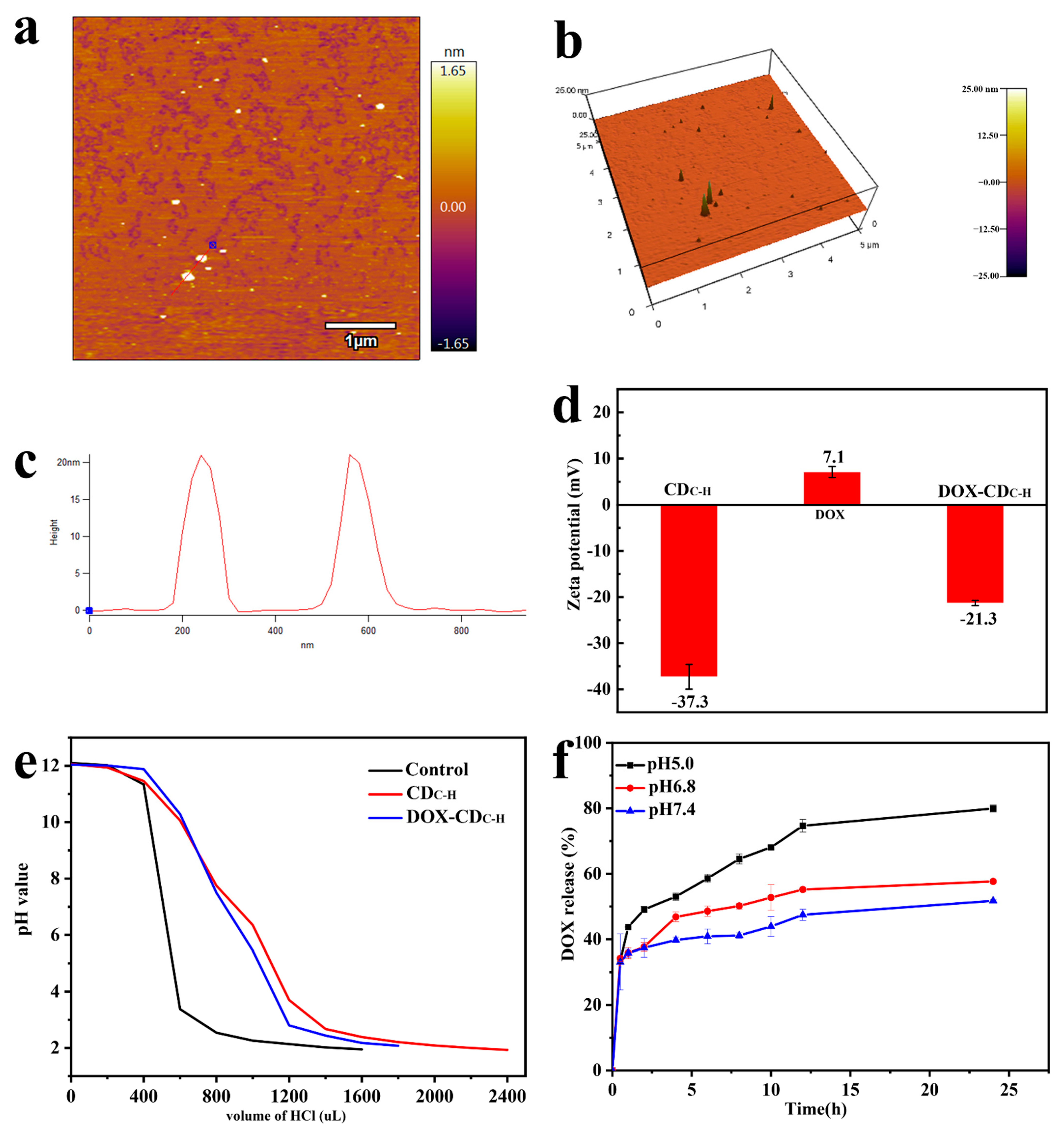
3.1.2. Buffering Ability of CDC-H and DOX–CDC-H
3.1.3. Drug-Loading and Release
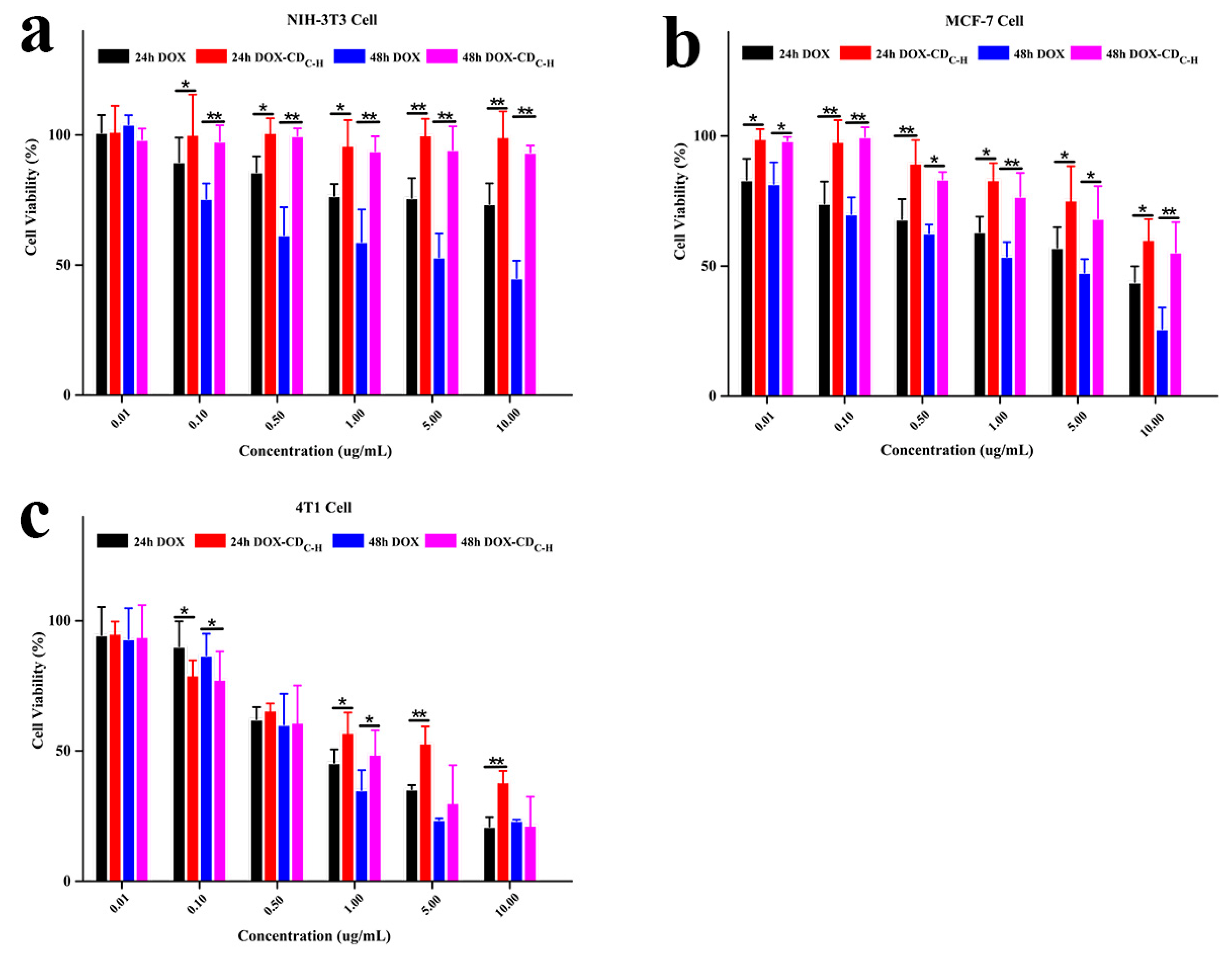
3.1.4. Cell Viability
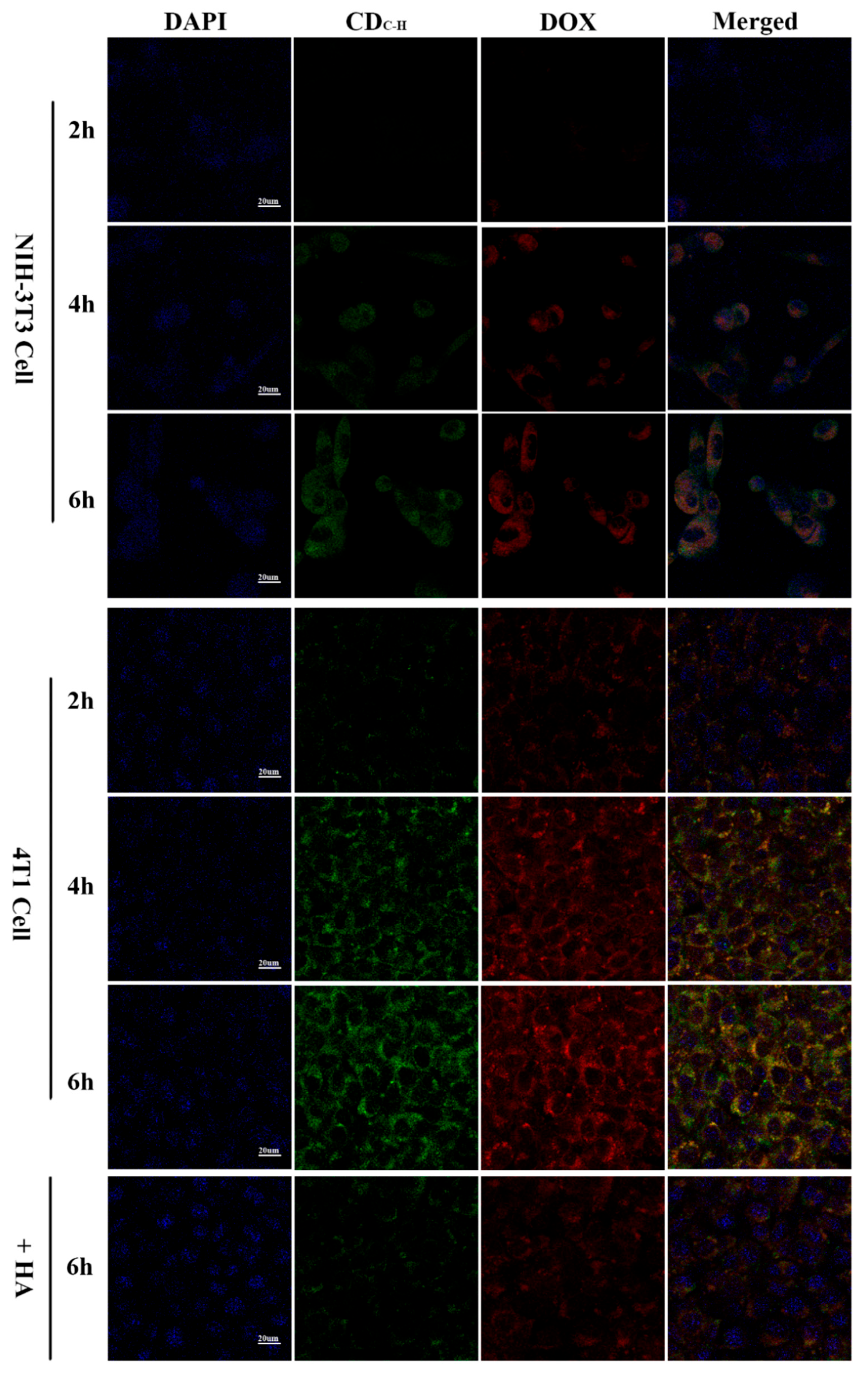
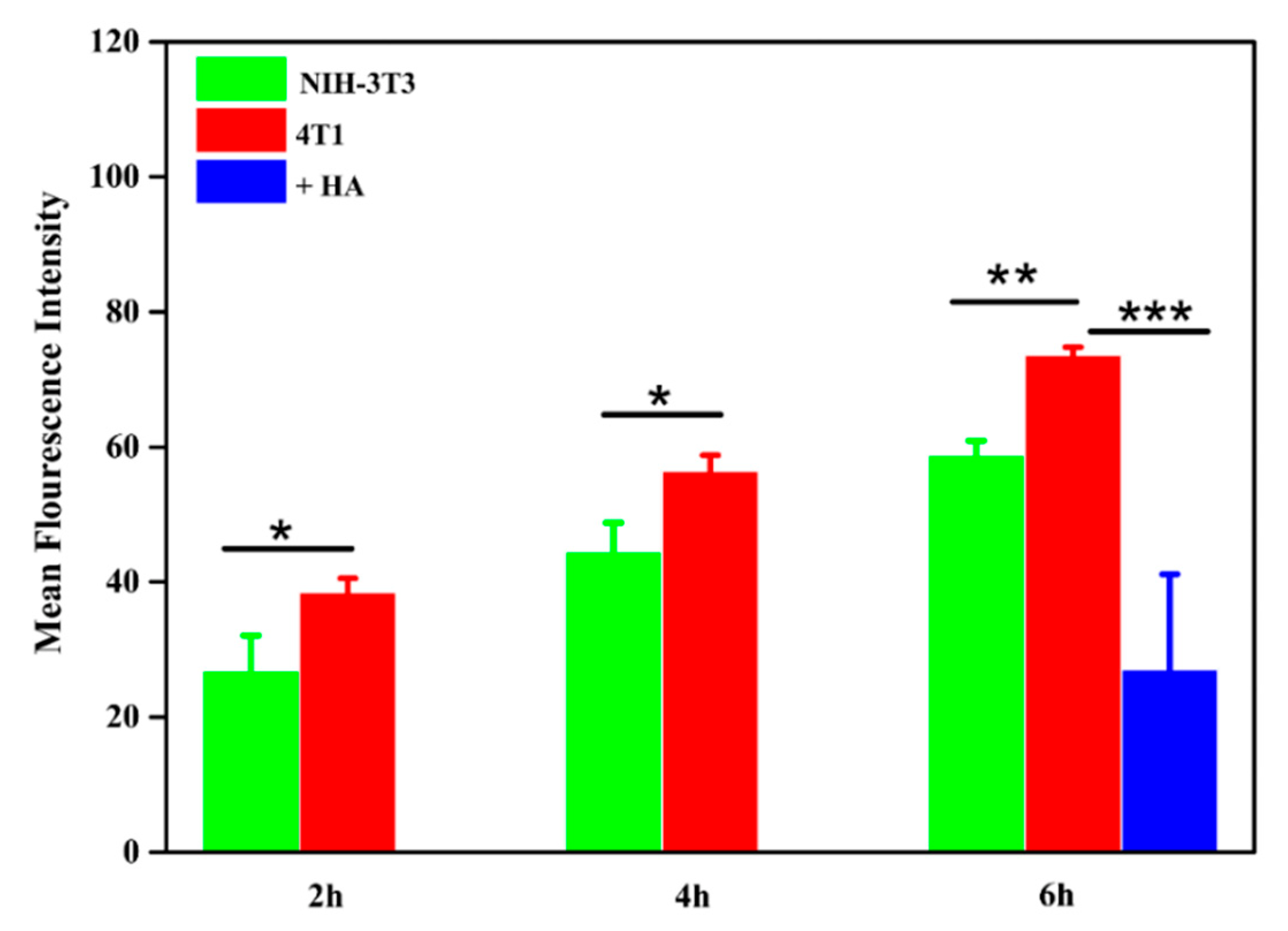
3.1.5. Cell Imaging and Cellular Uptake
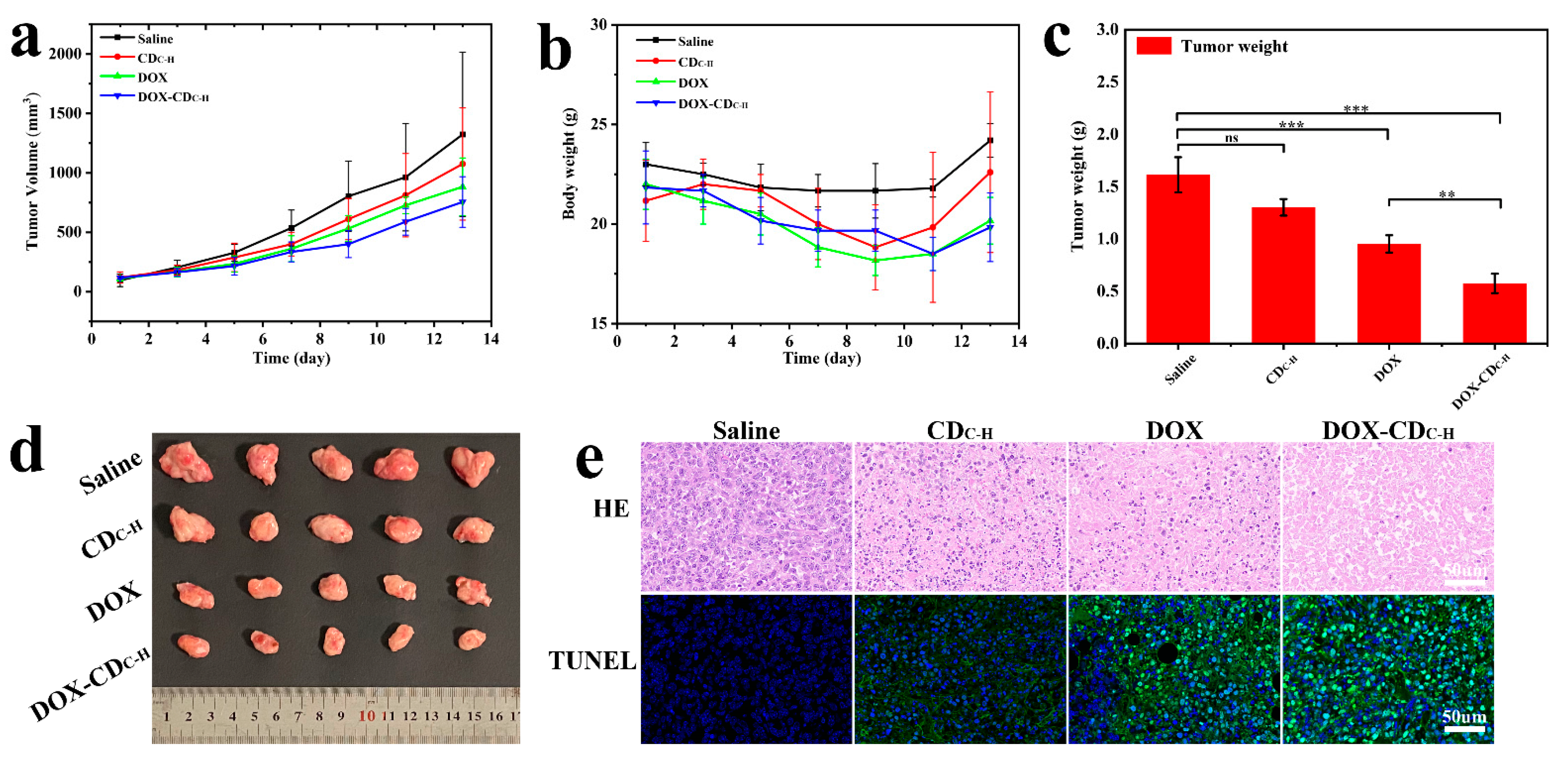
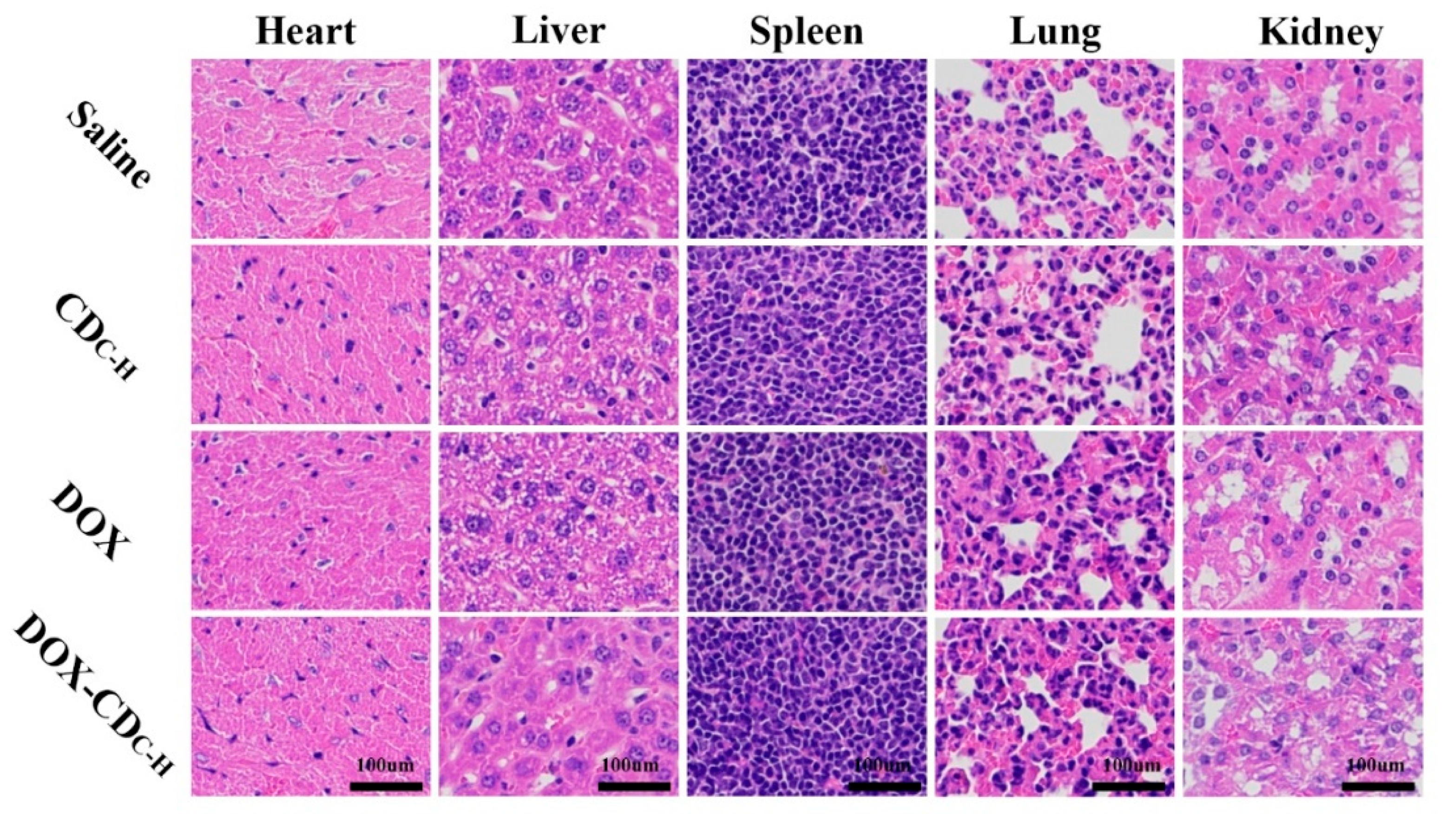
3.1.6. In Vivo Antitumor Therapy
3.2. Formatting of Mathematical Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zairov, R.R.; Dovzhenko, A.P.; Sarkanich, K.A.; Nizameev, I.R.; Luzhetskiy, A.V.; Sudakova, S.N.; Podyachev, S.N.; Burilov, V.A.; Vatsouro, I.M.; Vomiero, A.; et al. Single Excited Dual Band Luminescent Hybrid Carbon Dots-T erbium Chelate Nanothermometer. Nanomaterials 2021, 11, 3080. [Google Scholar] [CrossRef] [PubMed]
- Gopika, A.R.; Varsha, L.J.; Aiswarya, P.S.; Athira Krishnan, N.A.; Vinod, T.P. Carbon Dots from Natural Sources for Biomedical Applications. Part. Part. Syst. Charact. 2022, 39, 2200017. [Google Scholar] [CrossRef]
- Bochkova, O.; Dovjenko, A.; Zairov, R.; Kholin, K.; Biktimirova, R.; Fedorenko, S.; Nizameev, I.; Laskin, A.; Voloshina, A.; Lyubina, A.; et al. Silica-Supported Assemblage of CuII Ions with Carbon Dots for Self-Boosting and Glutathione-Induced ROS Generation. Coatings 2022, 12, 97. [Google Scholar] [CrossRef]
- Aparajita, G.; Anup, K.G.; Monalisa, C.; Prasanta, K.D. Folic Acid-Functionalized Carbon Dot-Enabled Starvation Therapy in Synergism with Paclitaxel against Breast Cancer. ACS Appl. Bio Mater. 2022, 5, 2389–2402. [Google Scholar] [CrossRef]
- Yu, M.; Guo, X.; Lu, H.; Li, P.; Huang, R.; Xu, C.; Gong, X.; Xiao, Y.; Xing, X. Carbon dots derived from folic acid as an ultra-succinct smart antimicrobial nanosystem for selective killing of S. aureus and biofilm eradication. Carbon 2022, 199, 395–406. [Google Scholar] [CrossRef]
- Mohandoss, S.; Palanisamy, S.; Priya, V.V.; Mohan, S.K.; Shim, J.-J.; Yelithao, K.; You, S.; Lee, Y.R. Excitation-dependent multiple luminescence emission of nitrogen and sulfur co-doped carbon dots for cysteine sensing, bioimaging, and photoluminescent ink applications. Microchem. J. 2021, 167, 106280. [Google Scholar] [CrossRef]
- Sonaimuthu, M.; Subramanian, P.; SangGuan, Y.; Jae-Jin, S.; Yong, R.L. Rapid detection of silver ions based on luminescent carbon nanodots for multicolor patterning, smartphone sensors, and bioimaging applications. Anal. Methods 2021, 13, 5719–5726. [Google Scholar] [CrossRef]
- Sonaimuthu, M.; Hari, D.K.; Subramanian, P.; SangGuan, Y.; Jae-Jin, S.; Yong, R.L. Multiple heteroatom-doped photoluminescentcarbon dots for ratiometric detection of Hg2+ ions in cell imaging and environmental applications. Anal. Methods 2022, 14, 635–642. [Google Scholar] [CrossRef]
- Anirudh, S.; Joydeep, D. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Natural-Product-Derived Carbon Dots: From Natural Products to Functional Materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, B.; Hao, L.; Liu, N.; Lin, Y.; Guo, W.; Li, X. Doxorubicin-loaded environmentally friendly carbon dots as a novel drug delivery system for nucleus targeted cancer therapy. Colloids Surf. B Biointerfaces 2017, 159, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gunjal, D.B.; Gurav, Y.M.; Gore, A.H.; Naik, V.M.; Waghmare, R.D.; Patil, C.S.; Sohn, D.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt. Mater. 2019, 98, 109484. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.C. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; He, Q.; Sun, R.; Fang, L.; Song, H.; Li, L.; Li, Z.; Tian, Y.; Cui, H.; Zhang, J. Preparation and application of carbon dots derived from cherry blossom flowers. Chem. Phys. Lett. 2019, 731, 136586. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Yuan, Y.; Wu, Y. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Hu, X.; An, X.; Li, L. Easy synthesis of highly fluorescent carbon dots from albumin and their photoluminescent mechanism and biological imaging applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 730–736. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S.; et al. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860. [Google Scholar] [CrossRef]
- Gong, P.; Sun, L.; Wang, F.; Liu, X.; Yan, Z.; Wang, M.; Zhang, L.; Tian, Z.; Liu, Z.; You, J. Highly fluorescent N-doped carbon dots with two-photon emission for ultrasensitive detection of tumor marker and visual monitor anticancer drug loading and delivery. Chem. Eng. J. 2019, 356, 994–1002. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, F.; Mansournia, M.A.; Mirhashemi, S.M. The potential role of chitosan-based nanoparticles as drug delivery systems in pancreatic cancer. IUBMB Life 2020, 72, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Li, L.; He, J. The performances of carboxymethyl chitosan in wash-off reactive dyeings. Carbohydr. Polym. 2009, 75, 203–207. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Lyu, Q.; Peng, L.; Hong, X.; Fan, T.; Li, J.; Cui, Y.; Zhang, H.; Zhao, J. Smart nano-micro platforms for ophthalmological applications: The state-of-the-art and future perspectives. Biomaterials 2021, 270, 120682. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Mizrahy, S.; Raz, S.R.; Hasgaard, M.; Liu, H.; Soffer-Tsur, N.; Cohen, K.; Dvash, R.; Landsman-Milo, D.; Bremer, M.G.; Moghimi, S.M.; et al. Hyaluronan-coated nanoparticles: The influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J. Control. Release: Off. J. Control. Release Soc. 2011, 156, 231–238. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Fang, Z.; Niu, Y.; Lou, J.; Wu, Y.; Zou, S.; Xia, S.; Sun, M.; Du, F. Fabrication of HA/PEI-functionalized carbon dots for tumor targeting, intracellular imaging and gene delivery. RSC Adv. 2017, 7, 3369–3375. [Google Scholar] [CrossRef]
- Tao, J.; Zou, H.; Liao, X.; Lu, X.; Cao, J.; Pan, J.; Li, C.; Zheng, Y. Fabrication of FA/HA-functionalized carbon dots for human breast cancer cell targeted imaging. Photodiagnosis Photodyn. Ther. 2022, 40, 103099. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.; Yu, Y.X.; Jiang, B.P.; Shen, X.C. Multifunctional hyaluronic acid-derived carbon dots for self-targeted imaging-guided photodynamic therapy. J. Mater. Chem. B 2018, 6, 6534–6543. [Google Scholar] [CrossRef]
- Wang, H.-J.; Zhang, J.; Liu, Y.-H.; Luo, T.-Y.; He, X.; Yu, X.-Q. Hyaluronic acid-based carbon dots for efficient gene delivery and cell imaging. RSC Adv. 2017, 7, 15613–15624. [Google Scholar] [CrossRef]
- Gao, N.; Yang, W.; Nie, H.; Gong, Y.; Jing, J.; Gao, L.; Zhang, X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Lee, J.M.; Youn, Y.S.; Na, K.; Lee, E.S. Hyaluronate dots for highly efficient photodynamic therapy. Carbohydr Polym 2018, 181, 10–18. [Google Scholar] [CrossRef]
- Hailing, Y.; Xiufang, L.; Lili, W.; Baoqiang, L.; Kaichen, H.; Yongquan, H.; Qianqian, Z.; Chaoming, M.; Xiaoshuai, R.; Rui, Z.; et al. Doxorubicin-loaded fluorescent carbon dots with PEI passivation as a drug delivery system for cancer therapy. Nanoscale 2020, 12, 17222–17237. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.; Wang, Y.; Lv, Y.; Wu, H.; Ma, X.; Tan, M. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol. Lett. 2016, 38, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tingting, K.; Liying, H.; Yuanyuan, W.; Xiaoxiao, C.; Bofeng, Z. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef]
- Fu, Y.; Jang, M.-S.; Wu, T.; Lee, J.H.; Li, Y.; Lee, D.S.; Yang, H.Y. Multifunctional hyaluronic acid-mediated quantum dots for targeted intracellular protein delivery and real-time fluorescence imaging. Carbohydr. Polym. 2019, 224, 115174. [Google Scholar] [CrossRef]
- Wen, Q.-L.; Pu, Z.-F.; Yang, Y.-J.; Wang, J.; Wu, B.-C.; Hu, Y.-L.; Liu, P.; Ling, J.; Cao, Q. Hyaluronic acid as a material for the synthesis of fluorescent carbon dots and its application for selective detection of Fe3+ ion and folic acid. Microchem. J. 2020, 159, 105364. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence carbon dots with excitation independent emission for pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Tian, L.; Qiu, Y.; Yu, Q.; Wang, X.; Guo, R.; He, Q. Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int. J. Pharm. 2020, 578, 119122. [Google Scholar] [CrossRef]

| CDHA (200 mg) | CDC-H (100:200, mg) | CDC-H (200:200, mg) | CDC-H (300:200, mg) | |
|---|---|---|---|---|
| Փ% | 8.03 ± 0.41 | 8.86 ± 0.37 | 11.64 ± 0.58 | 9.73 ± 0.47 |
| Kinetic Models | R2 | ||
|---|---|---|---|
| pH 5.0 | pH 6.8 | pH 7.4 | |
| Zero order | 0.598 | 0.460 | 0.419 |
| First order | 0.775 | 0.546 | 0.461 |
| Higuchi | 0.721 | 0.559 | 0.483 |
| Langmuir | 0.940 | 0.934 | 0.959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Gu, D.; Su, Y.; Ji, D.; Yang, Y.; Chen, K.; Pan, H.; Pan, W. Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy. Pharmaceutics 2022, 14, 2423. https://doi.org/10.3390/pharmaceutics14112423
Wang L, Gu D, Su Y, Ji D, Yang Y, Chen K, Pan H, Pan W. Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy. Pharmaceutics. 2022; 14(11):2423. https://doi.org/10.3390/pharmaceutics14112423
Chicago/Turabian StyleWang, Lijie, Donghao Gu, Yupei Su, Dongxu Ji, Yue Yang, Kai Chen, Hao Pan, and Weisan Pan. 2022. "Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy" Pharmaceutics 14, no. 11: 2423. https://doi.org/10.3390/pharmaceutics14112423
APA StyleWang, L., Gu, D., Su, Y., Ji, D., Yang, Y., Chen, K., Pan, H., & Pan, W. (2022). Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy. Pharmaceutics, 14(11), 2423. https://doi.org/10.3390/pharmaceutics14112423