Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy
Abstract
1. Introduction
2. Stimuli in the TME
2.1. Acidity
2.2. High GSH Concentration
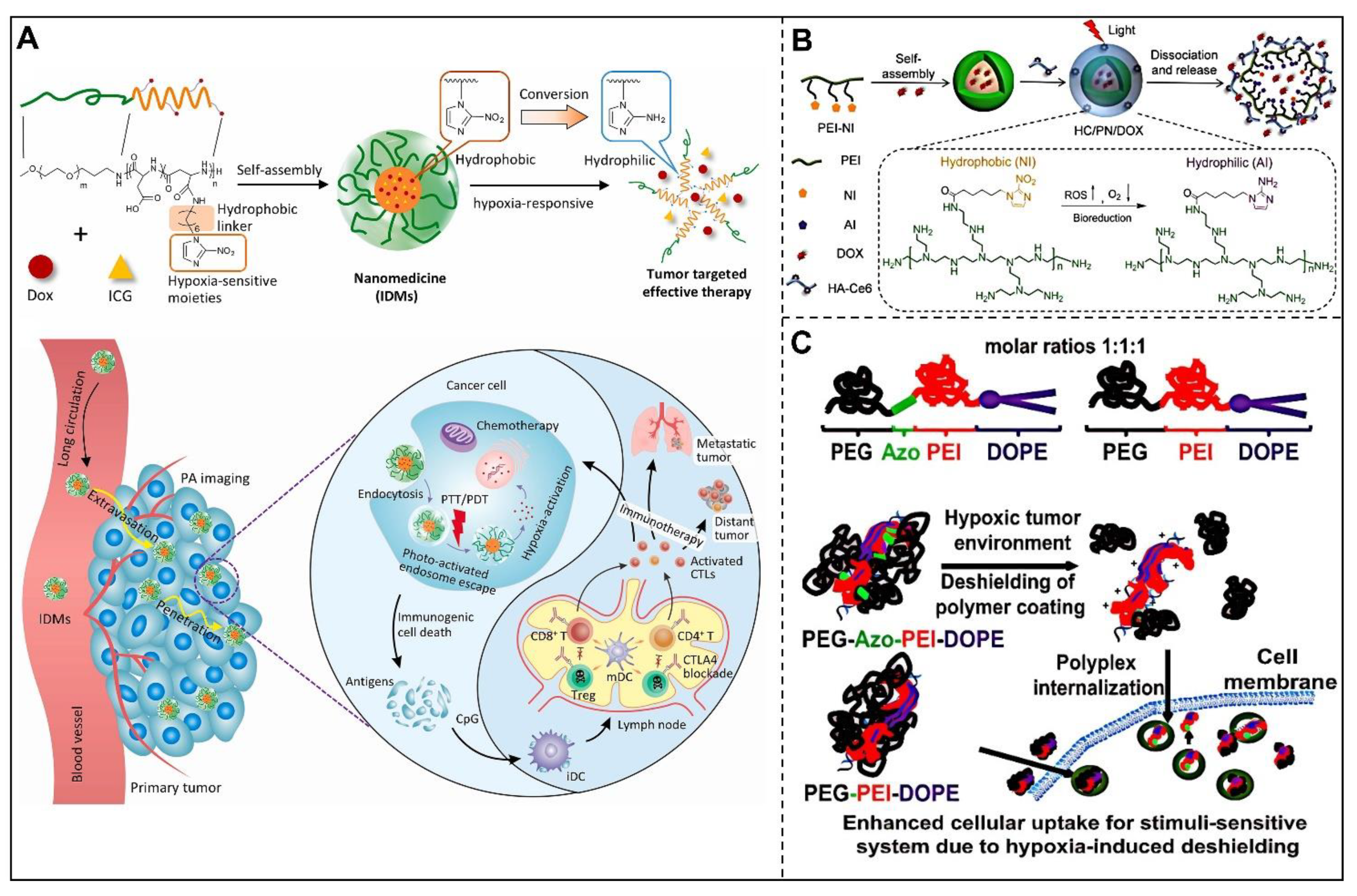
2.3. Hypoxia
2.4. Overexpressed Specific Enzyme
2.5. Excessive ROS
3. Stimuli-Responsive Nanoparticles
3.1. pH-Responsive Nanocarriers
3.1.1. Hydrophobic-to-Hydrophilic Transition
3.1.2. Acid-Labile Bond Cleavage

| Responsive Moiety | Nanoplatform | Cargos | Application | Tumor Model | Refs. |
|---|---|---|---|---|---|
| polyHis | poly(L-lactic acid)-b-PEG-b-polyHis micelles | DOX | pH-dependent drug release | MCF-7 | [40] |
| polymeric micelles constitute of two block copolymers of poly(L-lactic acid)-b-PEG-b-poly(L-histidine)-TAT and polyHis-b-PEG | DOX | pH-dependent drug release and tumor targeted chemotherapy | A2780/AD, MCF-7, and A549 | [42] | |
| A mixed-micelle system composed of polyHis-co-phenylalanine-b-poly(PEG) and poly(L-lactic acid)-b-PEG-folate | DOX | Reversal of multidrug resistance of cancer | A2780/DOXR | [43] | |
| a mixture of polyHis/PEG-folate and poly(L-lactic acid)-b-PEG-folate | DOX | Reversal of resistant MCF-7 tumor | MCF-7/DOXR | [44] | |
| A micelle composed of polyHis-b- PEG and poly(L-lactic acid)-b-PEG-b-polyHis-biotin | DOX | Increase of endocytosis. | MCF-7 | [45] | |
| tertiary amine | mPEG/HCou-g-MPCL micelles | DOX | pH-sensitive drug delivery | HeLa | [49] |
| GDA/EGFP | EGFP | pH-responsive cytosolic protein delivery | 143B | [50] | |
| sulfonamide | DNA/PEI/poly(methacyloyl sulfadimethoxine)-b-PEG | DNA | Tumor specific gene delivery | A2780 | [51] |
| Oligomeric sulfonamides-incorporated poly(L-lysine)/DNA | DNA | enhancement of nucleic acid delivery. | HEK293 | [52] | |
| hydrazone | HPMA | DOX | pH-sensitive drug release | EL4 | [62] |
| HPMA | DOX β-sitosterol | pH-sensitive tumor chemotherapy | Hep G2, A549 and H22 | [64] | |
| HA-hyd-DOX | DOX | pH-dependent drug release and tumor targeted chemotherapy | Hela | [65] | |
| orthoester | PEG-b-PtNEA27/56/73 | Nile Red. | Acid-sensitive and thermoresponsive drug release | NA | [66] |
| PMAOE | DNA | pH-modulated release of gene | NA | [68] | |
| imine | Dex-DOX | DOX | pH-sensitive tumor chemotherapy | B16F10 | [70] |
| benzoic-imine | benzoic-imine-containing PEI-g-mPEG | ICG | Acid-triggered photoinitiation release | NA | [71] |
| acetals | MSN−R848−OVAp | R848 and OVA | pH-sensitive tumor immunotherapy | NA | [73] |
| Ac-DEX | pyrene | pH-dependent drug release | NA | [74] | |
| pHLIP | HauNS-pHLIP-Ce6 | Ce6 | Tumor targeted PTT/PDT | Hela | [80] |
| MONs | DOX | Tumor targeted chemotherapy | MDA-MB-231, MCF-7 | [81] |
3.1.3. pH(Low) Insertion Peptides
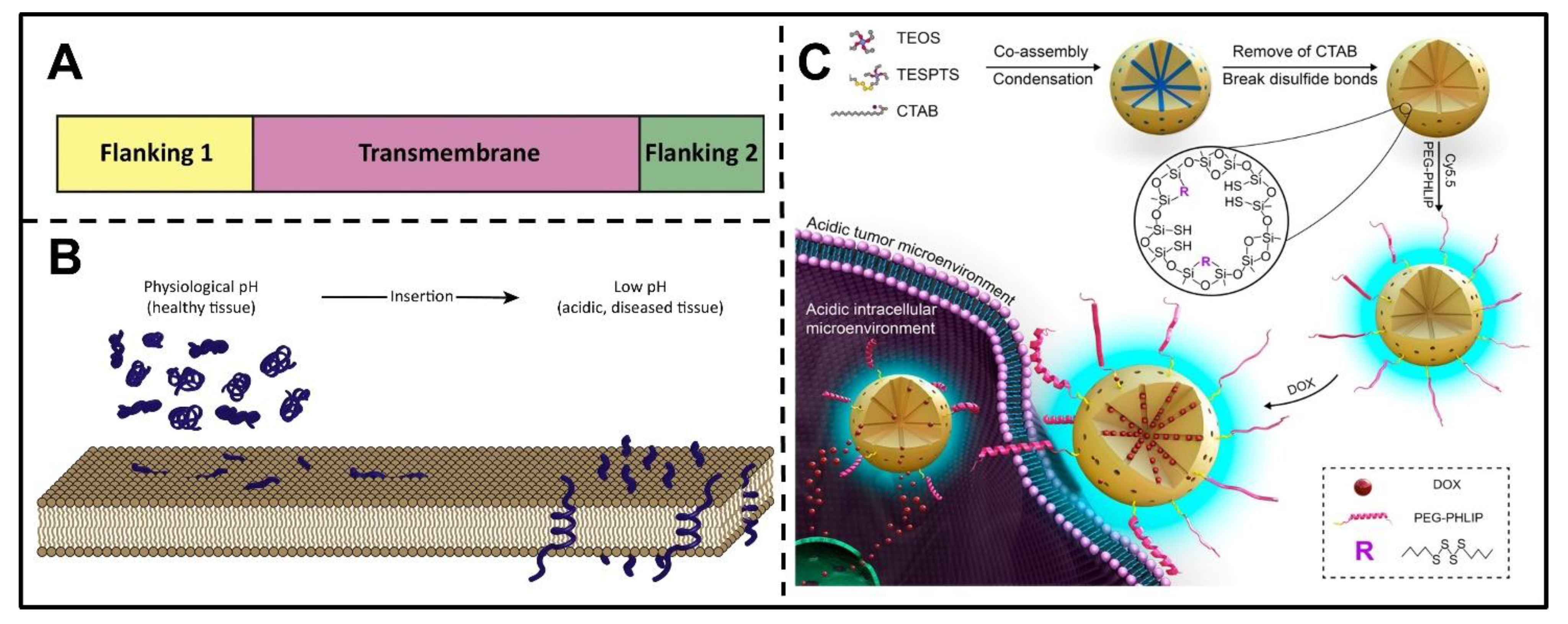
3.2. GSH-Responsive Nanocarriers
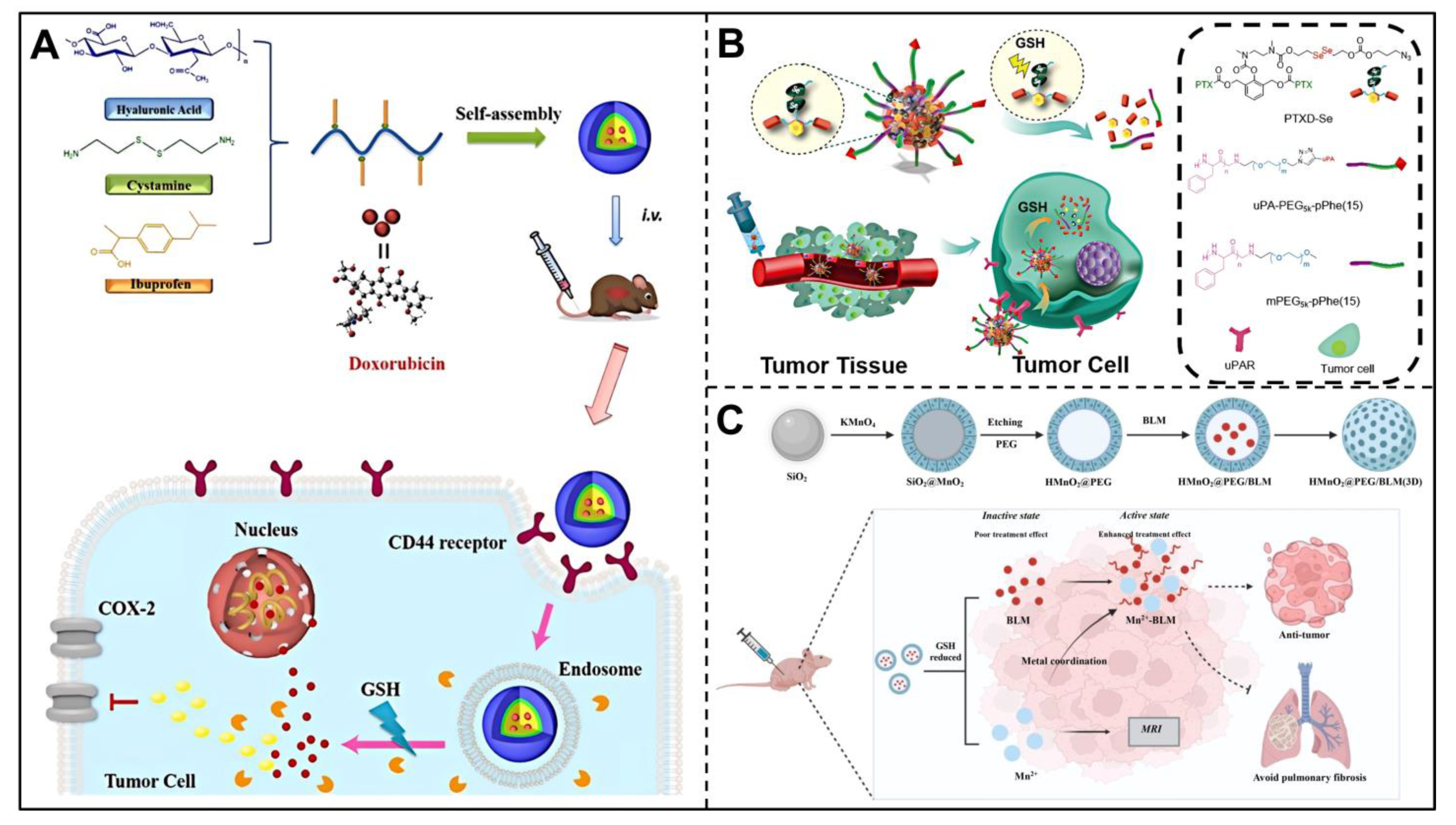
3.3. Hypoxia-Responsive Nanocarriers
3.4. Enzyme-Responsive Nanocarriers
3.4.1. Cathepsin B
3.4.2. Matrix Metalloproteinases
3.4.3. Phospholipase
3.4.4. Glycosidases
| Stimulus | Responsive Moiety | Nanoplatform | Cargos | Application | Tumor Model | Refs. |
|---|---|---|---|---|---|---|
| Cathepsin B | GFLG | DOX@MSN-GFLGR7RGDS/α-CD | DOX | Tumor targeted chemotherapy | HeLa | [106] |
| RH-(GFLG)3 | DOX | Tumor targeted chemotherapy | HeLa | [110] | ||
| FRRG | FRRG-DOX | DOX | Tumor targeted chemotherapy | HT-29 | [108] | |
| FRRG-MMAE | MMAE | Tumor targeted chemotherapy | 4T1 | [109] | ||
| MMP-2 | GPLGIAGQ | PEG2000-peptide-PTX | PTX | MMP-2-sensitive drug release | A549 | [115] |
| GPLGLAG | MPV-HOAD | OXA pheophorbide a | MMP-2-sensitive PDT and cancer immunotherapy | CT26 | [116] | |
| MMP-9 | GFFLG PhAc-FFAG | MMP-9 responsive peptides in conjunction with DOX | DOX | MMP-9-triggered drug release and chemotherapy | MDA-MB-231-luc-D3H2LN | [117] |
| MMP-13 | PLGLAR | MSNs-PLGLAR-BSA-LA@DOX | DOX | MMP-13-triggered drug release and chemotherapy | HepG2 | [118] |
| sPLA2 | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine | liposome shells and TRAIL-loaded DNA NCs cores | TRAIL | Targeted Delivery of Cytokine | COLO 205 cells | [120] |
| ester bonds in proAEL | proAEL | AEL | Tumor specific drug release for cancer therapy | KATO III | [121] | |
| phosphate | UCNP-loaded phosphate micelles | UCNP | Bioimaging of prostate cancer cells | 22Rv1 | [122] | |
| DSPC/DSPG/DSPE | DSPC/DSPG/DSPE liposomes | PNA | tumor targeted drug release for cancer therapy | Hela | [123] | |
| Galactosidase | Saccharides | SMPS modified with lactose or starch derivatives | [Ru(bipy)3]2+ dye | Glycosidase-responsive intracellular controlled release of drug | HeLa | [126] |
| Galactosidase | Carbohydrate unit | folate-DOX conjugate | DOX | Glycosidase-responsive chemotherapy | KG-1 and HL-60 | [127] |
| α-glucosidase | β-CD | β-CD-MNPs | prodigiosin | Anticancer drug delivery | MCF-7/GFPHepG2 | [128] |
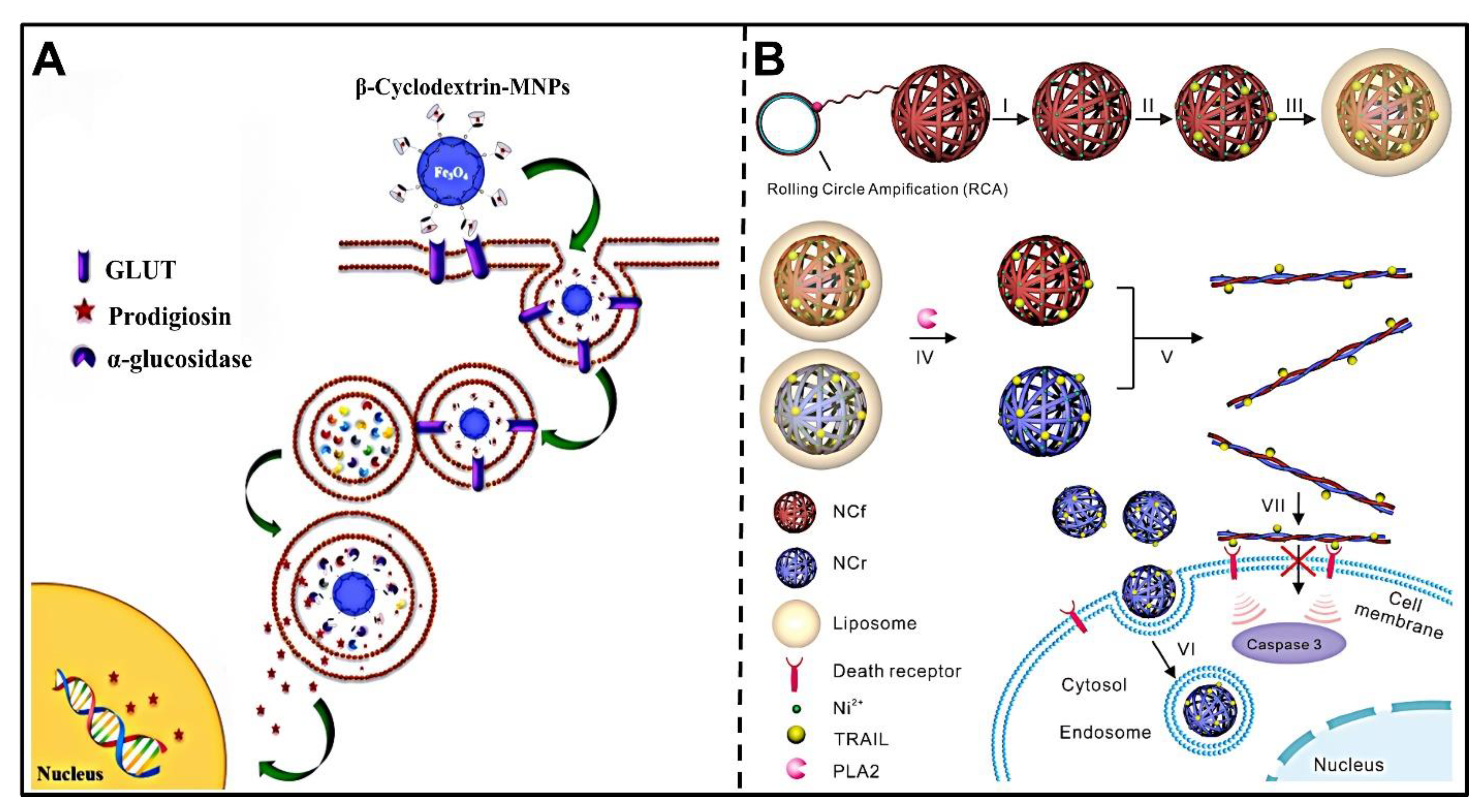
3.5. ROS-Responsive Nanocarriers
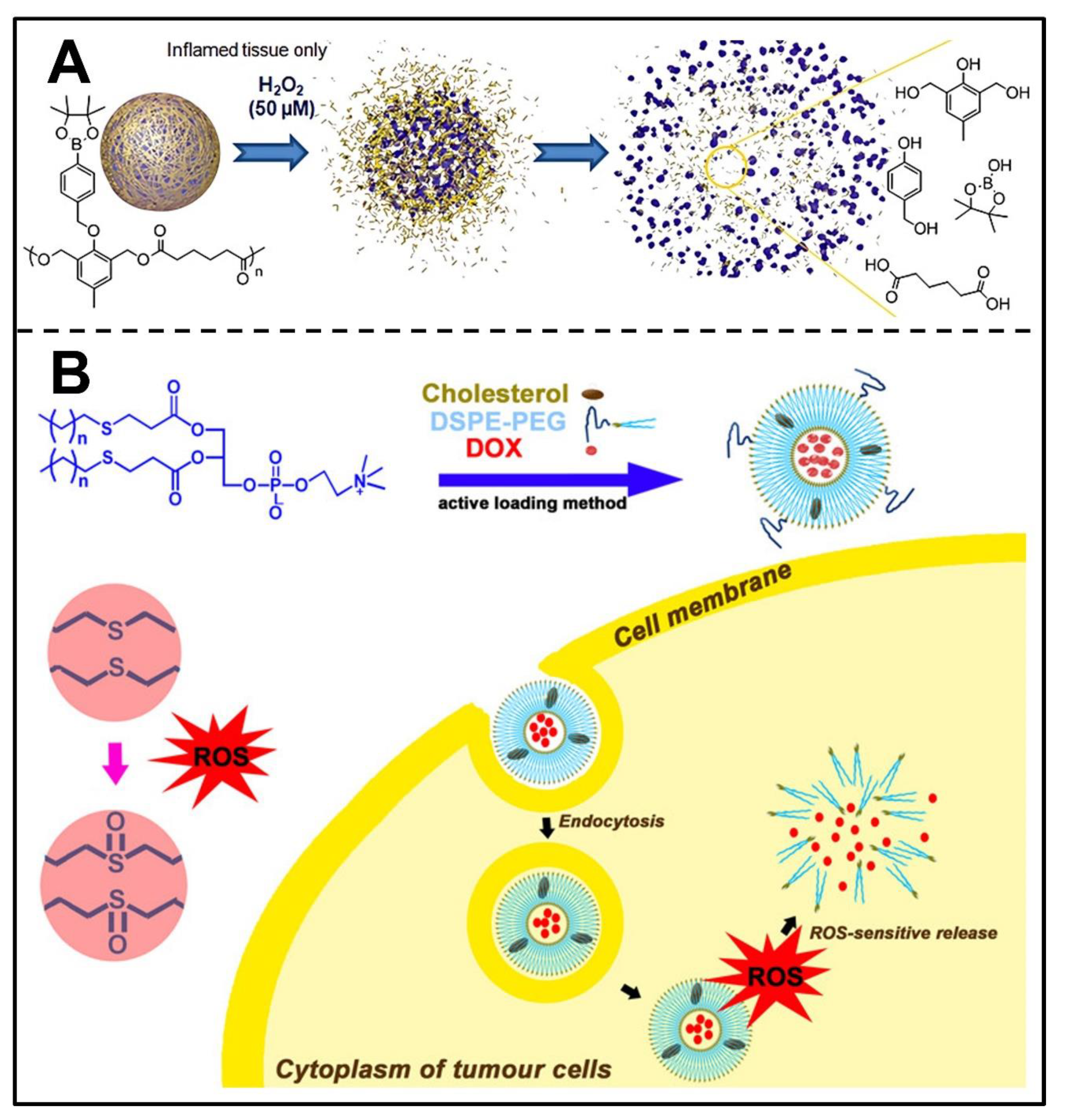
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Y.; Cheng, Y.-J.; Lei, Q.; Zhang, A.-Q.; Zhang, X.-Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125 Pt 23, 5591–5596. [Google Scholar] [CrossRef]
- Yang, S.; Gao, H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108. [Google Scholar] [CrossRef]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid Production in Glycolysis-impaired Tumors Provides New Insights into Tumor Metabolism. Clin. Cancer Res. 2002, 8, 1284. [Google Scholar]
- De Milito, A.; Iessi, E.; Logozzi, M.; Lozupone, F.; Spada, M.; Marino, M.L.; Federici, C.; Perdicchio, M.; Matarrese, P.; Lugini, L.; et al. Proton Pump Inhibitors Induce Apoptosis of Human B-Cell Tumors through a Caspase-Independent Mechanism Involving Reactive Oxygen Species. Cancer Res. 2007, 67, 5408. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J.; Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.-B.; Li, J.-S.; Durkan, C.; Wang, N.; Wang, G.-X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038. [Google Scholar] [CrossRef]
- Wojtkowiak, J.W.; Verduzco, D.; Schramm, K.J.; Gillies, R.J. Drug Resistance and Cellular Adaptation to Tumor Acidic pH Microenvironment. Mol. Pharm. 2011, 8, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Ramos, M.J. Theoretical Insights into the Mechanism for Thiol/Disulfide Exchange. Chem.-A Eur. J. 2004, 10, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. 2006, 43, 143–181. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.H.; Son, S.; Jo, D.G.; Ahn, C.H.; Suh, Y.D.; Kim, K. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 1, 16075. [Google Scholar] [CrossRef]
- Tao, J.; Yang, G.; Zhou, W.; Qiu, J.; Chen, G.; Luo, W.; Zhao, F.; You, L.; Zheng, L.; Zhang, T. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 2021, 14, 14. [Google Scholar] [CrossRef]
- Cowman, S.J.; Koh, M.Y. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer 2022, 8, 28–42. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-X.; Chen, S.-Y.; Yi, N.-B.; Li, X.; Chen, S.-L.; Lei, Z.; Cheng, D.-B.; Sun, T. Research progress on tumor hypoxia-associative nanomedicine. J. Control. Release 2022, 350, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery. Top. Curr. Chem. 2017, 375, 27. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Gao, J.; Liu, Q.; Wang, J.; Shen, Y. Enzyme-Responsive Charge-Reversal Polymer Mediated Effective Gene Therapy for Intraperitoneal Tumors. Biomacromolecules 2018, 19, 2308–2319. [Google Scholar] [CrossRef]
- Lee, S.H.; Gupta, M.K.; Bang, J.B.; Bae, H.; Sung, H.J. Current progress in Reactive Oxygen Species (ROS)-Responsive materials for biomedical applications. Adv. Healthc. Mater. 2013, 2, 908–915. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Peng, X.; Gandhi, V. ROS-activated anticancer prodrugs: A new strategy for tumor-specific damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Peng, H.; Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 22. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Y.; Huang, X.; Luby-Phelps, K.; Sumer, B.D.; Gao, J. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew. Chem. 2011, 50, 6109–6114. [Google Scholar] [CrossRef]
- Du, J.Z.; Mao, C.Q.; Yuan, Y.Y.; Yang, X.Z.; Wang, J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014, 32, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Andreev, O.A.; Engelman, D.M.; Reshetnyak, Y.K. Targeting diseased tissues by pHLIP insertion at low cell surface pH. Front. Physiol. 2014, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.M.; Mann, S.K.; Czuba, E.; Sahut, A.; Liu, H.; Suekama, T.C.; Bickerton, T.; Johnston, A.P.R.; Such, G.K. Self-assembling dual component nanoparticles with endosomal escape capability. Soft Matter 2015, 11, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, S.T.; Liang, K.; Such, G.K.; Johnston, A.P.; Leung, M.K.; Cui, J.; Caruso, F. Engineering enzyme-cleavable hybrid click capsules with a pH-sheddable coating for intracellular degradation. Small 2014, 10, 4080–4086. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Tziveleka, L.-A.; Varlas, S.; Iatrou, H. pH-Sensitive nanogates based on poly(L-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, K.T.; Kim, D.; Youn, Y.S.; Bae, Y.H. Tumor pH-responsive flower-like micelles of poly (L-lactic acid)-b-poly (ethylene glycol)-b-poly (L-histidine). J. Control. Release 2007, 123, 19–26. [Google Scholar] [CrossRef]
- Yin, H.; Lee, E.S.; Kim, D.; Lee, K.H.; Oh, K.T.; Bae, Y.H. Physicochemical characteristics of pH-sensitive poly (L-histidine)-b-poly (ethylene glycol)/poly (L-lactide)-b-poly (ethylene glycol) mixed micelles. J. Control. Release 2008, 126, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, E.S.; Oh, K.T.; Gao, Z.G.; Bae, Y.H. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small 2008, 4, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, X.; Zhang, J.; Pan, S.; Yang, C.; Wei, Y.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. pH-responsive hybrid nanoparticle with enhanced dissociation characteristic for siRNA delivery. Int. J. Nanomed. 2018, 13, 6885. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Huang, Z.; Chen, Y.; Pan, S.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Stimuli-responsive release and efficient siRNA delivery in non-small cell lung cancer by a poly (l-histidine)-based multifunctional nanoplatform. J. Mater. Chem. B 2020, 8, 1616–1628. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.; Zhang, W.; Wang, X.; Geng, Y.; Zhang, Y.; Wang, L.; Zhang, W.; Zhang, Y.-J.; Xiao, S. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat. Nanotechnol. 2022, 17, 541–551. [Google Scholar] [CrossRef]
- Yang, X.-L.; Wu, W.-X.; Li, J.; Hu, Z.-E.; Wang, N.; Yu, X.-Q. A facile strategy to construct fluorescent pH-sensitive drug delivery vehicle. Polymer 2020, 197, 122496. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J.; Gao, P.; Feng, Q.; Wang, H.; Cheng, Y. A pH-responsive phase-transition polymer with high serum stability in cytosolic protein delivery. Nano Lett. 2021, 21, 7855–7861. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Na, K.; Bae, Y.H. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: An in vitro study. Biomacromolecules 2006, 7, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Bae, Y.H. pH-tunable endosomolytic oligomers for enhanced nucleic acid delivery. Adv. Funct. Mater. 2007, 17, 1263–1272. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Kuang, Y.; Li, Q.; Zheng, D.-W.; Song, Q.; Chen, H.; Chen, X.; Xu, Y.; Li, C. Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int. J. Biol. Macromol. 2017, 98, 691–700. [Google Scholar] [CrossRef]
- Huang, L.; Tao, K.; Liu, J.; Qi, C.; Xu, L.; Chang, P.; Gao, J.; Shuai, X.; Wang, G.; Wang, Z. Design and fabrication of multifunctional sericin nanoparticles for tumor targeting and pH-responsive subcellular delivery of cancer chemotherapy drugs. ACS Appl. Mater. Interfaces 2016, 8, 6577–6585. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Deepagan, V.G.; Chang, K.Y.; Park, J.H. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer 2011, 52, 4753–4759. [Google Scholar] [CrossRef]
- Zha, Q.; Wang, X.; Cheng, X.; Fu, S.; Yang, G.; Yao, W.; Tang, R. Acid–degradable carboxymethyl chitosan nanogels via an ortho ester linkage mediated improved penetration and growth inhibition of 3-D tumor spheroids in vitro. Mater. Sci. Eng. C 2017, 78, 246–257. [Google Scholar] [CrossRef]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Cell-specific and pH-sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. Struct. Funct. Interact. 2018, 110, 437–448. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A pH-Responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Suarez, S.L.; Muñoz, A.; Mitchell, A.C.; Braden, R.L.; Luo, C.; Cochran, J.R.; Almutairi, A.; Christman, K.L. Degradable acetalated dextran microparticles for tunable release of an engineered hepatocyte growth factor fragment. ACS Biomater. Sci. Eng. 2016, 2, 197–204. [Google Scholar] [CrossRef]
- Shim, M.S.; Kim, C.S.; Ahn, Y.-C.; Chen, Z.; Kwon, Y.J. Combined multimodal optical imaging and targeted gene silencing using stimuli-transforming nanotheragnostics. J. Am. Chem. Soc. 2010, 132, 8316–8324. [Google Scholar] [CrossRef][Green Version]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.; Such, G.K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [PubMed]
- Etrych, T.; Jelinkova, M.A.; Ríhová, B.; Ulbrich, K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: Synthesis and preliminary in vitro and in vivo biological properties. J. Control. Release 2001, 73, 89–102. [Google Scholar] [CrossRef]
- Chytil, P.; Koziolová, E.; Etrych, T.; Ulbrich, K. HPMA Copolymer–Drug Conjugates with Controlled Tumor-Specific Drug Release. Macromol. Biosci. 2018, 18, 1700209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, L.; Yang, Y.; Xu, X.; Huang, Y. Tumor targeting by pH-sensitive, biodegradable, cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micelles. Biomaterials 2014, 35, 6622–6635. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef]
- Huang, X.; Du, F.; Cheng, J.; Dong, Y.; Liang, D.; Ji, S.; Lin, S.-S.; Li, Z. Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups. Macromolecules 2009, 42, 783–790. [Google Scholar] [CrossRef]
- Huang, X.; Du, F.; Ju, R.; Li, Z. Novel acid-labile, Thermoresponsive poly (methacrylamide) s with pendent Ortho Ester moieties. Macromol. Rapid Commun. 2007, 28, 597–603. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, J.; Tang, R.; Ji, W.; Wang, R.; Wang, J.; Wang, C. Synthesis and Characterization of Homopolymers Bearing Acid-Cleavable Cationic Side-Chains for pH-Modulated Release of DNA. Macromol. Biosci. 2014, 14, 1015–1024. [Google Scholar] [CrossRef]
- Li, Y.; Song, L.; Lin, J.; Pan, Z.; Ma, J.; Zhang, Y.; Su, G.; Ye, S.; Luo, F.H.; Zhu, X. Programmed Nanococktail Based on pH-Responsive Function Switch for Self-Synergistic Tumor-Targeting Therapy. Acs Appl. Mater. Interfaces 2017, 45, 39127–39142. [Google Scholar] [CrossRef]
- Feng, X.; Li, D.; Han, J.; Zhuang, X.; Ding, J. Schiff base bond-linked polysaccharide–doxorubicin conjugate for upregulated cancer therapy. Mater. Sci. Eng. C 2017, 76, 1121–1128. [Google Scholar] [CrossRef]
- Liao, S.-C.; Ting, C.-W.; Chiang, W.-H. Functionalized polymeric nanogels with pH-sensitive benzoic-imine cross-linkages designed as vehicles for indocyanine green delivery. J. Colloid Interface Sci. 2020, 561, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J. Acetals as pH-sensitive linkages for drug delivery. Bioconjugate Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef]
- Wagner, J.; Gößl, D.E.; Ustyanovska, N.; Xiong, M.; Hauser, D.; Zhuzhgova, O.; Hocevar, S.; Taskoparan, B.L.; Poller, L.; Datz, S. Mesoporous silica nanoparticles as pH-responsive carrier for the immune-activating drug resiquimod enhance the local immune response in mice. ACS Nano 2021, 15, 4450–4466. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, E.M.; Beaudette, T.T.; Broaders, K.E.; Dashe, J.; Fréchet, J.M. Acetal-Derivatized Dextran: An Acid-Responsive Biodegradable Material for Therapeutic Applications. J. Am. Chem. Soc. 2008, 130, 10494–10495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.; Chen, L.; Yu, S.; Cao, Y.; He, C.; Chen, X. Intracellular pH-sensitive PEG-block-acetalated-dextrans as efficient drug delivery platforms. ACS Appl. Mater. Interfaces 2013, 5, 10760–10766. [Google Scholar] [CrossRef]
- Cohen, J.A.; Beaudette, T.T.; Cohen, J.L.; Broaders, K.E.; Bachelder, E.M.; Frechet, J.M.J. Acetal-Modified Dextran Microparticles with Controlled Degradation Kinetics and Surface Functionality for Gene Delivery in Phagocytic and Non-Phagocytic Cells. Adv. Mater. 2010, 22, 3593–3597. [Google Scholar] [CrossRef]
- Bachelder, E.M.; Pino, E.N.; Ainslie, K.M. Acetalated dextran: A tunable and acid-labile biopolymer with facile synthesis and a range of applications. Chem. Rev. 2017, 117, 1915–1926. [Google Scholar] [CrossRef]
- Cui, L.; Cohen, J.L.; Chu, C.K.; Wich, P.R.; Kierstead, P.H.; FréChet, J.M. Conjugation chemistry through acetals toward a dextran-based delivery system for controlled release of siRNA. J. Am. Chem. Soc. 2012, 134, 15840–15848. [Google Scholar] [CrossRef]
- Ornelas-Megiatto, C.; Shah, P.N.; Wich, P.R.; Cohen, J.L.; Tagaev, J.A.; Smolen, J.A.; Wright, B.D.; Panzner, M.J.; Youngs, W.J.; Fréchet, J.M.J. Aerosolized antimicrobial agents based on degradable dextran nanoparticles loaded with silver carbene complexes. Mol. Pharm. 2012, 9, 3012–3022. [Google Scholar] [CrossRef]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. A pH-Driven and photoresponsive nanocarrier: Remotely-controlled by near-infrared light for stepwise antitumor treatment. Biomaterials 2016, 79, 25–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, M.; Tian, Y.; Zhu, Y.; Liu, W.; Tian, W.; Su, Y.; Ni, Q.; Xu, C.; Lu, N. Tumor acidic microenvironment targeted drug delivery based on pHLIP-modified mesoporous organosilica nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 30543–30552. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, D.; Moshnikova, A.; Thakur, M.S.; Moshnikova, V.; Daniels, J.; Engelman, D.M.; Andreev, O.A.; Reshetnyak, Y.K. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. USA 2013, 110, 5834–5839. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.C.; Lewis, J.S.; Andreev, O.A.; Reshetnyak, Y.K.; Engelman, D.M. Applications of pHLIP technology for cancer imaging and therapy. Trends Biotechnol. 2017, 35, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, Y.K.; Andreev, O.A.; Lehnert, U.; Engelman, D.M. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl. Acad. Sci. USA 2006, 103, 6460–6465. [Google Scholar] [CrossRef]
- Thévenin, D.; An, M.; Engelman, D.M. pHLIP-Mediated Translocation of Membrane-Impermeable Molecules into Cells. Chem. Biol. 2009, 16, 754–762. [Google Scholar] [CrossRef]
- Andreev, O.A.; Karabadzhak, A.G.; Weerakkody, D.; Andreev, G.O.; MEngelman, D.; Reshetnyak, Y.K. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl. Acad. Sci. USA 2010, 107, 4081–4086. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Han, X.; Liang, C.; Liu, J.; Song, X.; Ge, Z.; Liu, Z. Redox-Sensitive Nanoscale Coordination Polymers for Drug Delivery and Cancer Theranostics. Acs Appl. Mater. Interfaces 2017, 9, 23555–23563. [Google Scholar] [CrossRef]
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C. Doxorubicin delivered by redox-responsive Hyaluronic Acid–Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef]
- Liu, J.; Wu, T.; Lu, X.; Wu, X.; Liu, S.; Zhao, S.; Xu, X.; Ding, B. A self-assembled platform based on branched DNA for sgRNA/Cas9/antisense delivery. J. Am. Chem. Soc. 2019, 141, 19032–19037. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Raza, F.; Zafar, H.; Huang, G.; Yang, Y.; Shi, F.; Wang, D.; He, X. Design of GSH-Responsive Curcumin Nanomicelles for Oesophageal Cancer Therapy. Pharmaceutics 2022, 14, 1802. [Google Scholar] [CrossRef]
- He, X.; Zhang, J.; Li, C.; Zhang, Y.; Lu, Y.; Zhang, Y.; Liu, L.; Ruan, C.; Chen, Q.; Chen, X. Enhanced bioreduction-responsive diselenide-based dimeric prodrug nanoparticles for triple negative breast cancer therapy. Theranostics 2018, 8, 4884. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Nie, Y.; Cheng, G.; Xie, L.; Shen, Y.; Gu, Z. Gene-Delivery Vectors: Viral Mimicking Ternary Polyplexes: A Reduction-Controlled Hierarchical Unpacking Vector for Gene Delivery. Adv. Mater. 2014, 26, 1632. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, W.; Zheng, X.; He, S.; Xie, Z. Comparing Effects of Redox Sensitivity of Organic Nanoparticles to Photodynamic Activity. Chem. Mater. 2017, 29, 1856–1863. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, K.; Chen, Y.; Luo, X. Redox-responsive comparison of diselenide micelles with disulfide micelles. Colloid Polym. Sci. 2019, 297, 225–238. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. 2018, 130, 4996–5000. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, C.; Zhuang, J.; Hou, Y.-Y.; He, X.-W.; Li, W.-Y.; Bai, G.; Zhang, Y.-K. GSH-Responsive Drug Delivery System for Active Therapy and Reducing the Side Effects of Bleomycin. ACS Appl. Mater. Interfaces 2022, 14, 417–427. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- He, H.; Zhu, R.; Sun, W.; Cai, K.; Chen, Y.; Yin, L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale 2018, 10, 2856–2865. [Google Scholar] [CrossRef]
- Liu, J.; Ai, X.; Cabral, H.; Liu, J.; Huang, Y.; Mi, P. Tumor hypoxia-activated combinatorial nanomedicine triggers systemic antitumor immunity to effectively eradicate advanced breast cancer. Biomaterials 2021, 273, 120847. [Google Scholar] [CrossRef]
- Perche, F.; Biswas, S.; Wang, T.; Zhu, L.; Torchilin, V. Hypoxia-targeted siRNA delivery. Angew. Chem. 2014, 126, 3430–3434. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Lim, W.Q.; Zhang, R.; Feng, L.; Liu, G.; Wu, H.; Bindra, A.K.; Jana, D.; Liu, Z.; et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv. Mater. 2019, 31, 1901513. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Haldar, M.K.; You, S.; Choi, Y.; Mallik, S. Hypoxia-responsive polymersomes for drug delivery to hypoxic pancreatic cancer cells. Biomacromolecules 2016, 17, 2507–2513. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, W.; Guo, N.; Huangfu, M.; Liu, H.; Lin, M.; Xu, W.; Chen, J.; Wang, T.; Wei, Q.; et al. Targeting tumor hypoxia with stimulus-responsive nanocarriers in overcoming drug resistance and monitoring anticancer efficacy. Acta Biomater. 2018, 71, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.; Tung, C.H.; Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001, 7, 743–748. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Luo, G.-F.; Zhu, J.-Y.; Xu, X.-D.; Zeng, X.; Cheng, D.-B.; Li, Y.-M.; Wu, Y.; Zhang, X.-Z.; Zhuo, R.-X. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef]
- Gu, L.; Duan, Z.; Chen, X.; Li, X.; Luo, Q.; Bhamra, A.; Pan, D.; Zhu, H.; Tian, X.; Chen, R.; et al. A Transformable Amphiphilic and Block Polymer−Dendron Conjugate for Enhanced Tumor Penetration and Retention with Cellular Homeostasis Perturbation via Membrane Flow. Adv. Mater. 2022, 34, 2200048. [Google Scholar] [CrossRef]
- Shim, M.K.; Park, J.; Yoon, H.Y.; Lee, S.; Um, W.; Kim, J.-H.; Kang, S.-W.; Seo, J.-W.; Hyun, S.-W.; Park, J.H.; et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J. Control. Release 2019, 294, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shim, M.K.; Moon, Y.; Song, S.; Kim, J.; Choi, J.; Kim, J.; Lee, Y.; Park, J.Y.; Kim, Y.; et al. Tumor-Specific Monomethyl Auristatin E (MMAE) Prodrug Nanoparticles for Safe and Effective Chemotherapy. Pharmaceutics 2022, 14, 2131. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, J.S. Enzyme-Responsive Amphiphilic Peptide Nanoparticles for Biocompatible and Efficient Drug Delivery. Pharmaceutics 2022, 14, 143. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2017, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Ma, Y.; Wang, S.; Li, Y. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv. Mater. 2019, 31, 1805888. [Google Scholar] [CrossRef]
- Kalafatovic, D.; Nobis, M.; Son, J.; Anderson, K.I.; Ulijn, R.V. MMP-9 triggered self-assembly of doxorubicin nanofiber depots halts tumor growth. Biomaterials 2016, 98, 192–202. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Li, J.; Luo, Z.; Cai, K. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology 2015, 26, 145102. [Google Scholar] [CrossRef]
- Hansen, A.H.; Mouritsen, O.G.; Arouri, A. Enzymatic action of phospholipase A2 on liposomal drug delivery systems. Int. J. Pharm. 2015, 491, 49–57. [Google Scholar] [CrossRef]
- Sun, W.; Ji, W.; Hu, Q.; Yu, J.; Wang, C.; Qian, C.; Hochu, G.; Gu, Z. Transformable DNA nanocarriers for plasma membrane targeted delivery of cytokine. Biomaterials 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Andresen, T.L.; Jensen, S.S.; Jørgensen, K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef]
- Sharipov, M.; Tawfik, S.M.; Gerelkhuu, Z.; Huy, B.T.; Lee, Y.-I. Phospholipase A2-responsive phosphate micelle-loaded UCNPs for bioimaging of prostate cancer cells. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Enzyme-triggered release of the antisense octaarginine-pna conjugate from phospholipase A2 sensitive liposomes. ACS Appl. Bio. Mater. 2020, 3, 1018–1025. [Google Scholar] [CrossRef]
- Cao, L.; Huang, C.; Zhou, D.C.; Hu, Y.; Lih, T.M.; Savage, S.R.; Krug, K.; Clark, D.J.; Schnaubelt, M.; Chen, L.; et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 2021, 184, 5031–5052.e5026. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Huang-Fu, M.-Y.; Xu, W.-H.; Han, M. Stimulus-responsive nanoscale delivery systems triggered by the enzymes in the tumor microenvironment. Eur. J. Pharm. Biopharm. 2019, 137, 122–130. [Google Scholar] [CrossRef]
- Bernardos, A.; Mondragon, L.; Aznar, E.; Marcos, M.D.; Martinez-Mañez, R.; Sancenon, F.; Soto, J.; Barat, J.M.; Perez-Paya, E.; Guillem, C.; et al. Enzyme-Responsive Intracellular Controlled Release Using Nanometric Silica Mesoporous Supports Capped with “Saccharides”. Acs Nano 2010, 4, 6353–6368. [Google Scholar] [CrossRef]
- Clarhaut, J.; Fraineau, S.; Guilhot, J.; Peraudeau, E.; Tranoy-Opalinski, I.; Thomas, M.; Renoux, B.; Randriamalala, E.; Bois, P.; Chatelier, A.; et al. A galactosidase-responsive doxorubicin-folate conjugate for selective targeting of acute myelogenous leukemia blasts. Leuk Res. 2013, 37, 948–955. [Google Scholar] [CrossRef]
- Rastegari, B.; Karbalaei-Heidari, H.R.; Zeinali, S.; Sheardown, H. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: Synthesis, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2017, 158, 589–601. [Google Scholar] [CrossRef]
- Lee, J.; Jenjob, R.; Davaa, E.; Yang, S.-G. NIR-responsive ROS generating core and ROS-triggered 5′-Deoxy-5-fluorocytidine releasing shell structured water-swelling microgel for locoregional combination cancer therapy. J. Control. Release 2019, 305, 120–129. [Google Scholar] [CrossRef]
- Oddone, N.; Pederzoli, F.; Duskey, J.T.; De Benedictis, C.A.; Grabrucker, A.M.; Forni, F.; Vandelli, M.A.; Ruozi, B.; Tosi, G. ROS-responsive “smart” polymeric conjugate: Synthesis, characterization and proof-of-concept study. Int. J. Pharm. 2019, 570, 118655. [Google Scholar] [CrossRef]
- Luo, C.; Sun, B.; Wang, C.; Zhang, X.; Chen, Y.; Chen, Q.; Yu, H.; Zhao, H.; Sun, M.; Li, Z.; et al. Self-facilitated ROS-responsive nanoassembly of heterotypic dimer for synergistic chemo-photodynamic therapy. J. Control. Release 2019, 302, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, K.; Zhang, D.; Sun, B.; Ji, B.; Wei, L.; Li, Z.; Wang, M.; Zhang, X.; Zhang, H.; et al. Light-activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy. Biomater. Sci. 2018, 6, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, J.; Chen, T.; Tu, C.; Zhu, L.; Yan, D. A self-amplified ROS-responsive chemodrug–inhibitor conjugate for multi-drug resistance tumor therapy. Biomater. Sci. 2022, 10, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Du, L.; Liu, Y. A reactive oxygen species-responsive dendrimer with low cytotoxicity for efficient and targeted gene delivery. Chin. Chem. Lett. 2020, 31, 275–280. [Google Scholar] [CrossRef]
- Du, Y.; He, W.; Xia, Q.; Zhou, W.; Yao, C.; Li, X. Thioether phosphatidylcholine liposomes: A novel ROS-responsive platform for drug delivery. ACS Appl. Mater. Interfaces 2019, 11, 37411–37420. [Google Scholar] [CrossRef]
- Heppner, G.H.; Miller, B.E. Tumor heterogeneity: Biological implications and therapeutic consequences. Cancer Metastasis Rev. 1983, 2, 5–23. [Google Scholar] [CrossRef]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer Stem Cells: An Old Idea--A Paradigm Shift. Cancer Res. 2006, 66, 1883–1890; discussion 1895–1886. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Liu, F.; Wu, Y.; Peng, X.; Song, F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J. Control. Release 2021, 332, 269–284. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Nanomater. Neoplasms 2021, 53, 31–142. [Google Scholar]
- Crommelin, D.J.A.; Florence, A.T. Towards more effective advanced drug delivery Syst. J. Pharm. 2013, 454, 496–511. [Google Scholar]
- Yang, Y.; Liu, X.; Ma, W.; Xu, Q.; Chen, G.; Wang, Y.; Xiao, H.; Li, N.; Liang, X.-J.; Yu, M.; et al. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials 2021, 265, 120456. [Google Scholar] [CrossRef]
- Winkler, D.A. Role of artificial intelligence and machine learning in nanosafety. Small 2020, 16, 2001883. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhu, L.; Zhu, X.; Sun, M.; Yan, D. Drug-polymer hybrid macromolecular engineering: Degradable PEG integrated by platinum (IV) for cancer therapy. Matter 2019, 1, 1618–1630. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. https://doi.org/10.3390/pharmaceutics14112346
Zhou W, Jia Y, Liu Y, Chen Y, Zhao P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics. 2022; 14(11):2346. https://doi.org/10.3390/pharmaceutics14112346
Chicago/Turabian StyleZhou, Weixin, Yujie Jia, Yani Liu, Yan Chen, and Pengxuan Zhao. 2022. "Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy" Pharmaceutics 14, no. 11: 2346. https://doi.org/10.3390/pharmaceutics14112346
APA StyleZhou, W., Jia, Y., Liu, Y., Chen, Y., & Zhao, P. (2022). Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics, 14(11), 2346. https://doi.org/10.3390/pharmaceutics14112346





