Antibiofilm Combinatory Strategy: Moxifloxacin-Loaded Nanosystems and Encapsulated N-Acetyl-L-Cysteine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
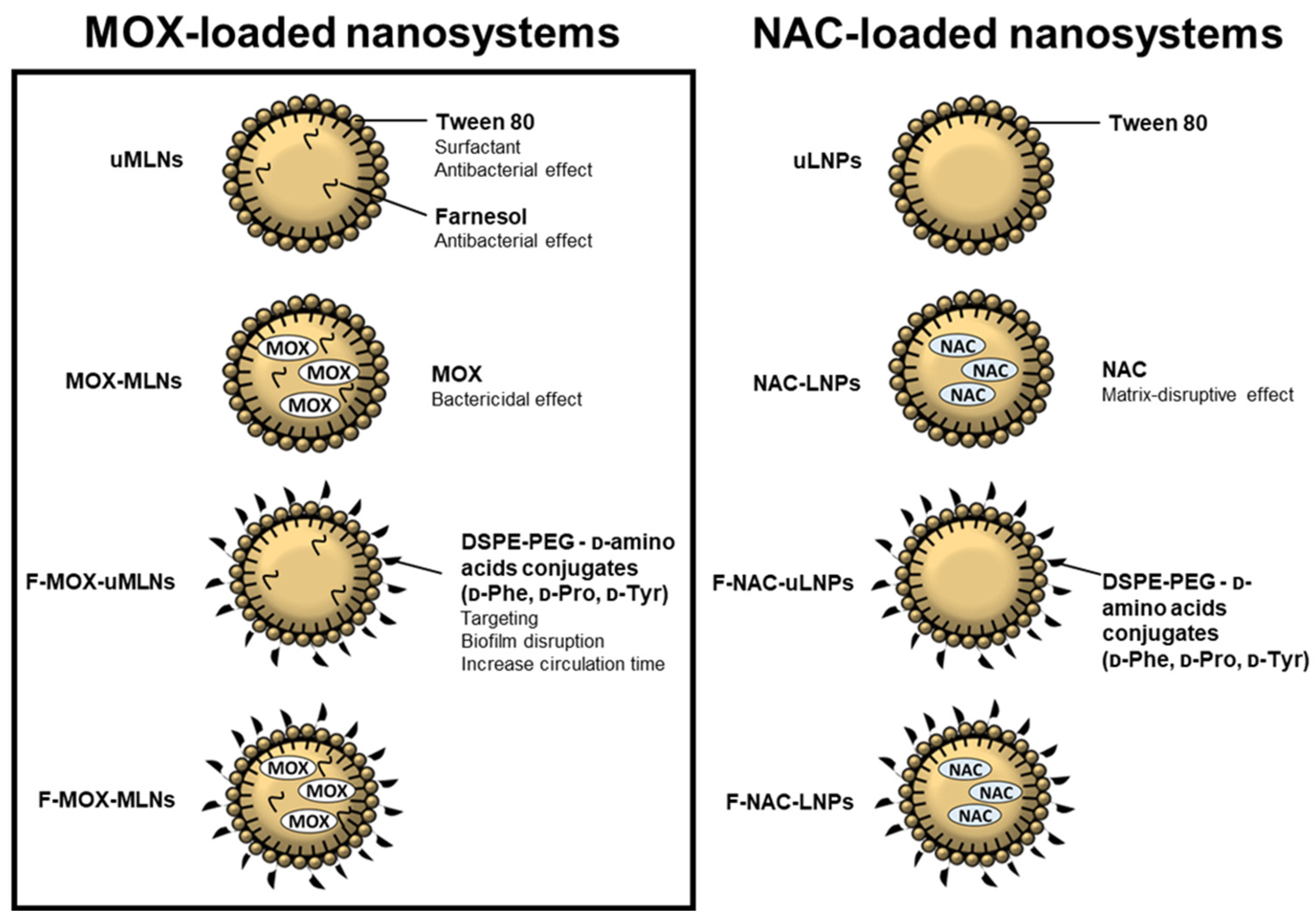
2.2. Preparation of MOX-Loaded MLNs
2.3. Physical Characterization of MLNs
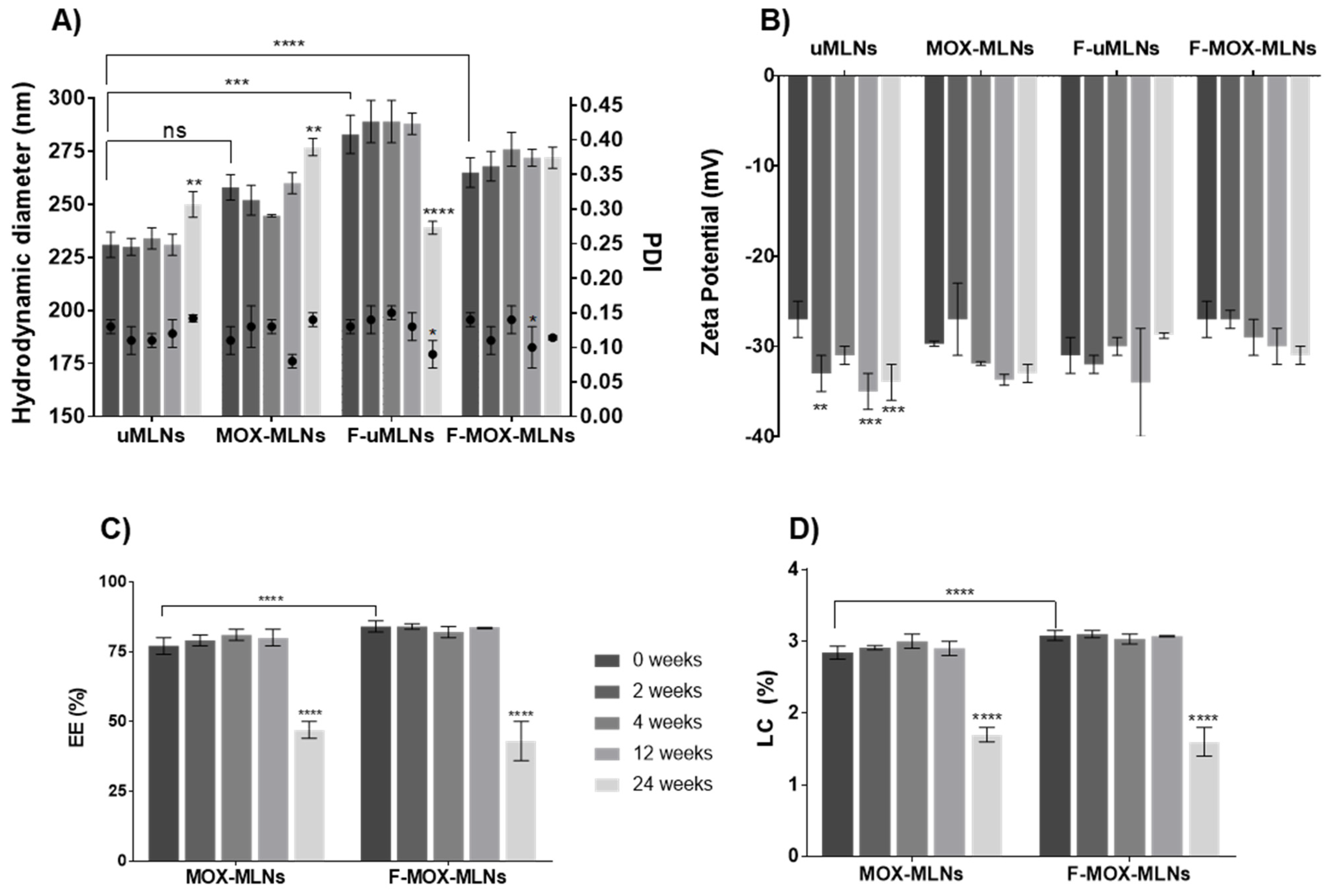
2.4. Determination of the Encapsulation Efficiency and Loading Capacity
2.5. Evaluation of the Storage Stability
2.6. In Vitro Cytocompatibility Studies
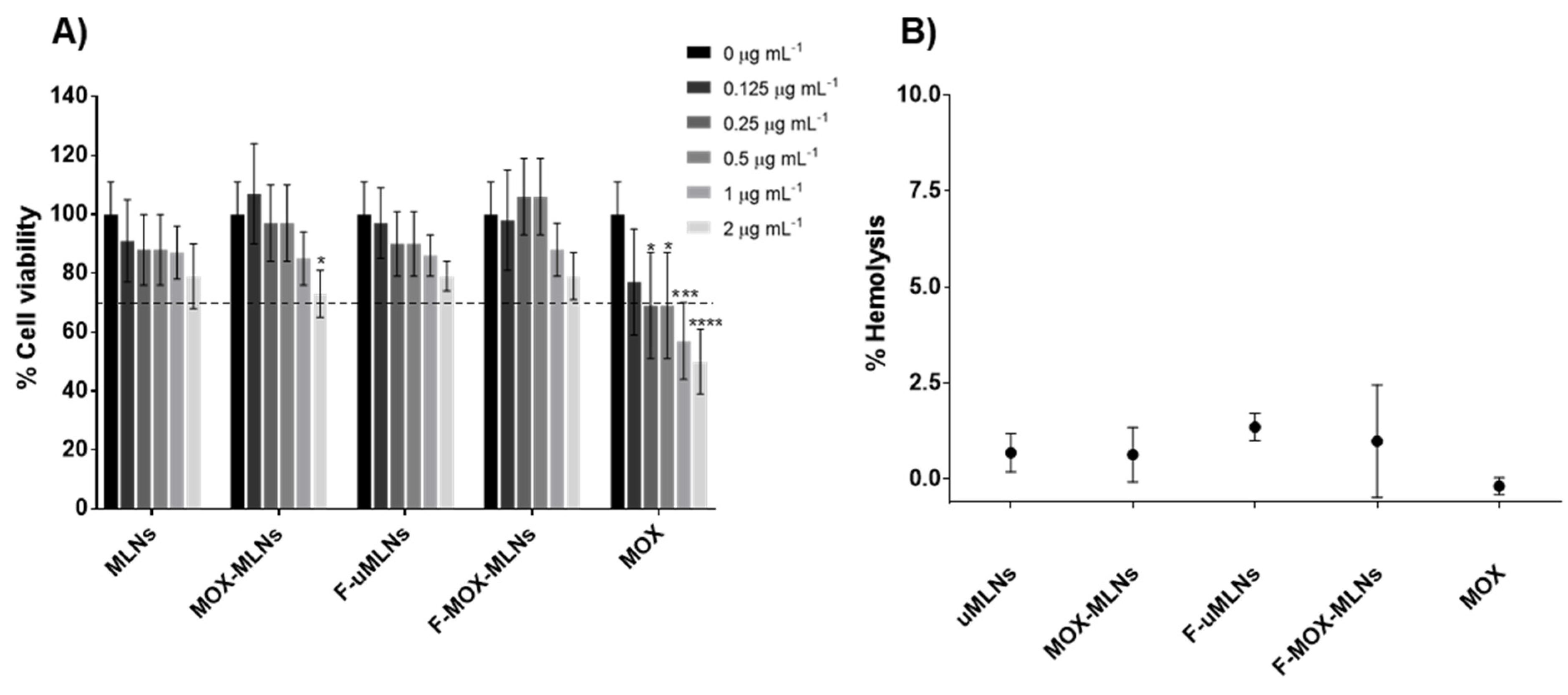
2.6.1. Cell Viability Assessment
2.6.2. Hemolysis Activity Assessment
2.7. In Vitro Antibacterial Studies
2.8. In Vitro Antibiofilm Studies
2.8.1. Microtiter Plate Biofilm Model
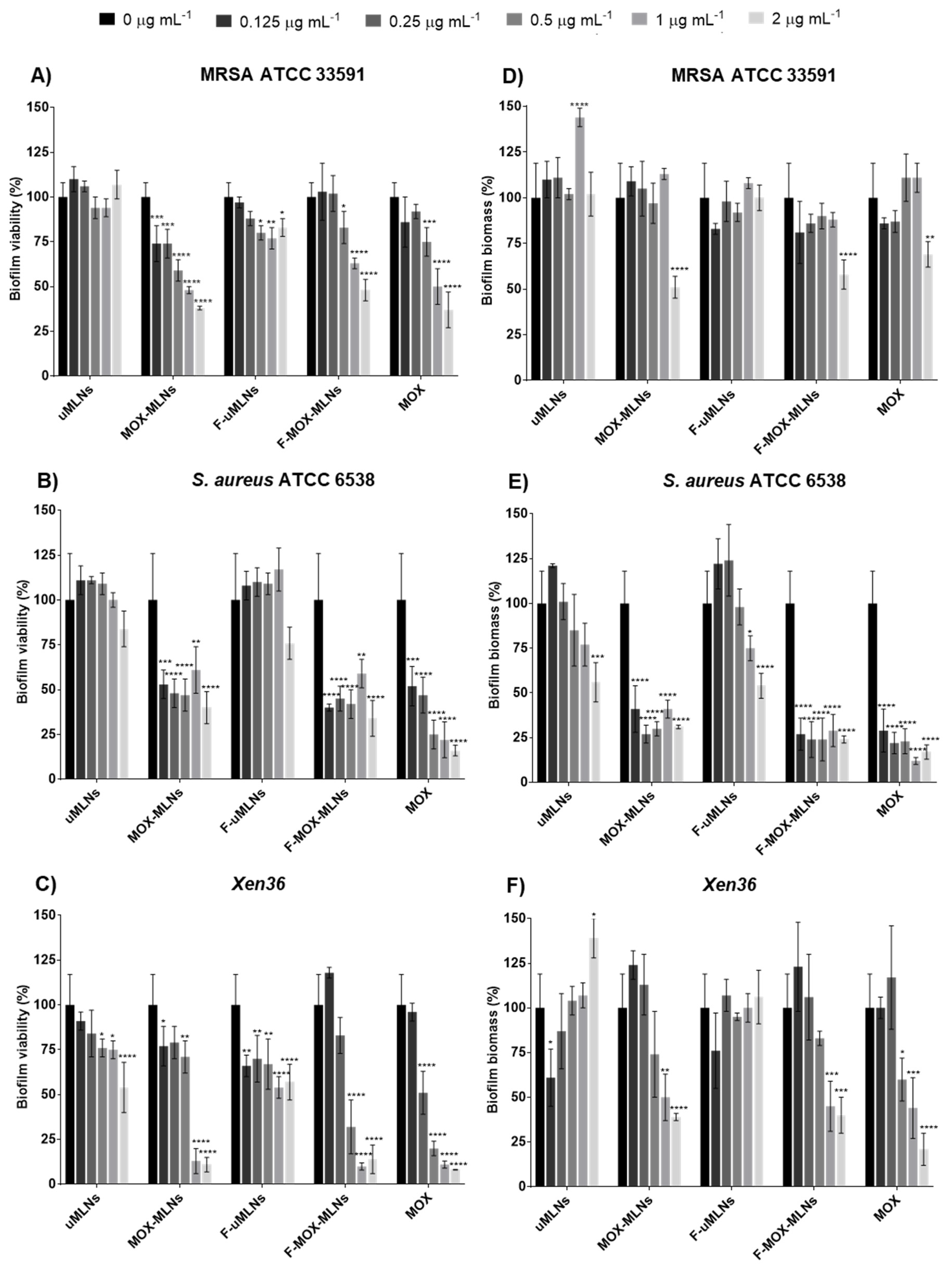
Biofilm Biomass Study
Biofilm Viability Study
2.8.2. Confocal Laser Scanning Microscopy (CLSM) Biofilm Analysis
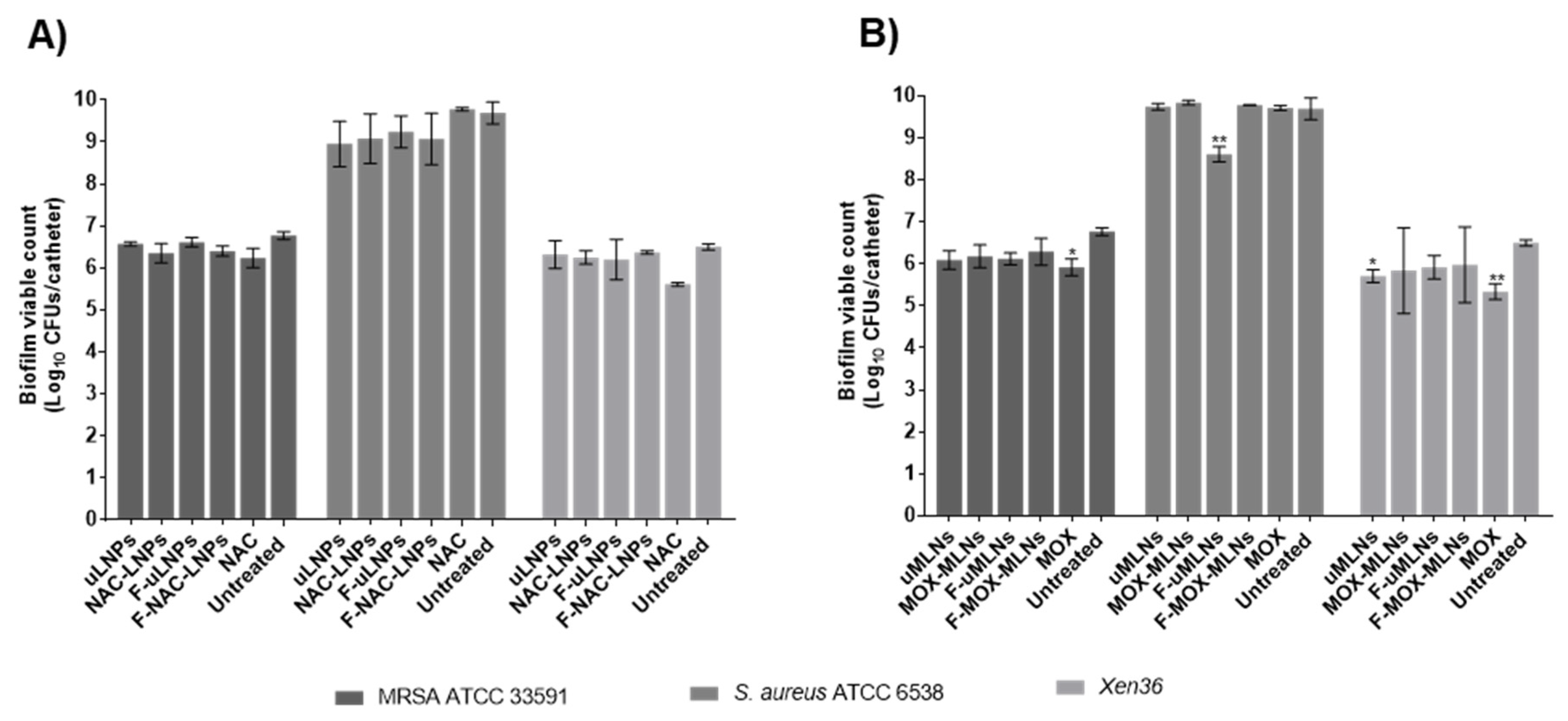
2.8.3. Catheter Biofilm Model
2.9. Statistical Analysis
3. Results
3.1. Physical Characterization of MLNs and Storage Stability
3.2. In Vitro Cytocompatibility Studies
3.3. In Vitro Antibacterial Studies
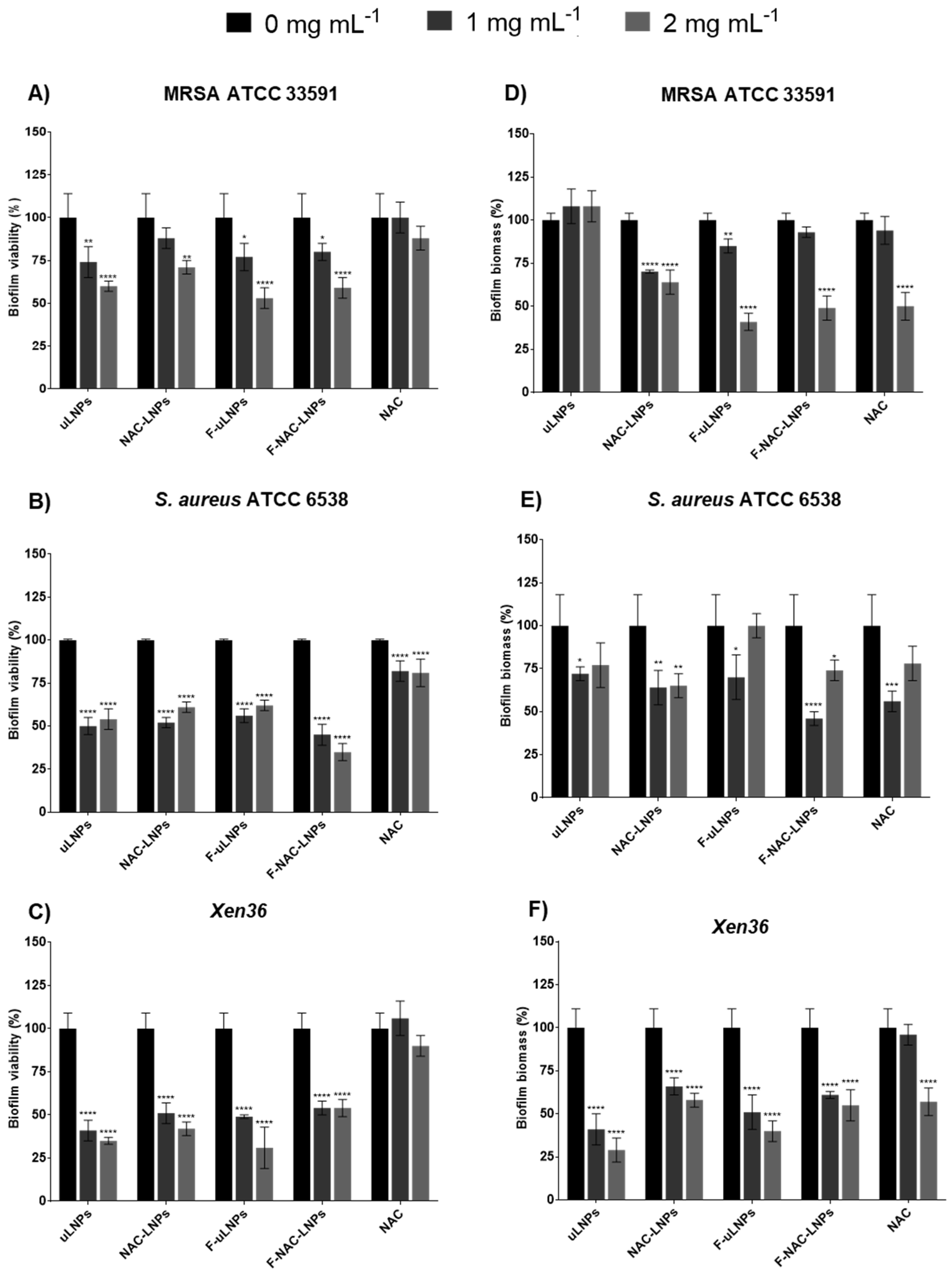
3.4. In Vitro Antibiofilm Studies
3.4.1. Biofilm Viability and Biomass Studies
3.4.2. Confocal Laser Scanning Microscopy (CLSM) Biofilm Analysis
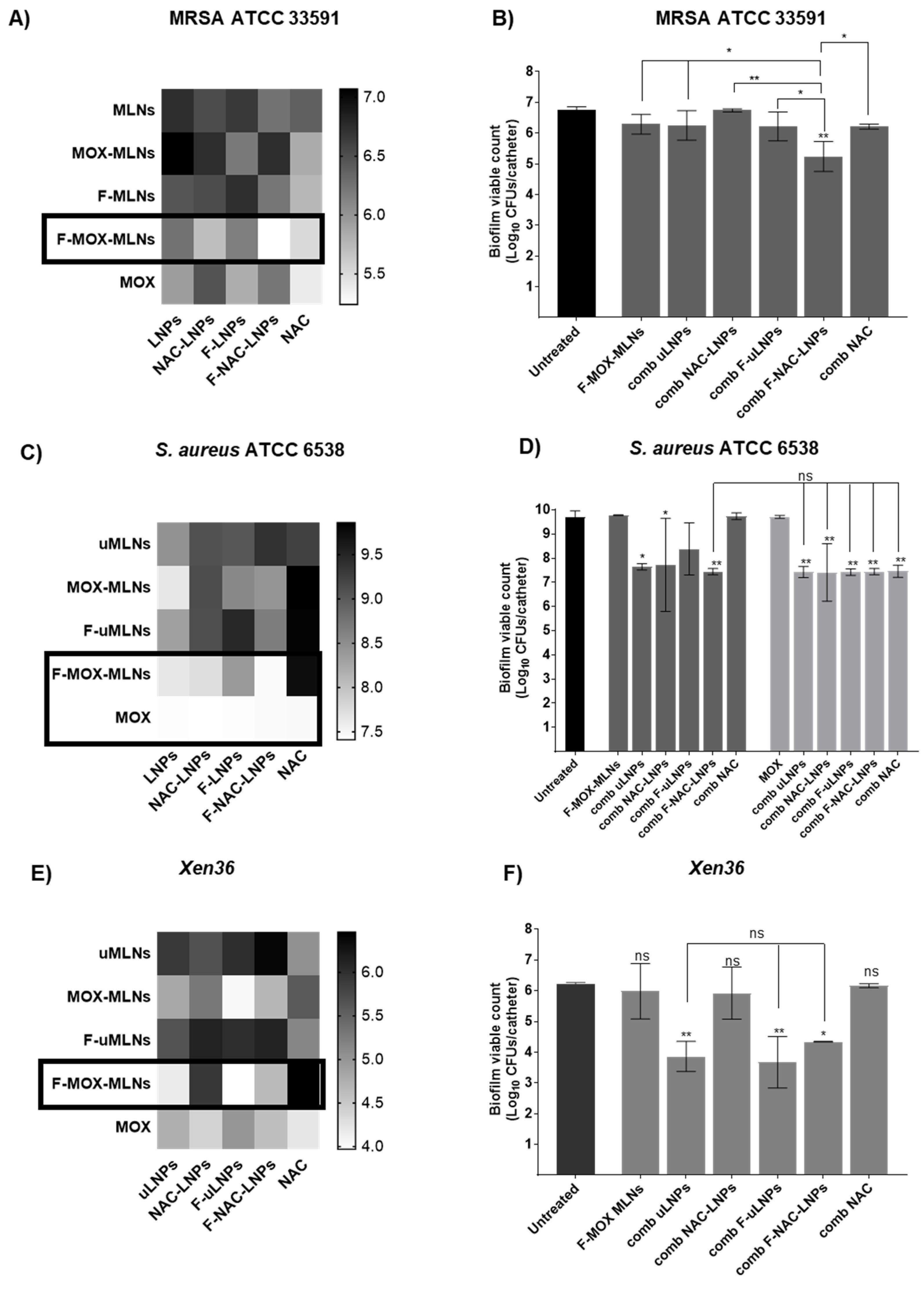
3.4.3. Combined Nano Therapy of MOX-Loaded and NAC-Loaded Nanosystems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti-infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Balfour, J.A.B.; Lamb, H.M. Moxifloxacin. Drugs 2000, 59, 115–139. [Google Scholar] [CrossRef]
- Bauer, J.; Siala, W.; Tulkens, P.M.; Van Bambeke, F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2013, 57, 2726–2737. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Vidaillac, C.; Rose, W.E.; Rybak, M.J. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2010, 54, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Greimel, F.; Scheuerer, C.; Gessner, A.; Simon, M.; Kalteis, T.; Grifka, J.; Benditz, A.; Springorum, H.-R.; Schaumburger, J. Efficacy of antibiotic treatment of implant-associated Staphylococcus aureus infections with moxifloxacin, flucloxacillin, rifampin, and combination therapy: An animal study. Drug Des. Dev. Ther. 2017, 11, 1729–1736. [Google Scholar] [CrossRef]
- Pinto, R.M.; Monteiro, C.; Costa Lima, S.A.; Casal, S.; Van Dijck, P.; Martins, M.C.L.; Nunes, C.; Reis, S. N-acetyl-l-cysteine-loaded nanosystems as a promising therapeutic approach toward the eradication of Pseudomonas aeruginosa biofilms. ACS Appl. Mater. Interfaces 2021, 13, 42329–42343. [Google Scholar] [CrossRef]
- Marchese, A.; Bozzolasco, M.; Gualco, L.; Debbia, E.A.; Schito, G.C.; Schito, A.M. Effect of Fosfomycin Alone and in Combination with N-Acetylcysteine on E. coli Biofilms. Int. J. Antimicrob. Agents 2003, 22, 95–100. [Google Scholar] [CrossRef]
- Manoharan, A.; Das, T.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Manos, J. The effect of N-acetylcysteine in a combined antibiofilm treatment against antibiotic-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2020, 75, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, Y. N-Acetylcysteine Inhibit Biofilms Produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl-L-Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef]
- Stass, H.; Kubitza, D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 1999, 43, 83–90. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Nafee, N. Nanocarriers Against Bacterial Biofilms: Current Status and Future Perspectives. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Kon, K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 167–189. [Google Scholar]
- Wang, Z.; Liu, X.; Duan, Y.; Huang, Y. Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials 2021, 280, 121249. [Google Scholar] [CrossRef]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.; Silva, A.M.; Souto, E.B. Current State-of-Art and New Trends on Lipid Nanoparticles (SLN and NLC) for Oral Drug Delivery. J. Drug Deliv. 2012, 2012, 750891. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory Effects of D-Amino Acids on Staphylococcus aureus Biofilm Development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. D-Amino Acids Enhance the Activity of Antimicrobials against Biofilms of Clinical Wound Isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, S.M.T.; Nunes, C.; Lima, S.A.C.; Soares-Sobrinho, J.L.; Reis, S. Multiple Lipid Nanoparticles (MLN), a New Generation of Lipid Nanoparticles for Drug Delivery Systems: Lamivudine-MLN Experimental Design. Pharm. Res. 2017, 34, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Pribaz, J.R.; Bernthal, N.M.; Billi, F.; Cho, J.S.; Ramos, R.I.; Guo, Y.; Cheung, A.L.; Francis, K.P.; Miller, L.S. Mouse model of chronic post-arthroplasty infection: Noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Francis, K.P.; Joh, D.; Bellinger-Kawahara, C.; Hawkinson, M.J.; Purchio, T.F.; Contag, P.R. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun. 2000, 68, 3594–3600. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Lopes-de-Campos, D.; Pinto, R.M.; Lima, S.A.C.; Santos, T.; Sarmento, B.; Nunes, C.; Reis, S. Delivering Amoxicillin at the Infection Site-a Rational Design through Lipid Nanoparticles. Int. J. Nanomed. 2019, 14, 2781–2795. [Google Scholar] [CrossRef]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, Y.; Dai, X.; Zhang, T.; Yu, Y.; Zhang, X.; Li, C. Functional Silver Nanocomposites as Broad-Spectrum Antimicrobial and Biofilm-Disrupting Agents. ACS Appl. Mater. Interfaces 2017, 9, 16834–16847. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef]
- Siala, W.; Kucharikova, S.; Braem, A.; Vleugels, J.; Tulkens, P.M.; Mingeot-Leclercq, M.P.; Van Dijck, P.; Van Bambeke, F. The Antifungal Caspofungin Increases Fluoroquinolone Activity Against Staphylococcus aureus Biofilms by Inhibiting N-Acetylglucosamine Transferase. Nat. Commun. 2016, 7, 13286. [Google Scholar] [CrossRef]
- Vande Velde, G.; Kucharíková, S.; Van Dijck, P.; Himmelreich, U. Bioluminescence imaging increases in vivo screening efficiency for antifungal activity against device-associated Candida albicans biofilms. Int. J. Antimicrob. Agents 2018, 52, 42–51. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Vande Velde, G.; Kucharíková, S.; Schrevens, S.; Himmelreich, U.; Van Dijck, P. Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell Microbiol. 2014, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: http://www.eucast.org (accessed on 8 February 2022).
- Drago, L.; De Vecchi, E.; Mattina, R.; Romanò, C.L. Activity of N-acetyl-L-cysteine against biofilm of Staphylococcus aureus and Pseudomonas aeruginosa on orthopedic prosthetic materials. Int. J. Artif. Organs 2013, 36, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-Amino Acids Trigger Biofilm Disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Rumbo, C.; Vallejo, J.A.; Cabral, M.P.; Martínez-Guitián, M.; Pérez, A.; Beceiro, A.; Bou, G. Assessment of antivirulence activity of several d-amino acids against Acinetobacter baumannii and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2016, 71, 3473–3481. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef]
- Liesse Iyamba, J.M.; Seil, M.; Devleeschouwer, M.; Takaisi Kikuni, N.B.; Dehaye, J.P. Study of the formation of a biofilm by clinical strains of Staphylococcus aureus. Biofouling 2011, 27, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory Effects of Polysorbate 80 on MRSA Biofilm Formed on Different Substrates Including Dermal Tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef] [PubMed]
- Toutain-Kidd, C.M.; Kadivar, S.C.; Bramante, C.T.; Bobin, S.A.; Zegans, M.E. Polysorbate 80 Inhibition of Pseudomonas aeruginosa Biofilm Formation and its Cleavage by the Secreted Lipase LipA. Antimicrob. Agents Chemother. 2009, 53, 136–145. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on Growth and Biofilm Formation in Laboratory Media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef] [PubMed]
- Warraich, A.A.; Mohammed, A.R.; Perrie, Y.; Hussain, M.; Gibson, H.; Rahman, A. Evaluation of anti-biofilm activity of acidic amino acids and synergy with ciprofloxacin on Staphylococcus aureus biofilms. Sci. Rep. 2020, 10, 9021. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, S.; Lou, X.; Cheng, S.; Liu, X.; Zheng, W.; Zheng, Z.; Wang, H. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. Microbiologyopen 2020, 9, e00946. [Google Scholar] [CrossRef] [PubMed]
- Sloup, R.E.; Cieza, R.J.; Needle, D.B.; Abramovitch, R.B.; Torres, A.G.; Waters, C.M. Polysorbates prevent biofilm formation and pathogenesis of Escherichia coli O104:H4. Biofouling 2016, 32, 1131–1140. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, R.M.; Seabra, C.L.; De Jonge, M.; Martins, M.C.L.; Van Dijck, P.; Reis, S.; Nunes, C. Antibiofilm Combinatory Strategy: Moxifloxacin-Loaded Nanosystems and Encapsulated N-Acetyl-L-Cysteine. Pharmaceutics 2022, 14, 2294. https://doi.org/10.3390/pharmaceutics14112294
Pinto RM, Seabra CL, De Jonge M, Martins MCL, Van Dijck P, Reis S, Nunes C. Antibiofilm Combinatory Strategy: Moxifloxacin-Loaded Nanosystems and Encapsulated N-Acetyl-L-Cysteine. Pharmaceutics. 2022; 14(11):2294. https://doi.org/10.3390/pharmaceutics14112294
Chicago/Turabian StylePinto, Rita M., Catarina Leal Seabra, Martine De Jonge, M. Cristina L. Martins, Patrick Van Dijck, Salette Reis, and Cláudia Nunes. 2022. "Antibiofilm Combinatory Strategy: Moxifloxacin-Loaded Nanosystems and Encapsulated N-Acetyl-L-Cysteine" Pharmaceutics 14, no. 11: 2294. https://doi.org/10.3390/pharmaceutics14112294
APA StylePinto, R. M., Seabra, C. L., De Jonge, M., Martins, M. C. L., Van Dijck, P., Reis, S., & Nunes, C. (2022). Antibiofilm Combinatory Strategy: Moxifloxacin-Loaded Nanosystems and Encapsulated N-Acetyl-L-Cysteine. Pharmaceutics, 14(11), 2294. https://doi.org/10.3390/pharmaceutics14112294







