Pharmacological Validation of Long-Term Treatment with Antiretroviral Drugs in a Model of SIV-Infected Non-Human Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antiretroviral Drug Formulations
2.3. Single-Dose Antiretroviral Drug Plasma Pharmacokinetic Study
2.4. Repeated Antiretroviral Doses in Long-Term ART-Treated SIV-Infected NHP
2.5. Blood Samples and Antiretroviral Drug Assays
2.6. Efficacy and Safety Assessments
2.7. Pharmacokinetic Analysis
2.8. Statistical Analysis
3. Results
3.1. Single-Dose Antiretroviral Drug Plasma Pharmacokinetic Study
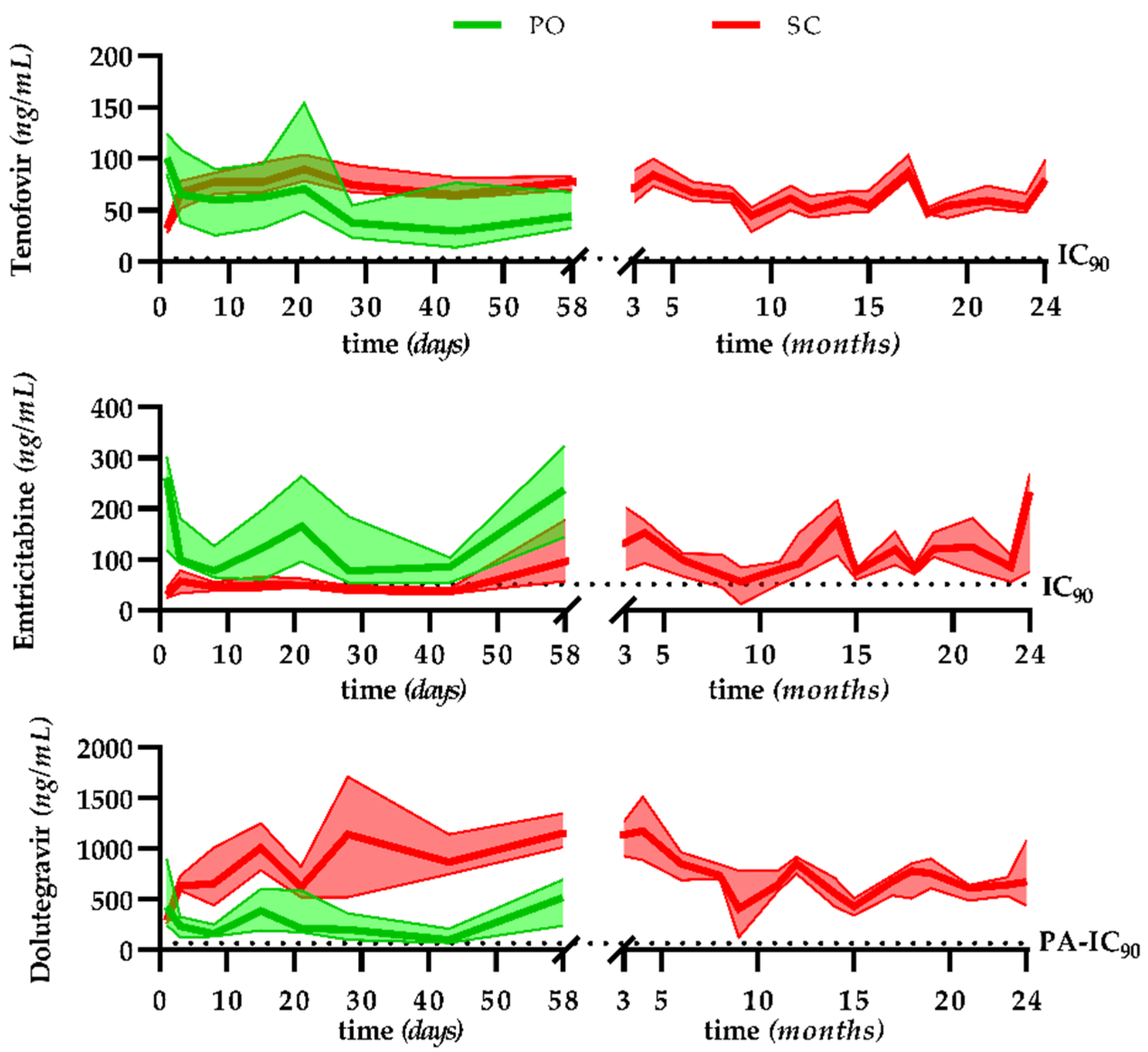
3.2. Pharmacokinetics, Efficacy, and Safety in the Long-Term Treatment Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perelson, A.S.; Essunger, P.; Cao, Y.; Vesanen, M.; Hurley, A.; Saksela, K.; Markowitz, M.; Ho, D.D. Decay Characteristics of HIV-1-Infected Compartments during Combination Therapy. Nature 1997, 387, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.J.; Reoma, L.B.; Kovacs, J.A.; Nath, A. Advances toward Curing HIV-1 Infection in Tissue Reservoirs. J. Virol. 2020, 94, e00375-19. [Google Scholar] [CrossRef] [PubMed]
- Cory, T.J.; Schacker, T.W.; Stevenson, M.; Fletcher, C.V. Overcoming Pharmacologic Sanctuaries. Curr. Opin. HIV AIDS 2013, 8, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 Replication Is Associated with Lower Antiretroviral Drug Concentrations in Lymphatic Tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.-Y.; Archer, J.; Kosakovsky Pond, S.L.; Chung, Y.-S.; Penugonda, S.; Chipman, J.G.; Fletcher, C.V.; et al. Persistent HIV-1 Replication Maintains the Tissue Reservoir during Therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef]
- Mzingwane, M.L.; Tiemessen, C.T. Mechanisms of HIV Persistence in HIV Reservoirs. Rev. Med. Virol. 2017, 27, e1924. [Google Scholar] [CrossRef]
- Deeks, S.G.; Autran, B.; Berkhout, B.; Benkirane, M.; Cairns, S.; Chomont, N.; Chun, T.-W.; Churchill, M.; Mascio, M.D.; Katlama, C.; et al. Towards an HIV Cure: A Global Scientific Strategy. Nat. Rev. Immunol. 2012, 12, 607–614. [Google Scholar] [CrossRef]
- Kline, C.; Ndjomou, J.; Franks, T.; Kiser, R.; Coalter, V.; Smedley, J.; Piatak, M.; Mellors, J.W.; Lifson, J.D.; Ambrose, Z. Persistence of Viral Reservoirs in Multiple Tissues after Antiretroviral Therapy Suppression in a Macaque RT-SHIV Model. PLoS ONE 2013, 8, e84275. [Google Scholar] [CrossRef]
- Wong, J.K.; Yukl, S.A. Tissue Reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef]
- Labarthe, L.; Gelé, T.; Gouget, H.; Benzemrane, M.-S.; Le Calvez, P.; Legrand, N.; Lambotte, O.; Le Grand, R.; Bourgeois, C.; Barrail-Tran, A. Pharmacokinetics and Tissue Distribution of Tenofovir, Emtricitabine and Dolutegravir in Mice. J. Antimicrob. Chemother. 2022, 77, 1094–1101. [Google Scholar] [CrossRef]
- Bruel, T.; Hamimi, C.; Dereuddre-Bosquet, N.; Cosma, A.; Shin, S.Y.; Corneau, A.; Versmisse, P.; Karlsson, I.; Malleret, B.; Targat, B.; et al. Long-Term Control of Simian Immunodeficiency Virus (SIV) in Cynomolgus Macaques Not Associated with Efficient SIV-Specific CD8+ T-Cell Responses. J. Virol. 2015, 89, 3542–3556. [Google Scholar] [CrossRef]
- Terrade, G.; Huot, N.; Petitdemange, C.; Lazzerini, M.; Orta Resendiz, A.; Jacquelin, B.; Müller-Trutwin, M. Interests of the Non-Human Primate Models for HIV Cure Research. Vaccines 2021, 9, 958. [Google Scholar] [CrossRef]
- Harding, J.D. Nonhuman Primates and Translational Research: Progress, Opportunities, and Challenges. ILAR J. 2017, 58, 141–150. [Google Scholar] [CrossRef]
- Bender, A.M.; Simonetti, F.R.; Kumar, M.R.; Fray, E.J.; Bruner, K.M.; Timmons, A.E.; Tai, K.Y.; Jenike, K.M.; Antar, A.A.R.; Liu, P.-T.; et al. The Landscape of Persistent Viral Genomes in ART Treated SIV, SHIV, and HIV-2 Infections. Cell Host Microbe 2019, 26, 73–85.e4. [Google Scholar] [CrossRef]
- Burgunder, E.; Fallon, J.K.; White, N.; Schauer, A.P.; Sykes, C.; Remling-Mulder, L.; Kovarova, M.; Adamson, L.; Luciw, P.; Garcia, J.V.; et al. Antiretroviral Drug Concentrations in Lymph Nodes: A Cross-Species Comparison of the Effect of Drug Transporter Expression, Viral Infection, and Sex in Humanized Mice, Nonhuman Primates, and Humans. J. Pharmacol. Exp. Ther. 2019, 370, 360–368. [Google Scholar] [CrossRef]
- Harper, J.; Ribeiro, S.P.; Chan, C.N.; Aid, M.; Deleage, C.; Micci, L.; Pino, M.; Cervasi, B.; Raghunathan, G.; Rimmer, E.; et al. Interleukin-10 Contributes to Reservoir Establishment and Persistence in SIV-Infected Macaques Treated with Antiretroviral Therapy. J. Clin. Investig. 2022, 132, e155251. [Google Scholar] [CrossRef]
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; del Rio, C.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society–USA Panel. JAMA 2020, 324, 1651. [Google Scholar] [CrossRef]
- Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; Department of Health and Human Services: Washington, DC, USA, 2021.
- European AIDS Clinical Society. European AIDS Clinical Society Guidelines V11; European AIDS Clinical Society: Brussels, Belgium, 2021. [Google Scholar]
- Organisation Mondiale de la Santé. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; Organisation Mondiale de la Santé: Geneva, Switzerland, 2021; p. 592. [Google Scholar]
- Del Prete, G.Q.; Smedley, J.; Macallister, R.; Jones, G.S.; Li, B.; Hattersley, J.; Zheng, J.; Piatak, M.; Keele, B.F.; Hesselgesser, J.; et al. Short Communication: Comparative Evaluation of Coformulated Injectable Combination Antiretroviral Therapy Regimens in Simian Immunodeficiency Virus-Infected Rhesus Macaques. AIDS Res. Hum. Retrovir. 2016, 32, 163–168. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Guidance for Industry|Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Food and Drug Administration: Silver Spring, MD, USA, 2005.
- Karlsson, I.; Malleret, B.; Brochard, P.; Delache, B.; Calvo, J.; Le Grand, R.; Vaslin, B. Dynamics of T-Cell Responses and Memory T Cells during Primary Simian Immunodeficiency Virus Infection in Cynomolgus Macaques. J. Virol. 2007, 81, 13456–13468. [Google Scholar] [CrossRef]
- Gouget, H.; Noé, G.; Barrail-Tran, A.; Furlan, V. UPLC–MS/MS Method for the Simultaneous Quantification of Bictegravir and 13 Others Antiretroviral Drugs plus Cobicistat and Ritonavir Boosters in Human Plasma. J. Pharm. Biomed. Anal. 2020, 181, 113057. [Google Scholar] [CrossRef]
- Cooper, R.D.; Wiebe, N.; Smith, N.; Keiser, P.; Naicker, S.; Tonelli, M. Systematic Review and Meta-Analysis: Renal Safety of Tenofovir Disoproxil Fumarate in HIV-Infected Patients. Clin. Infect. Dis. 2010, 51, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.M.; Neary, M.; Owen, A. The Role of Drug Transporters in the Kidney: Lessons from Tenofovir. Front. Pharmacol. 2014, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.G.; Rosen, E.P.; Prince, H.M.A.; White, N.; Sykes, C.; de la Cruz, G.; Mathews, M.; Deleage, C.; Estes, J.D.; Charlins, P.; et al. Heterogeneous Antiretroviral Drug Distribution and HIV/SHIV Detection in the Gut of Three Species. Sci. Transl. Med. 2019, 11, eaap8758. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.M.; Mirochnick, M.; Shapiro, D.E.; Bardeguez, A.; Rodman, J.; Robbins, B.; Huang, S.; Fiscus, S.A.; Van Rompay, K.K.A.; Rooney, J.F.; et al. Pharmacokinetics and Safety of Single-Dose Tenofovir Disoproxil Fumarate and Emtricitabine in HIV-1-Infected Pregnant Women and Their Infants. Antimicrob. Agents Chemother. 2011, 55, 5914–5922. [Google Scholar] [CrossRef][Green Version]
- Min, S.; Song, I.; Borland, J.; Chen, S.; Lou, Y.; Fujiwara, T.; Piscitelli, S.C. Pharmacokinetics and Safety of S/GSK1349572, a Next-Generation HIV Integrase Inhibitor, in Healthy Volunteers. Antimicrob. Agents Chemother. 2010, 54, 254–258. [Google Scholar] [CrossRef]
- Barditch-Crovo, P.; Deeks, S.G.; Collier, A.; Safrin, S.; Coakley, D.F.; Miller, M.; Kearney, B.P.; Coleman, R.L.; Lamy, P.D.; Kahn, J.O.; et al. Phase i/Ii Trial of the Pharmacokinetics, Safety, and Antiretroviral Activity of Tenofovir Disoproxil Fumarate in Human Immunodeficiency Virus-Infected Adults. Antimicrob. Agents Chemother. 2001, 45, 2733–2739. [Google Scholar] [CrossRef]
- Min, S.; Sloan, L.; DeJesus, E.; Hawkins, T.; McCurdy, L.; Song, I.; Stroder, R.; Chen, S.; Underwood, M.; Fujiwara, T.; et al. Antiviral Activity, Safety, and Pharmacokinetics/Pharmacodynamics of Dolutegravir as 10-Day Monotherapy in HIV-1-Infected Adults. AIDS 2011, 25, 1737–1745. [Google Scholar] [CrossRef]
- Zong, J.; Chittick, G.E.; Wang, L.H.; Hui, J.; Begley, J.A.; Blum, M.R. Pharmacokinetic Evaluation of Emtricitabine in Combination With Other Nucleoside Antivirals in Healthy Volunteers. J. Clin. Pharmacol. 2007, 47, 877–889. [Google Scholar] [CrossRef]
- Han, W.-L.; Shang, J.-C.; Yan, B.; Tan, R.; Huang, W.-X.; Zhong, X.-N.; Yang, J.-Q.; Huang, A.-L. Pharmacokinetics of Single- and Multiple-Dose Emtricitabine in Healthy Male Chinese Volunteers. PHA 2014, 93, 166–171. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Remsberg, C.M.; Sayre, C.L.; Forrest, M.L.; Davies, N.M. Flip-Flop Pharmacokinetics—Delivering a Reversal of Disposition: Challenges and Opportunities during Drug Development. Ther. Deliv. 2011, 2, 643–672. [Google Scholar] [CrossRef]
- Adams, J.L.; Patterson, K.B.; Prince, H.M.A.; Sykes, C.; Greener, B.N.; Dumond, J.B.; Kashuba, A.D.M. Single and Multiple Dose Pharmacokinetics of Dolutegravir in the Genital Tract of HIV-Negative Women. Antivir. Ther. 2013, 18, 1005–1013. [Google Scholar] [CrossRef]
- Greener, B.N.; Patterson, K.B.; Prince, H.M.A.; Sykes, C.S.; Adams, J.L.; Dumond, J.B.; Shaheen, N.J.; Madanick, R.D.; Dellon, E.S.; Cohen, M.S.; et al. Dolutegravir Pharmacokinetics in the Genital Tract and Colorectum of HIV-Negative Men after Single and Multiple Dosing. J. Acquir. Immune Defic. Syndr. 2013, 64, 39–44. [Google Scholar] [CrossRef]
- Koo, B.-S.; Lee, D.-H.; Kang, P.; Jeong, K.-J.; Lee, S.; Kim, K.; Lee, Y.; Huh, J.-W.; Kim, Y.-H.; Park, S.-J.; et al. Reference Values of Hematological and Biochemical Parameters in Young-Adult Cynomolgus Monkey (Macaca Fascicularis) and Rhesus Monkey (Macaca Mulatta) Anesthetized with Ketamine Hydrochloride. Lab. Anim. Res. 2019, 35, 7. [Google Scholar] [CrossRef]
- van Lunzen, J.; Maggiolo, F.; Arribas, J.R.; Rakhmanova, A.; Yeni, P.; Young, B.; Rockstroh, J.K.; Almond, S.; Song, I.; Brothers, C.; et al. Once Daily Dolutegravir (S/GSK1349572) in Combination Therapy in Antiretroviral-Naive Adults with HIV: Planned Interim 48 Week Results from SPRING-1, a Dose-Ranging, Randomised, Phase 2b Trial. Lancet Infect. Dis. 2012, 12, 111–118. [Google Scholar] [CrossRef]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.-C.; Rockstroh, J.K.; Girard, P.-M.; Sievers, J.; et al. Dolutegravir plus Lamivudine versus Dolutegravir plus Tenofovir Disoproxil Fumarate and Emtricitabine in Antiretroviral-Naive Adults with HIV-1 Infection (GEMINI-1 and GEMINI-2): Week 48 Results from Two Multicentre, Double-Blind, Randomised, Non-Inferiority, Phase 3 Trials. Lancet 2019, 393, 143–155. [Google Scholar] [CrossRef]
- Reese, M.J.; Savina, P.M.; Generaux, G.T.; Tracey, H.; Humphreys, J.E.; Kanaoka, E.; Webster, L.O.; Harmon, K.A.; Clarke, J.D.; Polli, J.W. In Vitro Investigations into the Roles of Drug Transporters and Metabolizing Enzymes in the Disposition and Drug Interactions of Dolutegravir, a HIV Integrase Inhibitor. Drug Metab. Dispos. 2013, 41, 353–361. [Google Scholar] [CrossRef]
- Urakami, Y.; Kimura, N.; Okuda, M.; Inui, K. Creatinine Transport by Basolateral Organic Cation Transporter HOCT2 in the Human Kidney. Pharm. Res. 2004, 21, 976–981. [Google Scholar] [CrossRef]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K.-I. Substrate Specificity of MATE1 and MATE2-K, Human Multidrug and Toxin Extrusions/H+-Organic Cation Antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef]
- Van Rompay, K.K.A.; Durand-Gasselin, L.; Brignolo, L.L.; Ray, A.S.; Abel, K.; Cihlar, T.; Spinner, A.; Jerome, C.; Moore, J.; Kearney, B.P.; et al. Chronic Administration of Tenofovir to Rhesus Macaques from Infancy through Adulthood and Pregnancy: Summary of Pharmacokinetics and Biological and Virological Effects. Antimicrob. Agents Chemother. 2008, 52, 3144–3160. [Google Scholar] [CrossRef]
- Lemaitre, J.; Desjardins, D.; Gallouët, A.-S.; Gomez-Pacheco, M.; Bourgeois, C.; Favier, B.; Sáez-Cirión, A.; Le Grand, R.; Lambotte, O. Expansion of Immature Neutrophils During SIV Infection Is Associated With Their Capacity to Modulate T-Cell Function. Front. Immunol. 2022, 13, 781356. [Google Scholar] [CrossRef] [PubMed]
- Mausoléo, A.; Olivo, A.; Desjardins, D.; Sáez-Cirión, A.; Barrail-Tran, A.; Avettand-Fenoel, V.; Noël, N.; Lagathu, C.; Béréziat, V.; Le Grand, R.; et al. Prolonged Antiretroviral Treatment Induces Adipose Tissue Remodelling Associated with Mild Inflammation in SIV-Infected Macaques. Cells 2022, 11, 3104. [Google Scholar] [CrossRef] [PubMed]
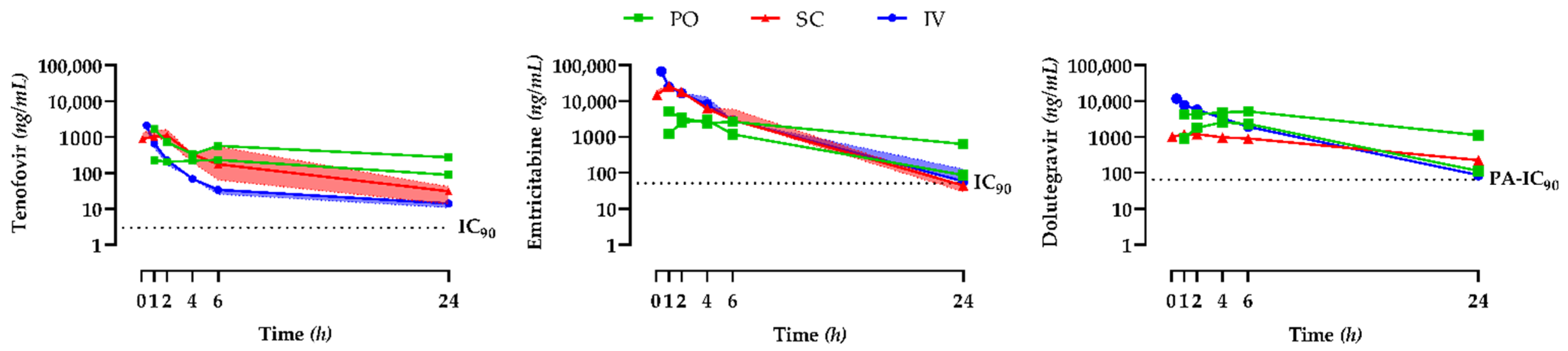

| Cmax (ng/mL) | Tmax (h) | C24h (ng/mL) | t½ (h) | AUC0→24h (ng·h/mL) | |
|---|---|---|---|---|---|
| Tenofovir | |||||
| PO (n = 2) | 1668 231 | 1.0 6.0 | 272 88 | 31.0 14.2 | 11,115 3857 |
| SC (n = 7) | 1103 (954–1654) | 1.0 (1.0–2.0) | 32 (16–50) | 4.7 (1.2–6.1) | 4391 (2326–8598) |
| IV (n = 4) | 2096 (1583–2352) | 0.25 (0.25–0.25) | 21 (12–38) | 5.1 (1.4–14.1) | 1983 (1825–2570) |
| Emtricitabine | |||||
| PO (n = 2) | 5062 2957 | 1.0 4.0 | 632 87 | 9.6 4.8 | 42,768 19,280 |
| SC (n = 7) | 25,882 (19,607–28,634) | 1.0 (1.0–1.0) | 44 (29–61) | 3.1 (2.6–3.2) | 79,578 (76,106–96,001) |
| IV (n = 4) | 67,732 (62,920–70,569) | 0.25 (0.25–0.25) | 58 (54–127) | 3.0 (2.8–3.2) | 111,530 (106,512–116,484) |
| Dolutegravir | |||||
| PO (n = 2) | 5105 2550 | 6.0 4.0 | 1109 113 | 8.2 4.1 | 72,426 23,956 |
| SC (n = 7) | 1229 (1082–1467) | 1.0 (1.0–2.0) | 224 (180–281) | 9.2 (8.8–9.7) | 15,352 (11,401–16,540) |
| IV (n = 4) | 11,643 (10,995–12,583) | 0.25 (0.25–0.25) | 86 (82–102) | 3.8 (3.7–4.2) | 39,601 (37,865–42,241) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelé, T.; Gouget, H.; Dereuddre-Bosquet, N.; Furlan, V.; Le Grand, R.; Lambotte, O.; Desjardins, D.; Barrail-Tran, A. Pharmacological Validation of Long-Term Treatment with Antiretroviral Drugs in a Model of SIV-Infected Non-Human Primates. Pharmaceutics 2022, 14, 2282. https://doi.org/10.3390/pharmaceutics14112282
Gelé T, Gouget H, Dereuddre-Bosquet N, Furlan V, Le Grand R, Lambotte O, Desjardins D, Barrail-Tran A. Pharmacological Validation of Long-Term Treatment with Antiretroviral Drugs in a Model of SIV-Infected Non-Human Primates. Pharmaceutics. 2022; 14(11):2282. https://doi.org/10.3390/pharmaceutics14112282
Chicago/Turabian StyleGelé, Thibaut, Hélène Gouget, Nathalie Dereuddre-Bosquet, Valérie Furlan, Roger Le Grand, Olivier Lambotte, Delphine Desjardins, and Aurélie Barrail-Tran. 2022. "Pharmacological Validation of Long-Term Treatment with Antiretroviral Drugs in a Model of SIV-Infected Non-Human Primates" Pharmaceutics 14, no. 11: 2282. https://doi.org/10.3390/pharmaceutics14112282
APA StyleGelé, T., Gouget, H., Dereuddre-Bosquet, N., Furlan, V., Le Grand, R., Lambotte, O., Desjardins, D., & Barrail-Tran, A. (2022). Pharmacological Validation of Long-Term Treatment with Antiretroviral Drugs in a Model of SIV-Infected Non-Human Primates. Pharmaceutics, 14(11), 2282. https://doi.org/10.3390/pharmaceutics14112282






