In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
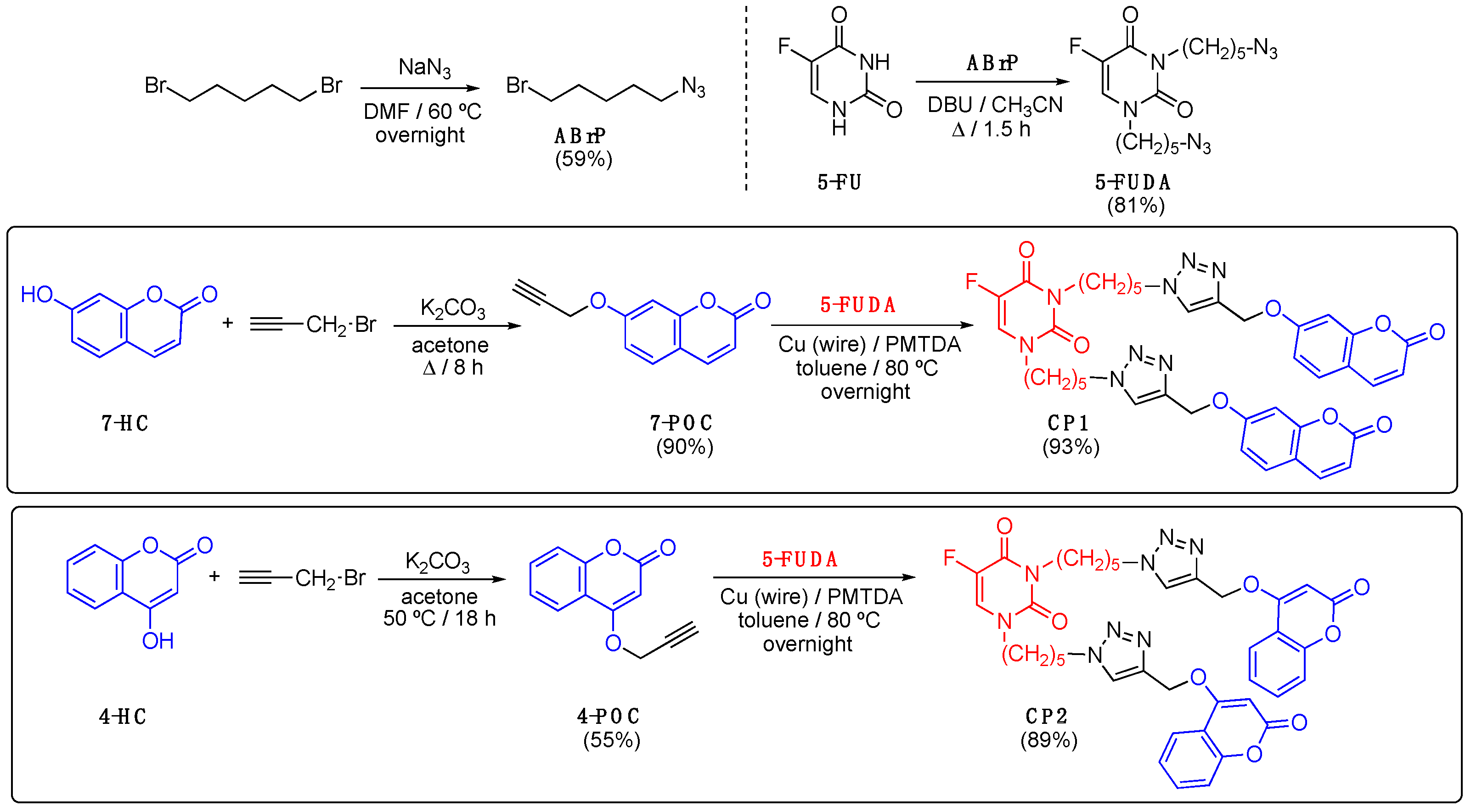
2.3. Synthesis
2.3.1. 1-Azido-5-bromopentane (ABrP)
2.3.2. 7-Propargyloxycoumarin (7-POC)
2.3.3. Click Product CP1
2.3.4. 4-Propargyloxycoumarin (4-POC)
2.3.5. Click Product CP2
2.4. In Vitro Antioxidant Activity
2.5. Preparation of CP1 and CP2 Nanoparticles
2.6. Determination of the Critical Aggregation Concentration (CAC)
2.7. Dynamic Light Scattering (DLS)
2.8. High Resolution Scanning Electron Microscopy (HR-SEM)
2.9. Viability Assays: Cell Culture
2.10. Viability Assays: MTT Assay
2.11. Viability Assays: Morphology Analysis—Optical Microscopy Images
2.12. Viability Assays: Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of CP1 and CP2
3.2. In Vitro Antioxidant Evaluation
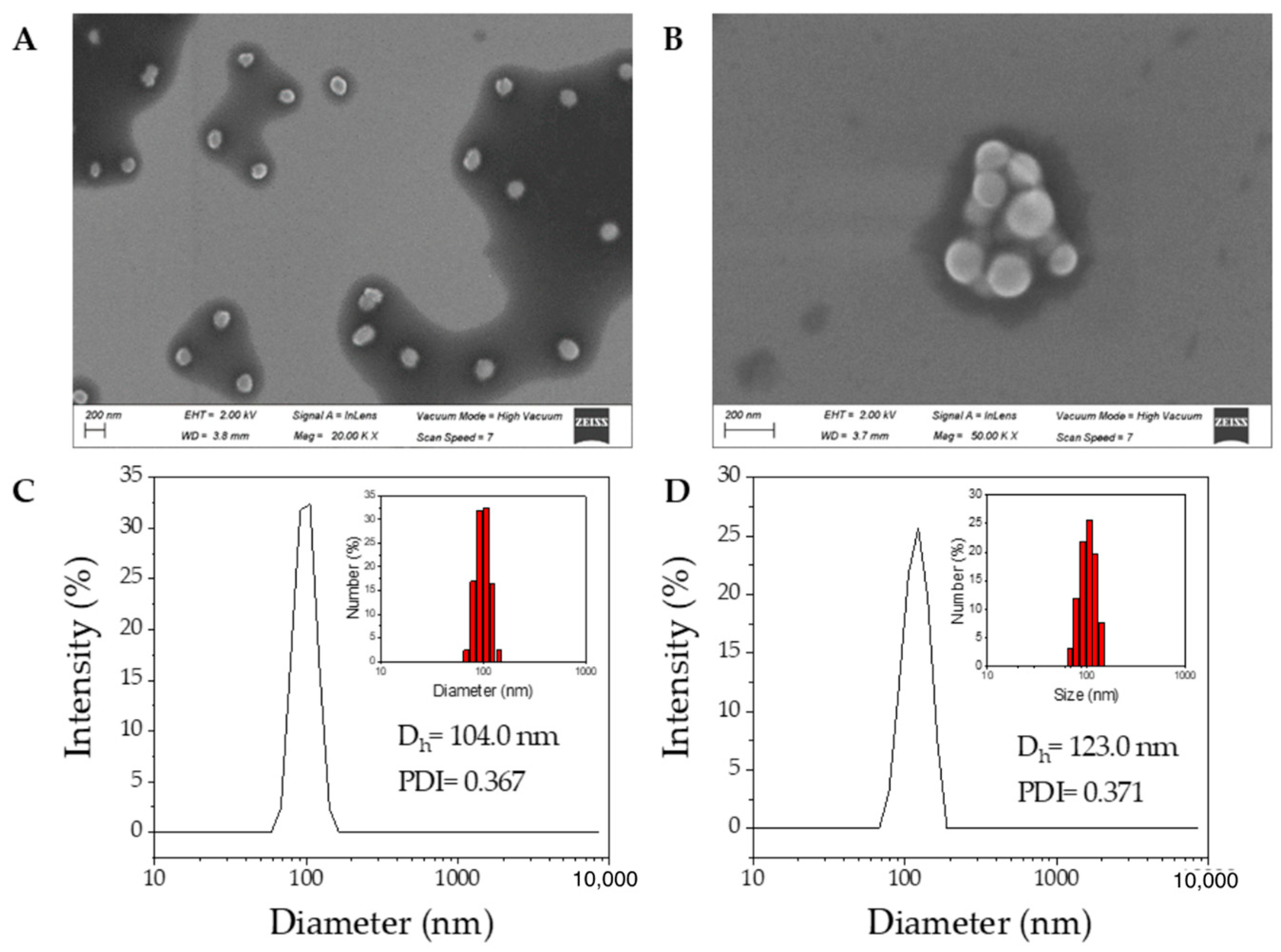
3.3. Preparation and Characterization of CP1 and CP2 Nanoparticles
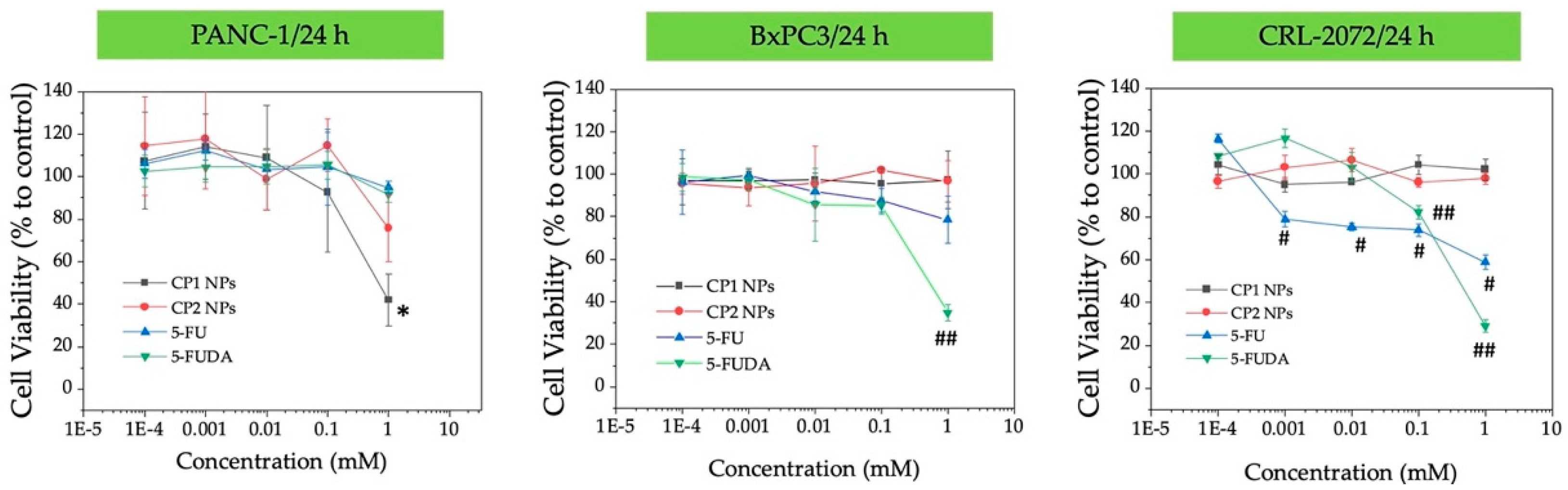
3.4. Preliminary Efficacy Assessment in Cell Culture: Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Deaths in 2018; International Agency for Research on Cancer (IARC): Geneva, Switzerland, 2018; Press Release. Available online: https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf (accessed on 24 November 2021).
- World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2014. [Google Scholar]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug combination in cancer treatment—From cocktails to conjugated combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Ramos Campos, E.V.; Rodríguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef]
- Dong, S.; He, J.; Sun, Y.; Li, D.; Li, L.; Zhang, M.; Ni, P. Efficient click synthesis of a protonized and reduction-sensitive amphiphilic small-molecule prodrug containing camptothecin and gemcitabine for a drug self-delivery system. Mol. Pharm. 2019, 16, 3770–3779. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Shen, J.; Zhao, G.; Wang, P.G. The impact of click chemistry in medicinal chemistry. Expert Opin. Drug Discov. 2012, 7, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater. Res. 2018, 22, 13. [Google Scholar] [CrossRef]
- Mashayekh, K.; Shiri, P. An overview of recent advances in the applications of click chemistry in the synthesis of bioconjugates with anticancer activities. ChemistrySelect 2019, 4, 13459–13478. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, homogeneous and heterogeneous catalysts, and novel developments and applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- Nebra, N.; García-Álvarez, J. Recent progress of Cu-catalyzed azide-alkyne cycloaddition reactions (CuAAC) in sustainable solvents: Glycerol, deep eutectic solvents, and aqueous media. Molecules 2020, 25, 2015. [Google Scholar] [CrossRef]
- Rečnik, L.-M.; Kandioller, W.; Mindt, T.L. 1,4-Disubstituted 1,2,3-triazoles as amide bond surrogates for the stabilisation of linear peptides with biological activity. Molecules 2020, 25, 3576. [Google Scholar] [CrossRef] [PubMed]
- Agouram, N.; El Hadrami, E.M.; Bentama, A. 1,2,3-Triazoles as biomimetics in peptide science. Molecules 2021, 26, 2937. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4- and 1,5-disubstituted 1,2,3-triazoles good pharmacophoric groups? ChemMedChem 2014, 9, 2497–2508. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Serafini, M.; Pirali, T.; Tron, G.C. Click 1,2,3-triazoles in drug discovery and development: From the flask to the clinic? Adv. Heterocycl. Chem. 2020, 134, 101–148. [Google Scholar] [CrossRef]
- Jain, A.; Piplani, P. Exploring the chemistry and therapeutic potential of triazoles: A comprehensive literature. Mini Rev. Med. Chem. 2019, 19, 1298–1368. [Google Scholar] [CrossRef] [PubMed]
- Slanova, K.; Todorov, L.; Belskaya, N.P.; Palafox, M.A.; Kostova, I.P. Developments in the application of 1,2,3-triazoles in cancer treatment. Recent Pat. Anticancer Drug Discov. 2020, 15, 92–112. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.H.; Subhedar, D.D.; Nawale, L.; Sarkar, D.; Khan, F.A.K.; Sangshetti, J.N.; Shingate, B.B. 1,2,3-Triazole derivatives as antitubercular agents: Synthesis, biological evaluation and molecular docking study. MedChemComm 2015, 6, 1104–1116. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumar, N.; Sehrawat, H.; Yadav, N.; Mishra, V. Click triazole as a linker for drug repurposing against SARs-CoV-2: A greener approach in race to find COVID-19 therapeutic. Curr. Res. Green Sustain. Chem. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Pałasz, A.; Cież, D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef] [PubMed]
- Diasio, R.B.; Harris, B.E. Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- O’Connor, O.A. Pharmacological Modulation of Fluoropyrimidines: Building on the Lessons of the Past. In Combination Cancer Therapy: Modulators and Potentiators; Schwartz, G.K., Ed.; Humana Press: Totowa, NJ, USA, 2005; Chapter 6; pp. 133–174. [Google Scholar]
- Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadin, F. Recent advances in designing 5-fluorouracil delivery systems: A stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Ciaffaglione, V.; Modica, M.N.; Pittalà, V.; Romeo, G.; Salerno, L.; Intagliata, S. Mutual prodrugs of 5-fluorouracil: From a classic chemotherapeutic agent to novel potential anticancer drugs. ChemMedChem 2021, 16, 3496–3512. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Sanduja, M.; Gupta, J.; Singh, H.; Pagare, P.P.; Rana, A. Uracil-coumarin based hybrid molecules as potent anti-cancer and anti-bacterial agents. J. Saudi Chem. Soc. 2020, 24, 251–256. [Google Scholar] [CrossRef]
- López, S.; Rodríguez-López, J.; García, M.T.; Rodríguez, J.F.; Pérez-Ortiz, J.M.; Ramos, M.J.; Gracia, I. Self-assembled coumarin- and 5-fluorouracil-PEG micelles as multifunctional drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 74, 103582. [Google Scholar] [CrossRef]
- Gerken, P.A.; Wolstenhulme, J.R.; Tumber, A.; Hatch, S.B.; Zhang, Y.; Müller, S.; Chandler, S.A.; Mair, B.; Li, F.; Nijman, S.M.B.; et al. Discovery of a highly selective cell-active inhibitor of the histone lysine demethylases KDM2/7. Angew. Chem. Int. Ed. 2017, 56, 15555–15559. [Google Scholar] [CrossRef]
- Caldarelli, S.A.; El Fangour, S.; Wein, S.; Tran van Ba, C.; Périgaud, C.; Pellet, A.; Vial, H.J.; Peyrottes, S. New bis-thiazolium analogues as potential antimalarial agents: Design, synthesis, and biological evaluation. J. Med. Chem. 2013, 56, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Scalvini, L.; Bartolini, M.; Belluti, F.; Gobbi, S.; Andrisano, V.; Ligresti, A.; Di Marzo, V.; Rivara, S.; Mor, M.; et al. Fatty acid amide hydrolase (FAAH), acetylcholinesterase (AChE), and butyrylcholinesterase (BuChE): Networked targets for the development of carbamates as potential anti-Alzheimer’s disease agents. J. Med. Chem. 2016, 59, 6387–6406. [Google Scholar] [CrossRef]
- Kosiova, I.; Kovackova, S.; Kois, P. Synthesis of coumarin–nucleoside conjugates via Huisgen 1,3-dipolar cycloaddition. Tetrahedron 2007, 63, 312–320. [Google Scholar] [CrossRef]
- Prasad Rao, C.; Srimannarayana, G. Claisen rearrangement of 4-propargloxycoumarins: Formation of 2H,5H-pyrano [3,2-c][1]benzopyran-5-ones. Synth. Commun. 1990, 20, 535–540. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Subhedar, D.D.; Shingate, B.B.; Kalam Khan, F.A.; Sangshetti, J.N.; Khedkar, V.M.; Nawale, L.; Sarkar, D.; Navale, G.R.; Shinde, S.S. Synthesis, biological evaluation and molecular docking of novel coumarin incorporated triazoles as antitubercular, antioxidant and antimicrobial agents. Med. Chem. Res. 2016, 25, 790–804. [Google Scholar] [CrossRef]
- Wafa, N.; Sofiane, G. In-Vitro antioxidant and anti-inflammatory activities valorisation of tannin crude extract of Helianthemum helianthemoïdes (Desf.) Grosser. J. Drug. Deliv. Ther. 2020, 10, 135–139. [Google Scholar] [CrossRef]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J. Nanoparticle Res. 2008, 10, 823–829. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols, 2nd ed.; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Cancer biology and hormesis: Human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit. Rev. Toxicol. 2005, 35, 463–582. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Paculacox, C.M.; Hursey, M.L.; Rubinstein, L.R.; Boyd, M.R. Morphometric and colorimetric analyses of human tumor-cell line growth and drug sensitivity in soft agar culture. Cancer Res. 1991, 51, 1247–1256. [Google Scholar]
- Lee, J.H.; Lee, H.-B.; Jung, G.O.; Oh, J.T.; Park, D.E.; Chae, K.M. Effect of quercetin on apoptosis of PANC-1 cells. J. Korean Surg. Soc. 2013, 85, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, B.; Sun, S.; Lan, L.; Chen, Y.; Han, S.; Li, X.; Li, Z. Downregulation of miR-486-5p enhances the anti-tumor effect of 5-fluorouracil on pancreatic cancer cells. Onco. Targets Ther. 2020, 13, 1649–1659. [Google Scholar] [CrossRef]
- El-Mahdy, H.A.; El-Husseiny, A.A.; Kandil, Y.I.; Gamal El-Din, A.M. Diltiazem potentiates the cytotoxicity of gemcitabine and 5-fluorouracil in PANC-1 human pancreatic cancer cells through inhibition of P-glycoprotein. Life Sci. 2020, 262, 118518. [Google Scholar] [CrossRef] [PubMed]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef]
- Luo, G.; Muyaba, M.; Lyu, W.; Tang, Z.; Zhao, R.; Xu, Q.; You, Q.; Xiang, H. Design, synthesis and biological evaluation of novel 3-substituted 4-anilino-coumarin derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2017, 27, 867–874. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef]



| Compound | 10 µM | 20 µM | 30 µM | 40 µM | 50 µM | IC50 (µM) |
|---|---|---|---|---|---|---|
| 7-HC | 50.42 ± 0.23 | 51.08 ± 0.32 | 52.67 ± 0.08 | 50.32 ± 0.16 | 67.39 ± 0.04 | 13.82 ± 0.06 |
| 7-POC | 48.37 ± 0.12 | 48.53 ± 0.04 | 49.36 ± 0.04 | 50.04 ± 0.42 | 50.33 ± 0.03 | 2.95 ± 0.15 |
| CP1 | 52.28 ± 0.30 | 53.6. ± 0.12 | 55.17 ± 0.24 | 55.31 ± 0.75 | 56.80 ± 0.46 | 2.82 ± 0.32 |
| 4-HC | 71.98 ± 0.01 | 79.98 ± 0.01 | 85.42 ± 0.06 | 88.07 ± 0.04 | 90.95 ± 0.01 | 1.55 ± 0.01 |
| 4-POC | 59.71 ± 0.44 | 60.73 ± 0.12 | 61.82 ± 0.07 | 62.38 ± 0.05 | 63.07 ± 0.17 | 1.34 ± 0.25 |
| CP2 | 59.14 ± 0.14 | 69.48 ± 0.67 | 75.38 ± 0.23 | 79.30 ± 0.05 | 83.15 ± 0.59 | 5.38 ± 0.38 |
| Ascorbic acid | 45.78 ± 0.37 | 67.73 ± 0.29 | 78.91 ± 0.98 | 87.45 ± 0.41 | 95.42 ± 0.45 | 11.42 ± 0.17 |
Time (Days) | CP1 NPs | CP2 NPs | ||||
|---|---|---|---|---|---|---|
| DLS (nm) | PDI | ζ (mV) | DLS (nm) | PDI | ζ (mV) | |
| 1 | 104.0 ± 15.4 | 0.367 | −31.8 ± 3.0 | 123.0 ± 4.0 | 0.371 | −30.6 ± 3.2 |
| 2 | 137.8 ± 9.3 | 0.546 | −30.1 ± 3.6 | 129.2 ± 1.9 | 0.436 | −30.4 ± 3.6 |
| 3 | 117.4 ± 82.3 | 0.506 | −31.8 ± 3.4 | 110.4 ± 14.0 | 0.473 | −30.8 ± 3.7 |
| 4 | 201.9 ± 46.7 | 0.733 | −30.1 ± 3.5 | 131.9 ± 4.3 | 0.539 | −30.2 ± 3.2 |
| 5 | 117.3 ± 17.9 | 0.899 | −31.8 ± 4.7 | 124.8 ± 29.7 | 0.496 | −30.6 ± 3.0 |
| 6 | 210.5 ± 60.3 | 0.556 | −30.1 ± 5.5 | 115.3 ± 24.3 | 0.543 | −31.1 ± 2.0 |
| 7 | 178.3 ± 19.1 | 0.369 | −31.8 ± 3.0 | 234.1 ± 20.2 | 1.000 | −9.78 ± 3.6 |
| 8 | 104.6 ± 92.4 | 0.437 | −30.1 ± 3.8 | 299.5 ± 25.7 | 1.000 | −9.91 ± 3.0 |
| 9 | 228.0 ± 36.9 | 0.522 | −15.0 ± 4.1 | 368.9 ± 70.7 | 0.872 | −9.89 ± 3.6 |
| 10 | 380.4 ± 67.2 | 0.553 | −17.2 ± 4.7 | 360.1 ± 104.8 | 0.502 | −3.72 ± 3.0 |
| 11 | 394.9 ± 76.4 | 0.553 | −14.5 ± 5.0 | 304.6 ± 38.3 | 0.308 | −3.90 ± 3.3 |
| 12 | 381.0 ± 70.6 | 0.492 | −7.04 ± 5.8 | 405.3 ± 58.9 | 0.388 | −3.76 ± 3.5 |
| 13 | 321.6 ± 80.6 | 0.624 | −12.1 ± 3.0 | 317.1 ± 59.1 | 0.588 | −13.2 ± 3.9 |
| 14 | 214.9 ± 95.8 | 0.424 | −11.3 ± 5.1 | 311.8 ± 186.9 | 0.588 | −12.6 ± 4.0 |
| 15 | 248.4 ± 98.2 | 0.620 | −11.7 ± 2.6 | 597.7 ± 234.1 | 0.533 | −12.5 ± 3.4 |
| 16 | 469.2 ± 96.7 | 0.490 | −3.69 ± 5.1 | 609.9 ± 215.0 | 0.385 | −4.41 ± 2.9 |
| 17 | 304.6 ± 130.7 | 0.423 | −2.92 ± 3.6 | 683.1 ± 321.7 | 0.340 | −4.03 ± 3.2 |
| 18 | 405.3 ± 201.8 | 0.608 | −3.69 ± 4.3 | 661.4 ± 178.3 | 0.416 | −4.07 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Gracia, I.; Plaza-Pedroche, R.; Rodríguez, J.F.; Pérez-Ortiz, J.M.; Rodríguez-López, J.; Ramos, M.J. In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates. Pharmaceutics 2022, 14, 2152. https://doi.org/10.3390/pharmaceutics14102152
López S, Gracia I, Plaza-Pedroche R, Rodríguez JF, Pérez-Ortiz JM, Rodríguez-López J, Ramos MJ. In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates. Pharmaceutics. 2022; 14(10):2152. https://doi.org/10.3390/pharmaceutics14102152
Chicago/Turabian StyleLópez, Sonia, Ignacio Gracia, Rodrigo Plaza-Pedroche, Juan Francisco Rodríguez, José Manuel Pérez-Ortiz, Julián Rodríguez-López, and María Jesús Ramos. 2022. "In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates" Pharmaceutics 14, no. 10: 2152. https://doi.org/10.3390/pharmaceutics14102152
APA StyleLópez, S., Gracia, I., Plaza-Pedroche, R., Rodríguez, J. F., Pérez-Ortiz, J. M., Rodríguez-López, J., & Ramos, M. J. (2022). In Vitro Antioxidant and Pancreatic Anticancer Activity of Novel 5-Fluorouracil-Coumarin Conjugates. Pharmaceutics, 14(10), 2152. https://doi.org/10.3390/pharmaceutics14102152








