Development of Nasal Vaccines and the Associated Challenges
Abstract
1. Introduction
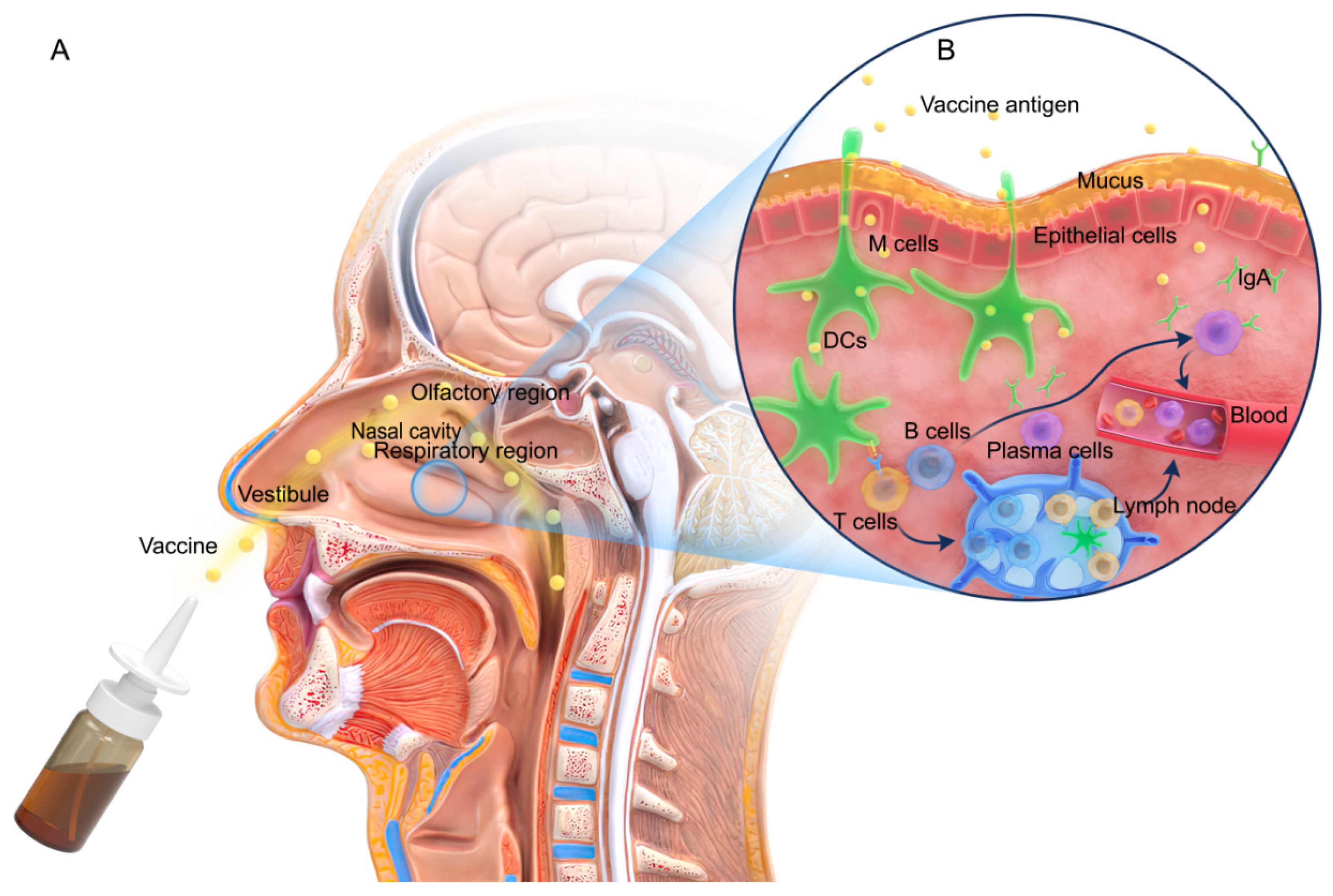
2. Nasal Structure and Immune Responses
3. Insights from the Currently Available Licensed Influenza Nasal Vaccine
4. Challenges of Nasal Vaccine
5. Mode of Mucosal Vaccination
6. Live-Attenuated Vaccines
7. Nasal Vaccine Adjuvants
7.1. Immunostimulatory Adjuvants
7.1.1. Toxoid Adjuvants
7.1.2. Cytokine Adjuvants
7.1.3. TLR Agonist Adjuvants
7.1.4. STING Agonist Adjuvants
7.1.5. Bioadhesive Adjuvants
7.1.6. Cell-Targeted Adjuvants
7.2. Delivery System Adjuvants
7.2.1. Viral Vectors
7.2.2. Liposome Delivery System
7.2.3. VLP Delivery System
7.2.4. Virosome-Mediated Delivery System
7.2.5. ISCOMs
7.2.6. NE-Based Adjuvants
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Churdboonchart, V.; Bhamarapravati, N.; Sirisidhi, K. Immune response in rabbits to dengue viral proteins. Southeast Asian J. Trop. Med. Public Health 1990, 21, 621–629. [Google Scholar]
- Golden, G. Smallpox vaccination: The Minnesota story. Minn. Med. 2003, 86, 20–25. [Google Scholar]
- Yin, L.-T.; Hao, H.-X.; Wang, H.-L.; Zhang, J.-H.; Meng, X.-L.; Yin, G.-R. Intranasal Immunisation with Recombinant Toxoplasma gondii Actin Partly Protects Mice against Toxoplasmosis. PLoS ONE 2013, 8, e82765. [Google Scholar] [CrossRef]
- Almeida, A.J.; Alpar, H.O. Nasal Delivery of Vaccines. J. Drug Target. 1996, 3, 455–467. [Google Scholar] [CrossRef]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal Drug Delivery: How, Why and What for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kumar, M.; Pathak, K. A review on mucoadhesive polymer used in nasal drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 215–222. [Google Scholar] [CrossRef]
- Xing, Y.; Lu, P.; Xue, Z.; Liang, C.; Zhang, B.; Kebebe, D.; Liu, H.; Liu, Z. Nano-Strategies for Improving the Bioavailability of Inhaled Pharmaceutical Formulations. Mini-Rev. Med. Chem. 2020, 20, 1258–1271. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Immune Functions of Nasopharyngeal Lymphoid Tissue. Adv. Oto-Rhino-Laryngol. 2011, 72, 20–24. [Google Scholar] [CrossRef]
- Date, Y.; Ebisawa, M.; Fukuda, S.; Shima, H.; Obata, Y.; Takahashi, D.; Kato, T.; Hanazato, M.; Nakato, G.; Williams, I.R.; et al. NALT M cells are important for immune induction for the common mucosal immune system. Int. Immunol. 2017, 29, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Koornstra, P.J.; Hameleers, D.M.; Biewenga, J.; Spit, B.J.; Duijvestijn, A.M.; Vriesman, P.J.V.B.; Sminia, T. The role of nasopharyngeal lymphoid tissue. Immunol. Today 1992, 13, 219–224. [Google Scholar] [CrossRef]
- Fujimura, Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. Int. J. Pathol. 2000, 436, 560–566. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.-S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp. Mol. Med. 2014, 46, e85. [Google Scholar] [CrossRef]
- Yamamoto, M.; Pascual, D.W.; Kiyono, H. M Cell-Targeted Mucosal Vaccine Strategies. Curr. Top. Microbiol. Immunol. 2011, 354, 39–52. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Pabst, R. Let’s go mucosal: Communication on slippery ground. Trends Immunol. 2004, 25, 570–577. [Google Scholar] [CrossRef]
- Dunne, P.J.; Moran, B.; Cummins, R.C.; Mills, K.H.G. CD11c+CD8alpha+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J. Immunol. 2009, 183, 400–410. [Google Scholar] [CrossRef]
- Kiyono, H.; Fukuyama, S. NALT- versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Farstad, I.N.; Haraldsen, G. Regional specialization in the mucosal immune system: Primed cells do not always home along the same track. Immunol. Today 1999, 20, 267–277. [Google Scholar] [CrossRef]
- McGhee, J.R.; Fujihashi, K. Inside the Mucosal Immune System. PLOS Biol. 2012, 10, e1001397. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Diniz, M.O.; Roussel, H.; Tran, T.; Ferreira, L.C.; Badoual, C.; Tartour, E. Mucosal vaccines: Novel strategies and applications for the control of pathogens and tumors at mucosal sites. Hum. Vaccin. Immunother. 2014, 10, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Potential of Nasopharynx-associated Lymphoid Tissue for Vaccine Responses in the Airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef]
- Tan, G.; McKenna, P.M.; Koser, M.L.; McLinden, R.; Kim, J.H.; McGettigan, J.P.; Schnell, M.J. Strong cellular and humoral anti-HIV Env immune responses induced by a heterologous rhabdoviral prime-boost approach. Virology 2005, 331, 82–93. [Google Scholar] [CrossRef]
- Woodrow, K.A.; Bennett, K.M.; Lo, D.D. Mucosal Vaccine Design and Delivery. Annu. Rev. Biomed. Eng. 2012, 14, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting Microbial Pathogens at Epithelial Borders. Immunol. Investig. 2010, 39, 383–406. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Price, G.E.; Soboleski, M.R.; Lo, C.-Y.; Misplon, J.A.; Quirion, M.R.; Houser, K.V.; Pearce, M.B.; Pappas, C.; Tumpey, T.M.; Epstein, S.L. Single-Dose Mucosal Immunization with a Candidate Universal Influenza Vaccine Provides Rapid Protection from Virulent H5N1, H3N2 and H1N1 Viruses. PLoS ONE 2010, 5, e13162. [Google Scholar] [CrossRef]
- Hemann, E.A.; Kang, S.-M.; Legge, K.L. Protective CD8 T Cell-Mediated Immunity against Influenza A Virus Infection following Influenza Virus-like Particle Vaccination. J. Immunol. 2013, 191, 2486–2494. [Google Scholar] [CrossRef]
- Stolley, J.M.; Johnston, T.S.; Soerens, A.G.; Beura, L.K.; Rosato, P.C.; Joag, V.; Wijeyesinghe, S.P.; Langlois, R.A.; Osum, K.C.; Mitchell, J.S.; et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 2020, 217, e20192197. [Google Scholar] [CrossRef]
- Son, Y.M.; Cheon, I.S.; Wu, Y.; Li, C.; Wang, Z.; Gao, X.; Chen, Y.; Takahashi, Y.; Fu, Y.X.; Dent, A.L.; et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol. 2021, 6, eabb6852. [Google Scholar] [CrossRef] [PubMed]
- Swarnalekha, N.; Schreiner, D.; Litzler, L.C.; Iftikhar, S.; Kirchmeier, D.; Künzli, M.; Son, Y.M.; Sun, J.; Moreira, E.A.; King, C.G. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021, 6, eabb6808. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Curran, M.P. Live attenuated influenza vaccine (FluMist®; Fluenz™): A review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.; Luke, C.; Coelingh, K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influ. Other Respir. Viruses 2008, 2, 193–202. [Google Scholar] [CrossRef]
- Turner, P.J.; Abdulla, A.F.; Cole, M.E.; Javan, R.R.; Gould, V.; O’Driscoll, M.E.; Southern, J.; Zambon, M.; Miller, E.; Andrews, N.J.; et al. Differences in nasal immunoglobulin A responses to influenza vaccine strains after live attenuated influenza vaccine (LAIV) immunization in children. Clin. Exp. Immunol. 2020, 199, 109–118. [Google Scholar] [CrossRef]
- Maassab, H.F.; Bryant, M.L. The development of live attenuated cold-adapted influenza virus vaccine for humans. Rev. Med. Virol. 1999, 9, 237–244. [Google Scholar] [CrossRef]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. 2017, 24, e00414-16. [Google Scholar] [CrossRef]
- Lartey, S.; Zhou, F.; Brokstad, K.A.; Mohn, K.G.-I.; Slettevoll, S.A.; Pathirana, R.D.; Cox, R.J. Live-Attenuated Influenza Vaccine Induces Tonsillar Follicular T Helper Cell Responses That Correlate With Antibody Induction. J. Infect. Dis. 2020, 221, 21–32. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Richert, L.; Al-Tawil, N.; Storsaeter, J.; Colin, C.; Bauduin, C.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N.; et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: A phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect. Dis. 2020, 20, 1290–1301. [Google Scholar] [CrossRef]
- Lin, A.; Apostolovic, D.; Jahnmatz, M.; Liang, F.; Ols, S.; Tecleab, T.; Wu, C.; Van Hage, M.; Solovay, K.; Rubin, K.; et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J. Clin. Investig. 2020, 130, 2332–2346. [Google Scholar] [CrossRef]
- Bull, N.; Stylianou, E.; Kaveh, D.A.; Pinpathomrat, N.; Pasricha, J.; Harrington-Kandt, R.; Garcia-Pelayo, M.C.; Hogarth, P.J.; McShane, H. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+ KLRG1− CD4+ T cells. Mucosal Immunol. 2019, 12, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Haller, A.A.; Mitiku, M.; MacPhail, M. Bovine parainfluenza virus type 3 (PIV3) expressing the respiratory syncytial virus (RSV) attachment and fusion proteins protects hamsters from challenge with human PIV3 and RSV. J. Gen. Virol. 2003, 84, 2153–2162. [Google Scholar] [CrossRef]
- Garg, R.; Brownlie, R.; Latimer, L.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. A chimeric glycoprotein formulated with a combination adjuvant induces protective immunity against both human respiratory syncytial virus and parainfluenza virus type 3. Antivir. Res. 2018, 158, 78–87. [Google Scholar] [CrossRef]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Eiting, K.; Cui, K.; Leonard, A.K.; Morris, D.; Li, C.-Y.; Farber, K.; Sileno, A.P.; Houston, M.E.; Johnson, P.H.; et al. Therapeutic utility of a novel tight junction modulating peptide for enhancing intranasal drug delivery. J. Pharm. Sci. 2006, 95, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Y.; Gungor, S.; Cevher, E. Nasal Delivery of High Molecular Weight Drugs. Molecules 2009, 14, 3754–3779. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017, 8, 151–167. [Google Scholar] [CrossRef]
- Napolioni, V.; MacMurray, J. Infectious diseases, IL6 −174G > C polymorphism, and human development. Brain Behav. Immun. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A.; Crommelin, D.J.; Jiskoot, W. N-Trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: Biological properties and immunogenicity in a mouse model. Vaccine 2007, 25, 144–153. [Google Scholar] [CrossRef]
- Gilmore, J.L.; Yi, X.; Quan, L.; Kabanov, A.V. Novel Nanomaterials for Clinical Neuroscience. J. Neuroimmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef]
- Garmise, R.J.; Staats, H.; Hickey, A.J. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech 2007, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcão, A. Intranasal delivery of systemic-acting drugs: Small-molecules and biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef]
- de Haan, A.; Geerligs, H.; Huchshorn, J.; van Scharrenburg, G.; Palache, A.; Wilschut, J. Mucosal immunoadjuvant activity of liposomes: Induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine 1995, 13, 155–162. [Google Scholar] [CrossRef]
- Lobaina, Y.; Urquiza, D.; Garay, H.; Perera, Y.; Yang, K. Evaluation of Cell-Penetrating Peptides as Mucosal Immune Enhancers for Nasal Vaccination. Int. J. Pept. Res. Ther. 2021, 27, 2873–2882. [Google Scholar] [CrossRef]
- Mielcarek, N.; Debrie, A.-S.; Raze, D.; Bertout, J.; Rouanet, C.; Ben Younes, A.; Creusy, C.; Engle, J.; Goldman, E.W.; Locht, C. Live Attenuated B. pertussis as a Single-Dose Nasal Vaccine against Whooping Cough. PLOS Pathog. 2006, 2, e65. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef]
- Newly described cell type may prove valuable in development of plague vaccines against biowarfare. Expert Rev. Vaccines 2011, 10, 259.
- Hiremath, G.S.; Omer, S.B. A Meta-Analysis of Studies Comparing the Respiratory Route with the Subcutaneous Route of Measles Vaccine Administration. Hum. Vaccines 2005, 1, 30–36. [Google Scholar] [CrossRef]
- Weniger, B.G.; Papania, M.J. Alternative vaccine delivery methods. Vaccines 2012, 1357–1392. [Google Scholar] [CrossRef]
- Demirel, T.; Orhan, K.S.; Keleş, N.; Değer, K. Comparison of the efficacy of nasal drop and nasal spray applications of fluticasone propionate in nasal polyps. Kulak Burun Bogaz Ihtis. Derg. Kbb = J. Ear Nose Throat 2008, 18, 1–6. [Google Scholar]
- Primosch, R.E.; Guelmann, M. Comparison of drops versus spray administration of intranasal midazolam in two- and three-year-old children for dental sedation. Pediatr. Dent. 2005, 27, 401–408. [Google Scholar]
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Low, N.; Kraemer, S.; Schneider, M.; Restrepo, A.M.H. Immunogenicity and safety of aerosolized measles vaccine: Systematic review and meta-analysis. Vaccine 2008, 26, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Dilraj, A.; Sukhoo, R.; Cutts, F.T.; Bennett, J.V. Aerosol and subcutaneous measles vaccine: Measles antibody responses 6 years after re-vaccination. Vaccine 2007, 25, 4170–4174. [Google Scholar] [CrossRef]
- Bennett, J.V.; De Castro, J.F.; Valdespino-Gomez, J.L.; Garcia-Garcia, M.D.L.; Islas-Romero, R.; Echaniz-Aviles, G.; Jimenez-Corona, A.; Sepulveda-Amor, J. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: Randomized trials in Mexican schoolchildren. Bull. World Health Organ. 2002, 80, 806–812. [Google Scholar] [PubMed]
- de Castro, J.F.; Bennett, J.V.; Rincon, H.G.; Munoz, M.A.Y.; Sanchez, L.A.E.P.; Santos, J.I. Evaluation of immunogenicity and side effects of triple viral vaccine (MMR) in adults, given by two routes: Subcutaneous and respiratory (aerosol). Vaccine 2005, 23, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H.; Griffin, D.E.; Rota, P.A.; Papania, M.; Cape, S.P.; Bennett, D.; Quinn, B.; Sievers, R.E.; Shermer, C.; Powell, K.; et al. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc. Natl. Acad. Sci. USA 2011, 108, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Agarkhedkar, S.; Kulkarni, P.S.; Winston, S.; Sievers, R.; Dhere, R.M.; Gunale, B.; Powell, K.; Rota, P.A.; Papania, M. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase I clinical trial. Vaccine 2014, 32, 6791–6797. [Google Scholar] [CrossRef]
- Patil, H.P.; Murugappan, S.; De Vries-Idema, J.; Meijerhof, T.; De Haan, A.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.; Huckriede, A. Comparison of adjuvants for a spray freeze-dried whole inactivated virus influenza vaccine for pulmonary administration. Eur. J. Pharm. Biopharm. 2015, 93, 231–241. [Google Scholar] [CrossRef]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef]
- Yuki, Y.; Nochi, T.; Kong, I.G.; Takahashi, H.; Sawada, S.-I.; Akiyoshi, K.; Kiyono, H. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 2013, 29, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Yuki, Y.; Katakai, Y.; Harada, N.; Takahashi, H.; Takeda, S.; Mejima, M.; Joo, S.; Kurokawa, S.; Sawada, S.; et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 2015, 8, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Uchida, Y.; Yuki, Y.; Katakai, Y.; Yamanoue, T.; Ogawa, H.; Munesue, Y.; Nakano, N.; Hanari, K.; Miyazaki, T.; et al. A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci. Vaccine 2021, 39, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, O.; Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Rochlin, E.; Eliyahu, H.; Goldwaser, I.; Balasingam, S.; Mann, A.J.; Lambkin-Williams, R.; et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine 2011, 29, 2474–2486. [Google Scholar] [CrossRef]
- Salunke, S.R.; Patil, S.B. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef]
- Bedford, J.G.; Caminschi, I.; Wakim, L.M. Intranasal Delivery of a Chitosan-Hydrogel Vaccine Generates Nasal Tissue Resident Memory CD8+ T Cells That Are Protective against Influenza Virus Infection. Vaccines 2020, 8, 572. [Google Scholar] [CrossRef]
- Pastor, Y.; Ting, I.; Martínez, A.L.; Irache, J.M.; Gamazo, C. Intranasal delivery system of bacterial antigen using thermosensitive hydrogels based on a Pluronic-Gantrez conjugate. Int. J. Pharm. 2020, 579, 119154. [Google Scholar] [CrossRef]
- Kavanagh, H.; Noone, C.; Cahill, E.; English, K.; Locht, C.; Mahon, B.P. Attenuated Bordetella pertussis vaccine strain BPZE1 modulates allergen-induced immunity and prevents allergic pulmonary pathology in a murine model. Clin. Exp. Allergy 2010, 40, 933–941. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Mateo, J.S.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Safety and Immunogenicity of the Respiratory Syncytial Virus Vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-Seronegative Children. J. Infect. Dis. 2020, 222, 82–91. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Song, Y.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102775118. [Google Scholar] [CrossRef]
- Feunou, P.F.; Kammoun, H.; Debrie, A.-S.; Mielcarek, N.; Locht, C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine 2010, 28, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Feunou, P.F.; Kammoun, H.; Debrie, A.-S.; Locht, C. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine 2014, 32, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Jahnmatz, M.; Amu, S.; Ljungman, M.; Wehlin, L.; Chiodi, F.; Mielcarek, N.; Locht, C.; Thorstensson, R. B-cell responses after intranasal vaccination with the novel attenuated Bordetella pertussis vaccine strain BPZE1 in a randomized phase I clinical trial. Vaccine 2014, 32, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Thorstensson, R.; Trollfors, B.; Al-Tawil, N.; Jahnmatz, M.; Bergström, J.; Ljungman, M.; Törner, A.; Wehlin, L.; Van Broekhoven, A.; Bosman, F.; et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE 2014, 9, e83449. [Google Scholar] [CrossRef]
- Debrie, A.-S.; Coutte, L.; Raze, D.; Mooi, F.; Alexander, F.; Gorringe, A.; Mielcarek, N.; Locht, C. Construction and evaluation of Bordetella pertussis live attenuated vaccine strain BPZE1 producing Fim3. Vaccine 2018, 36, 1345–1352. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Brekke, K.; Lind, A.; Holm-Hansen, C.; Haugen, I.L.; Sørensen, B.; Sommerfelt, M.; Kvale, D. Intranasal Administration of a Therapeutic HIV Vaccine (Vacc-4x) Induces Dose-Dependent Systemic and Mucosal Immune Responses in a Randomized Controlled Trial. PLoS ONE 2014, 9, e112556. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Van Ginkel, F.W.; Jackson, R.J.; Yuki, Y.; McGhee, J.R. Cutting Edge: The Mucosal Adjuvant Cholera Toxin Redirects Vaccine Proteins into Olfactory Tissues. J. Immunol. 2000, 165, 4778–4782. [Google Scholar] [CrossRef]
- Kurono, Y. The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines. Auris Nasus Larynx 2021, 49, 1–10. [Google Scholar] [CrossRef]
- Kraan, H.; Soema, P.; Amorij, J.-P.; Kersten, G. Intranasal and sublingual delivery of inactivated polio vaccine. Vaccine 2017, 35, 2647–2653. [Google Scholar] [CrossRef]
- Valli, E.; Harriett, A.J.; Nowakowska, M.K.; Baudier, R.; Provosty, W.B.; McSween, Z.; Lawson, L.; Nakanishi, Y.; Norton, E.B. LTA1 is a safe, intranasal enterotoxin-based adjuvant that improves vaccine protection against influenza in young, old and B-cell-depleted (μMT) mice. Sci. Rep. 2019, 9, 15128. [Google Scholar] [CrossRef]
- Pan, S.C.; Hsu, W.T.; Lee, W.S.; Wang, N.C.; Chen, T.J.; Liu, M.C.; Pai, H.C.; Hsu, Y.S.; Chang, M.; Hsieh, S.M. A Double-blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh(αK): A Phase II study. Vaccine 2020, 38, 1048–1056. [Google Scholar] [CrossRef]
- Pan, S.C.; Hsieh, S.M.; Lin, C.F.; Hsu, Y.S.; Chang, M.; Chang, S.C. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): A Phase I study. Vaccine 2019, 37, 1994–2003. [Google Scholar] [CrossRef]
- Bernasconi, V.; Norling, K.; Gribonika, I.; Ong, L.C.; Burazerovic, S.; Parveen, N.; Schön, K.; Stensson, A.; Bally, M.; Larson, G.; et al. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 2021, 14, 523–536. [Google Scholar] [CrossRef]
- Li, H.; Ren, H.; Zhang, Y.; Cao, L.; Xu, W. Intranasal vaccination with a recombinant protein CTA1-DD-RBF protects mice against hRSV infection. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Erume, J.; Partidos, C.D. Evaluation of the LTK63 adjuvant effect on cellular immune responses to measles virus nucleoprotein. Afr. Health Sci. 2011, 11, 151–157. [Google Scholar]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef]
- Williams, C.M.; Roy, S.; Califano, D.; McKenzie, A.N.J.; Metzger, D.W.; Furuya, Y. The Interleukin-33–Group 2 Innate Lymphoid Cell Axis Represents a Potential Adjuvant Target To Increase the Cross-Protective Efficacy of Influenza Vaccine. J. Virol. 2021, 95, e0059821. [Google Scholar] [CrossRef]
- Maeto, C.; Rodríguez, A.M.; Holgado, M.P.; Falivene, J.; Gherardi, M.M. Novel Mucosal DNA-MVA HIV Vaccination in Which DNA-IL-12 Plus Cholera Toxin B Subunit (CTB) Cooperates to Enhance Cellular Systemic and Mucosal Genital Tract Immunity. PLoS ONE 2014, 9, e107524. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Zhang, R.; Prabhu, S.K.; Andersen, H.; Venzon, D.; Cook, A.; Brown, R.; Teow, E.; Velasco, J.; et al. Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight 2021, 6, e148494. [Google Scholar] [CrossRef]
- Wang, X.; Meng, D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell 2015, 6, 170–184. [Google Scholar] [CrossRef]
- Hou, B.; Reizis, B.; DeFranco, A.L. Toll-like Receptors Activate Innate and Adaptive Immunity by using Dendritic Cell-Intrinsic and -Extrinsic Mechanisms. Immunity 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid a modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Xu, H.; Alzhrani, R.F.; Warnken, Z.N.; Thakkar, S.G.; Zeng, M.; Smyth, H.D.C.; Iii, R.O.W.; Cui, Z. Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration. Mol. Pharm. 2020, 17, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Bian, L.; Zhao, H.; Liu, Y.; Lu, J.; Liu, D.; Zhang, K.; Song, Y.; Luo, Y.; Jiang, C.; et al. Respiratory Syncytial Virus F Subunit Vaccine With AS02 Adjuvant Elicits Balanced, Robust Humoral and Cellular Immunity in BALB/c Mice. Front. Immunol. 2020, 11, 526965. [Google Scholar] [CrossRef]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Habibi, M.; Karam, M.R.A.; Shokrgozar, M.A.; Oloomi, M.; Jafari, A.; Bouzari, S. Intranasal immunization with fusion protein MrpH·FimH and MPL adjuvant confers protection against urinary tract infections caused by uropathogenic Escherichia coli and Proteus mirabilis. Mol. Immunol. 2015, 64, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Hamaoka, S.; Kinoshita, M.; Kainuma, A.; Shimizu, M.; Katoh, H.; Moriyama, K.; Ishii, K.J.; Sawa, T. The protective effects of nasal PcrV-CpG oligonucleotide vaccination against Pseudomonas aeruginosa pneumonia. Microbiol. Immunol. 2018, 62, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Hinkula, J.; Nyström, S.; Devito, C.; Bråve, A.; Applequist, S.E. Long-Lasting Mucosal and Systemic Immunity against Influenza A Virus Is Significantly Prolonged and Protective by Nasal Whole Influenza Immunization with Mucosal Adjuvant N3 and DNA-Plasmid Expressing Flagellin in Aging In- and Outbred Mice. Vaccines 2019, 7, 64. [Google Scholar] [CrossRef]
- Renu, S.; Feliciano-Ruiz, N.; Lu, F.; Ghimire, S.; Han, Y.; Schrock, J.; Dhakal, S.; Patil, V.; Krakowka, S.; HogenEsch, H.; et al. A Nanoparticle-Poly(I:C) Combination Adjuvant Enhances the Breadth of the Immune Response to Inactivated Influenza Virus Vaccine in Pigs. Vaccines 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, L.S.; Hjelm, B.E.; Arntzen, C.J.; Herbst-Kralovetz, M.M. An Intranasally Delivered Toll-Like Receptor 7 Agonist Elicits Robust Systemic and Mucosal Responses to Norwalk Virus-Like Particles. Clin. Vaccine Immunol. 2010, 17, 1850–1858. [Google Scholar] [CrossRef]
- Hjelm, B.E.; Kilbourne, J.; Herbst-Kralovetz, M.M. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum. Vaccines Immunother. 2014, 10, 410–416. [Google Scholar] [CrossRef]
- Van Dis, E.S.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef]
- Matos, M.N.; Cazorla, S.I.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Malchiodi, E. Immunization with Tc52 or its amino terminal domain adjuvanted with c-di-AMP induces Th17+Th1 specific immune responses and confers protection against Trypanosoma cruzi. PLOS Neglected Trop. Dis. 2017, 11, e0005300. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Su, Y.; Cai, L.; Li, J.; Liang, J.; Gu, Q.; Sun, M.; Shi, L. Intranasal Immunization With a c-di-GMP-Adjuvanted Acellular Pertussis Vaccine Provides Superior Immunity Against Bordetella pertussis in a Mouse Model. Front. Immunol. 2022, 13, 878832. [Google Scholar] [CrossRef] [PubMed]
- Hosny, E.A.; Elkheshen, S.A.; Saleh, S.I. Buccoadhesive tablets for insulin delivery: In-vitro and in-vivo studies. Boll. Chim. Farm. 2002, 141, 210–217. [Google Scholar] [PubMed]
- Shim, S.; Yoo, H. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Marine drugs 2020, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Moran, H.B.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Westerink, M.J.; Smithson, S.L.; Srivastava, N.; Blonder, J.; Coeshott, C.; Rosenthal, G.J. ProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine 2001, 20, 711–723. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Hajam, I.A.; Senevirathne, A.; Hewawaduge, C.; Kim, J.; Lee, J.H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Kumar, U.S.; Afjei, R.; Ferrara, K.; Massoud, T.F.; Paulmurugan, R. Gold-Nanostar-Chitosan-Mediated Delivery of SARS-CoV-2 DNA Vaccine for Respiratory Mucosal Immunization: Development and Proof-of-Principle. ACS Nano 2021, 15, 17582–17601. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef]
- Manocha, M.; Pal, P.C.; Chitralekha, K.; Thomas, B.E.; Tripathi, V.; Gupta, S.D.; Paranjape, R.; Kulkarni, S.; Rao, D.N. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine 2005, 23, 5599–5617. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccines Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.-L.; Rejman, J.; Demeester, J.; Irvine, D.J.; Gander, B.; De Smedt, S.C. Particulate vaccines: On the quest for optimal delivery and immune response. Drug Discov. Today 2011, 16, 569–582. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Ramos, M.; Tak, P.; Lebre, M. Fms-like tyrosine kinase 3 ligand-dependent dendritic cells in autoimmune inflammation. Autoimmun. Rev. 2014, 13, 117–124. [Google Scholar] [CrossRef]
- Kataoka, K.; McGhee, J.R.; Kobayashi, R.; Fujihashi, K.; Shizukuishi, S.; Fujihashi, K. Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 2004, 172, 3612–3619. [Google Scholar] [CrossRef]
- Kataoka, K.; Kawabata, S.; Koyanagi, K.; Hashimoto, Y.; Miyake, T.; Fujihashi, K. Respiratory FimA-Specific Secretory IgA Antibodies Upregulated by DC-Targeting Nasal Double DNA Adjuvant Are Essential for Elimination of Porphyromonas gingivalis. Front. Immunol. 2021, 12, 634923. [Google Scholar] [CrossRef]
- Borg, N.A.; Wun, K.S.; Kjer-Nielsen, L.; Wilce, M.C.J.; Pellicci, D.G.; Koh, R.; Besra, G.S.; Bharadwaj, M.; Godfrey, D.I.; McCluskey, J.; et al. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 2007, 448, 44–49. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, K.A.; Lee, J.Y.; Kang, M.H.; Song, Y.C.; Baek, D.J.; Kim, S.; Kang, C.Y. An α-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 2011, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Sun, R.; Song, G.; Zhu, S.; Nie, Z.; Lin, L.; Yi, R.; Wu, S.; Wang, G.; He, Y.; et al. A Glycolipid α-GalCer Derivative, 7DW8-5 as a Novel Mucosal Adjuvant for the Split Inactivated Influenza Vaccine. Viruses 2022, 14, 1174. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Weaver, B.T.; Choi, H.W.; Yang, H.; Granek, J.A.; Chan, C.; Abraham, S.N.; Staats, H.F. Nasal Immunization With Small Molecule Mast Cell Activators Enhance Immunity to Co-Administered Subunit Immunogens. Front. Immunol. 2021, 12, 730346. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Wang, H.; Liao, P.; Song, Z.; Gao, S.; Wu, Y.; Zhang, X.; Yin, Y.; Xu, W. Compound 48/80 acts as a potent mucosal adjuvant for vaccination against Streptococcus pneumoniae infection in young mice. Vaccine 2015, 33, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Chandrudu, S.; Giddam, A.K.; Reiman, J.; Skwarczynski, M.; McPhun, V.; Moyle, P.M.; Batzloff, M.R.; Good, M.F.; Toth, I. Group A Streptococcal vaccine candidate: Contribution of epitope to size, antigen presenting cell interaction and immunogenicity. Nanomedicine 2014, 9, 2613–2624. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Ohtsuka, J.; Imai, M.; Fukumura, M.; Maeda, M.; Eguchi, A.; Ono, R.; Maemura, T.; Ito, M.; Yamayoshi, S.; Kataoka, Y.; et al. Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. Iscience 2021, 24, 103379. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccine Nasal Spray. JAMA 2021, 326, 1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, B.; Ngwuta, J.; Liu, X.; Surman, S.; Lingemann, M.; Kwong, P.D.; Graham, B.S.; Collins, P.L.; Munir, S. Attenuated Human Parainfluenza Virus Type 1 Expressing the Respiratory Syncytial Virus (RSV) Fusion (F) Glycoprotein from an Added Gene: Effects of Prefusion Stabilization and Packaging of RSV F. J. Virol. 2017, 91, e01101-17–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, B.; Liu, X.; Amaro-Carambot, E.; Surman, S.; Kwong, P.D.; Graham, B.S.; Collins, P.L.; Munir, S. Human parainfluenza virus type 3 expressing the respiratory syncytial virus pre-fusion F protein modified for virion packaging yields protective intranasal vaccine candidates. PLoS ONE 2020, 15, e0228572. [Google Scholar] [CrossRef] [PubMed]
- Koonpaew, S.; Kaewborisuth, C.; Srisutthisamphan, K.; Wanitchang, A.; Thaweerattanasinp, T.; Saenboonrueng, J.; Poonsuk, S.; Jengarn, J.; Viriyakitkosol, R.; Kramyu, J.; et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines 2021, 9, 850. [Google Scholar] [CrossRef]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-N.; Kim, K.-H.; Lee, Y.; Jeeva, S.; Park, B.R.; Kang, S.-M. Recombinant Live Attenuated Influenza Virus Expressing Conserved G-Protein Domain in a Chimeric Hemagglutinin Molecule Induces G-Specific Antibodies and Confers Protection against Respiratory Syncytial Virus. Vaccines 2020, 8, 716. [Google Scholar] [CrossRef]
- Matyushenko, V.; Kotomina, T.; Kudryavtsev, I.; Mezhenskaya, D.; Prokopenko, P.; Matushkina, A.; Sivak, K.; Muzhikyan, A.; Rudenko, L.; Isakova-Sivak, I. Conserved T-cell epitopes of respiratory syncytial virus (RSV) delivered by recombinant live attenuated influenza vaccine viruses efficiently induce RSV-specific lung-localized memory T cells and augment influenza-specific resident memory T-cell responses. Antivir. Res. 2020, 182, 104864. [Google Scholar] [CrossRef]
- Wang, J.; Shu, T.; Deng, W.; Zheng, Y.; Liao, M.; Ye, X.; Han, L.; He, P.; Zheng, X.; Li, T.; et al. Mucosal Priming with a Recombinant Influenza A Virus-Vectored Vaccine Elicits T-Cell and Antibody Responses to HIV-1 in Mice. J. Virol. 2021, 95, e00059-21–21. [Google Scholar] [CrossRef]
- Fragoso-Saavedra, M.; Vega-López, M.A. Induction of mucosal immunity against pathogens by using recombinant baculoviral vectors: Mechanisms, advantages, and limitations. J. Leukoc. Biol. 2020, 108, 835–850. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Richardson, J.S.; Pillet, S.; Bello, A.J.; Kobinger, G.P. Airway Delivery of an Adenovirus-Based Ebola Virus Vaccine Bypasses Existing Immunity to Homologous Adenovirus in Nonhuman Primates. J. Virol. 2013, 87, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Patel, A.; Tran, K.N.; Gray, M.; Zhang, Y.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Nasal Delivery of an Adenovirus-Based Vaccine Bypasses Pre-Existing Immunity to the Vaccine Carrier and Improves the Immune Response in Mice. PLoS ONE 2008, 3, e3548. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Livermore, D.M.; Ornelles, D.A.; Bayer, W.; Marques, E.; Czerkinsky, C.; Dolzhikova, I.V.; Ertl, H.C. COVID-19 vaccination and HIV-1 acquisition. Lancet 2022, 399, e34–e35. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, Phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Kotelkin, A.; Yang, L.; Elankumaran, S.; Murphy, B.R.; Samal, S.K.; Collins, P.L.; Bukreyev, A. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. USA 2007, 104, 9788–9793. [Google Scholar] [CrossRef]
- Díaz, M.F.; Calderón, K.; Rojas-Neyra, A.; Vakharia, V.N.; Choque-Guevara, R.; Montalvan-Avalos, A.; Poma-Acevedo, A.; Rios-Matos, D.; Agurto-Arteaga, A.; Cauti-Mendoza, M.G.; et al. Intranasal vaccination of hamsters with a Newcastle disease virus vector expressing the S1 subunit protects animals against SARS-CoV-2 disease. Sci. Rep. 2022, 12, 10359. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Amanat, F.; González-Domínguez, I.; McCroskery, S.; Slamanig, S.; Coughlan, L.; Rosado, V.; Lemus, N.; Jangra, S.; et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 2021, 12, 6197. [Google Scholar] [CrossRef]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H., III; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. Ebiomedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Park, H.S.; Matsuoka, Y.; Luongo, C.; Yang, L.; Santos, C.; Liu, X.; Ahlers, L.R.; Moore, I.N.; Afroz, S.; Johnson, R.F.; et al. Intranasal immunization with avian paramyxovirus type 3 expressing SARS-CoV-2 spike protein protects hamsters against SARS-CoV-2. NPJ Vaccines 2022, 7, 72. [Google Scholar] [CrossRef]
- Durrani, Z.; McInerney, T.L.; McLain, L.; Jones, T.; Bellaby, T.; Brennan, F.R.; Dimmock, N.J. Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J. Immunol. Methods 1998, 220, 93–103. [Google Scholar] [CrossRef]
- Bernasconi, V.; Norling, K.; Bally, M.; Höök, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Ali, A.A.; Orr, N.; Tunney, M.M.; McCarthy, H.O.; Kett, V.L. Novel freeze-dried DDA and TPGS liposomes are suitable for nasal delivery of vaccine. Int. J. Pharm. 2017, 533, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal Immunization with DOTAP Cationic Liposomes Combined with DC-Cholesterol Induces Potent Antigen-Specific Mucosal and Systemic Immune Responses in Mice. PLoS ONE 2015, 10, e0139785. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of class B CpG ODN onto DOTAP/DC-chol liposome in nasal vaccine formulations augments antigen-specific immune responses in mice. BMC Res. Notes 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef]
- Christensen, D.; Foged, C.; Rosenkrands, I.; Lundberg, C.V.; Andersen, P.; Agger, E.M.; Nielsen, H.M. CAF01 liposomes as a mucosal vaccine adjuvant: In vitro and in vivo investigations. Int. J. Pharm. 2010, 390, 19–24. [Google Scholar] [CrossRef]
- Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Flasterstein, O.; Eliyahu, H.; Simberg, D.; Goldwaser, I.; Barenholz, Y.; Kedar, E. A new intranasal influenza vaccine based on a novel polycationic lipid—Ceramide carbamoyl-spermine (CCS) I. immunogenicity and efficacy studies in mice. Vaccine 2006, 24, 3990–4006. [Google Scholar] [CrossRef]
- Even-Or, O.; Avniel-Polak, S.; Barenholz, Y.; Nussbaum, G. The cationic liposome CCS/C adjuvant induces immunity to influenza independently of the adaptor protein MyD88. Hum. Vaccines Immunother. 2020, 16, 3146–3154. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhang, M.; Chen, R.; Niu, R.; Deng, Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 2014, 88, 194–206. [Google Scholar] [CrossRef]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Peng, K.; Wang, Y.; Gong, T.; Zhang, Z.; He, Q.; Sun, X. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017, 64, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.A.; Paul, S.; Dellosa, G.K.Y.; Jaraquemada, D.; Bello, M.B. Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. npj Vaccines 2022, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Jandus, C.; Maurer, P.; Hammann-Haenni, A.; Schwarz, K.; Bachmann, M.F.; Speiser, D.; Romero, P. Virus-like particles induce robust human T-helper cell responses. Eur. J. Immunol. 2012, 42, 330–340. [Google Scholar] [CrossRef]
- Zabel, F.; Mohanan, D.; Bessa, J.; Link, A.; Fettelschoss-Gabriel, A.; Saudan, P.; Kündig, T.M.; Bachmann, M.F. Viral Particles Drive Rapid Differentiation of Memory B Cells into Secondary Plasma Cells Producing Increased Levels of Antibodies. J. Immunol. 2014, 192, 5499–5508. [Google Scholar] [CrossRef]
- Jain, N.K.; Sahni, N.; Kumru, O.S.; Joshi, S.B.; Volkin, D.B.; Middaugh, C.R. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv. Drug Deliv. Rev. 2015, 93, 42–55. [Google Scholar] [CrossRef]
- Atmar, R.L.; Bernstein, D.I.; Harro, C.D.; Al-Ibrahim, M.S.; Chen, W.H.; Ferreira, J.; Estes, M.K.; Graham, D.Y.; Opekun, A.R.; Richardson, C.; et al. Norovirus Vaccine against Experimental Human Norwalk Virus Illness. N. Engl. J. Med. 2011, 365, 2178–2187. [Google Scholar] [CrossRef]
- Herbst-Kralovetz, M.; Mason, H.S.; Chen, Q. Norwalk virus-like particles as vaccines. Expert Rev. Vaccines 2010, 9, 299–307. [Google Scholar] [CrossRef]
- Kaneda, Y. Virosome: A novel vector to enable multi-modal strategies for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 730–738. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; Clement, F.; Van Engelenburg, F.; Dobbelsteen, M.V.D.; Adler, M.; Amacker, M.; Lopalco, L.; Bomsel, M.; Chalifour, A.; et al. Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes. PLoS ONE 2013, 8, e55438. [Google Scholar] [CrossRef]
- Lambkin, R.; Oxford, J.S.; Bossuyt, S.; Mann, A.; Metcalfe, I.C.; Herzog, C.; Viret, J.-F.; Glück, R. Strong local and systemic protective immunity induced in the ferret model by an intranasal virosome-formulated influenza subunit vaccine. Vaccine 2004, 22, 4390–4396. [Google Scholar] [CrossRef] [PubMed]
- Durrer, P.; Glück, U.; Spyr, C.; Lang, A.B.; Zurbriggen, R.; Herzog, C.; Glück, R. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine 2003, 21, 4328–4334. [Google Scholar] [CrossRef]
- Fernandes, B.; Castro, R.; Bhoelan, F.; Bemelman, D.; Gomes, R.A.; Costa, J.; Gomes-Alves, P.; Stegmann, T.; Amacker, M.; Alves, P.M.; et al. Insect Cells for High-Yield Production of SARS-CoV-2 Spike Protein: Building a Virosome-Based COVID-19 Vaccine Candidate. Pharmaceutics 2022, 14, 854. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.; Harris, R.; Davis, R.; Drane, D.; Cox, J.; Ryan, D.; Sutton, P.; Rockman, S.; Pearse, M. Intranasal vaccination with ISCOMATRIX® adjuvanted influenza vaccine. Vaccine 2003, 21, 946–949. [Google Scholar] [CrossRef]
- Hu, K.-F.; Lövgren-Bengtsson, K.; Morein, B. Immunostimulating complexes (ISCOMs) for nasal vaccination. Adv. Drug Deliv. Rev. 2001, 51, 149–159. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.; Baars, M.; van Amerongen, G.; van Beek, R.; Osterhaus, A. A single dose of an ISCOM influenza vaccine induces long-lasting protective immunity against homologous challenge infection but fails to protect Cynomolgus macaques against distant drift variants of influenza A (H3N2) viruses. Vaccine 2001, 20, 158–163. [Google Scholar] [CrossRef]
- Rivera-Patron, M.; Moreno, M.; Baz, M.; Roehe, P.M.; Cibulski, S.P.; Silveira, F. ISCOM-like Nanoparticles Formulated with Quillaja brasiliensis Saponins Are Promising Adjuvants for Seasonal Influenza Vaccines. Vaccines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Eliasson, D.G.; Helgeby, A.; Schön, K.; Nygren, C.; El-Bakkouri, K.; Fiers, W.; Saelens, X.; Lövgren, K.B.; Nyström, I.; Lycke, N.Y. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine 2011, 29, 3951–3961. [Google Scholar] [CrossRef]
- Andersen, C.S.; Dietrich, J.; Agger, E.M.; Lycke, N.Y.; Lövgren, K.; Andersen, P. The Combined CTA1-DD/ISCOMs Vector Is an Effective Intranasal Adjuvant for Boosting Prior Mycobacterium bovis BCG Immunity to Mycobacterium tuberculosis. Infect. Immun. 2007, 75, 408–416. [Google Scholar] [CrossRef]
- Hu, K.-F.; Elvander, M.; Merza, M.; Åkerblom, L.; Brandenburg, A.; Morein, B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin. Exp. Immunol. 1998, 113, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.F.; Regner, M.; Siegrist, C.A.; Lambert, P.; Chen, M.; Bengtsson, K.L.; Morein, B. The immunomodulating properties of human respiratory syncytial virus and immunostimulating complexes containing Quillaja saponin components QH-A, QH-C and ISCOPREP703. FEMS Immunol. Med. Microbiol. 2005, 43, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Hirano, T.; Noda, K.; Umemoto, S.; Suzuki, M. Nasal immunization with plasmid DNA encoding P6 protein and immunostimulatory complexes elicits nontypeable Haemophilus influenzae-specific long-term mucosal immune responses in the nasopharynx. Vaccine 2011, 29, 1881–1890. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Janczak, K.W.; Landers, J.J.; Makidon, P.; Sower, L.E.; Peterson, J.W.; Baker, J.R. Mucosal Immunization with a Novel Nanoemulsion-Based Recombinant Anthrax Protective Antigen Vaccine Protects against Bacillus anthracis Spore Challenge. Infect. Immun. 2007, 75, 4020–4029. [Google Scholar] [CrossRef]
- Ahmed, M.; Smith, D.M.; Hamouda, T.; Rangel-Moreno, J.; Fattom, A.; Khader, S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 2017, 35, 4983–4989. [Google Scholar] [CrossRef]
- Das, S.C.; Hatta, M.; Wilker, P.R.; Myc, A.; Hamouda, T.; Neumann, G.; Baker, J.R.; Kawaoka, Y. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine 2012, 30, 6871–6877. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, C.-C.; Huang, M.-H.; Huang, C.-H.; Jan, J.-T.; Wu, S.-C. Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine. Vaccines 2020, 8, 240. [Google Scholar] [CrossRef]
- Hamouda, T.; Sutcliffe, J.A.; Ciotti, S.; Baker, J.R. Intranasal Immunization of Ferrets with Commercial Trivalent Influenza Vaccines Formulated in a Nanoemulsion-Based Adjuvant. Clin. Vaccine Immunol. 2011, 18, 1167–1175. [Google Scholar] [CrossRef]
- Schulze, K.; Ebensen, T.; Chandrudu, S.; Skwarczynski, M.; Toth, I.; Olive, C.; Guzman, C.A. Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2463–2474. [Google Scholar] [CrossRef] [PubMed]

| Disease | Vaccine Identification | ClinicalTrials.gov Identifier | Phase | Sponsor | Status |
|---|---|---|---|---|---|
| Pertussis | BPZE1 | NCT01188512 NCT02453048 | 1 | Inserm | Completed |
| Vaccine GamLPV | NCT04036526 | 1/2 | Gamaleya Research Institute of Epidemiology and Microbiology | Unknown | |
| BPZE1 | NCT03541499 | 2 | NIAID | Completed | |
| BPZE1 | NCT03942406 NCT05116241 | 2 | ILiAD Biotechnologies | Completed/Recruiting | |
| COVID-19 | COVI-VAC | NCT05233826 NCT04619628 | 1 | Codagenix, Inc. | Active, not recruiting |
| RSV | RSV ΔNS2/Δ1313/I1314L RSV D46/NS2/N/ΔM2-2-HindIII RSV LID ΔM2-2 1030s RSV 6120/∆NS1 RSV 6120/F1/G2 RSV 6120/∆NS2/1030s RSV cps2 Vaccine RSV MEDI ΔM2-2 | NCT03227029 NCT03102034 NCT02794870 NCT02952339 NCT03916185 NCT03422237 NCT03099291 NCT04520659 NCT03596801 NCT03387137 NCT01968083 NCT01852266 NCT02237209 NCT01893554 NCT01459198 | 1/2 | NIAID | Completed/Recruiting/ Active, not recruiting |
| MV-012-968 | NCT04227210 | 1 | Meissa Vaccines, Inc. | Recruiting | |
| RSV vaccine formulation 1 | NCT04491877 | 2 | Sanofi Pasteur, a Sanofi Company | Recruiting | |
| Parainfluenza Virus Diseases | rHPIV1 84/del170/942A Standard Dose HPIV2 rHPIV3cp45 HPIV3/ΔHNF/EbovZ rB/HPIV3 HPIV3-EbovZ GP | NCT00641017 NCT01139437 NCT01021397 NCT03462004 NCT01254175 NCT00366782 NCT00308412 NCT02564575 | 1 | NIAID | Completed |
| RSV and PIV3 | MEDI-534 | NCT00345670 NCT00493285 NCT00686075 | 1/2 | MedImmune LLC | Completed |
| Influenza | H2N3 MO 2003/AA ca Vaccine Live-attenuated H7N9 A/Anhui/13 ca influenza virus vaccine Live Influenza A Vaccine H7N3 (6-2) AA ca H9N2 (6-2) AA ca H2N2 1960 AA ca H6N1 Teal HK 97/AA Influenza A H7N7 H5N1 (6-2) AA ca | NCT01175122 NCT01995695 NCT02251288 NCT02151344 NCT00516035 NCT01854632 NCT00853255 NCT00380237 NCT00722774 NCT00734175 NCT00922259 NCT00110279 NCT01534468 NCT00488046 | 1/3 | NIAID | Completed |
| SIIL LAIV cH8/1N1 LAIV LAIV H7N3 | NCT01625689 NCT03300050 NCT01511419 | 1/2 | PATH | Completed/Recruiting | |
| GHB16L2 | NCT01369862 | 1/2 | AVIR Green Hills Biotechnology AG | Completed | |
| CAIV-T UniFluVec | NCT00224783 NCT00192309 NCT00192413 NCT04650971 | 1/2/3 | MedImmune LLC | Completed | |
| A/17/CA/2009/38 (H1N1) | NCT01666262 | 1/2 | Mahidol University | Completed | |
| Lactobacillus rhamnosus | NCT00620412 | 1 | Tufts Medical Center | Completed | |
| Human metapneumovirus | rHMPV-Pa vaccine | NCT01255410 | 1 | NIAID | Completed |
| Meissa | MV-012-968 | NCT04690335 | 2 | Meissa Vaccines, Inc. | Completed |
| Sendai virus | Sendai virus vaccine | NCT00186927 | 1 | St. Jude Children’s Research Hospital | Active, not recruiting |
| Disease | Vaccine Identification | Type | Adjuvant | ClinicalTrials.gov Identifier | Phase | Sponsor | Status |
|---|---|---|---|---|---|---|---|
| Influenza | Vaccination | Subunit | Liposomal | NCT00197301 | 1/2 | Hadassah Medical Organization | Completed |
| OVX836 | Subunit | NCT03594890 | 1 | Osivax | Completed | ||
| BW-1014 | Subunit | Nanoemulsion Adjuvanted | NCT05397119 | 1 | BlueWillow Biologics | Recruiting | |
| Trivalent inactivated influenza virus vaccine Flucelvax(R) BPL-1357 | Inactivated | Type 1 interferon | NCT00436046 NCT03845231 NCT05027932 | 1/2 | NIAID | Completed | |
| NB-1008 | Inactivated | W805EC | NCT01354379 NCT01333462 | 1 | NanoBio Corporation | Completed | |
| H5N1 Influenza Vietnam 1194 Hemagglutinin (HA) | Adenoviral-vectored | NCT01806909 | 1 | NIAID | Completed | ||
| Hemagglutinin (HA) | Inactivated | DCB07010 | NCT03293732 | 1 | Advagene Biopharma Co., Ltd. | Completed | |
| AD07030 | Inactivated | LTh(αK) | NCT03784885 | 2 | Advagene Biopharma Co., Ltd. | Completed | |
| GelVac™ nasal powder H5N1 | Inactivated H5N1 | NCT01258062 | 1 | Ology Bioservices | Completed | ||
| HIV | EN41-FPA2 HIV | NCT01509144 | 1 | PX’Therapeutics | Completed | ||
| Ad4-mgag Ad4-Env150KN | Ad4-based | NCT01989533 NCT03878121 | 1 | NIAID | Completed/Recruiting | ||
| Human Immunodeficiency Virus glycoprotein 140 (vaccine) | Glycoprotein 140 | LTK63 | NCT00369031 | 1 | St George’s, University of London | Terminated | |
| Vacc-4x | Peptides | Endocine | NCT01473810 | 1 | Oslo University Hospital | Completed | |
| MYM-V101 | Subunit | virosome | NCT01084343 | 1 | Mymetics Corporation | Completed | |
| COVID-19 | Gam-COVID-Vac | Adenoviral- vectored | NCT05248373 | 1/2 | Gamaleya Research Institute of Epidemiology and Microbiology | Not yet recruiting | |
| AZD1222 | Adenoviral- vectored | NCT05007275 | 1 | Imperial College London | Recruiting | ||
| AD17002 | Subunit | LTh(αK) | NCT05069610 | 1 | Advagene Biopharma Co., Ltd. | Recruiting | |
| MV-014-212 | RSV-vectored | NCT04798001 | 1 | Meissa Vaccines, Inc. | Recruiting | ||
| DelNS1-nCoV-RBD LAIV | Influenza virus Vectored | NCT04809389 | 1 | The University of Hong Kong | Active, not recruiting | ||
| DelNS1-2019-nCoV-RBD-OPT1 | Influenza virus Vectored | NCT05200741 | 1 | The University of Hong Kong | Not yet recruiting | ||
| ACM-001 | Subunit | CpG | NCT05385991 | 1 | ACM Biolabs | Not yet recruiting | |
| SC-Ad6-1 | Ad6-vectored | NCT04839042 | 1 | Tetherex Pharmaceuticals Corporation | Recruiting | ||
| AVX/COVID-12 Vaccine | NDV Vectored | NCT05205746 | 2 | Laboratorio Avi-Mex, S.A. de C.V. | Recruiting | ||
| NDV-HXP-S | NDV Vectored | NCT05181709 | 1 | Sean Liu | Recruiting | ||
| RSV | SeVRSV | Sendai Virus Vectored | NCT03473002 | 1 | NIAID | Completed | |
| Ad26.RSV.preF | Ad26-vectored | NCT03334695 | 2 | Janssen Vaccines and Prevention B.V. | Completed | ||
| RSV001 | PanAd3-RSV | NCT01805921 | 1 | ReiThera Srl | Completed | ||
| Ebolavirus Zaire Glycoprotein | HPIV3/ΔHNF/EbovZ GP | HPIV3-vectored | NCT03462004 | 1 | NIAID | Suspended | |
| Allergen | CYT005-AllQbG10 | Immunomodulatory principle (QbG10) | NCT00293904 | 1 | Cytos Biotechnology AG | Completed | |
| Anthrax | BW-1010 | Recombinant Anthrax Vaccine | Nanoemulsion Adjuvanted | NCT04148118 | 1 | BlueWillow Biologics | Completed |
| Norwalk | Norwalk VLP Vaccine | VLP | NCT00806962 | 1 | LigoCyte Pharmaceuticals, Inc. | Completed | |
| Cholera | Cholera Toxin B Subunit (CTB) | Subunit | NCT00820144 | 1 | Centre Hospitalier Universitaire de Nice | Completed | |
| Tuberculosis | Ag85B-ESAT6 fusion protein H1 | Subunit | LTK63 | NCT00440544 | 1 | St George’s, University of London | Terminated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. https://doi.org/10.3390/pharmaceutics14101983
Nian X, Zhang J, Huang S, Duan K, Li X, Yang X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics. 2022; 14(10):1983. https://doi.org/10.3390/pharmaceutics14101983
Chicago/Turabian StyleNian, Xuanxuan, Jiayou Zhang, Shihe Huang, Kai Duan, Xinguo Li, and Xiaoming Yang. 2022. "Development of Nasal Vaccines and the Associated Challenges" Pharmaceutics 14, no. 10: 1983. https://doi.org/10.3390/pharmaceutics14101983
APA StyleNian, X., Zhang, J., Huang, S., Duan, K., Li, X., & Yang, X. (2022). Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics, 14(10), 1983. https://doi.org/10.3390/pharmaceutics14101983







