Abstract
Viruses, bacteria, fungi, and several other pathogenic microorganisms usually infect the host via the surface cells of respiratory mucosa. Nasal vaccination could provide a strong mucosal and systemic immunity to combat these infections. The intranasal route of vaccination offers the advantage of easy accessibility over the injection administration. Therefore, nasal immunization is considered a promising strategy for disease prevention, particularly in the case of infectious diseases of the respiratory system. The development of a nasal vaccine, particularly the strategies of adjuvant and antigens design and optimization, enabling rapid induction of protective mucosal and systemic responses against the disease. In recent times, the development of efficacious nasal vaccines with an adequate safety profile has progressed rapidly, with effective handling and overcoming of the challenges encountered during the process. In this context, the present report summarizes the most recent findings regarding the strategies used for developing nasal vaccines as an efficient alternative to conventional vaccines.
1. Introduction
The respiratory system in mammals performs the essential physiological function of the exchange of gases between the organism and the environment. As a consequence, the respiratory system inevitably encounters infections with various foreign pathogens [1], such as the new severe acute respiratory syndrome coronavirus (SARS-CoV-2), which is transmitted mainly via the respiratory tract, was responsible for the pandemic that began at the end of the year 2019, and has emerged as a serious threat to public health. Vaccination is one of the most effective immunological approaches currently available for enhancing the lifespan and quality of health in humans. The conventional mode of vaccination is immunization through an injection. However, this dates back to the 10th century AD in China, in which the scabs on the rashes of smallpox patients were crushed into powder and blown into the nostrils of healthy individuals for vaccination against the smallpox [2]. In the recent history of vaccine development, many clinical trials have been conducted on respiratory mucosal vaccines. The nasal route of vaccination prevents the hepatic first-pass effect and/or gastrointestinal decomposition, while only little enzymatic degradation of the vaccine antigens occurs in the nasal cavity, thereby requiring a lower dose of antigens compared to the parenteral and oral routes of immunization [3]. In addition, nasal mucosal vaccination reduces syringe usage and medical waste, due to which it is regarded as a resource-saving and environment-friendly strategy suitable for a sustainable healthcare model. Moreover, the mucosal vaccines are convenient for use as self-help vaccination, which ensures the comfort of individuals and enhances individuals compliance, thereby being suitable for mass vaccinations in the general population [4]. Since nasal mucosal vaccines have the potential to induce heightened protective immune responses at the primary site of pathogen infection, including the secretion of antibodies and tissue-resident T cell responses, these vaccines allow for blocking the epithelial cells and other cells to pathogen infection, thereby preventing the infection during the beginning stages rather than only reducing infection in later stages and preventing the further development of disease symptoms [5,6]. Meanwhile, nasal mucosal immunization has also been demonstrated to be ideal for SARS-CoV-2 vaccination [7].
2. Nasal Structure and Immune Responses
Several factors influence nasal vaccine design and mucosal immune responses. One of these factors is the physiological structure of the nasal cavity. The nasal cavity is divided into vestibular, respiratory, and olfactory compartments. The lining of the nasal cavity comprises the epithelium, the basement membrane, and the lamina propria, all of which are covered by a layer of tissue structure known as nasal mucosa. The human nasal mucosa has a wide surface area of 150 cm2, which provides a greater effective area for antigen absorption. The basal layer of the endothelium is referred to as the submucosa, which is rich in blood vessels and, therefore, facilitates the absorption of the vaccine antigens [8]. On the surface of the nasal cavity, the epithelial cells are tightly connected, forming a special physical barrier that prevents the invasion of antigenic particles, similar to the active defense against pathogenic microorganisms [9].
The mucosal immune system is the key to a host’s resistance to pathogen invasion [10]. Functionally, the nasal mucosal immune system is divided into inductive sites and effector sites, as illustrated in Figure 1. An inductive site is where the induction of the immune response begins. This is the site where the antigen first reaches the respiratory tract, then crosses the mucus layer, and is finally absorbed. The inductive site mainly comprises mucosal lymphoid follicles located within the respiratory zone, which are collectively referred to as the nasal-associated lymphoid tissue (NALT) [11,12]. NALT constitutes the Waldeyer ring in humans and can be considered to correspond to the NALT in rodents, which includes the palatine tonsils, nasopharyngeal tonsils, bilateral lingual tonsils, bilateral pharyngeal isthmus, and bilateral pharyngeal lymphatic rings [13]. NALT is the only structurally intact mucosa-associated lymphoid tissue in the upper respiratory tract. In addition, NALT is encompassed by epithelial cells and a small number of microfold (M) cells, which are invaginated to form pockets. The basal site of M cells (i.e., inside the pockets) is a region where the areas rich in B cells, T cells, macrophages, and dendritic cells (DCs) form a specialized lymphoid tissue [14,15]. M cells serve as a flexible transmembrane transporter for the non-specific as well as specific endocytosis of the antigens presented on their outer membranes. Thereafter, these antigen-containing vesicles release an extracellular secretion on the basolateral side of M cells, thereby enabling the transportation of the antigens to the antigen-presenting cells (APCs), including macrophages, DCs, and B cells, for processing and presentation [16]. In addition, certain intraepithelial or subepithelial DCs are capable of capturing antigens and migrating to the local/regional lymph nodes via the draining lymphatics [17]. The APCs that have taken up the antigen migrate to the follicular B cell zone and interfollicular T cell zone, where these cells present the antigens to the neighboring naive T cells for initiating adaptive cellular immunity [18].
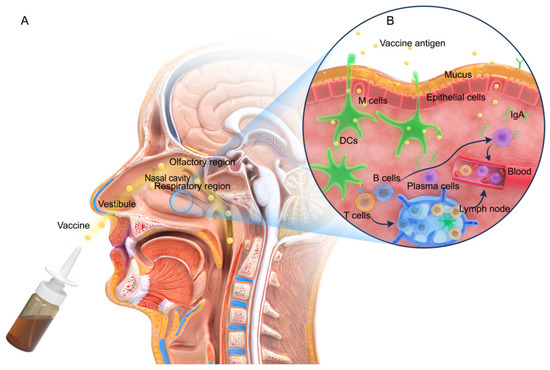
Figure 1.
Nasal structure and the mucosal immune response. (A) Structure of the nasal cavity. (B) The immune response mechanism in the nasal mucosa. M cells or DCs (green) take up the antigens at the induction site, migrate to NALT, and interact with T cells (yellow) to activate B cells (blue) into plasma cells (purple). A few of the plasma cells migrate to the effector site and secrete IgA antibodies. In addition, the remaining B cells and the activated T cells pass through the blood, induce cellular and IgG-based systemic immune responses, and may even differentiate into memory B and T cells.
An effector site in the nasal mucosal immune system is where the immune responses of B and T cells occur. The effector site includes the lamina propria (LP) and the intraepithelial layer of the respiratory mucosa [19] and is connected to the inductive site mainly through lymphocyte homing. The majority of the immune cells migrate to the inductive site to exert their effects, allowing the mucosal immunity to be relatively independent of systemic immunity and, therefore, exhibiting relative limitation [20]. This functional connection system, comprising the mucosal induction sites migrating to effector sites, is referred to as the common mucosal immune system (CMIS) [21]. CMIS may be driven by specific integrin and chemokine receptor programs [22,23]. In addition, the immune cells enter other mucosal sites and exert their effector functions, thereby linking the immune responses in different mucosal sites [24]. Consequently, antigen-specific immunity is not restricted to only the induction site and may occur even in distant mucosal zones [25]. Moreover, the antigen-activated immune cells may migrate via the bloodstream in the body and participate in systemic immune responses, thereby inducing mucosal and systemic immune responses, which are characterized by the production of IgA and IgG, respectively [6]. The secretion of local IgA antibodies block the binding of pathogens to nasal epithelial receptors [26,27]. The systemic and mucosal immune responses elicit potent humoral and cellular immune responses that may provide cross-protection [28,29].
Nasal mucosal vaccines function by inducing local tissue-resident memory (TRM) T cells, which form an essential part of mucosal surface immunity. Studies have suggested that the nasal mucosal immunization with live-attenuated influenza vaccine (LAIV) produces lung tissue-specific CD4+ TRM T cells and viral CD8+ TRM T cells with a phenotype similar to those produced upon infection with the influenza virus. On the contrary, intranasal immunization with inactivated influenza vaccines (IIV) or intramuscular immunization with LAIV reportedly could not elicit T cell responses or provide protection against viral infection. These findings suggested that both respiratory tract targeting and live-attenuated strains were required for the induction of TRM T cells [5]. Recent studies in mouse models demonstrated that while TRM cells migrate from the lung to the mediastinal lymph nodes during infection, a process referred to as “retrograde migration”, the TRM cell phenotype is maintained and provides long-term protection [30]. The T-helper cells induced after infection with the influenza virus are considered critical for the subsequent vaccination-based induction of long-term cellular and humoral immunity in the respiratory tract [31,32], further corroborating the effectiveness of mucosal vaccines.
A thorough understanding of the interactions between the structure of nasal mucosa and nasal mucosal immunity would provide valuable insights into the design of mucosal vaccine. In particular, a comprehensive exploration of mucosal APCs, innate lymphocytes, and TRM cells at mucosal sites could reveal attractive targets for vaccine design. These insights would assist in designing rational nasal vaccines against various infectious viruses (including SARS-CoV-2) and cancers using novel vaccine technologies.
3. Insights from the Currently Available Licensed Influenza Nasal Vaccine
Various types of injectable vaccines (including adjuvant inactivated vaccines, subunit antigen, and RNA vaccines) are currently available in the market; however, these vaccines have not yet been licensed as nasal vaccines. The only approved intranasal trivalent LAIV is the FluMist® (Fluenz®), which has been in clinical use in the United States since 2003 [33]. In February 2012, the FDA approved FluMist® Quadrivalent for people aged between 2 and 49 years [34], which was indicative of the completion of its transition from a trivalent to a quadrivalent nasal LAIV [35]. The genetically engineered LAIV virus in FluMist® is a cold-adapted (ca), temperature-sensitive (ts), and attenuated (att) influenza virus. This virus is capable of replicating at 25 °C and not at 37 °C, suggesting that the LAIV form of this virus could only infect and replicate in the nasopharyngeal mucosal cells, which enhances antigen capture and delivery, thereby inducing a pattern of immunity similar to the one induced upon natural infection [34,36].
The immune response elicited by FluMist® and its demonstrated safety are the main reasons for its success as a vaccine. Currently, nasal immunization with LAIV is an important mode of influenza vaccination [37,38]. In the last few decades, various other intranasal vaccine candidates against a range of human diseases have been conducted. For instance, the nasal immunization vaccine against Bordetella pertussis has successfully completed phase I clinical trial and entered phase II clinical trial [39,40]. Promising preclinical data have been generated for nasal immunization with Bacillus Calmette-Guérin (BCG) vaccine for the treatment of mycobacterium tuberculosis [41]. Chimeric RSV/PIV3 vaccine candidates (respiratory syncytial virus (RSV) attachment (G) and fusion (F) genes chimeric to the attenuated parainfluenza virus type 3 (PIV3)) or recombinant bivalent subunit vaccines (containing the RSV F protein and PIV3 haemagglutinin-neuraminidase (HN) protein and combined with adjuvant) have demonstrated effectiveness as nasal immunization vaccines in animal models [42,43]. Nine SARS-CoV-2 nasal vaccines, including the inactivated, live attenuated, protein subunit, nucleic acid, viral vector, and other vaccines, are currently in clinical trials, which further corroborates the feasibility of nasal vaccines [44].
4. Challenges of Nasal Vaccine
In the development of inactivated virus nasal vaccine, split nasal vaccine, subunit nasal vaccine, peptide nasal vaccine, and nucleic acid nasal vaccine, many factors limit the antigen absorption and bioavailability. The lipophilicity and size of vaccine molecules are important in nasal permeation. Lipophilic molecules usually diffuse via transcellular pathways, while hydrophilic molecules, particularly those with a high molecular weight, have low membrane permeability [45]. Nasal volume is another limiting factor that restricts the feasible volume for nasal administration to 25–250 µL [46]. Nasal mucosal cilia clearance and mucus barrier obstruct the antigen uptake of the vaccine, the enzymatic environment, and the local pH of the nasal mucosa, all of which would ultimately exert a negative effect on the stability of the vaccine [6]. These obstacles prevent adequate antigen delivery and the subsequent uptake and presentation of APCs residing in the nasal cavity, thereby hindering the initiation of protective immunity [47,48].
To overcome these obstacles, various strategies have been proposed to improve the efficacy of nasal vaccine in the last few years. Different antigen formulations have been developed, such as mucoadhesives (chitosan), to reduce the effects of nasal mucosal cilia on antigen clearance [49,50]. Sodium alginate (SA) and carboxymethylcellulose-high molecular weight (CMC-HMW) are reported to prolong the retention of antigens at the mucosal sites [51]. Phosphatidylcholine and cell-penetrating peptides (CPP) promote the uptake of antigens at mucosal sites [52,53,54]. Moreover, genetic optimization of the vaccines based on viruses, proteins/peptides, and nucleic acids and the use of delivery system (e.g., liposomes, nanoemulsions, and virosome) has enabled enhanced vaccine stability [55]. For instance, the use of immune enhancers, such as toxoid, cytokines, and Toll-like receptor (TLR) agonist adjuvants, reportedly promote antigen uptake and enhance the local immune response [47,56], while specific ligands or antibodies targeting APCs or M cells improve antigen uptake [57]. These strategies aim to stabilize the antigenic components, break the mucosal barrier, induce effective antigen presentation to the immune system, and activate mucosal immunity [47,52]. In this context, the present report describes the most recent findings in the development of nasal vaccines (Figure 2).
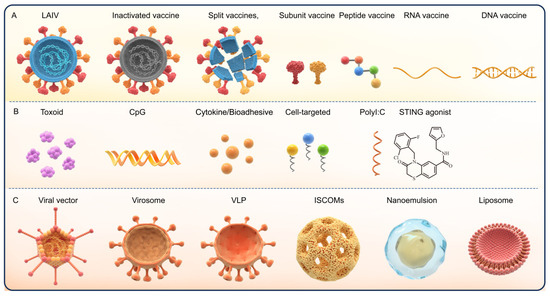
Figure 2.
The different types of nasal vaccines and adjuvants. (A) The subtypes of nasal vaccines. (B) The subtypes of immune enhancement adjuvants. (C) Adjuvant delivery system.
5. Mode of Mucosal Vaccination
Respiratory vaccination primarily targets the nasal or the lower respiratory tract. While nasal sprays, drops, and gels mainly act on the nasal cavity, the atomized aerosols and dry powder inhalation formulations act mainly on the lower respiratory tract. Nasal spray immunization is the most common method of nasal mucosal immunization compared to other nasal formulations [58]. To prevent infections of the upper respiratory tract, the particles of the nasal spray vaccine should be approximately 5 µm or above in size. Currently, the only approved vaccine for the respiratory tract is the nasal influenza vaccine, which is delivered directly to the nasal cavity as a large spray of particles to prevent deposition in the lower respiratory tract [59]. Few studies have compared the nasal spray immunization and nasal drop immunization. One study found that nasal drops have demonstrated greater effectiveness compared to nasal sprays in reducing the symptoms of nasal polyps [60], while the other study reported that nasal administration of midazolam is an excellent alternative for sedation in pediatric nuclear medicine and nasal sprays are superior to nasal drops [61], suggesting that the preferred routes of administration could vary with different drugs.
Small-particle aerosols (≤3 µm) are capable of entering the lower respiratory tract via aerosol inhalation and protecting against lower respiratory tract infections [62]. Inhalation of aerosols requires certain aerosol inhalation devices. For instance, vaccines for liquid measles may be formulated into aerosols using early modified atomizers, and have undergone extensive clinical trials. A systematic meta-analysis has confirmed that inhaled liquid aerosol vaccine against measles is safe, immunogenic, and generally well tolerated [63]. Aerosol vaccines against measles exhibit an immune response that is equivalent to or superior to injectable vaccines, while the antibody response of the former is relatively strong and durable using even a small dose of vaccine, thereby providing effective and durable protection against measles [63,64,65,66]. Inhaled dry powder live-attenuated measles vaccine (MVDP) showed superior storage stability compared to liquid vaccines. After nasal immunization, MVDP induces robust measles virus (MeV)-specific humoral and T cell responses in macaques, which has been reported to be safe, effective, and conducive to measles control [67]. Phase I clinical trial of MVDP has confirmed the safety and immunogenicity of this vaccine [68]. Dry-powder nasal mucosal inactivated influenza virus vaccine has also partially entered the clinic trial stage (ClinicalTrials.gov Identifier: NCT01488188). The nasal powder agent prolongs the retention of powder formulations on the nasal mucosa, potentially increasing local and systemic immune responses and providing better efficacy in protective immunity [51,69]. The above reports suggest that a mucosal vaccine may be used in various modes of immunization.
Special application techniques are required due to the high viscosity of nasal gel vaccines. Various gel polymers-based formulations have been developed, such as cationic cholesterol-based amylopectin (CHP) nanogels, which serve as antigen carriers for nasal vaccines to induce effective immune protection [70,71,72,73], such as the hydrogels constituted of branched polyethyleneimine and oxidized dextran or constituted of the polymer Gantrez® AN119 and Pluronic® F127 (PF127), the gels prepared from deacetylated gellan gum, chitosan [74,75,76,77]. All of these formulations enhance the immune response, demonstrating that nasal gels are a promising novel alternative for use as a nasal mucosal delivery system.
6. Live-Attenuated Vaccines
The success of attenuated nasal vaccines against influenza has inspired the development of other attenuated nasal vaccines. One example is the vaccine containing live-attenuated Bordetella pertussis strain BPZE1, which colonizes the respiratory tract and induces a robust protective immune response against Bordetella pertussis infection upon only a single nasal administration. In addition, it has demonstrated promising results in phase I clinical trials [40,55,78] (NCT02453048, NCT00870350). Another example is the attenuated RSV/ΔNS2/Δ1313/I1314L vaccine, with an application of genetic engineering technology, the interferon antagonist NS2 gene and the RSV polymerase gene codon 1313 were knocked out and the missense mutation I1314L was introduced, the resulting attenuated virus vaccine was temperature-sensitive, genetically stable, replication-deficient, and immunogenic in non-human primates [79]. Clinical trials demonstrated that this attenuated vaccine had an acceptable safety level and good immunogenicity in RSV-seronegative children, although further clinical trials in a large population are warranted [79]. An attenuated vaccine against SARS-CoV-2 named COVI-VAC was constructed by re-encoding a segment of the virus spike protein with a suboptimal synonymous codon pair (codon pair de-optimization) and introducing 283 silent (site) mutations. In animal models, COVI-VAC provided protection with a single nasal mucosal immunization [80]. The phase I clinical trials were about to be conducted (NCT05233826; NCT04619628).
Nasal administration of live-attenuated B. pertussis (BPZE1) by genetic inactivation or removal of three major toxins provides protection not only early in life (even at birth) in mice, but up to 1 year after immunization [81]. Another study also found that mice given intranasal BPZE1 and boosted with the acellular pertussis vaccine showed T cells and antibody responses [82]. Meanwhile, the BPZE1 also completed the phase I clinical trial [83,84]. BPZE1f3, a derivative of BPZE1 that contains both serotype 2 (Fim2) and serotype 3 (Fim3), produced significantly stronger protection against Fim3-only producing B. pertussis in mice than BPZE1 [85].
Table 1 presents the clinical status of nasal mucosal attenuated vaccines that are currently under development. In addition, the already marketed attenuated nasal vaccines against influenza, most of the other attenuated nasal vaccines under development (vaccines against RSV, parainfluenza virus, and avian pandemic influenza) are in the phase I clinical stage, and only a few are in the phase II clinical stage. Therefore, the nasal mucosal attenuated vaccines have demonstrated great potential, although further investigation is warranted prior to their availability to the general population.

Table 1.
The current progress in the clinical development of nasal attenuated vaccines.
7. Nasal Vaccine Adjuvants
The replication-competent viral vector-based nasal vaccines mimic the cell membrane fusion and replication capabilities of the virus during natural infections, thereby providing enhanced mucosal and humoral immunity [86]. The other nasal vaccines under development, including inactivated virus vaccine, split vaccine, subunit vaccine, peptide vaccine, and nucleic acid vaccine, require adjuvants to address their poor immunogenicity or improve delivery. The peptide-based vaccines require antigen optimization and adjuvant application, such as the HIV therapeutic vaccine Vacc-4x, which comprises four slightly modified HIV Gag p24 shared peptides and has to be used in combination with the Endocine adjuvant. In phase I clinical trials, Vacc-4x induced a dose-dependent vaccine-specific T cell response as well as the mucosal and systemic humoral responses (NCT01473810) [87]. The subunit and inactivated vaccines may also require adjuvants to enhance immune responses. Figure 2 presents the common nasal vaccines and adjuvants, and Table 2 presents the clinical progress of peptide, subunit, inactivated, and adenovirus-vectored nasal vaccines. Most of the vaccines under phase I/II clinical trials were the vector vaccine or the inactivated and subunit nasal vaccine with nasal adjuvants, such as the relatively potential LTh(αK), or nanoemulsion, liposomal, and type 1 interferon. There are no phase III clinical trials on nasal vaccines. Therefore, in-depth research is required on the development of a nasal vaccine.

Table 2.
Clinical progress in the development of peptide, subunit, inactivated, and viral vector-based nasal vaccines.
The specific structure and immune response of the nasal mucosa render several of the vaccine adjuvants applicable to intramuscular or subcutaneous administration non-applicable to mucosal vaccines. The classification of the nasal mucosal adjuvants resembles mucosal adjuvants and comprises the following two categories: Immunostimulatory adjuvants and immune delivery system adjuvants. While immunostimulatory adjuvants, e.g., E. coli heat-labile enterotoxin (LT), cholera toxin (CT), TLR ligands, chitosan, and cytokines, activate the natural immunity directly, the immune delivery system adjuvants comprise viral vectors, virosomes, liposomes, nanoemulsions (nEs), virus-like particles (VLPs), immune-stimulating complexes (ISCOMs), and a variety of polymers, including poly(D,L-lactide-coglycolide) (PLGA), poloxamers, and alginate [88,89].
7.1. Immunostimulatory Adjuvants
The nasal mucosa is constantly exposed to an enormous number of pathogenic microorganisms. Generally, the endogenous cellular and molecular mechanisms in the mucosa downregulate the mucosal immune response to foreign antigens [90], probably to prevent inducing excessive immune response against harmless antigens that are frequently encountered by the mucosa. The physical barrier and the weaker immune response of the nasal mucosa render it difficult for most protein or nucleic acid-based vaccines to induce a robust protective antigen-specific mucosal immune response. However, the immunosuppressive activity of the mucosa may be evaded using mucosal adjuvants.
7.1.1. Toxoid Adjuvants
The currently used mucosal adjuvants include cholera toxin (CT) and heat-labile enterotoxins (LT), in which the ADP-ribosyltransferase activity and the structural properties of the A subunit combined with the membrane-bound activity of the B subunit contribute to the adjuvant activity of LT and CT. It is believed that CT and LT induce mucosal immune responses by: (1) Increasing the permeability of epithelial cells and enhancing their antigen uptake; (2) enhancing the antigen presentation on different APCs; (3) regulating B cell differentiation and promoting IgA secretion; (4) regulating T cell proliferation and cytokine production [56,86]. However, the target cells and the underlying specific molecular mechanisms remain elusive to date.
Phosphocholine (PC) is a widely studied broad-spectrum mucosal vaccine. When conjugated with keyhole limpet hemocyanin (PC-KLH) and CT adjuvant, it induced and enhanced the IgA titer in nasal wash and saliva. In addition, it increased the response of IL-4 and IFN by CD4+ T cells. This finding indicated the feasibility of using PC combined with toxoid adjuvant as a mucosal vaccine against upper respiratory tract infections and allergic rhinitis [91]. However, nasal immunization may damage the olfactory nerve [92]. Although CT and LT have been demonstrated as powerful mucosal immune adjuvants in several studies, their application to humans is limited due to their toxicity, as evidenced in the case of CT adjuvants used in polio vaccines [93]. The generation of CT and LT mutants through genetic engineering could be one solution for eliminating or reducing their toxicity while retaining their adjuvant properties and using them in subunits or inactivated nasal vaccine. For instance, isolated LT enzyme A1 (LTA1) has demonstrated excellent efficacy and safety as an immune adjuvant for inactivated influenza vaccines [94]. LThαK lacks adenosine diphosphate (ADP)-ribosyltransferase activity and is a detoxified E. coli heat-unstable toxin derivative that has been studied extensively as a nasal mucosal adjuvant owing to its prolonged nasal retention and safety. The use of LThαK (NCT03784885) as an adjuvant for a trivalent inactivated influenza vaccine in phase II clinical trial revealed an acceptable level of safety and higher antigen-specific IgA responses after two nasal vaccinations [95,96]. CTA1-DD is a nontoxic mucosal adjuvant with the enzymatic properties of CT combined with B cell targeting capability. In addition, no neurotoxicity was observed after nasal vaccination with CTA1-DD, which ensures its safety as an adjuvant for nasal vaccines [97]. The HRSV fusion pre-F protein with CTA1-DD as an adjuvant could serve as a potential nasal vaccine candidate against the hRSV infection in humans [98]. LTK63, another nontoxic mutant of LT, also demonstrated the safety and efficacy of nasal mucosal adjuvants [99]. Not only the amino acid substitutions of nontoxic mutants are needed for acquiring suitable, safe, and adjuvant-active toxoid adjuvants, but also the other strategies, such as combining with other adjuvants and reducing dosage, could be considered. A similar case is the AS01 that contains the MPLA and the saponin QS-21, where the toxicity of QS-21 is reduced when combined with MPLA [100].
7.1.2. Cytokine Adjuvants
The type I IFN has been used as the adjuvant for the inactivated nasal influenza vaccine in phase I clinical trial (NCT00436046), which has been successfully completed. The IL-1 family cytokines, including IL-1A, IL-1B, IL-18, and IL-33, have been used as adjuvants for the recombinant influenza virus hemagglutinin (RHA) vaccine. This vaccine-adjuvant combination could significantly stimulate the production of nasal mucosal IgA and system anti-RHA IgG after nasal vaccination in BALB/c mice [101,102]. Furthermore, DNA-IL-12 plus CTB was used as a mucosal adjuvant for DNA prime/MVA boost nasal vaccinations, which resulted in enhanced cellular systemic and mucosal genital tract immunity [103]. IL-15 has been proposed as a nasal mucosal adjuvant when developing a novel SARS-CoV-2 vaccine [104]. Other cytokines, such as those from the GM-CSF family and TNF family, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and IL-18, are also used as mucosal adjuvants to enhance the sIgA and systemic immune responses. They were studied as candidate nasal vaccine adjuvants [105]. Overall, cytokine adjuvants were studied as mucosal adjuvants for inactivated and subunit vaccines, and preliminary research results were presented.
7.1.3. TLR Agonist Adjuvants
TLRs are a group of pattern recognition receptors (PRRs), which recognize microbial pathogens and initiate host response to infection. The activation of TLRs induces a robust and immediate innate immune response, which leads to various adaptive immune responses [106]. Therefore, TLRs are ideal targets when developing effective adjuvants, especially for subunit or inactivated nasal vaccine. Monophospholipid A (MPL), the toxicity of which is 1/1000th of the toxicity of lipopolysaccharide (LPS) [107], has been used as a component of the injectable vaccine adjuvants AS01, AS02, AS04, and AS15 [108,109,110,111]. In a study, nasal administration of mice with the novel fusion protein MRPH-FIMH significantly increased the IgG and IgA contents in serum, nasal washings, vaginal washes, and urine samples, while the addition of MPL as an adjuvant further enhanced the specific humoral and cellular responses against FIMH and MRPH [112]. In another study, nasal vaccination of Pseudomonas aeruginosa PCRV with TLR9 agonist CpG ODN as the adjuvant significantly increased the titers of PCRV-specific IgA, which is probably a component of the disease protection mechanism [113]. The use of N3 cationic adjuvant for inactivated influenza virus vaccine significantly enhanced the systemic and mucosal-specific immune responses against influenza upon immunization, while the combination of N3 cationic adjuvant with a TLR5 agonist further enhanced these responses and durably protected against the heterologous influenza A/H1N1/CA09PDM virus [114]. The TLR3 agonist PolyI:C and the TLR7/8 agonist resiquimod (R848) are also reported to be effective in inducing mucosal immune responses [115,116,117], suggesting that TLR agonists have the potential to be adjuvants for nasal vaccines.
7.1.4. STING Agonist Adjuvants
Cyclic dinucleotides that activate the stimulator of interferon genes (STING) have been evaluated as mucosal adjuvants. Nasal administration of a subunit vaccine with a synthetic cyclic dinucleotide (cyclodiguanide) adjuvant could induce protective immunity against Mycobacterium tuberculosis in mice, and this response was associated with the effective induction of TH17 cells [118]. Other cyclic dinucleotides, such as cyclic adenosine diphosphate (cyclic di-AMP) and cyclic adenosine diphosphate (cyclic di-GMP), have also demonstrated potential as mucosal adjuvants in previous studies [119,120]. The above reports provide a strong theoretical basis for the further development of nasal mucosal adjuvants targeting the STING pathway.
7.1.5. Bioadhesive Adjuvants
Bioadhesive-based nasal vaccines are preferred for achieving nasal clearance [121]. In this context, chitosan is recognized as an established mucosal adjuvant/delivery system that has been extensively studied owing to its low toxicity, adhesion, pro-permeability, immunostimulation, ability to be absorbed in human tissues, and excellent histocompatibility with human tissues and organs [122,123,124]. In addition, the effectiveness of chitosan as a nasal adjuvant has been confirmed in a mouse model. Nasal vaccination of chitosan and pneumococcal surface protein A (PspA) reportedly induced lung PspA-specific IFNγ and STING signaling-dependent IgG1, IgG2c, and IgA responses [125]. The non-ionic block copolymer named Pluronic F127 (F127), when used with chitosan, enhanced mucosal IgA secretion [126]. Moreover, EG-coated polylactic acid 80 enhanced IgG and IgA immune responses. PEG-grafted chitosan nanoparticles reportedly enhanced the nasal absorption of insulin [127]. These studies suggested that chitosan may be used in combination with other mucosal adjuvants for protein nasal vaccine to synergistically enhance the immune effect.
Nasal vaccination of protein-coated chitosan nanoparticles encapsulating the influenza mRNA molecules reportedly protected chickens against avian influenza [128]. The nasal mucosal immunization with SC2-spike DNA vaccine transported on a modified gold-chitosan nanocarrier resulted in an immune response with high levels of antibodies (IgG, IgA, and IgM) and lung mucosal and TRM T cells [129]. Recently, chitosan-based nasal vaccines have emerged as a research hotspot, and with continued research, chitosan is expected to play a further important role as an immune adjuvant combined with protein and nucleic acid-based nasal vaccines.
Another substance that exhibits mucoadhesive properties is PLGA. PLGA and PLGA/chitosan (chit) nanoparticles (NPs) with mucoadhesive properties may be used to encapsulate ropinirole hydrochloride (RH), a dopaminergic agonist against Parkinson’s disease, to facilitate RH delivery [130]. The feasibility of mucoadhesion adjuvants for clinical use needs to be validated by further research.
7.1.6. Cell-Targeted Adjuvants
M cell-targeting vaccines are based on the endocytic uptake of foreign antigens by M cells. UEA-1 is a plant lectin that is used as an M cell-specific ligand for targeting α-l-fucose (murine PP M cells). The HIV peptide and UEA-1 entrapped in PLG microparticles reportedly enhanced the mucosal and systemic immune responses through nasal immunization, which validated UEA-1 as an M cell-targeting nasal vaccine component [131]. Certain specific molecules expressed highly on the surface of M cells, such as glycoprotein 2, uromodulin, the cellular prion protein (PrPC), α(1,2)-fucose-containing carbohydrate, C5aR, α-2,3 sialic acid, and β1 integrin, are also used as receptors in M cell targeting [132,133].
To induce an immune response in the respiratory tract, the antigens must be taken up by APCs [134]. The activation, maturation, and proliferation of DCs are regulated by a range of molecules, including viral components, antigenic molecules of bacterial origin, growth factors, and cytokines [135,136]. For instance, FMS-like tyrosine kinase 3 (FLT3) is an important receptor tyrosine kinase in cell signaling. The binding between FLT3 and the ligand (FL) of FLT3 is reported to induce conformational changes in the pre-existing homodimer and consequently activate the tyrosine kinase, thereby inducing a DC response [137]. Nasal immunization of mice with ovalbumin (OVA) along with a plasmid encoding FL (pFL) as the nasal DC-targeting adjuvant reportedly induced OVA-specific SIgA and systemic IgG and IgA Ab responses [138]. The nasal administration of a combination of DNA plasmids encoding the FLT3 ligand (pFL) and CpG oligodeoxynucleotide 1826 (CpG ODN) (FL/CpG), as the nasal mucosal adjuvant, effectively enhanced the DC responses, balanced the Th1 and Th2 type cellular responses, and provided rFimA-specific IgA-based protection in the respiratory tract against Porphyromonas gingivalis [139].
Furthermore, the synthetic glycoside named α-galactosylceramide (also referred to as α-GalCer; composed of an α-linked sugar and a lipid fraction) binds to the non-classical MHC I molecule CD1d, thereby inducing the proliferation of natural killer T (NKT) cells and secretion of IFNγ, which results in the expression of the semi-invariant Vk14 T cell receptor and ultimately the induction of innate and adaptive immunity [140]. The optimized α-GalCer exhibited better solubility and was effective in stimulating the NKT cells, thereby inducing the release of Th1/Th2 cytokines, which significantly elevated the titers of systemic IgG and mucosal IgA antibody and enhanced the production of cytotoxic T lymphocytes in mouse and in vitro human system. These findings suggest that α-GalCer analogs with branched acyl chains could serve as effective mucosal adjuvants for inducing protective immune responses against influenza virus infection [141,142].
Activated mast cells contribute to stimulating inflammatory mediators and releasing immune cells. The mast cell activators named polymeric compound 48/80 (C48/80) and the cationic peptide mastoparan 7 (M7) have been used as adjuvants in nasal mucosal immunization [143], for example, pneumonia vaccine in combination with C48/80 could combat lethal pneumococcal infection [144]. Certain small molecule mast cell activators have also exhibited certain mucosal adjuvant effects and may be novel mucosal adjuvants.
7.2. Delivery System Adjuvants
The size of the delivery particles affects the cellular uptake and pharmacokinetics of the delivered molecules. In addition, the surface modification of the delivery particles alters the specificity and effectiveness of the ligand–APC interactions [145]. Viral vectors, when used in nasal vaccine, proliferate in the host tissues after nasal mucosal immunization, offering greater probability of stimulating immune responses. In the case of delivery vectors other than the virus-based system, factors such as size, biodegradability, adhesion, internalization rate, pH sensitivity, antigen release rate, and adjuvant activity have to be considered, as well.
7.2.1. Viral Vectors
Different vectors usually elicit different immune responses and, therefore, provide different degrees of protection. Therefore, certain vectors may be preferred when developing nasal mucosal-targeted vaccines owing to their unique properties. For instance, adenovirus type 5 (Ad5) is a common respiratory virus, and Ad5-based recombinant vectors have been used widely as vaccine candidates against SARS-CoV-2, influenza, Ebola, HIV-1, and other infectious diseases [146,147]. Notably, nasal mucosal immunization with an Ad5 vector-based vaccine provides better mucosal immunity and protection, which can effectively reduce viral load in the upper respiratory tract. Therefore, this vaccine is a suitable candidate for preventing SARS-CoV-2 infection and transmission and mitigating the impact of the pandemic [146,148,149]. Another nasal vaccine developed against SARS-CoV-2 was based on the replication-incompetent human parainfluenza virus type 2 (HPIV2) [150]. This vaccine delivers ectopic genes as stable RNA molecules and ectopic proteins to the membrane, thereby inducing high levels of neutralizing IgG and mucosal IgA antibodies in mice for the neutralization of SARS-CoV-2 spike proteins. The vaccine is currently in clinical trial on healthy human volunteers [151]. Human parainfluenza virus types 1 and 3 (HPIV1 and HPIV3) may be modified through genetic engineering to express the RSV fusion pre-F proteins, which serve as nasal vaccine candidates with protective effects [152,153]. Similarly, the influenza virus is a promising vaccine vector, which is being used for developing a novel nasal vaccine against SARS-CoV-2. This novel vaccine induces serum neutralizing antibodies comparable to the levels generated in the natural infection [154,155]. Recombinant attenuated influenza viruses expressing the structural domain of the RSV G protein reportedly induced robust IgA-specific immune responses and TRM T cell responses in the lung and bronchoalveolar fluid of mice, thereby protecting the mice from RSV attack [156,157]. Nasal immunization with HIV vaccine plus BCG or influenza virus-based vectors was reported to promote HIV-specific cellular and humoral immune responses in the airway and vagina of mice [158]. Nasal or oral administration of the baculovirus-vectored human papillomavirus (HPV) vaccine demonstrated protective effects against vaginal HPV infection [159].
However, the immunoprotection of the Ad5 vector-based vaccines may be reduced due to the pre-existing anti-Ad5 immunity in humans resulting from natural exposure or prior vaccination. The vector-specific serum antibodies were reported to severely impede the seroconversion (change from seronegative to seropositive condition) of neutralizing antibodies against SARS-CoV-2 in volunteers with intramuscular vaccination [160]. The nasal mucosal immunization against Ad5-S-nb2 in macaques was reported to induce a significantly delayed antibody production against Ad5 and lower serum antibody titers compared to intramuscular administration [148]. This finding suggested that nasal mucosal immunization with adenoviral vector-based vaccines is less affected by pre-existing Ad5 antibodies [161,162]. Despite the scientific concerns regarding an increased risk of HIV infection upon using adenoviral vector-based vaccines [163], the HAdV-26 COVID-19 vaccine has been used widely in large-scale trials or after emergency authorizations, and no increase in the HIV-1 infection rates was reported [163]. The LAIV vector-based SARS-CoV-2 attenuated vaccine (dNS1-RBD) has completed phase I and II clinic trials, which revealed weak T cell immunity in the peripheral blood and weak humoral and mucosal immune responses against SARS-CoV-2 in the vaccinated recipients. However, further studies are warranted to validate the safety and efficacy of the nasal vaccine as an alternative immunization route to the currently used intramuscular SARS-CoV-2 vaccine [164].
In clinical trials, viral vector-based vaccines may prove to be significantly ineffective when the same vector or similar vectors are used, as the efficacy of human vaccines is reduced in individuals that have been previously infected with these viruses. Several non-homologous viral vectors have been studied extensively, among which is the Newcastle disease virus (NDV), which was used as a vaccine vector for SARS in 2003 and SARS-CoV-2 in 2019 [165,166,167]. NDV vectors expressing wild-type S or membrane-anchored S and without polybasic cleavage sites may also be used as vaccine vectors against SARS-CoV-2. These COVID-19 vaccine candidates reportedly protected mice from mouse-adapted SARS-CoV-2 attack without any detectable NDV titers and viral antigens in the lung [168]. Another example is the avian paramyxovirus type 3 (APMV3), which was used as the vaccine vector against SARS-CoV-2 [169]. In addition, the rabies virus (RV) vaccine strain, a recombinant vesicular stomatitis virus (VSV), and cowpea mosaic virus (CPMV) have been used as candidate vaccine vectors [24,170]. However, the mutations and biosafety of these candidate viral vectors remain unclear to date, warranting further investigation prior to application.
7.2.2. Liposome Delivery System
Liposomes are spherical vesicles with a bilayer structure formed by the phospholipids dispersed in an aqueous solution. Liposomes have diameters ranging from 10 nm to several μm and contain an aqueous phase encapsulated inside their structures [171]. The encapsulated aqueous phase contains water-soluble compounds (e.g., proteins, peptides, and nucleic acids), while the lipophilic compounds (e.g., antigens and adjuvants) are embedded in the lipid bilayer of the liposome structures. Liposomes offer the advantages of preventing antigen degradation, delivering poorly soluble drugs, and reducing drug toxicity. In addition, the encapsulation of the drug inside a liposome allows for the customization of the localization and distribution of the drug at the target site. These advantages facilitate the effect of vaccine antigens in the nasal mucosal environment.
Cationic liposomes to be used as a vaccine adjuvant-delivery system may be prepared using 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), dimethyl dioctadecyl ammonium bromide (DDA), and dimethylaminoethane-carbamoyl-cholesterol to further enhance the immunogenicity of vaccines [171]. In comparison to non-cationic lipid vesicles, DDA- and PEG-based cationic liposomes used as a vaccine adjuvant-delivery system further enhance the immune responses [172]. In addition, nasal vaccination with cationic liposomes prepared from DOTAP and carbamoyl-cholesterol are efficiently internalized by DCs present in the NALT, which induces the production of antigen-specific IgA and T cell responses in the nasal mucosa tissues [173,174]. Nasal mucosal vaccination with pneumococcal surface protein A plus cationic liposomes composed of DOTAP and cholesteryl 3β-N-(dimethylaminoethyl)-carbamate (DC-chol) (DOTAP/DC-chol liposome) reportedly induced protective immunity against Streptococcus pneumoniae infection in mice [175]. CAF01 is a novel liposomal adjuvant system comprising cationic liposomal carriers (DDA and glycolipid immunomodulators (alginate 6,6-dibehenate (TDB)) with a stable structure [176]. Studies have demonstrated that nasal vaccination with CAF01-based vaccines for the prevention of influenza or Streptococcus pyogenes was effective in inducing the production of mucosal effector T cells and IgA immune responses and also protected the vaccinated model animals. In addition, the spleen of CAF01-vaccinated mice produced four-fold higher levels of antigen-specific IFN-γ responses compared to the mice with non-adjuvanted vaccination. This finding demonstrated that CAF01 significantly enhanced the levels of vaccine-specific serum IgG [177]. Endocine™, which consists of the lipids mono-olein and oleic acid, has been used in a nasal vaccine (Vacc-4x). The clinical trial confirmed the safety and mucosal and systemic antibody responses, as well as the dose-dependent vaccine-specific T cell responses [87]. VaxiSomeTM, a liposome composed of a novel polycationic sphingolipid complexed with cholesterol is another effective adjuvant/carrier system for nasal mucosal immunization against influenza in mice [178,179]. Furthermore, the stabilization of liposomes through the layer-by-layer deposition of polyelectrolytes is reported to increase antigen-specific IgA and IgG antibody levels and T cell-based immune responses [180]. This finding suggests that the optimization of liposomes significantly impacts their stability and vaccine-induced immune responses.
Cationic cyclodextrin-polyethylenimine 2 k conjugate (CP 2 k) complexed with anionic mRNA encoding HIV gp120 reportedly overcame the epithelial physical barrier, prolonged nasal retention, enhanced the paracellular delivery of mRNA, minimized toxin absorption in the nasal cavity, induced balanced Th1/Th2/Th17 types, and achieved a robust systemic and mucosal anti-HIV immune response [181]. Cationic cyclodextrin-PEI conjugated to the IVT mRNA encoding OVA, significantly promoted nasal mRNA vaccine delivery in mice after nasal vaccination and also stimulated DC maturation and migration, which further enhanced the humoral and cellular immune responses [182]. The advent of mRNA vaccines against SARS-CoV-2 may further facilitate the development of lipid nanoparticle nasal mucosal RNA vaccines against respiratory infectious diseases [183]. This suggests that liposomes could be used for delivering several forms of antigens.
7.2.3. VLP Delivery System
VLPs are self-assembling biomolecules that closely resemble native viruses. VLPs do not contain any genetic material, which is beneficial for APC recognition and uptake and also for BCR crosslinking [184,185]. In comparison to recombinant protein vaccines, VLPs exhibit excellent stability, which is conducive to the development of mucosal vaccines [186]. For instance, VLP-based norovirus vaccine used with chitosan adjuvant has exhibited effective nasal immunization [187]. In addition, nasal immunization with Norwalk VLP was reported to induce antibody production at distal mucosal sites [188].
7.2.4. Virosome-Mediated Delivery System
Virosomes are vesicles with a monolayer or a bilayer phospholipid membrane that encapsulates virus-derived proteins, although these could not replicate. Virosomes are able to fuse with target cells, and owing to their delivery effectiveness and demonstrated safety, virosomes are considered to be used for directly delivering the vaccine antigens inside the host cells [189]. Virosomes containing surface HIV-1 gp41-derived P1 lipidated peptide (MYM-V101) were reported to induce mucosal anti-gp41 antibodies against conserved gp41 motifs. In addition, these virosomes are expected to possess the HIV-1 transcytosis inhibition activity and may, therefore, contribute to the reduction in sexually transmitted HIV-1 (NCT01084343) [190]. Nasal immunization with nasal virosome-formulated influenza subunit vaccine in a ferret model prevented viral shedding almost entirely and also protected against homologous viral attack compared to parenteral immunization [191]. The results of phase II clinical trial of an influenza vaccine plus HLT and nasal virosomes as adjuvants revealed an efficient induction of IgA-neutralizing antibodies in the mucosa [192]. Moreover, Bárbara Fernandes reported the successful construction of a virosome-based COVID-19 vaccine candidate [193], which indicates good prospects for using virosomes as a vaccine delivery system.
7.2.5. ISCOMs
ISCOMs are negatively charged nanoparticles with cage-like structures. ISCOMs are composed of phospholipids, cholesterol, and Quil A (the saponin derived from the bark of the Quillaja saponaria plant), all of which are capable of accommodating a wide range of antigens. Hydrophobic antigens may be embedded or anchored directly into the colloidal structure of the lipid, while hydrophilic antigens require certain modifications prior to their effective loading into ISCOMs [194]. Initially, ISCOMs were used for injectable vaccination. Currently, these are being gradually introduced to mucosal vaccination (e.g., nasal and oral). ISCOMs-based vaccines induce an antigen-specific mucosal IgA response, producing serum IgG antibodies and cytotoxic T cells (CTL) [195]. Similar to CT and LT, ISCOMs break the immune tolerance and exert potent mucosal adjuvant activities, secreting IgA and inducing systemic IgG immune responses and CTL responses [196].
ISCOMs-based vaccines containing influenza virus, Mycobacterium tuberculosis, RSV, and measles antigen have been indicated to effectively induce an immune response in the body after nasal/pulmonary vaccination, producing high titers of IgA in the nasal cavity and the lung [197,198,199,200,201,202,203]. In certain cases, this local mucosal inoculation produces greater immunity compared to that produced upon injectable vaccination. ISCOMs prepared using Quilaja brasiliensis in place of Quil A may also be used to encapsulate the OVA antigen and then used as a vaccine adjuvant-delivery system to induce the production of local mucosal and distal IgA secretion after nasal vaccination [203]. Furthermore, the addition of DNA plasmids to ISCOMs reportedly induced local cellular and antibody responses against Haemophilus influenzae after nasal immunization [204]. The combination of CTA1-DD and ISCOMs was reported to enhance the immunity against BCG through the recruitment of antigen-specific effector cells to the infected site [200].
However, the use of ISCOMs as a mucosal vaccine adjuvant-delivery system for nasal vaccination has certain limitations. The hydrophilic antigens must be modified to improve lipophilicity for effective delivery, while certain saponin-based adjuvants are highly toxic when used at high doses. These limitations have curbed the development and application of ISCOMs in vaccine formulations to a certain extent.
7.2.6. NE-Based Adjuvants
Although emulsion-based adjuvants have demonstrated success in injectable vaccine applications, these adjuvants have not reached the standard of mucosal vaccine clinical application to date [205]. Nasal immunization of recombinant anthrax protein vaccine (BW-1010) in nanoemulsion (NE) adjuvant (oil-in-water emulsion) is in the currently ongoing phase I trial (NCT04148118) conducted by Bluewillow Biologics. The NE adjuvant was observed to promote Th1 cellular immunity and Th17 cellular immunity, which is consistent with the results obtained for the Mycobacterium tuberculosis vaccine plus NE adjuvant system [206,207]. W805EC is another oil-in-water NE adjuvant, when combined with the inactivated influenza vaccine, the protective hemagglutination inhibiting (HI) antibody and influenza-specific IgG and IgA were elicited [208]. PEG-b-PLACL (PELC) is a squalene-based oil-in-water NE adjuvant that reportedly promotes antigen penetration and uptake in the nasal mucosa and enhances protein interactions [209]. A preclinical study conducted on a guinea pig model of infection has confirmed the protection provided by this vaccine system, which was also correlated with systemic and mucosal antibody induction. NB-1008 is a surfactant-stabilized soybean oil-in-water NE adjuvant. The nasal mucosal immunization with the NB-1008 adjuvant comprising influenza virus antigens was reported to induce mucosal and serum antibody responses along with a robust cellular Th1 immune response [210]. Moreover, 5–15 nm NEs prepared using coupling techniques, such as lipopeptide coupled with polylysine nuclei, effectively promoted the systemic, mucosal, and cellular immune responses after nasal vaccination, thereby protecting against Streptococcus pyogenes infection [211].
8. Conclusions
Nasal mucosal immunization offers the advantage of inducing mucosal sIgA, cellular and systemic immune responses, and immune responses at distal mucosal sites, which would improve the effectiveness of vaccine against pathogens. The success of nasal LAIV has inspired the development of numerous other nasal attenuated vaccines, vector-based vaccines, and nasal mucosal adjuvant vaccines. The advent of genetic engineering further facilitated the success of attenuated vaccine strains, with several of the nasal mucosal attenuated vaccines currently in phase I, II, and III clinical trials, indicating good prospects for nasal mucosal attenuated vaccines. The viral vector-based vaccines result in greater stimulation of the immune response, although whether the vector-targeted immune response reduces the immunogenicity of the vaccine is still confusing. In the case of inactivated vaccines, subunits vaccines, and nucleic acid vaccines, the addition of mucosal adjuvants, such as toxoids, cytokines, and TLR agonists, enhances the immunogenicity of antigens, while ISCOMs, NEs, VLPs, and virosomes are effective in delivering antigens and ensuring antigenic stability. Some adjuvant-added nasal vaccines comprise both immune-enhancing and delivery-facilitating adjuvants to promote the delivery of vaccines to immune cells and also enhance the immunogenicity of vaccines. The establishment of novel adjuvanted nasal vaccines as safe and effective requires validation through extensive preclinical and clinical trials, and although the successful development of nasal vaccines has a long way to go, it owns positive implications. If the safety of the adjuvant-added nasal vaccines is guaranteed, the effective candidate for peptide, subunit, inactivated nasal vaccines would be enough to warrant success.
Author Contributions
Writing—original draft preparation, X.N., J.Z. and X.Y.; writing—review and editing, S.H., K.D., X.L. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the National Science and Technology Major Project titled “Major New Drug Manufacturing Program” of China (2016ZX09106003-008) (Wuhan Institute of Biological Products Co., Ltd.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest that could have influenced the present study in any manner.
References
- Churdboonchart, V.; Bhamarapravati, N.; Sirisidhi, K. Immune response in rabbits to dengue viral proteins. Southeast Asian J. Trop. Med. Public Health 1990, 21, 621–629. [Google Scholar]
- Golden, G. Smallpox vaccination: The Minnesota story. Minn. Med. 2003, 86, 20–25. [Google Scholar]
- Yin, L.-T.; Hao, H.-X.; Wang, H.-L.; Zhang, J.-H.; Meng, X.-L.; Yin, G.-R. Intranasal Immunisation with Recombinant Toxoplasma gondii Actin Partly Protects Mice against Toxoplasmosis. PLoS ONE 2013, 8, e82765. [Google Scholar] [CrossRef]
- Almeida, A.J.; Alpar, H.O. Nasal Delivery of Vaccines. J. Drug Target. 1996, 3, 455–467. [Google Scholar] [CrossRef]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal Drug Delivery: How, Why and What for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kumar, M.; Pathak, K. A review on mucoadhesive polymer used in nasal drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 215–222. [Google Scholar] [CrossRef]
- Xing, Y.; Lu, P.; Xue, Z.; Liang, C.; Zhang, B.; Kebebe, D.; Liu, H.; Liu, Z. Nano-Strategies for Improving the Bioavailability of Inhaled Pharmaceutical Formulations. Mini-Rev. Med. Chem. 2020, 20, 1258–1271. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Immune Functions of Nasopharyngeal Lymphoid Tissue. Adv. Oto-Rhino-Laryngol. 2011, 72, 20–24. [Google Scholar] [CrossRef]
- Date, Y.; Ebisawa, M.; Fukuda, S.; Shima, H.; Obata, Y.; Takahashi, D.; Kato, T.; Hanazato, M.; Nakato, G.; Williams, I.R.; et al. NALT M cells are important for immune induction for the common mucosal immune system. Int. Immunol. 2017, 29, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Koornstra, P.J.; Hameleers, D.M.; Biewenga, J.; Spit, B.J.; Duijvestijn, A.M.; Vriesman, P.J.V.B.; Sminia, T. The role of nasopharyngeal lymphoid tissue. Immunol. Today 1992, 13, 219–224. [Google Scholar] [CrossRef]
- Fujimura, Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. Int. J. Pathol. 2000, 436, 560–566. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.-S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp. Mol. Med. 2014, 46, e85. [Google Scholar] [CrossRef]
- Yamamoto, M.; Pascual, D.W.; Kiyono, H. M Cell-Targeted Mucosal Vaccine Strategies. Curr. Top. Microbiol. Immunol. 2011, 354, 39–52. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Pabst, R. Let’s go mucosal: Communication on slippery ground. Trends Immunol. 2004, 25, 570–577. [Google Scholar] [CrossRef]
- Dunne, P.J.; Moran, B.; Cummins, R.C.; Mills, K.H.G. CD11c+CD8alpha+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J. Immunol. 2009, 183, 400–410. [Google Scholar] [CrossRef]
- Kiyono, H.; Fukuyama, S. NALT- versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Farstad, I.N.; Haraldsen, G. Regional specialization in the mucosal immune system: Primed cells do not always home along the same track. Immunol. Today 1999, 20, 267–277. [Google Scholar] [CrossRef]
- McGhee, J.R.; Fujihashi, K. Inside the Mucosal Immune System. PLOS Biol. 2012, 10, e1001397. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Diniz, M.O.; Roussel, H.; Tran, T.; Ferreira, L.C.; Badoual, C.; Tartour, E. Mucosal vaccines: Novel strategies and applications for the control of pathogens and tumors at mucosal sites. Hum. Vaccin. Immunother. 2014, 10, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Potential of Nasopharynx-associated Lymphoid Tissue for Vaccine Responses in the Airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef]
- Tan, G.; McKenna, P.M.; Koser, M.L.; McLinden, R.; Kim, J.H.; McGettigan, J.P.; Schnell, M.J. Strong cellular and humoral anti-HIV Env immune responses induced by a heterologous rhabdoviral prime-boost approach. Virology 2005, 331, 82–93. [Google Scholar] [CrossRef]
- Woodrow, K.A.; Bennett, K.M.; Lo, D.D. Mucosal Vaccine Design and Delivery. Annu. Rev. Biomed. Eng. 2012, 14, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting Microbial Pathogens at Epithelial Borders. Immunol. Investig. 2010, 39, 383–406. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Price, G.E.; Soboleski, M.R.; Lo, C.-Y.; Misplon, J.A.; Quirion, M.R.; Houser, K.V.; Pearce, M.B.; Pappas, C.; Tumpey, T.M.; Epstein, S.L. Single-Dose Mucosal Immunization with a Candidate Universal Influenza Vaccine Provides Rapid Protection from Virulent H5N1, H3N2 and H1N1 Viruses. PLoS ONE 2010, 5, e13162. [Google Scholar] [CrossRef]
- Hemann, E.A.; Kang, S.-M.; Legge, K.L. Protective CD8 T Cell-Mediated Immunity against Influenza A Virus Infection following Influenza Virus-like Particle Vaccination. J. Immunol. 2013, 191, 2486–2494. [Google Scholar] [CrossRef]
- Stolley, J.M.; Johnston, T.S.; Soerens, A.G.; Beura, L.K.; Rosato, P.C.; Joag, V.; Wijeyesinghe, S.P.; Langlois, R.A.; Osum, K.C.; Mitchell, J.S.; et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 2020, 217, e20192197. [Google Scholar] [CrossRef]
- Son, Y.M.; Cheon, I.S.; Wu, Y.; Li, C.; Wang, Z.; Gao, X.; Chen, Y.; Takahashi, Y.; Fu, Y.X.; Dent, A.L.; et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol. 2021, 6, eabb6852. [Google Scholar] [CrossRef] [PubMed]
- Swarnalekha, N.; Schreiner, D.; Litzler, L.C.; Iftikhar, S.; Kirchmeier, D.; Künzli, M.; Son, Y.M.; Sun, J.; Moreira, E.A.; King, C.G. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021, 6, eabb6808. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Curran, M.P. Live attenuated influenza vaccine (FluMist®; Fluenz™): A review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.; Luke, C.; Coelingh, K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influ. Other Respir. Viruses 2008, 2, 193–202. [Google Scholar] [CrossRef]
- Turner, P.J.; Abdulla, A.F.; Cole, M.E.; Javan, R.R.; Gould, V.; O’Driscoll, M.E.; Southern, J.; Zambon, M.; Miller, E.; Andrews, N.J.; et al. Differences in nasal immunoglobulin A responses to influenza vaccine strains after live attenuated influenza vaccine (LAIV) immunization in children. Clin. Exp. Immunol. 2020, 199, 109–118. [Google Scholar] [CrossRef]
- Maassab, H.F.; Bryant, M.L. The development of live attenuated cold-adapted influenza virus vaccine for humans. Rev. Med. Virol. 1999, 9, 237–244. [Google Scholar] [CrossRef]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. 2017, 24, e00414-16. [Google Scholar] [CrossRef]
- Lartey, S.; Zhou, F.; Brokstad, K.A.; Mohn, K.G.-I.; Slettevoll, S.A.; Pathirana, R.D.; Cox, R.J. Live-Attenuated Influenza Vaccine Induces Tonsillar Follicular T Helper Cell Responses That Correlate With Antibody Induction. J. Infect. Dis. 2020, 221, 21–32. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Richert, L.; Al-Tawil, N.; Storsaeter, J.; Colin, C.; Bauduin, C.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N.; et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: A phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect. Dis. 2020, 20, 1290–1301. [Google Scholar] [CrossRef]
- Lin, A.; Apostolovic, D.; Jahnmatz, M.; Liang, F.; Ols, S.; Tecleab, T.; Wu, C.; Van Hage, M.; Solovay, K.; Rubin, K.; et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J. Clin. Investig. 2020, 130, 2332–2346. [Google Scholar] [CrossRef]
- Bull, N.; Stylianou, E.; Kaveh, D.A.; Pinpathomrat, N.; Pasricha, J.; Harrington-Kandt, R.; Garcia-Pelayo, M.C.; Hogarth, P.J.; McShane, H. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+ KLRG1− CD4+ T cells. Mucosal Immunol. 2019, 12, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Haller, A.A.; Mitiku, M.; MacPhail, M. Bovine parainfluenza virus type 3 (PIV3) expressing the respiratory syncytial virus (RSV) attachment and fusion proteins protects hamsters from challenge with human PIV3 and RSV. J. Gen. Virol. 2003, 84, 2153–2162. [Google Scholar] [CrossRef]
- Garg, R.; Brownlie, R.; Latimer, L.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. A chimeric glycoprotein formulated with a combination adjuvant induces protective immunity against both human respiratory syncytial virus and parainfluenza virus type 3. Antivir. Res. 2018, 158, 78–87. [Google Scholar] [CrossRef]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Eiting, K.; Cui, K.; Leonard, A.K.; Morris, D.; Li, C.-Y.; Farber, K.; Sileno, A.P.; Houston, M.E.; Johnson, P.H.; et al. Therapeutic utility of a novel tight junction modulating peptide for enhancing intranasal drug delivery. J. Pharm. Sci. 2006, 95, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Y.; Gungor, S.; Cevher, E. Nasal Delivery of High Molecular Weight Drugs. Molecules 2009, 14, 3754–3779. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017, 8, 151–167. [Google Scholar] [CrossRef]
- Napolioni, V.; MacMurray, J. Infectious diseases, IL6 −174G > C polymorphism, and human development. Brain Behav. Immun. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A.; Crommelin, D.J.; Jiskoot, W. N-Trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: Biological properties and immunogenicity in a mouse model. Vaccine 2007, 25, 144–153. [Google Scholar] [CrossRef]
- Gilmore, J.L.; Yi, X.; Quan, L.; Kabanov, A.V. Novel Nanomaterials for Clinical Neuroscience. J. Neuroimmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef]
- Garmise, R.J.; Staats, H.; Hickey, A.J. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech 2007, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcão, A. Intranasal delivery of systemic-acting drugs: Small-molecules and biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef]
- de Haan, A.; Geerligs, H.; Huchshorn, J.; van Scharrenburg, G.; Palache, A.; Wilschut, J. Mucosal immunoadjuvant activity of liposomes: Induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine 1995, 13, 155–162. [Google Scholar] [CrossRef]
- Lobaina, Y.; Urquiza, D.; Garay, H.; Perera, Y.; Yang, K. Evaluation of Cell-Penetrating Peptides as Mucosal Immune Enhancers for Nasal Vaccination. Int. J. Pept. Res. Ther. 2021, 27, 2873–2882. [Google Scholar] [CrossRef]
- Mielcarek, N.; Debrie, A.-S.; Raze, D.; Bertout, J.; Rouanet, C.; Ben Younes, A.; Creusy, C.; Engle, J.; Goldman, E.W.; Locht, C. Live Attenuated B. pertussis as a Single-Dose Nasal Vaccine against Whooping Cough. PLOS Pathog. 2006, 2, e65. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef]
- Newly described cell type may prove valuable in development of plague vaccines against biowarfare. Expert Rev. Vaccines 2011, 10, 259.
- Hiremath, G.S.; Omer, S.B. A Meta-Analysis of Studies Comparing the Respiratory Route with the Subcutaneous Route of Measles Vaccine Administration. Hum. Vaccines 2005, 1, 30–36. [Google Scholar] [CrossRef]
- Weniger, B.G.; Papania, M.J. Alternative vaccine delivery methods. Vaccines 2012, 1357–1392. [Google Scholar] [CrossRef]
- Demirel, T.; Orhan, K.S.; Keleş, N.; Değer, K. Comparison of the efficacy of nasal drop and nasal spray applications of fluticasone propionate in nasal polyps. Kulak Burun Bogaz Ihtis. Derg. Kbb = J. Ear Nose Throat 2008, 18, 1–6. [Google Scholar]
- Primosch, R.E.; Guelmann, M. Comparison of drops versus spray administration of intranasal midazolam in two- and three-year-old children for dental sedation. Pediatr. Dent. 2005, 27, 401–408. [Google Scholar]
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Low, N.; Kraemer, S.; Schneider, M.; Restrepo, A.M.H. Immunogenicity and safety of aerosolized measles vaccine: Systematic review and meta-analysis. Vaccine 2008, 26, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Dilraj, A.; Sukhoo, R.; Cutts, F.T.; Bennett, J.V. Aerosol and subcutaneous measles vaccine: Measles antibody responses 6 years after re-vaccination. Vaccine 2007, 25, 4170–4174. [Google Scholar] [CrossRef]
- Bennett, J.V.; De Castro, J.F.; Valdespino-Gomez, J.L.; Garcia-Garcia, M.D.L.; Islas-Romero, R.; Echaniz-Aviles, G.; Jimenez-Corona, A.; Sepulveda-Amor, J. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: Randomized trials in Mexican schoolchildren. Bull. World Health Organ. 2002, 80, 806–812. [Google Scholar] [PubMed]
- de Castro, J.F.; Bennett, J.V.; Rincon, H.G.; Munoz, M.A.Y.; Sanchez, L.A.E.P.; Santos, J.I. Evaluation of immunogenicity and side effects of triple viral vaccine (MMR) in adults, given by two routes: Subcutaneous and respiratory (aerosol). Vaccine 2005, 23, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H.; Griffin, D.E.; Rota, P.A.; Papania, M.; Cape, S.P.; Bennett, D.; Quinn, B.; Sievers, R.E.; Shermer, C.; Powell, K.; et al. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc. Natl. Acad. Sci. USA 2011, 108, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Agarkhedkar, S.; Kulkarni, P.S.; Winston, S.; Sievers, R.; Dhere, R.M.; Gunale, B.; Powell, K.; Rota, P.A.; Papania, M. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase I clinical trial. Vaccine 2014, 32, 6791–6797. [Google Scholar] [CrossRef]
- Patil, H.P.; Murugappan, S.; De Vries-Idema, J.; Meijerhof, T.; De Haan, A.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.; Huckriede, A. Comparison of adjuvants for a spray freeze-dried whole inactivated virus influenza vaccine for pulmonary administration. Eur. J. Pharm. Biopharm. 2015, 93, 231–241. [Google Scholar] [CrossRef]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef]
- Yuki, Y.; Nochi, T.; Kong, I.G.; Takahashi, H.; Sawada, S.-I.; Akiyoshi, K.; Kiyono, H. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 2013, 29, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Yuki, Y.; Katakai, Y.; Harada, N.; Takahashi, H.; Takeda, S.; Mejima, M.; Joo, S.; Kurokawa, S.; Sawada, S.; et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 2015, 8, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Uchida, Y.; Yuki, Y.; Katakai, Y.; Yamanoue, T.; Ogawa, H.; Munesue, Y.; Nakano, N.; Hanari, K.; Miyazaki, T.; et al. A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci. Vaccine 2021, 39, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, O.; Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Rochlin, E.; Eliyahu, H.; Goldwaser, I.; Balasingam, S.; Mann, A.J.; Lambkin-Williams, R.; et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine 2011, 29, 2474–2486. [Google Scholar] [CrossRef]
- Salunke, S.R.; Patil, S.B. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef]
- Bedford, J.G.; Caminschi, I.; Wakim, L.M. Intranasal Delivery of a Chitosan-Hydrogel Vaccine Generates Nasal Tissue Resident Memory CD8+ T Cells That Are Protective against Influenza Virus Infection. Vaccines 2020, 8, 572. [Google Scholar] [CrossRef]
- Pastor, Y.; Ting, I.; Martínez, A.L.; Irache, J.M.; Gamazo, C. Intranasal delivery system of bacterial antigen using thermosensitive hydrogels based on a Pluronic-Gantrez conjugate. Int. J. Pharm. 2020, 579, 119154. [Google Scholar] [CrossRef]
- Kavanagh, H.; Noone, C.; Cahill, E.; English, K.; Locht, C.; Mahon, B.P. Attenuated Bordetella pertussis vaccine strain BPZE1 modulates allergen-induced immunity and prevents allergic pulmonary pathology in a murine model. Clin. Exp. Allergy 2010, 40, 933–941. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Mateo, J.S.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Safety and Immunogenicity of the Respiratory Syncytial Virus Vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-Seronegative Children. J. Infect. Dis. 2020, 222, 82–91. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Song, Y.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102775118. [Google Scholar] [CrossRef]
- Feunou, P.F.; Kammoun, H.; Debrie, A.-S.; Mielcarek, N.; Locht, C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine 2010, 28, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Feunou, P.F.; Kammoun, H.; Debrie, A.-S.; Locht, C. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine 2014, 32, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Jahnmatz, M.; Amu, S.; Ljungman, M.; Wehlin, L.; Chiodi, F.; Mielcarek, N.; Locht, C.; Thorstensson, R. B-cell responses after intranasal vaccination with the novel attenuated Bordetella pertussis vaccine strain BPZE1 in a randomized phase I clinical trial. Vaccine 2014, 32, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Thorstensson, R.; Trollfors, B.; Al-Tawil, N.; Jahnmatz, M.; Bergström, J.; Ljungman, M.; Törner, A.; Wehlin, L.; Van Broekhoven, A.; Bosman, F.; et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE 2014, 9, e83449. [Google Scholar] [CrossRef]
- Debrie, A.-S.; Coutte, L.; Raze, D.; Mooi, F.; Alexander, F.; Gorringe, A.; Mielcarek, N.; Locht, C. Construction and evaluation of Bordetella pertussis live attenuated vaccine strain BPZE1 producing Fim3. Vaccine 2018, 36, 1345–1352. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Brekke, K.; Lind, A.; Holm-Hansen, C.; Haugen, I.L.; Sørensen, B.; Sommerfelt, M.; Kvale, D. Intranasal Administration of a Therapeutic HIV Vaccine (Vacc-4x) Induces Dose-Dependent Systemic and Mucosal Immune Responses in a Randomized Controlled Trial. PLoS ONE 2014, 9, e112556. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Van Ginkel, F.W.; Jackson, R.J.; Yuki, Y.; McGhee, J.R. Cutting Edge: The Mucosal Adjuvant Cholera Toxin Redirects Vaccine Proteins into Olfactory Tissues. J. Immunol. 2000, 165, 4778–4782. [Google Scholar] [CrossRef]
- Kurono, Y. The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines. Auris Nasus Larynx 2021, 49, 1–10. [Google Scholar] [CrossRef]
- Kraan, H.; Soema, P.; Amorij, J.-P.; Kersten, G. Intranasal and sublingual delivery of inactivated polio vaccine. Vaccine 2017, 35, 2647–2653. [Google Scholar] [CrossRef]
- Valli, E.; Harriett, A.J.; Nowakowska, M.K.; Baudier, R.; Provosty, W.B.; McSween, Z.; Lawson, L.; Nakanishi, Y.; Norton, E.B. LTA1 is a safe, intranasal enterotoxin-based adjuvant that improves vaccine protection against influenza in young, old and B-cell-depleted (μMT) mice. Sci. Rep. 2019, 9, 15128. [Google Scholar] [CrossRef]
- Pan, S.C.; Hsu, W.T.; Lee, W.S.; Wang, N.C.; Chen, T.J.; Liu, M.C.; Pai, H.C.; Hsu, Y.S.; Chang, M.; Hsieh, S.M. A Double-blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh(αK): A Phase II study. Vaccine 2020, 38, 1048–1056. [Google Scholar] [CrossRef]
- Pan, S.C.; Hsieh, S.M.; Lin, C.F.; Hsu, Y.S.; Chang, M.; Chang, S.C. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): A Phase I study. Vaccine 2019, 37, 1994–2003. [Google Scholar] [CrossRef]
- Bernasconi, V.; Norling, K.; Gribonika, I.; Ong, L.C.; Burazerovic, S.; Parveen, N.; Schön, K.; Stensson, A.; Bally, M.; Larson, G.; et al. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 2021, 14, 523–536. [Google Scholar] [CrossRef]
- Li, H.; Ren, H.; Zhang, Y.; Cao, L.; Xu, W. Intranasal vaccination with a recombinant protein CTA1-DD-RBF protects mice against hRSV infection. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Erume, J.; Partidos, C.D. Evaluation of the LTK63 adjuvant effect on cellular immune responses to measles virus nucleoprotein. Afr. Health Sci. 2011, 11, 151–157. [Google Scholar]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef]
- Williams, C.M.; Roy, S.; Califano, D.; McKenzie, A.N.J.; Metzger, D.W.; Furuya, Y. The Interleukin-33–Group 2 Innate Lymphoid Cell Axis Represents a Potential Adjuvant Target To Increase the Cross-Protective Efficacy of Influenza Vaccine. J. Virol. 2021, 95, e0059821. [Google Scholar] [CrossRef]
- Maeto, C.; Rodríguez, A.M.; Holgado, M.P.; Falivene, J.; Gherardi, M.M. Novel Mucosal DNA-MVA HIV Vaccination in Which DNA-IL-12 Plus Cholera Toxin B Subunit (CTB) Cooperates to Enhance Cellular Systemic and Mucosal Genital Tract Immunity. PLoS ONE 2014, 9, e107524. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Zhang, R.; Prabhu, S.K.; Andersen, H.; Venzon, D.; Cook, A.; Brown, R.; Teow, E.; Velasco, J.; et al. Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight 2021, 6, e148494. [Google Scholar] [CrossRef]
- Wang, X.; Meng, D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell 2015, 6, 170–184. [Google Scholar] [CrossRef]
- Hou, B.; Reizis, B.; DeFranco, A.L. Toll-like Receptors Activate Innate and Adaptive Immunity by using Dendritic Cell-Intrinsic and -Extrinsic Mechanisms. Immunity 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid a modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Xu, H.; Alzhrani, R.F.; Warnken, Z.N.; Thakkar, S.G.; Zeng, M.; Smyth, H.D.C.; Iii, R.O.W.; Cui, Z. Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration. Mol. Pharm. 2020, 17, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Bian, L.; Zhao, H.; Liu, Y.; Lu, J.; Liu, D.; Zhang, K.; Song, Y.; Luo, Y.; Jiang, C.; et al. Respiratory Syncytial Virus F Subunit Vaccine With AS02 Adjuvant Elicits Balanced, Robust Humoral and Cellular Immunity in BALB/c Mice. Front. Immunol. 2020, 11, 526965. [Google Scholar] [CrossRef]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Habibi, M.; Karam, M.R.A.; Shokrgozar, M.A.; Oloomi, M.; Jafari, A.; Bouzari, S. Intranasal immunization with fusion protein MrpH·FimH and MPL adjuvant confers protection against urinary tract infections caused by uropathogenic Escherichia coli and Proteus mirabilis. Mol. Immunol. 2015, 64, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Hamaoka, S.; Kinoshita, M.; Kainuma, A.; Shimizu, M.; Katoh, H.; Moriyama, K.; Ishii, K.J.; Sawa, T. The protective effects of nasal PcrV-CpG oligonucleotide vaccination against Pseudomonas aeruginosa pneumonia. Microbiol. Immunol. 2018, 62, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Hinkula, J.; Nyström, S.; Devito, C.; Bråve, A.; Applequist, S.E. Long-Lasting Mucosal and Systemic Immunity against Influenza A Virus Is Significantly Prolonged and Protective by Nasal Whole Influenza Immunization with Mucosal Adjuvant N3 and DNA-Plasmid Expressing Flagellin in Aging In- and Outbred Mice. Vaccines 2019, 7, 64. [Google Scholar] [CrossRef]
- Renu, S.; Feliciano-Ruiz, N.; Lu, F.; Ghimire, S.; Han, Y.; Schrock, J.; Dhakal, S.; Patil, V.; Krakowka, S.; HogenEsch, H.; et al. A Nanoparticle-Poly(I:C) Combination Adjuvant Enhances the Breadth of the Immune Response to Inactivated Influenza Virus Vaccine in Pigs. Vaccines 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, L.S.; Hjelm, B.E.; Arntzen, C.J.; Herbst-Kralovetz, M.M. An Intranasally Delivered Toll-Like Receptor 7 Agonist Elicits Robust Systemic and Mucosal Responses to Norwalk Virus-Like Particles. Clin. Vaccine Immunol. 2010, 17, 1850–1858. [Google Scholar] [CrossRef]
- Hjelm, B.E.; Kilbourne, J.; Herbst-Kralovetz, M.M. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum. Vaccines Immunother. 2014, 10, 410–416. [Google Scholar] [CrossRef]
- Van Dis, E.S.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef]
- Matos, M.N.; Cazorla, S.I.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Malchiodi, E. Immunization with Tc52 or its amino terminal domain adjuvanted with c-di-AMP induces Th17+Th1 specific immune responses and confers protection against Trypanosoma cruzi. PLOS Neglected Trop. Dis. 2017, 11, e0005300. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Su, Y.; Cai, L.; Li, J.; Liang, J.; Gu, Q.; Sun, M.; Shi, L. Intranasal Immunization With a c-di-GMP-Adjuvanted Acellular Pertussis Vaccine Provides Superior Immunity Against Bordetella pertussis in a Mouse Model. Front. Immunol. 2022, 13, 878832. [Google Scholar] [CrossRef] [PubMed]
- Hosny, E.A.; Elkheshen, S.A.; Saleh, S.I. Buccoadhesive tablets for insulin delivery: In-vitro and in-vivo studies. Boll. Chim. Farm. 2002, 141, 210–217. [Google Scholar] [PubMed]
- Shim, S.; Yoo, H. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Marine drugs 2020, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Moran, H.B.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Westerink, M.J.; Smithson, S.L.; Srivastava, N.; Blonder, J.; Coeshott, C.; Rosenthal, G.J. ProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine 2001, 20, 711–723. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Hajam, I.A.; Senevirathne, A.; Hewawaduge, C.; Kim, J.; Lee, J.H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Kumar, U.S.; Afjei, R.; Ferrara, K.; Massoud, T.F.; Paulmurugan, R. Gold-Nanostar-Chitosan-Mediated Delivery of SARS-CoV-2 DNA Vaccine for Respiratory Mucosal Immunization: Development and Proof-of-Principle. ACS Nano 2021, 15, 17582–17601. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef]
- Manocha, M.; Pal, P.C.; Chitralekha, K.; Thomas, B.E.; Tripathi, V.; Gupta, S.D.; Paranjape, R.; Kulkarni, S.; Rao, D.N. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine 2005, 23, 5599–5617. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccines Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.-L.; Rejman, J.; Demeester, J.; Irvine, D.J.; Gander, B.; De Smedt, S.C. Particulate vaccines: On the quest for optimal delivery and immune response. Drug Discov. Today 2011, 16, 569–582. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Ramos, M.; Tak, P.; Lebre, M. Fms-like tyrosine kinase 3 ligand-dependent dendritic cells in autoimmune inflammation. Autoimmun. Rev. 2014, 13, 117–124. [Google Scholar] [CrossRef]
- Kataoka, K.; McGhee, J.R.; Kobayashi, R.; Fujihashi, K.; Shizukuishi, S.; Fujihashi, K. Nasal Flt3 ligand cDNA elicits CD11c+CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 2004, 172, 3612–3619. [Google Scholar] [CrossRef]
- Kataoka, K.; Kawabata, S.; Koyanagi, K.; Hashimoto, Y.; Miyake, T.; Fujihashi, K. Respiratory FimA-Specific Secretory IgA Antibodies Upregulated by DC-Targeting Nasal Double DNA Adjuvant Are Essential for Elimination of Porphyromonas gingivalis. Front. Immunol. 2021, 12, 634923. [Google Scholar] [CrossRef]
- Borg, N.A.; Wun, K.S.; Kjer-Nielsen, L.; Wilce, M.C.J.; Pellicci, D.G.; Koh, R.; Besra, G.S.; Bharadwaj, M.; Godfrey, D.I.; McCluskey, J.; et al. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 2007, 448, 44–49. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, K.A.; Lee, J.Y.; Kang, M.H.; Song, Y.C.; Baek, D.J.; Kim, S.; Kang, C.Y. An α-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 2011, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Sun, R.; Song, G.; Zhu, S.; Nie, Z.; Lin, L.; Yi, R.; Wu, S.; Wang, G.; He, Y.; et al. A Glycolipid α-GalCer Derivative, 7DW8-5 as a Novel Mucosal Adjuvant for the Split Inactivated Influenza Vaccine. Viruses 2022, 14, 1174. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Weaver, B.T.; Choi, H.W.; Yang, H.; Granek, J.A.; Chan, C.; Abraham, S.N.; Staats, H.F. Nasal Immunization With Small Molecule Mast Cell Activators Enhance Immunity to Co-Administered Subunit Immunogens. Front. Immunol. 2021, 12, 730346. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Wang, H.; Liao, P.; Song, Z.; Gao, S.; Wu, Y.; Zhang, X.; Yin, Y.; Xu, W. Compound 48/80 acts as a potent mucosal adjuvant for vaccination against Streptococcus pneumoniae infection in young mice. Vaccine 2015, 33, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Chandrudu, S.; Giddam, A.K.; Reiman, J.; Skwarczynski, M.; McPhun, V.; Moyle, P.M.; Batzloff, M.R.; Good, M.F.; Toth, I. Group A Streptococcal vaccine candidate: Contribution of epitope to size, antigen presenting cell interaction and immunogenicity. Nanomedicine 2014, 9, 2613–2624. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Ohtsuka, J.; Imai, M.; Fukumura, M.; Maeda, M.; Eguchi, A.; Ono, R.; Maemura, T.; Ito, M.; Yamayoshi, S.; Kataoka, Y.; et al. Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. Iscience 2021, 24, 103379. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccine Nasal Spray. JAMA 2021, 326, 1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, B.; Ngwuta, J.; Liu, X.; Surman, S.; Lingemann, M.; Kwong, P.D.; Graham, B.S.; Collins, P.L.; Munir, S. Attenuated Human Parainfluenza Virus Type 1 Expressing the Respiratory Syncytial Virus (RSV) Fusion (F) Glycoprotein from an Added Gene: Effects of Prefusion Stabilization and Packaging of RSV F. J. Virol. 2017, 91, e01101-17–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, B.; Liu, X.; Amaro-Carambot, E.; Surman, S.; Kwong, P.D.; Graham, B.S.; Collins, P.L.; Munir, S. Human parainfluenza virus type 3 expressing the respiratory syncytial virus pre-fusion F protein modified for virion packaging yields protective intranasal vaccine candidates. PLoS ONE 2020, 15, e0228572. [Google Scholar] [CrossRef] [PubMed]
- Koonpaew, S.; Kaewborisuth, C.; Srisutthisamphan, K.; Wanitchang, A.; Thaweerattanasinp, T.; Saenboonrueng, J.; Poonsuk, S.; Jengarn, J.; Viriyakitkosol, R.; Kramyu, J.; et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines 2021, 9, 850. [Google Scholar] [CrossRef]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-N.; Kim, K.-H.; Lee, Y.; Jeeva, S.; Park, B.R.; Kang, S.-M. Recombinant Live Attenuated Influenza Virus Expressing Conserved G-Protein Domain in a Chimeric Hemagglutinin Molecule Induces G-Specific Antibodies and Confers Protection against Respiratory Syncytial Virus. Vaccines 2020, 8, 716. [Google Scholar] [CrossRef]
- Matyushenko, V.; Kotomina, T.; Kudryavtsev, I.; Mezhenskaya, D.; Prokopenko, P.; Matushkina, A.; Sivak, K.; Muzhikyan, A.; Rudenko, L.; Isakova-Sivak, I. Conserved T-cell epitopes of respiratory syncytial virus (RSV) delivered by recombinant live attenuated influenza vaccine viruses efficiently induce RSV-specific lung-localized memory T cells and augment influenza-specific resident memory T-cell responses. Antivir. Res. 2020, 182, 104864. [Google Scholar] [CrossRef]
- Wang, J.; Shu, T.; Deng, W.; Zheng, Y.; Liao, M.; Ye, X.; Han, L.; He, P.; Zheng, X.; Li, T.; et al. Mucosal Priming with a Recombinant Influenza A Virus-Vectored Vaccine Elicits T-Cell and Antibody Responses to HIV-1 in Mice. J. Virol. 2021, 95, e00059-21–21. [Google Scholar] [CrossRef]
- Fragoso-Saavedra, M.; Vega-López, M.A. Induction of mucosal immunity against pathogens by using recombinant baculoviral vectors: Mechanisms, advantages, and limitations. J. Leukoc. Biol. 2020, 108, 835–850. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Richardson, J.S.; Pillet, S.; Bello, A.J.; Kobinger, G.P. Airway Delivery of an Adenovirus-Based Ebola Virus Vaccine Bypasses Existing Immunity to Homologous Adenovirus in Nonhuman Primates. J. Virol. 2013, 87, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Patel, A.; Tran, K.N.; Gray, M.; Zhang, Y.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Nasal Delivery of an Adenovirus-Based Vaccine Bypasses Pre-Existing Immunity to the Vaccine Carrier and Improves the Immune Response in Mice. PLoS ONE 2008, 3, e3548. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Livermore, D.M.; Ornelles, D.A.; Bayer, W.; Marques, E.; Czerkinsky, C.; Dolzhikova, I.V.; Ertl, H.C. COVID-19 vaccination and HIV-1 acquisition. Lancet 2022, 399, e34–e35. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, Phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Kotelkin, A.; Yang, L.; Elankumaran, S.; Murphy, B.R.; Samal, S.K.; Collins, P.L.; Bukreyev, A. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. USA 2007, 104, 9788–9793. [Google Scholar] [CrossRef]
- Díaz, M.F.; Calderón, K.; Rojas-Neyra, A.; Vakharia, V.N.; Choque-Guevara, R.; Montalvan-Avalos, A.; Poma-Acevedo, A.; Rios-Matos, D.; Agurto-Arteaga, A.; Cauti-Mendoza, M.G.; et al. Intranasal vaccination of hamsters with a Newcastle disease virus vector expressing the S1 subunit protects animals against SARS-CoV-2 disease. Sci. Rep. 2022, 12, 10359. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Amanat, F.; González-Domínguez, I.; McCroskery, S.; Slamanig, S.; Coughlan, L.; Rosado, V.; Lemus, N.; Jangra, S.; et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 2021, 12, 6197. [Google Scholar] [CrossRef]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H., III; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. Ebiomedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Park, H.S.; Matsuoka, Y.; Luongo, C.; Yang, L.; Santos, C.; Liu, X.; Ahlers, L.R.; Moore, I.N.; Afroz, S.; Johnson, R.F.; et al. Intranasal immunization with avian paramyxovirus type 3 expressing SARS-CoV-2 spike protein protects hamsters against SARS-CoV-2. NPJ Vaccines 2022, 7, 72. [Google Scholar] [CrossRef]
- Durrani, Z.; McInerney, T.L.; McLain, L.; Jones, T.; Bellaby, T.; Brennan, F.R.; Dimmock, N.J. Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J. Immunol. Methods 1998, 220, 93–103. [Google Scholar] [CrossRef]
- Bernasconi, V.; Norling, K.; Bally, M.; Höök, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Ali, A.A.; Orr, N.; Tunney, M.M.; McCarthy, H.O.; Kett, V.L. Novel freeze-dried DDA and TPGS liposomes are suitable for nasal delivery of vaccine. Int. J. Pharm. 2017, 533, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal Immunization with DOTAP Cationic Liposomes Combined with DC-Cholesterol Induces Potent Antigen-Specific Mucosal and Systemic Immune Responses in Mice. PLoS ONE 2015, 10, e0139785. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of class B CpG ODN onto DOTAP/DC-chol liposome in nasal vaccine formulations augments antigen-specific immune responses in mice. BMC Res. Notes 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef]
- Christensen, D.; Foged, C.; Rosenkrands, I.; Lundberg, C.V.; Andersen, P.; Agger, E.M.; Nielsen, H.M. CAF01 liposomes as a mucosal vaccine adjuvant: In vitro and in vivo investigations. Int. J. Pharm. 2010, 390, 19–24. [Google Scholar] [CrossRef]
- Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Flasterstein, O.; Eliyahu, H.; Simberg, D.; Goldwaser, I.; Barenholz, Y.; Kedar, E. A new intranasal influenza vaccine based on a novel polycationic lipid—Ceramide carbamoyl-spermine (CCS) I. immunogenicity and efficacy studies in mice. Vaccine 2006, 24, 3990–4006. [Google Scholar] [CrossRef]
- Even-Or, O.; Avniel-Polak, S.; Barenholz, Y.; Nussbaum, G. The cationic liposome CCS/C adjuvant induces immunity to influenza independently of the adaptor protein MyD88. Hum. Vaccines Immunother. 2020, 16, 3146–3154. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhang, M.; Chen, R.; Niu, R.; Deng, Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur. J. Pharm. Biopharm. 2014, 88, 194–206. [Google Scholar] [CrossRef]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Peng, K.; Wang, Y.; Gong, T.; Zhang, Z.; He, Q.; Sun, X. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017, 64, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.A.; Paul, S.; Dellosa, G.K.Y.; Jaraquemada, D.; Bello, M.B. Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. npj Vaccines 2022, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Jandus, C.; Maurer, P.; Hammann-Haenni, A.; Schwarz, K.; Bachmann, M.F.; Speiser, D.; Romero, P. Virus-like particles induce robust human T-helper cell responses. Eur. J. Immunol. 2012, 42, 330–340. [Google Scholar] [CrossRef]
- Zabel, F.; Mohanan, D.; Bessa, J.; Link, A.; Fettelschoss-Gabriel, A.; Saudan, P.; Kündig, T.M.; Bachmann, M.F. Viral Particles Drive Rapid Differentiation of Memory B Cells into Secondary Plasma Cells Producing Increased Levels of Antibodies. J. Immunol. 2014, 192, 5499–5508. [Google Scholar] [CrossRef]
- Jain, N.K.; Sahni, N.; Kumru, O.S.; Joshi, S.B.; Volkin, D.B.; Middaugh, C.R. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv. Drug Deliv. Rev. 2015, 93, 42–55. [Google Scholar] [CrossRef]
- Atmar, R.L.; Bernstein, D.I.; Harro, C.D.; Al-Ibrahim, M.S.; Chen, W.H.; Ferreira, J.; Estes, M.K.; Graham, D.Y.; Opekun, A.R.; Richardson, C.; et al. Norovirus Vaccine against Experimental Human Norwalk Virus Illness. N. Engl. J. Med. 2011, 365, 2178–2187. [Google Scholar] [CrossRef]
- Herbst-Kralovetz, M.; Mason, H.S.; Chen, Q. Norwalk virus-like particles as vaccines. Expert Rev. Vaccines 2010, 9, 299–307. [Google Scholar] [CrossRef]
- Kaneda, Y. Virosome: A novel vector to enable multi-modal strategies for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 730–738. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; Clement, F.; Van Engelenburg, F.; Dobbelsteen, M.V.D.; Adler, M.; Amacker, M.; Lopalco, L.; Bomsel, M.; Chalifour, A.; et al. Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes. PLoS ONE 2013, 8, e55438. [Google Scholar] [CrossRef]
- Lambkin, R.; Oxford, J.S.; Bossuyt, S.; Mann, A.; Metcalfe, I.C.; Herzog, C.; Viret, J.-F.; Glück, R. Strong local and systemic protective immunity induced in the ferret model by an intranasal virosome-formulated influenza subunit vaccine. Vaccine 2004, 22, 4390–4396. [Google Scholar] [CrossRef] [PubMed]
- Durrer, P.; Glück, U.; Spyr, C.; Lang, A.B.; Zurbriggen, R.; Herzog, C.; Glück, R. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine 2003, 21, 4328–4334. [Google Scholar] [CrossRef]
- Fernandes, B.; Castro, R.; Bhoelan, F.; Bemelman, D.; Gomes, R.A.; Costa, J.; Gomes-Alves, P.; Stegmann, T.; Amacker, M.; Alves, P.M.; et al. Insect Cells for High-Yield Production of SARS-CoV-2 Spike Protein: Building a Virosome-Based COVID-19 Vaccine Candidate. Pharmaceutics 2022, 14, 854. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.; Harris, R.; Davis, R.; Drane, D.; Cox, J.; Ryan, D.; Sutton, P.; Rockman, S.; Pearse, M. Intranasal vaccination with ISCOMATRIX® adjuvanted influenza vaccine. Vaccine 2003, 21, 946–949. [Google Scholar] [CrossRef]
- Hu, K.-F.; Lövgren-Bengtsson, K.; Morein, B. Immunostimulating complexes (ISCOMs) for nasal vaccination. Adv. Drug Deliv. Rev. 2001, 51, 149–159. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.; Baars, M.; van Amerongen, G.; van Beek, R.; Osterhaus, A. A single dose of an ISCOM influenza vaccine induces long-lasting protective immunity against homologous challenge infection but fails to protect Cynomolgus macaques against distant drift variants of influenza A (H3N2) viruses. Vaccine 2001, 20, 158–163. [Google Scholar] [CrossRef]
- Rivera-Patron, M.; Moreno, M.; Baz, M.; Roehe, P.M.; Cibulski, S.P.; Silveira, F. ISCOM-like Nanoparticles Formulated with Quillaja brasiliensis Saponins Are Promising Adjuvants for Seasonal Influenza Vaccines. Vaccines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Eliasson, D.G.; Helgeby, A.; Schön, K.; Nygren, C.; El-Bakkouri, K.; Fiers, W.; Saelens, X.; Lövgren, K.B.; Nyström, I.; Lycke, N.Y. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine 2011, 29, 3951–3961. [Google Scholar] [CrossRef]
- Andersen, C.S.; Dietrich, J.; Agger, E.M.; Lycke, N.Y.; Lövgren, K.; Andersen, P. The Combined CTA1-DD/ISCOMs Vector Is an Effective Intranasal Adjuvant for Boosting Prior Mycobacterium bovis BCG Immunity to Mycobacterium tuberculosis. Infect. Immun. 2007, 75, 408–416. [Google Scholar] [CrossRef]
- Hu, K.-F.; Elvander, M.; Merza, M.; Åkerblom, L.; Brandenburg, A.; Morein, B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin. Exp. Immunol. 1998, 113, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.F.; Regner, M.; Siegrist, C.A.; Lambert, P.; Chen, M.; Bengtsson, K.L.; Morein, B. The immunomodulating properties of human respiratory syncytial virus and immunostimulating complexes containing Quillaja saponin components QH-A, QH-C and ISCOPREP703. FEMS Immunol. Med. Microbiol. 2005, 43, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Hirano, T.; Noda, K.; Umemoto, S.; Suzuki, M. Nasal immunization with plasmid DNA encoding P6 protein and immunostimulatory complexes elicits nontypeable Haemophilus influenzae-specific long-term mucosal immune responses in the nasopharynx. Vaccine 2011, 29, 1881–1890. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Janczak, K.W.; Landers, J.J.; Makidon, P.; Sower, L.E.; Peterson, J.W.; Baker, J.R. Mucosal Immunization with a Novel Nanoemulsion-Based Recombinant Anthrax Protective Antigen Vaccine Protects against Bacillus anthracis Spore Challenge. Infect. Immun. 2007, 75, 4020–4029. [Google Scholar] [CrossRef]
- Ahmed, M.; Smith, D.M.; Hamouda, T.; Rangel-Moreno, J.; Fattom, A.; Khader, S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 2017, 35, 4983–4989. [Google Scholar] [CrossRef]
- Das, S.C.; Hatta, M.; Wilker, P.R.; Myc, A.; Hamouda, T.; Neumann, G.; Baker, J.R.; Kawaoka, Y. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine 2012, 30, 6871–6877. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, C.-C.; Huang, M.-H.; Huang, C.-H.; Jan, J.-T.; Wu, S.-C. Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine. Vaccines 2020, 8, 240. [Google Scholar] [CrossRef]
- Hamouda, T.; Sutcliffe, J.A.; Ciotti, S.; Baker, J.R. Intranasal Immunization of Ferrets with Commercial Trivalent Influenza Vaccines Formulated in a Nanoemulsion-Based Adjuvant. Clin. Vaccine Immunol. 2011, 18, 1167–1175. [Google Scholar] [CrossRef]
- Schulze, K.; Ebensen, T.; Chandrudu, S.; Skwarczynski, M.; Toth, I.; Olive, C.; Guzman, C.A. Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).