The Interactions and Release Kinetics of Sodium Hyaluronate Implemented in Nonionic and Anionic Polymeric Hydrogels, Studied by Immunoenzymatic ELISA Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogels
2.3. Viscosity Test
2.4. Release Study of HA from Hydrogels
2.5. Mathematical Comparison Equations
2.6. FTIR Study
2.7. DSC Investigation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Viscosity Test
3.2. Kinetics of HA Release
3.3. The Difference Factor f1 and the Similarity Factor f2
3.4. Statistical Analysis
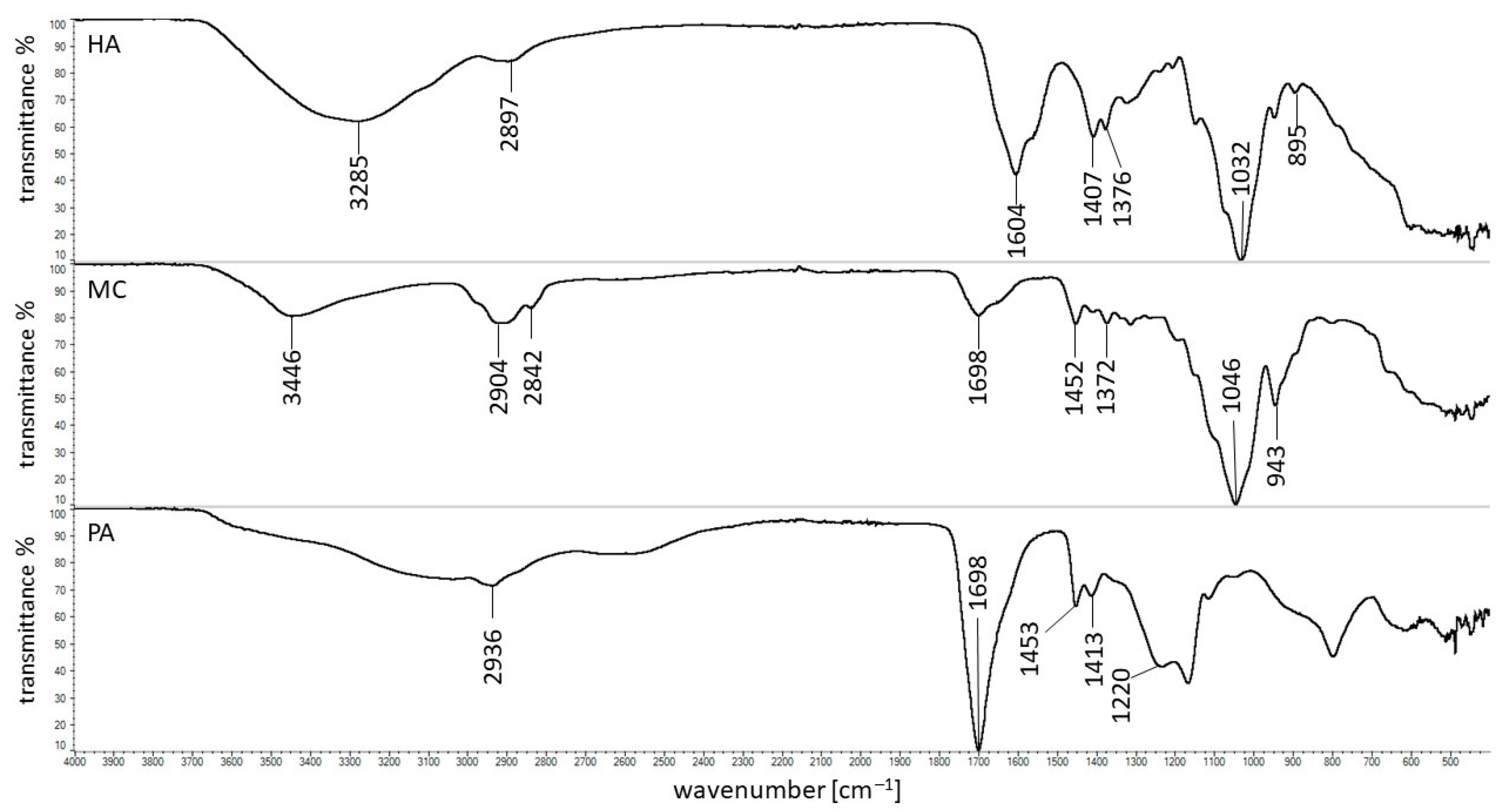
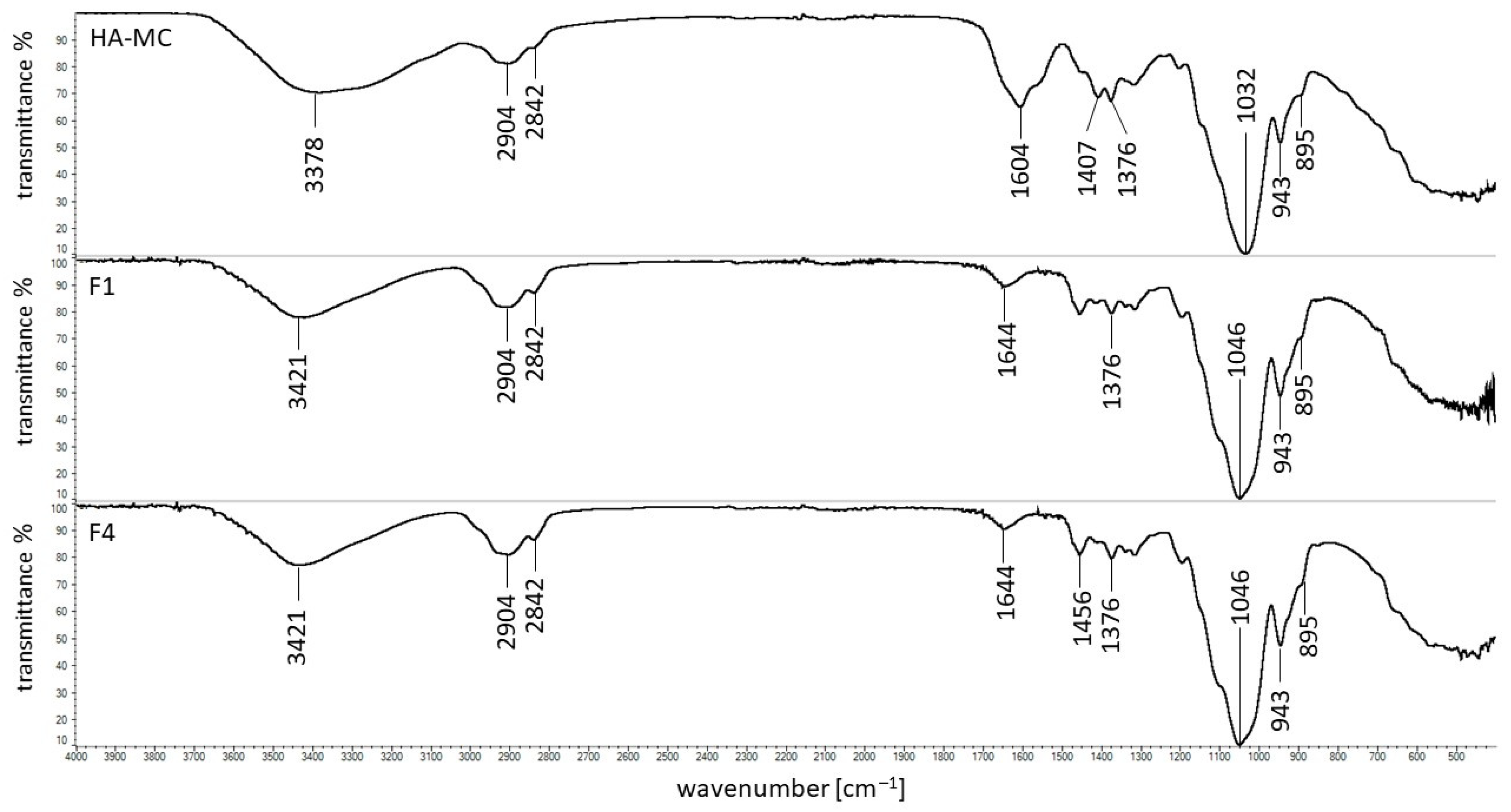
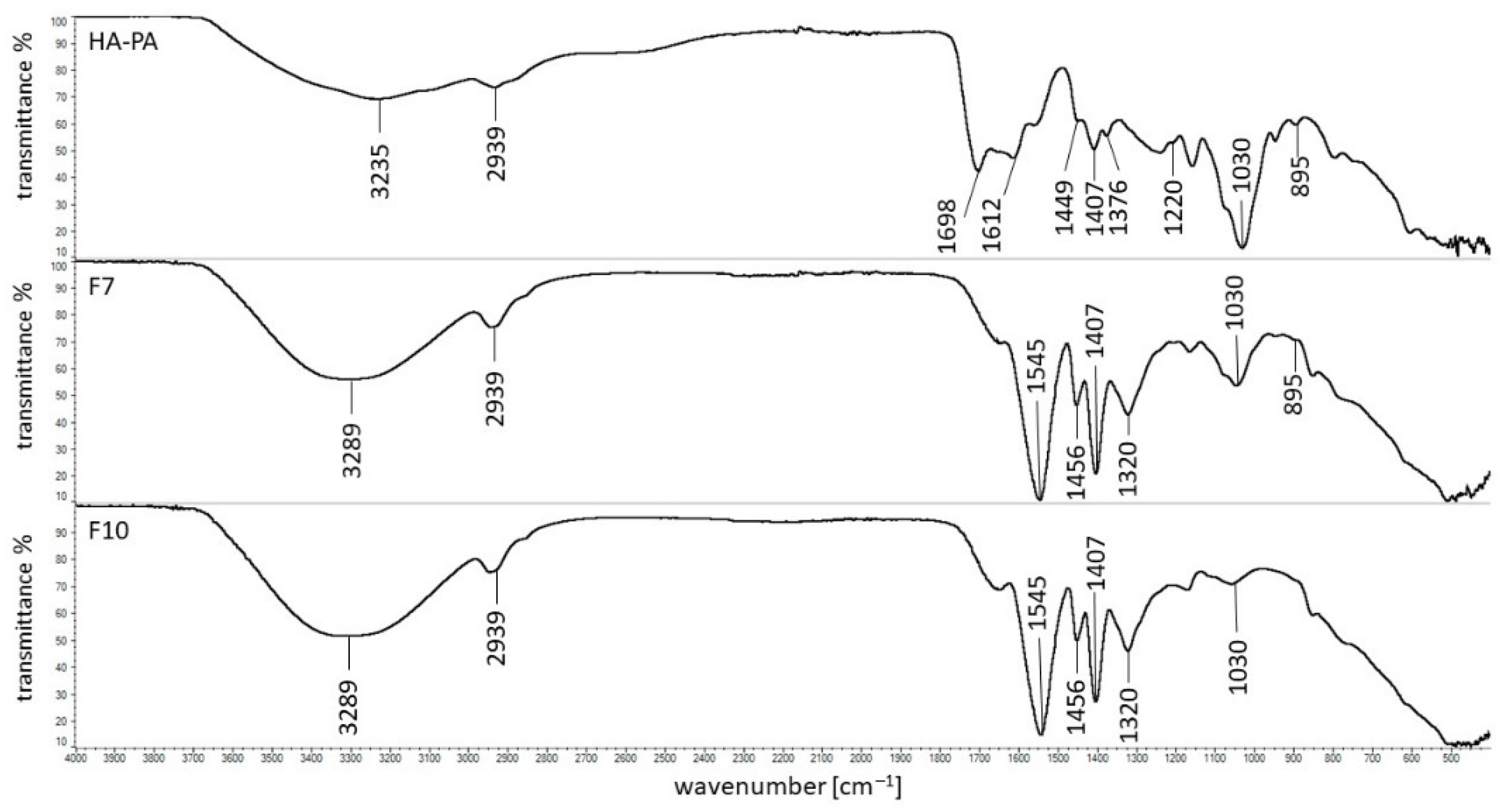
3.5. FTIR Study
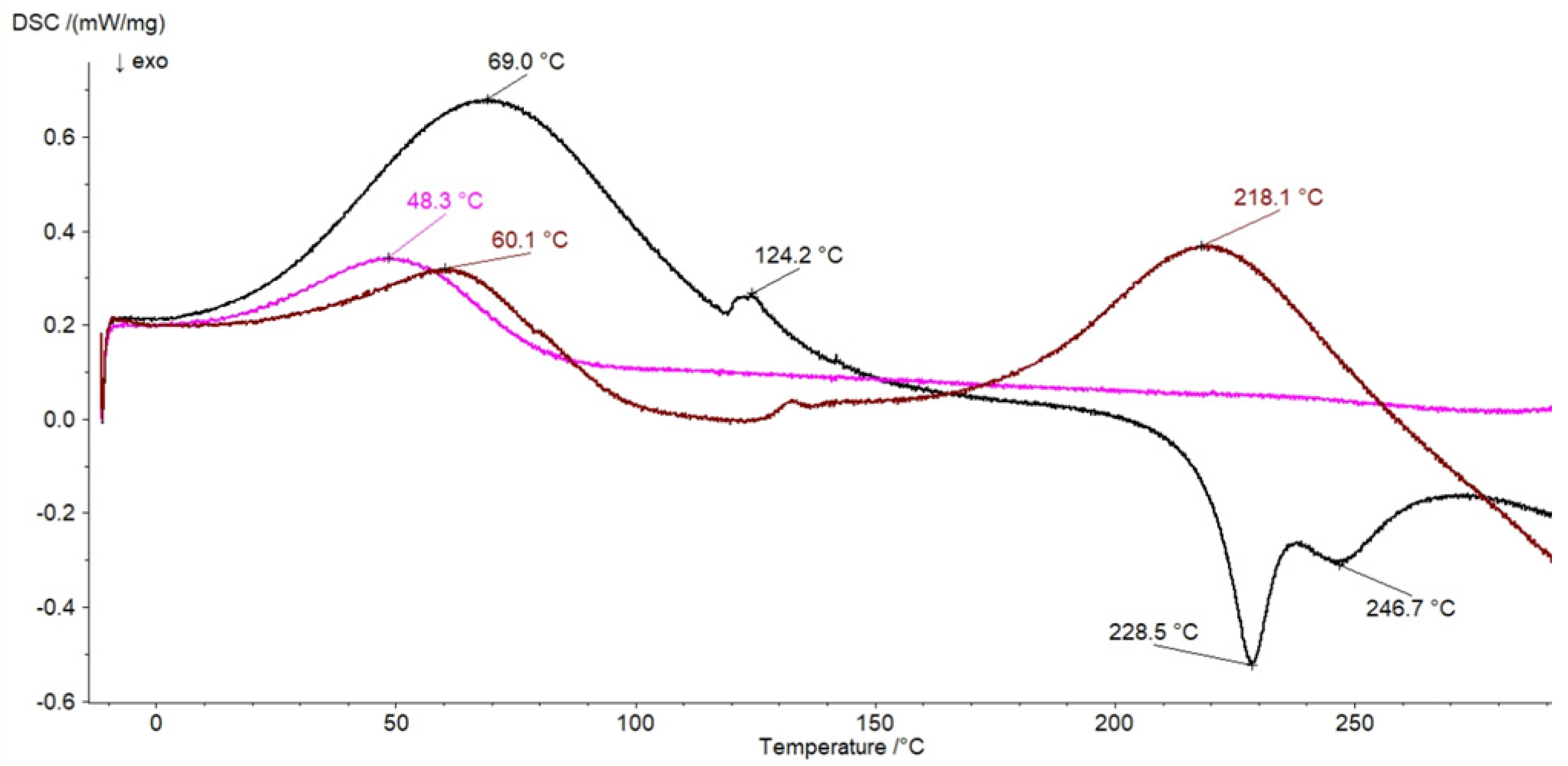
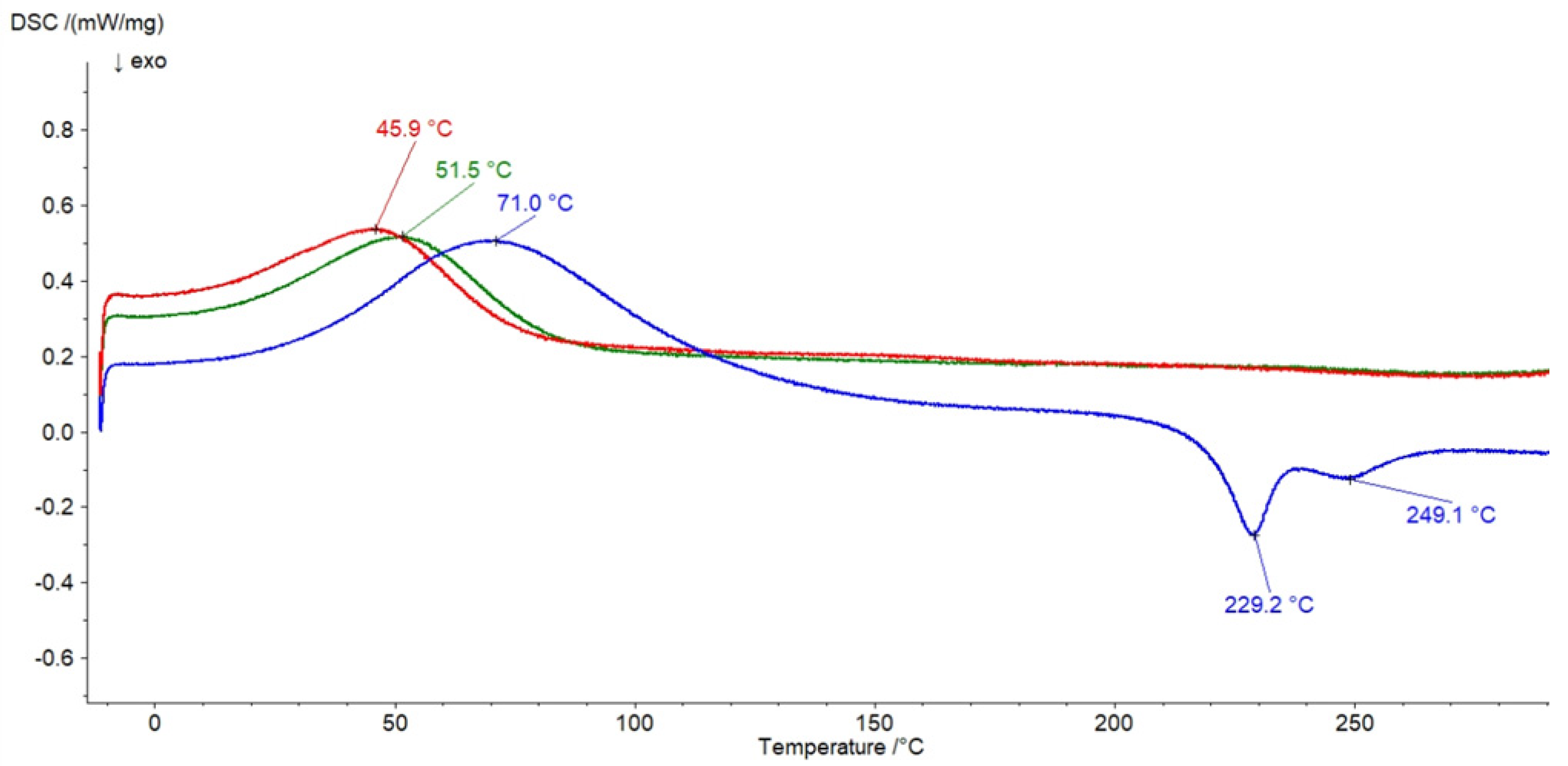
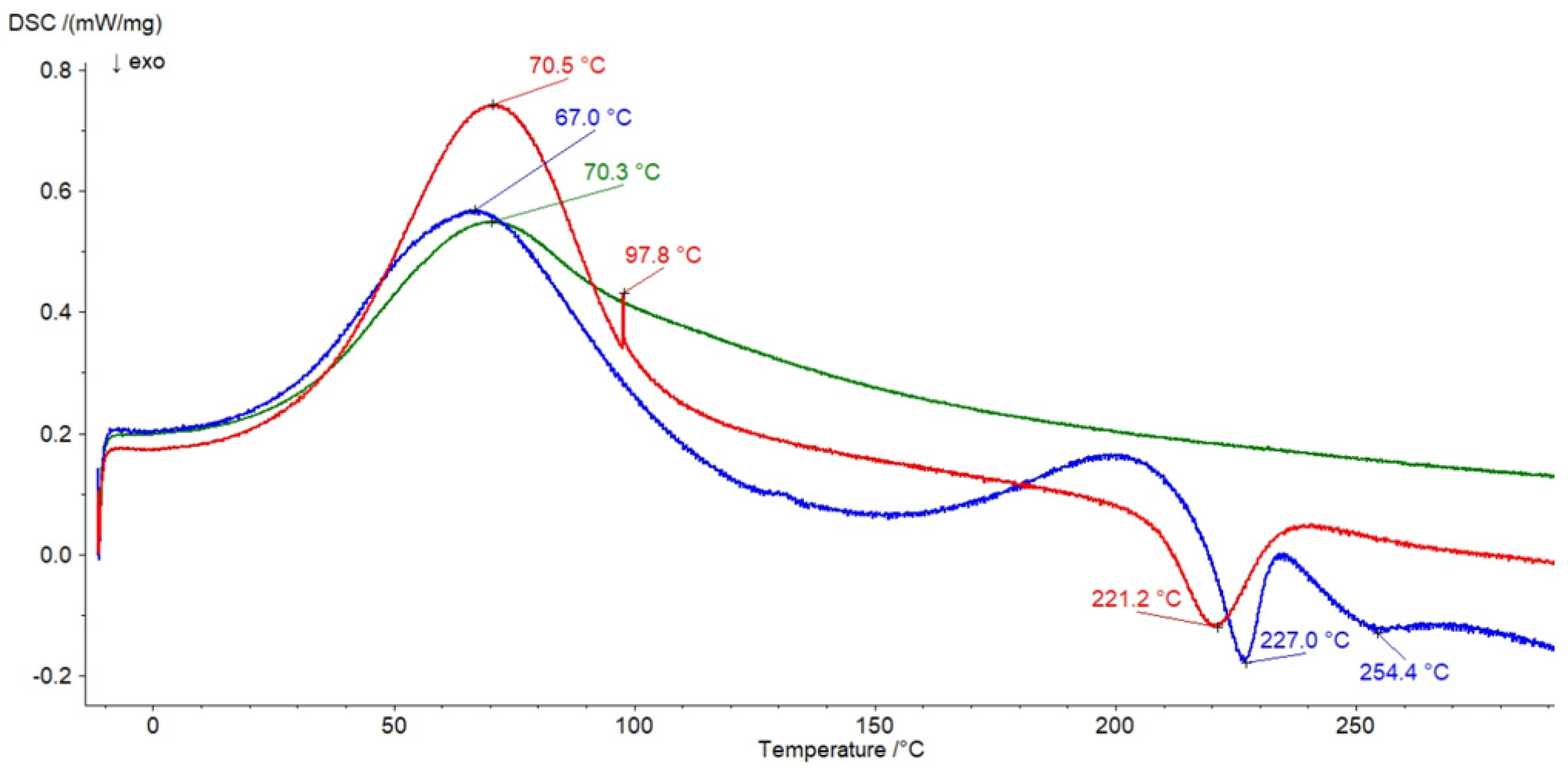
3.6. DSC Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Olczyk, K. Hialuronian-struktura, metabolizm, funkcje i rola w procesach gojenia ran Hyaluronan: Structure, metabolism, functions, and role in wound healing. Postępy Hig. Med. Dosw. 2008, 62, 651–659. [Google Scholar]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R. Hyaluronic Acid: The Influence of Molecular Weight and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, D.; Milner-Krawczyk, M.; Kazanecka, M. Kwas hialuronowy—Charakterystyka, otrzymywanie i zastosowanie. Biotechnol. Food Sci. 2011, 75, 55–70. [Google Scholar]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Ghazi, K.; Deng-Pichon, U.; Warnet, J.M.; Rat, P. Hyaluronan Fragments Improve Wound Healing on In Vitro Cutaneous Model through P2X7 Purinoreceptor Basal Activation: Role of Molecular Weight. PLoS ONE 2012, 7, e48351. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltes, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem. 2009, 2009, 1718–1745. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, M.; Słyż, J.; Winnicka, K. Rheological and textural properties of hydrogels, containing sulfur as a model drug, made using different polymers types. Polymers 2019, 64, 208–215. [Google Scholar] [CrossRef]
- Marques-Marinho, F.D.; Vianna-Soares, D.C. Cellulose and Its Derivatives Use in the Pharmaceutical Compounding Practice. In Bioorganic Chemistry; Van de ven Theo, G.M., Ed.; IntechOpen: London, UK; McGill University: Quebec City, QC, Canada, 2013; pp. 141–162. [Google Scholar]
- Catanzano, O.; D’Esposito, V.; Acierno, S.; Ambrosio, M.R.; de Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate-hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gomez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.5; European Directorate for the Quality of Medicines Council of Europe: Strasbourg, France, 2021.
- Chockalingam, P.S.; Zeng, W.; Morris, E.A.; Flannery, C.R. Release of hyaluronan and hyaladherins (aggrecan G1 domain and link proteins) from articular cartilage exposed to ADAMTS-4 (aggrecanase 1) or ADAMTS-5 (aggrecanase 2). Arthritis Rheum. 2004, 50, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles Paulo. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of Dissolution Profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms; FDA: Rockville, MD, USA, 1997; Volume 4, pp. 15–22. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070246.pdf (accessed on 15 December 2015).
- O’Hara, T.; Dunne, A.; Butler, J.; Devane, J. A review of methods used to compare dissolution profile data. Pharm. Sci. Technol. Today 1998, 1, 214–223. [Google Scholar] [CrossRef]
- Szcześniak, M.; Pluta, J. Wpływ substancji hydrofilizujących na właściwości hydrożeli sporządzonych na bazie Carbopolu Infuence of hydrophiling agents on gel formation rate on Carbopol base. Polim. Med. 2011, 41, 27–32. [Google Scholar]
- Liu, D.Z.; Sheu, M.T.; Chen, C.H.; Yang, Y.R.; Ho, H.O. Release characteristics of lidocaine from local implant of polyanionic and polycationic hydrogels. J. Control. Release 2007, 118, 333–339. [Google Scholar] [CrossRef]
- Khan, G.M.; Jiabi, Z. Formulation and in vitro evaluation of ibuprofen-carbopol 974(R)P-NF controlled release matrix tablets III: Influence of co-excipients on release rate of the drug. J. Control. Release 1998, 54, 185–190. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachova, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Soltes, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Valachova, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Soltes, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C 2018, 90, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/ Kaolin composite sponges loaded with penicillin—Streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Malik, K.; Singh, I. Formulation and evaluation of mucoadhesive matrix tablets of taro gum: Optimization using response surface methodology. Polim. Med. 2011, 41, 23–34. [Google Scholar] [PubMed]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.J.; Karunakaran, K.T. Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus. J. BioSci. Biotech. 2013, 2, 173–179. [Google Scholar]
- Alkrad, J.A.; Mrestani, Y.; Stroehl, D.; Wartewig, S.; Neubert, R. Characterization of enzymatically digested hyaluronic acid using NMR, Raman, IR, and UV-Vis spectroscopies. J. Pharm. Biomed. Anal. 2003, 31, 545–550. [Google Scholar] [CrossRef]
- Luan, T.; Wu, L.; Zhang, H.; Wang, Y. A study on the nature of intermolecular links in the cryotropic weak gels of hyaluronan. Carbohydr. Polym. 2012, 87, 2076–2085. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of methylcellulose on the properties and morphology of polysulfone membranes prepared by phase inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Pinotti, A.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocoll. 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Pastuszka, D.; Mazurek, K.L.; Szumny, A.J.; Alagoz, F.; Musiał, W.S. Properties of pectin based polymeric matrices for targeted drug delivery. Acta Pol. Pharm. Drug Res. 2017, 74, 1875–1885. [Google Scholar]
- Neira, A.; Tarraga, M.; Catalan, R. Degradation of polyacrylic acid by fenton’s reagent. J. Chil. Chem. Soc. 2007, 52, 1314–1317. [Google Scholar] [CrossRef]
- Alvarez-Gayosso, C.; Canseco, M.A.; Estrada, R.; Palacios-Alquisira, J.; Hinojosa, J.; Castano, V. Preparation and microstructure of cobalt(III) poly (acrylate) hybrid materials. Int. J. Basic Appl. Sci. 2015, 4, 255–263. [Google Scholar] [CrossRef]
- Elkheshen, S.A. Interaction of verapamil hydrochloride with Carbopol 934P and its effect on the release rate of the drug and the water uptake of the polymer matrix. Drug Dev. Ind. Pharm. 2001, 27, 925–934. [Google Scholar] [CrossRef]
- Villetti, M.A.; Crespo, J.S.; Soldi, M.S.; Pires, A.T.N.; Borsali, R.; Soldi, V. Thermal degradation of natural polymers. J. Therm. Anal. Calorim. 2002, 67, 295–303. [Google Scholar] [CrossRef]
- Benešová, K.; Pekař, M.; Lapčík, L.; Kučerík, J. Stability evaluation of n-alkyl hyaluronic acid derivates by DSC and TG measurement. J. Therm. Anal. Calorim. 2006, 83, 341–348. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Ebrahimnejad, P.; Amiri, F.T.; Nokhodchi, A. Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J. Drug Target. 2021, 29, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sgorla, D.; Almeida, A.; Azevedo, C.; Bunhak, É.J.; Sarmento, B.; Cavalcanti, O.A. Development and characterization of crosslinked hyaluronic acid polymeric films for use in coating processes. Int. J. Pharm. 2016, 511, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Gradys, A.; Kolbuk, D.; Krzton-Maziopa, A.; Sajkiewicz, P. Crosslinking kinetics of methylcellulose aqueous solution and its potential as a scaffold for tissue engineering. Polymers 2019, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
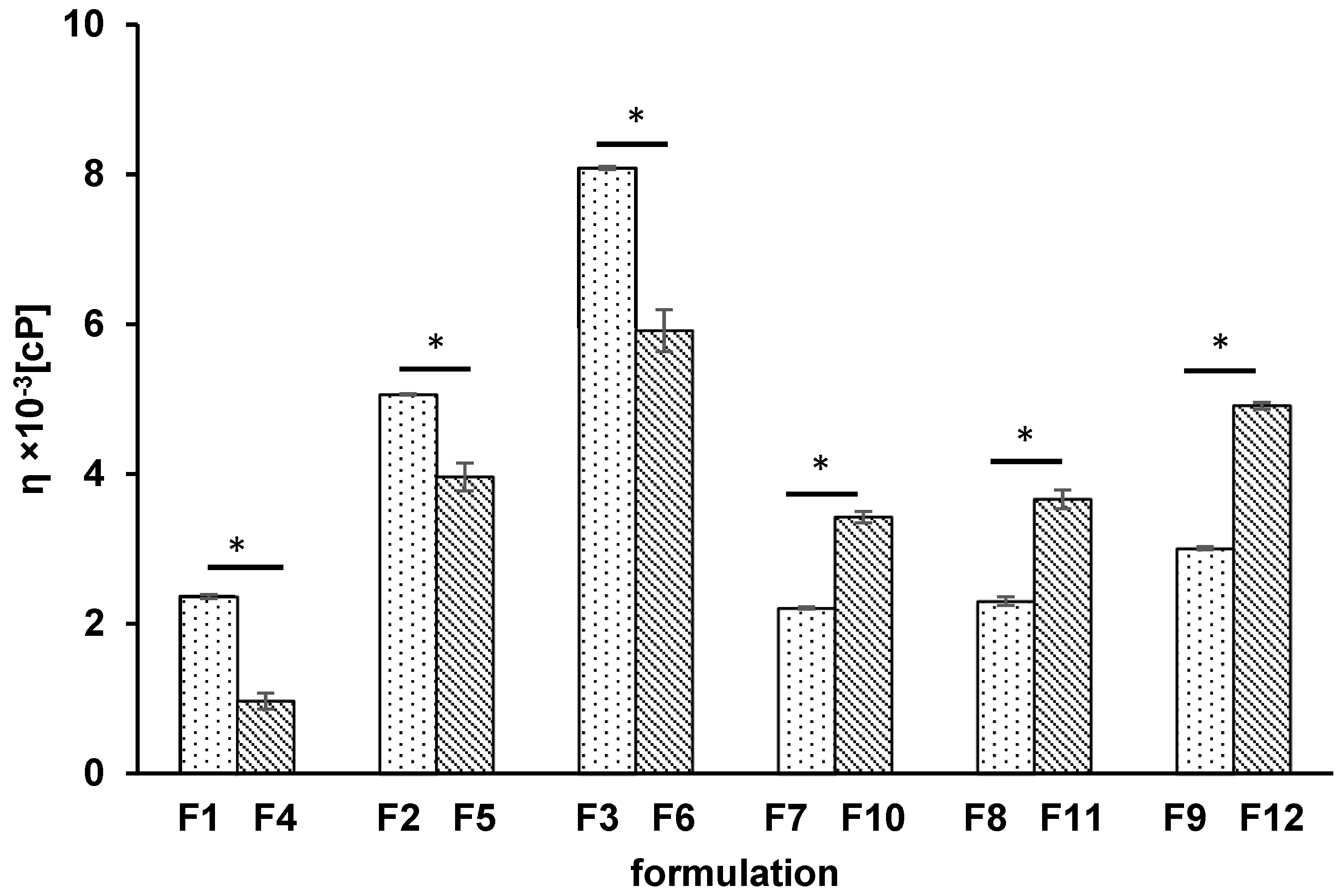
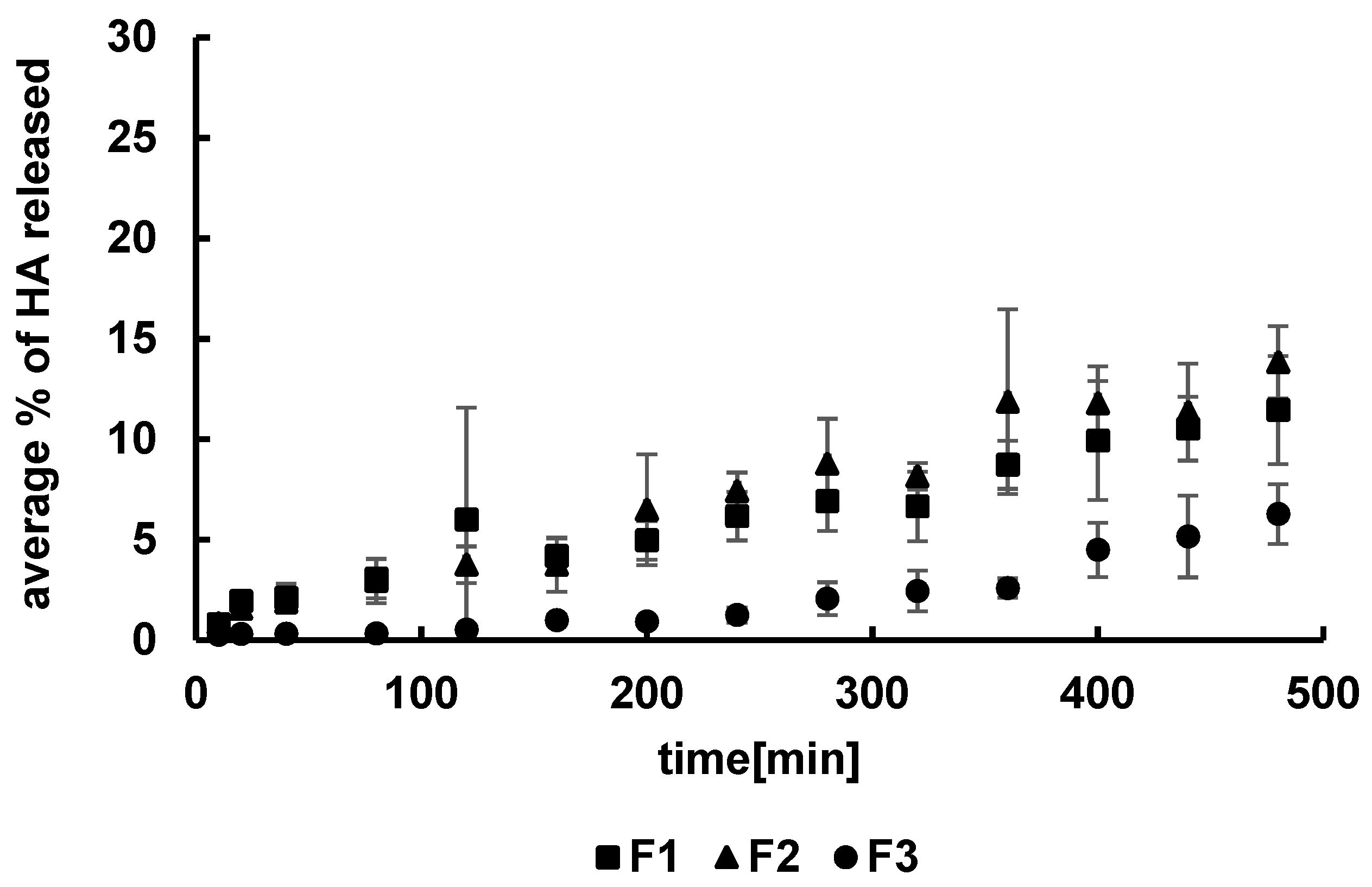
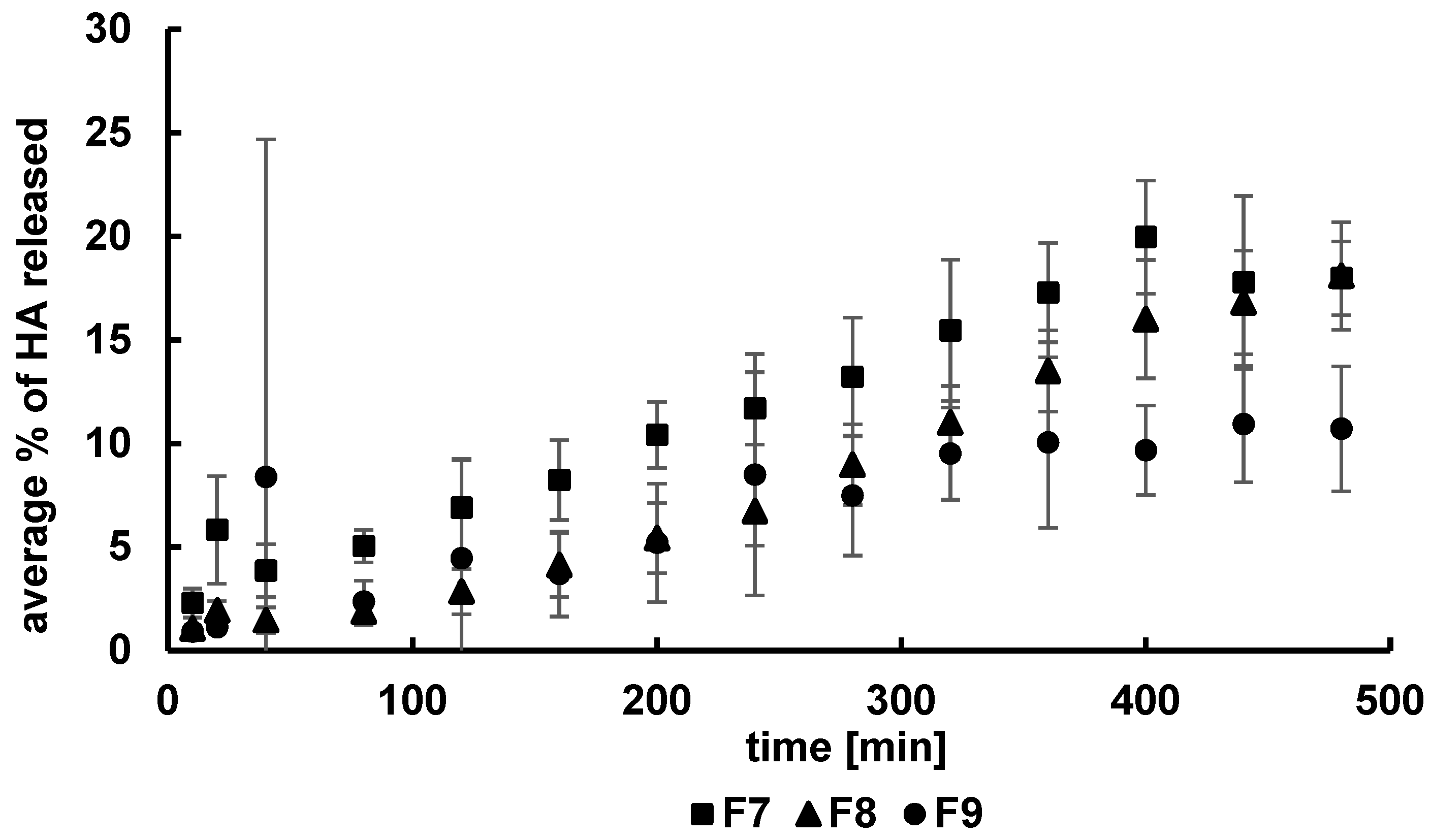
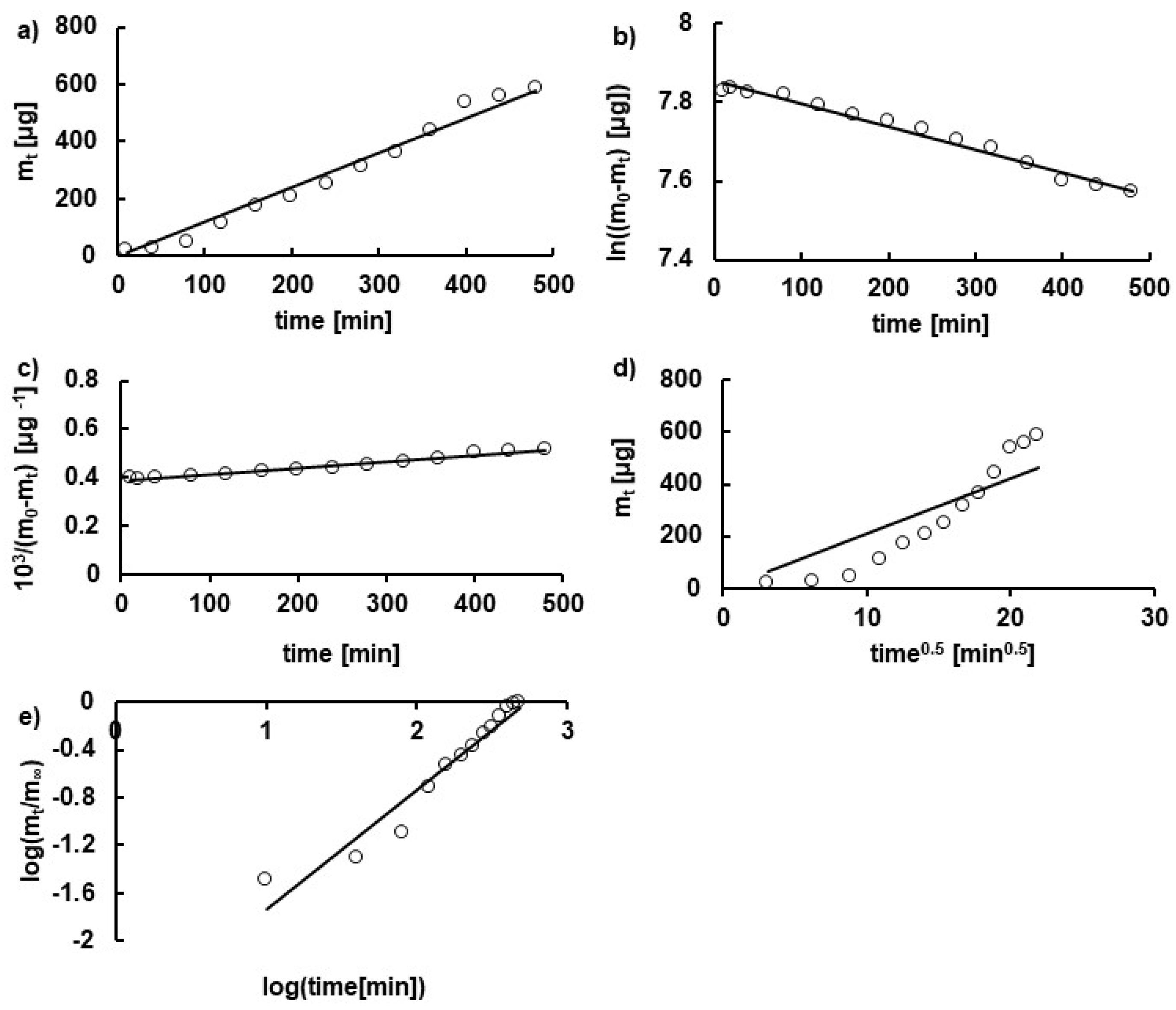
| Formulation | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC (g) | 5.00 | 6.25 | 7.50 | 5.00 | 6.25 | 7.50 | - | - | - | - | - | - |
| PA (g) | - | - | - | - | - | - | 0.56 | 0.66 | 0.75 | 0.56 | 0.66 | 0.75 |
| HA (g) | 0.125 | 0.125 | 0.125 | - | - | - | 0.125 | 0.125 | 0.125 | - | - | - |
| H2O (g) | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 2.702 | 5.722 | 1.396 | 1.598 | 3.552 | 0.151 | 0.060 | 0.645 | 1.061 | 1.300 | 2.548 |
| F2 | 3.020 | 4.098 | 1.103 | 0.850 | 2.853 | 2.762 | 2.057 | 1.641 | 1.402 | 0.153 | |
| F3 | 7.118 | 4.123 | 2.170 | 5.873 | 5.782 | 5.077 | 4.661 | 4.422 | 3.173 | ||
| F4 | 2.995 | 4.948 | 1.245 | 1.336 | 2.041 | 2.457 | 2.696 | 3.945 | |||
| F5 | 1.953 | 1.749 | 1.659 | 0.953 | 0.537 | 0.299 | 0.950 | ||||
| F6 | 3.703 | 3.612 | 2.907 | 2.491 | 2.252 | 1.003 | |||||
| F7 | 0.091 | 0.796 | 1.212 | 1.451 | 2.699 | ||||||
| F8 | 0.705 | 1.121 | 1.360 | 2.609 | |||||||
| F9 | 0.416 | 0.655 | 1.903 | ||||||||
| F10 | 0.239 | 1.487 | |||||||||
| F11 | 1.249 |
| Kinetic Model | Kinetic Parameters | F1 | F2 | F3 |
|---|---|---|---|---|
| Z-O | k0 (μg·min−1) | 0.60 ± 0.09 | 0.74 ± 0.10 | 0.24 ± 0.53 |
| t0.5 (min) | 2089 ± 295 | 1734 ± 233 | 5327 ± 1224 | |
| R2 | 0.93 ± 0.07 | 0.94 ± 0.03 | 0.86 ± 0.06 | |
| F-O | k1 × 104 (min−1) | 2.2 ± 0.7 | 2.9 ± 0.8 | 1.2 ± 0.4 |
| t0.5 (min) | 3316 ± 1137 | 2395 ± 683 | 5833 ± 2012 | |
| R2 | 0.78 ± 0.23 | 0.81 ± 0.10 | 0.75 ± 0.10 | |
| S-O | k2 × 108 (μg−1·min−1) | 9.5 ± 3.3 | 0.12 ± 0.40 | 5.2 ± 1.7 |
| t0.5 (min) | 4494 ± 1598 | 3192 ±946 | 8171 ± 2872 | |
| R2 | 0.77 ± 0.24 | 0.80 ± 0.10 | 0.75 ± 0.10 | |
| H | kH (μg·min−1/2) | 11.1 ± 1.8 | 13.3 ± 2.2 | 4.1 ± 1.3 |
| t0.5 (min) | 13,538 ± 3797 | 9368 ± 3104 | 101,671 ± 67,553 | |
| R2 | 0.72 ± 0.18 | 0.73 ± 0.07 | 0.51 ± 0.08 | |
| K–P | kK–P× 102 (min−n) | 2.1 ± 1.0 | 1.0 ± 0.5 | 0.3 ± 0.2 |
| t0.5 (min) | 203 ± 344 | 242 ± 471 | 506 ± 2428 | |
| R2 | 0.87 ± 0.08 | 0.87 ± 0.08 | 0.74 ± 0.08 | |
| n | 0.66 ± 0.14 | 0.74 ± 0.16 | 0.86 ± 0.30 | |
| best fit | Z-O | Z-O | Z-O, K–P | |
| Kinetic Model | Kinetic Parameters | F7 | F8 | F9 |
|---|---|---|---|---|
| Z-O | k0 (μg·min−1) | 1.2 ± 1.3 | 1.0 ± 0.08 | 0.66 ± 0.8 |
| t0.5 (min) | 1154 ± 129 | 1427 ± 117 | 2125 ± 236 | |
| R2 | 0.96 ± 0.02 | 0.98 ± 0.01 | 0.96 ± 0.03 | |
| F-O | k1 × 104 (min−1) | 4.1 ± 0.9 | 4.3 ± 0.5 | 2.6 ± 0.6 |
| t0.5 (min) | 1711 ± 385 | 1641 ± 211 | 2863 ± 620 | |
| R2 | 0.87 ± 0.07 | 0.95 ± 0.02 | 0.87 ± 0.08 | |
| S-O | k2 × 107 (μg−1·min−1) | 1.7 ± 0.4 | 1.7 ± 0.2 | 1.1 ± 0.2 |
| t0.5 (min) | 2198 ± 512 | 2149 ± 300 | 3883 ± 865 | |
| R2 | 0.86 ± 0.08 | 0.95 ± 0.02 | 0.87 ± 0.09 | |
| H | kH (μg·min−1/2) | 21.6 ± 2.3 | 17.8 ± 3.2 | 11.8 ± 1.8 |
| t0.5 (min) | 4064 ± 907 | 6826 ± 2613 | 14,880 ± 4827 | |
| R2 | 0.84 ± 0.04 | 0.73 ± 0.07 | 0.77 ± 0.09 | |
| K–P | kK–P × 102 (min−n) | 3.0 ± 1.3 | 0.3 ± 0.1 | 1.1 ± 0.6 |
| t0.5 (min) | 150 ± 216 | 287 ± 549 | 211 ± 457 | |
| R2 | 0.89 ± 0.09 | 0.88 ± 0.03 | 0.87 ± 0.07 | |
| n | 0.59 ± 0.1 | 0.93 ± 0.2 | 0.73 ± 0.2 | |
| best fit | Z-O | Z-O | Z-O | |
| f1 | f2 | |||
|---|---|---|---|---|
| F2 | F3 | F2 | F3 | |
| F1 | 21.2 | 66.5 | 86.5 | 67.8 |
| F2 | - | 70.4 | - | 63.0 |
| F8 | F9 | F8 | F9 | |
| F7 | 58.5 | 85.7 | 44.5 | 37.3 |
| F8 | - | 50.2 | - | 61.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Pastuszka, D.; Skrzypczyk, A.; Musiał, W. The Interactions and Release Kinetics of Sodium Hyaluronate Implemented in Nonionic and Anionic Polymeric Hydrogels, Studied by Immunoenzymatic ELISA Test. Pharmaceutics 2022, 14, 58. https://doi.org/10.3390/pharmaceutics14010058
Wójcik-Pastuszka D, Skrzypczyk A, Musiał W. The Interactions and Release Kinetics of Sodium Hyaluronate Implemented in Nonionic and Anionic Polymeric Hydrogels, Studied by Immunoenzymatic ELISA Test. Pharmaceutics. 2022; 14(1):58. https://doi.org/10.3390/pharmaceutics14010058
Chicago/Turabian StyleWójcik-Pastuszka, Dorota, Aleksandra Skrzypczyk, and Witold Musiał. 2022. "The Interactions and Release Kinetics of Sodium Hyaluronate Implemented in Nonionic and Anionic Polymeric Hydrogels, Studied by Immunoenzymatic ELISA Test" Pharmaceutics 14, no. 1: 58. https://doi.org/10.3390/pharmaceutics14010058
APA StyleWójcik-Pastuszka, D., Skrzypczyk, A., & Musiał, W. (2022). The Interactions and Release Kinetics of Sodium Hyaluronate Implemented in Nonionic and Anionic Polymeric Hydrogels, Studied by Immunoenzymatic ELISA Test. Pharmaceutics, 14(1), 58. https://doi.org/10.3390/pharmaceutics14010058






