Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Cocrystal
2.3. Differential Scanning Calorimetry (DSC)
2.4. Thermogravimetric Analysis (TGA)
2.5. Nuclear Magnetic Resonance (NMR)
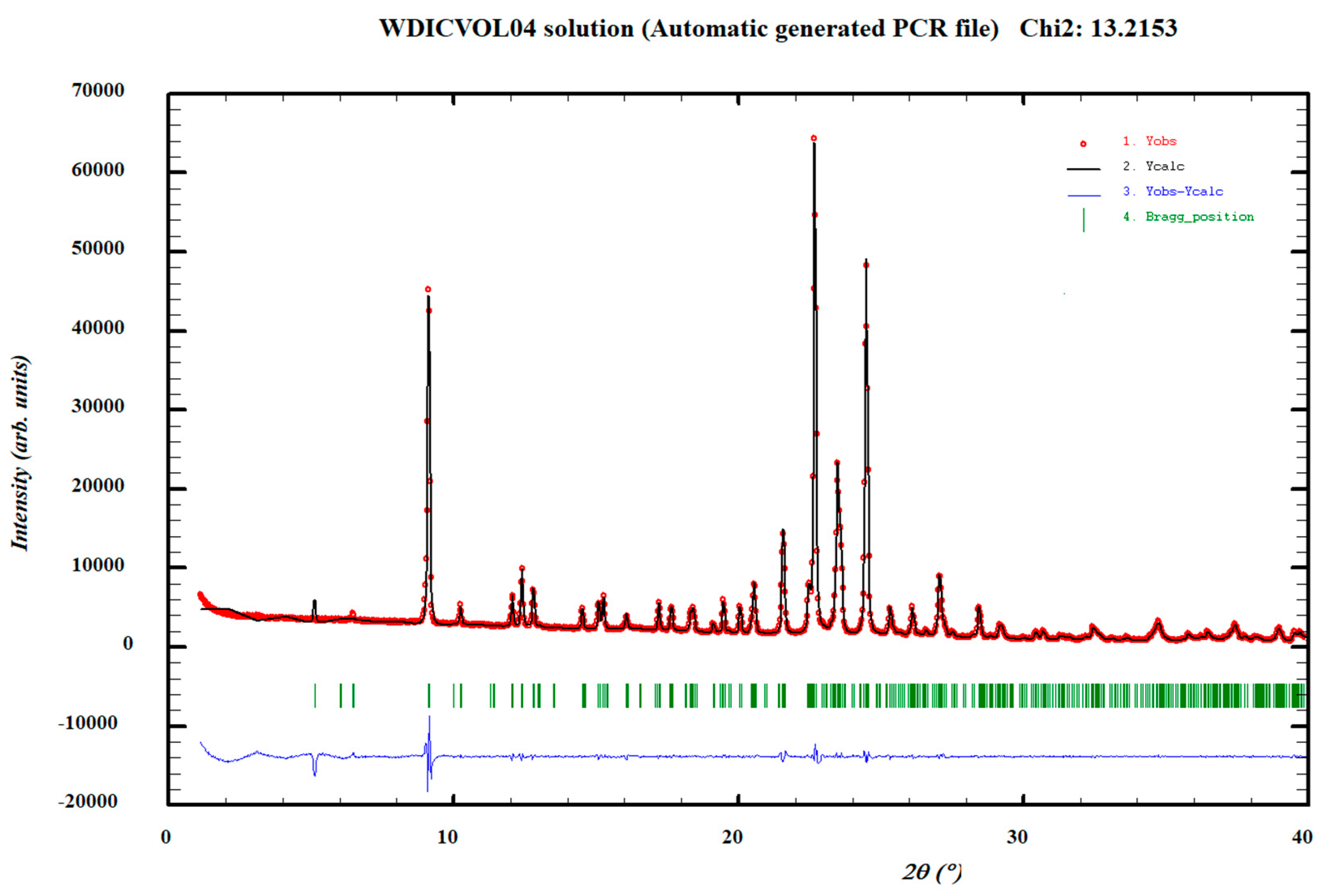
2.6. X-ray Crystallographic Analysis
2.7. Solubility
2.8. Intrinsic Dissolution Rate (IDR)
2.9. pKa Determination
3. Results and Discussion
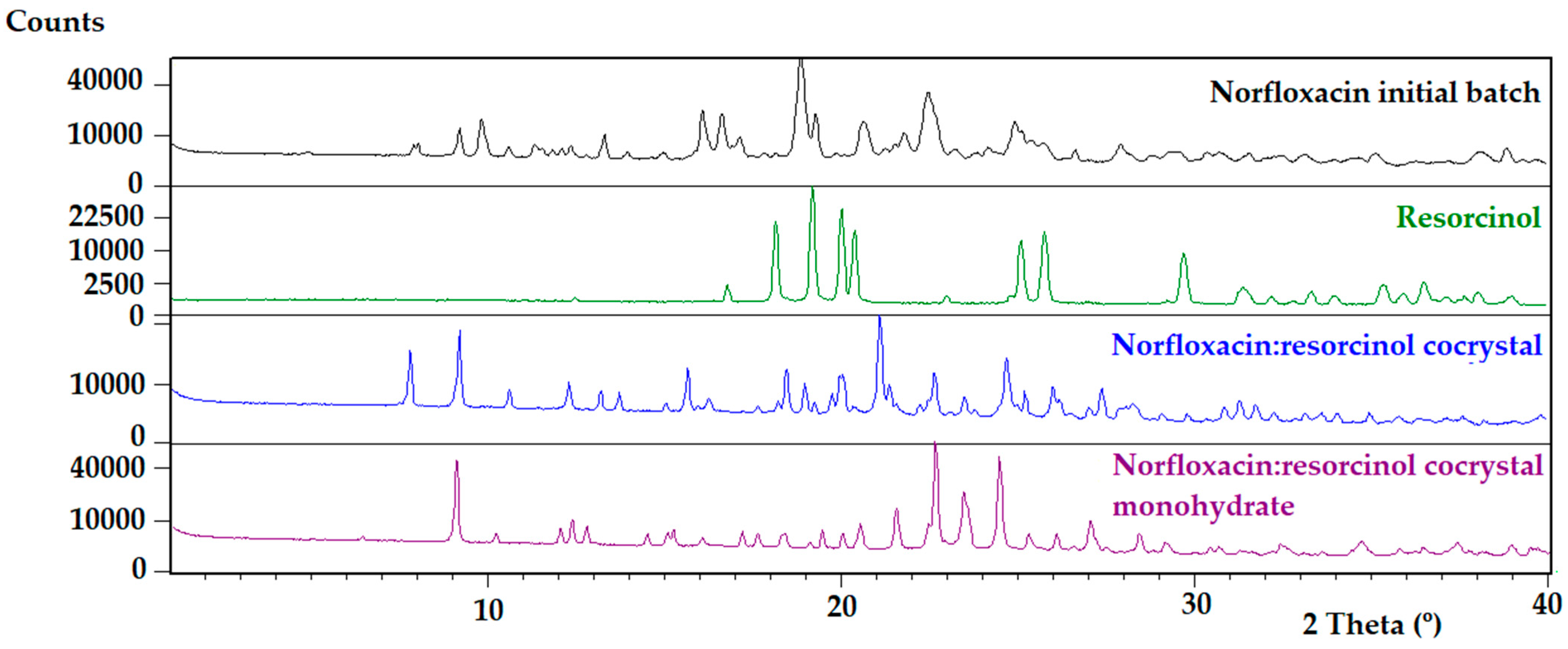
3.1. Characterization of the Cocrystals
3.2. Solubility Study
3.3. Intrinsic Dissolution Rate Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shargel, L.; Wu-Pong, S.; Yu, A. Applied Biopharmaceutics & Pharmacokinetics, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2012; p. 413. [Google Scholar]
- Merisko-Liversidge, E. Nanocrystals: Resolving pharmaceutical formulation issues associated with poorly water-soluble compounds. In Particles, 1st ed.; Marty, J.J., Ed.; Marcel Dekker: Orlando, FL, USA, 2002. [Google Scholar]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Kratochvíl, B. Pharmaceutical cocrystals. Procedia Chem. 2014, 10, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.; Motherwell, W.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Salmon, D. Building Co-Crystals with Molecular Sense and Supramolecular Sensibility Highlight. CrystEngComm 2005, 7, 439–448. [Google Scholar]
- Bhogala, B.R.; Nangia, A. Ternary and quaternary co-crystals of 1, 3-cis, 5-cis-cyclohexanetricarboxylic acid and 4,4′-bipyridines. New J. Chem. 2008, 32, 800–807. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, R.A.; Davey, R.J.; Peterson, M.L. Making co-crystals the utility of ternary phase diagrams. Cryst. Growth Des. 2007, 7, 1223–1226. [Google Scholar] [CrossRef]
- Childs, S.L.; Rodríguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. CrystEngComm 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Delori, A.; Friščić, T.; Jones, W. The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 2012, 14, 2350–2362. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friscic, T. Applying hot-stage microscopy to co-crystal screening: A study of nicotinamide with seven active pharmaceutical ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Noszál, B.; Hermecz, I.; Keresztúri, G.; Podányi, B.; Szasz, G. Protonation equilibria of quinolone antibacterials. J. Pharm. Sci. 1990, 79, 1023–1028. [Google Scholar] [CrossRef]
- Ross, D.L.; Riley, C.M. Aqueous solubilities of some variously substituted quinolone antimicrobials. Int. J. Pharm. 1990, 63, 237–250. [Google Scholar] [CrossRef]
- De Souza, M.V. New fluoroquinolones: A class of potent antibiotics. Mini-Rev. Med. Chem. 2005, 5, 1009–1017. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Pharmaceutical cocrystal and salts of norfloxacin. Cryst. Growth Des. 2010, 10, 2948–2953. [Google Scholar] [CrossRef]
- Velaga, S.P.; Basavoju, S.; Boström, D. Norfloxacin saccharinate–saccharin dihydrate cocrystal—A new pharmaceutical cocrystal with an organic counter ion. J. Mol. Struct. 2008, 889, 150–153. [Google Scholar] [CrossRef]
- Ferreira, P.O.; de Almeida, A.C.; dos Santos, É.C.; Droppa Junior, R.; Ferreira, F.F.; Kogawa, A.C.; Caires, F.J. A norfloxacin-nicotinic acid cocrystal: Mechanochemical synthesis, thermal and structural characterization and solubility assays. Thermochim. Acta 2020, 694, 178782. [Google Scholar] [CrossRef]
- Burdock, G.A. Encyclopedia of Food and Color Additives; CRC Press: Boca Raton, FL, USA; New York, NY, USA; London, UK; Tokyo, Japan, 1997; Available online: https://www.routledge.com/Encyclopedia-of-Food--Color-Additives/Burdock/p/book/9780849394164?gclid=EAIaIQobChMI5Iyxo4389AIV9hoGAB3q2gyWEAAYASAAEgLmifD_BwE# (accessed on 18 November 2021).
- European Food Safety Authority (EFSA). Scientific opinion on the use of resorcinol as a food additive. EFSA J. 2010, 8, 1411. [Google Scholar] [CrossRef]
- Barbas, R.; Font-Bardia, M.; Prohens, R. Polymorphism of sildenafil: A new metastable desolvate. Cryst. Growth Des. 2018, 18, 7618–7627. [Google Scholar] [CrossRef]
- Barbas, R.; Kumar, V.; Vallcorba, O.; Prohens, R.; Frontera, A. Sildenafil–resorcinol cocrystal: XRPD structure and DFT calculations. Crystals 2020, 10, 1126. [Google Scholar] [CrossRef]
- Bofill, L.; de Sande, D.; Barbas, R.; Prohens, R. A New and Highly Stable Cocrystal of Vitamin D3 for Use in Enhanced Food Supplements. Cryst. Growth Des. 2021, 21, 1418–1423. [Google Scholar] [CrossRef]
- Lee, M.J.; Chun, N.H.; Kim, H.C.; Kim, M.J.; Kim, P.; Cho, M.Y.; Choi, G.J. Agomelatine co-crystals with resorcinol and hydroquinone: Preparation and characterization. Korean J. Chem. Eng. 2018, 35, 984–993. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.R.; Nangia, A. Fast dissolving curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Ràfols, C.; Fael, H.; Fuguet, E.; Outhwaite, B.; Lee, S.; Ruiz, R. Dissolution rates of ciprofloxacin and its cocrystal with resorcinol. ADMET DMPK 2018, 6, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Barbas, R.; Prohens, R.; Puigjaner, C. A new polymorph of norfloxacin. J. Therm. Anal. Calorim. 2007, 89, 687–692. [Google Scholar] [CrossRef]
- Avdeef, A.; Fuguet, E.; Llinàs, A.; Ràfols, C.; Bosch, E.; Völgyi, G.; Verbić, T.; Boldyreva, E.; Takács-Novák, K. Equilibrium solubility measurement of ionizable drugs–consensus recommendations for improving data quality. ADMET DMPK 2016, 4, 117–178. [Google Scholar] [CrossRef] [Green Version]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef] [PubMed]
- EPA. EPA Product Properties Test Guidelines: OPPTS 830.7840, Water Solubility: Column Elution Method; Shake Flask Method; Environmental Protection Agency: Washington, DC, USA, 1998. Available online: https://nepis.epa.gov (accessed on 18 November 2021).
- Gravestock, T.; Box, K.; Comer, J.; Frake, E.; Judge, S.; Ruiz, R. The “GI dissolution” method: A low volume, in vitro apparatus for assessing the dissolution/precipitation behaviour of an active pharmaceutical ingredient under biorelevant conditions. Anal. Methods 2011, 3, 560–567. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 1991, 24, 987–993. [Google Scholar] [CrossRef]
- Ràfols, C. Departament d’Enginyeria Química i Química Analítica, Universitat de Barcelona. Martí i Franquès 1–11, 08028 Barcelona, Spain. pKa determination of norfloxacin and resorcinol. 2017; Unpublished work. [Google Scholar]
- Cabot, J.M.; Fuguet, E.; Ràfols, C.; Rosés, M. Determination of acidity constants by the capillary electrophoresis internal standard method. IV. Polyprotic compounds. J. Chromatogr. A 2013, 1279, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A. Multi-lab intrinsic solubility measurement reproducibility in CheqSol and shake-flask methods. ADMET DMPK 2019, 7, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Avdeef, A.; Tsinman, O. Miniaturized rotating disk intrinsic dissolution rate measurement: Effects of buffer capacity in comparisons to traditional Wood’s apparatus. Pharm. Res. 2008, 25, 2613–2627. [Google Scholar] [CrossRef]



| Compound | Starting Solid Form | pH | % Dissolved at t = 30 min | Dissolution Rate | Final Solid Form |
|---|---|---|---|---|---|
| Norfloxacin | Mixture of polymorphs | 2.0 | 101.2 (6.3) | 4.16 (0.73) | Tetrahydrate |
| 4.0 | 71.3 (6.9) | 0.79 (0.03) | |||
| 5.5 | 23.0 (5.2) | 0.19 (0.02) | |||
| 7.4 | 9.4 (1.0) | 0.06 (0.01) | |||
| Norfloxacin–resorcinol cocrystal | Anhydrous | 2.0 | 94.4 (1.1) | 2.53 (1.63) | Monohydrate |
| 4.0 | 91.2 (0.9) | 0.70 (0.15) | |||
| 5.5 | 48.0 (12.5) | 0.33 (0.04) | |||
| 7.4 | 9.6 (1.0) | 0.19 (0.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fael, H.; Barbas, R.; Prohens, R.; Ràfols, C.; Fuguet, E. Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile. Pharmaceutics 2022, 14, 49. https://doi.org/10.3390/pharmaceutics14010049
Fael H, Barbas R, Prohens R, Ràfols C, Fuguet E. Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile. Pharmaceutics. 2022; 14(1):49. https://doi.org/10.3390/pharmaceutics14010049
Chicago/Turabian StyleFael, Hanan, Rafael Barbas, Rafel Prohens, Clara Ràfols, and Elisabet Fuguet. 2022. "Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile" Pharmaceutics 14, no. 1: 49. https://doi.org/10.3390/pharmaceutics14010049
APA StyleFael, H., Barbas, R., Prohens, R., Ràfols, C., & Fuguet, E. (2022). Synthesis and Characterization of a New Norfloxacin/Resorcinol Cocrystal with Enhanced Solubility and Dissolution Profile. Pharmaceutics, 14(1), 49. https://doi.org/10.3390/pharmaceutics14010049









