1. Introduction
Diabetes mellitus is a chronic metabolic disease characterized by hyperglycemia, which is considered a serious threat to human health [
1,
2,
3]. Long-standing hyperglycemia is known to cause several complications, including blindness, cerebrovascular disease, and kidney failure [
4]. Insulin is the only hypoglycemic hormone in the human body and is essential for the treating patients with diabetes to reduce blood glucose levels (BGLs) [
5]. However, inadequate or excessive insulin injection can result in poor glycemic control [
6]. Compared with traditional insulin delivery systems, closed-loop insulin delivery systems can effectively regulate BGLs within the normal range. This type of system can discharge sufficient insulin during hyperglycemia and self-adjust to release a smaller insulin dose during normoglycemia; this is desirable for improving the quality of life of patients with diabetes [
7].
Notably, to construct a closed-loop insulin delivery system, glucose-sensitive elements need to be employed; these mainly include phenylboronic acid (PBA)-containing polymers, glucose oxidase (GOx), concanavalin A, and modified insulins [
4]. PBA can reversibly form boronic acid–diol complexes with 1,2- or 1,3-diols via covalent bonds [
8,
9]. Accordingly, PBA-containing polymers were designed and synthesized by chemical reactions, which were further used to construct glucose-responsive systems, including microcapsules [
10], microspheres [
11], gels [
12], and micelles [
13]. In these systems, the structures were destroyed when PBA combined with glucose under high glucose conditions, and the entrapped drugs were further released. However, it is difficult to synthesize PBA-containing polymers with high molecular weights, and their quality cannot be controlled. Conversely, PBA derivatives with low molecular weights are easily obtained and stable in quality, which could be employed to construct a new glucose-responsive system. GOx catalyzes the rapid and efficient conversion of glucose into gluconic acid and hydrogen peroxide (H
2O
2), accompanied by oxygen consumption. Thus, an environment composed of low pH, H
2O
2, or hypoxia offers strategies for constructing a glucose-responsive system [
14,
15,
16,
17]. Concanavalin A, known to possess high specificity and affinity for glucose, can form a three-dimensional cross-linked network structure with a glucose-containing polymer. The network structure of the insulin delivery system undergoes disintegration when glucose and glucose-containing polymers competitively bind to concanavalin A [
18,
19]. In addition, glucose-containing insulin [
20] or PBA-containing insulin [
21] can achieve rhythmic regulation of BGLs by altering the onset and duration of action. Collectively, significant progress has been made in this field; however, the challenge remains to construct a glucose-responsive delivery system that confers good glycemic control and biocompatibility.
Multivesicular liposomes (MVLs) consist of many nonconcentric polyhedral aqueous chambers separated by a network of lipid layers, with a typical particle size ranging from 1 to 100 μm [
22,
23]. Given their unique structure and large size, MVLs offer the probability of sustained and controlled release of both hydrophilic and lipophilic drugs. Moreover, the structure of the interior compartments can remain intact when exterior compartments are destroyed [
24]. Notably, neutral lipids, such as triglycerides, are essential for MVL formation. In MVLs, triglycerides are located in two regions, at bilayer intersection points and in intravesicular triglyceride droplets [
25,
26]. The release rate of entrapped drugs can be modified by utilizing triglycerides containing alkyl chains of different lengths [
24]. Accordingly, MVLs can present several advantages as a drug carrier, including high encapsulation efficiency (EE), high encapsulation volume, good biocompatibility, sustained-release effect, multiple administration methods, and adjustable release rate [
27,
28,
29]. Although MVLs encapsulating insulin have been reported, they are not glucose-responsive and cannot maintain blood glucose homeostasis [
23]. If the appropriate glucose-sensitive elements are introduced into MVLs, the resulting delivery system may possess both glucose responsiveness and MVL advantages. However, taking advantage of these glucose-sensitive elements to render MVLs glucose-responsive is challenging.
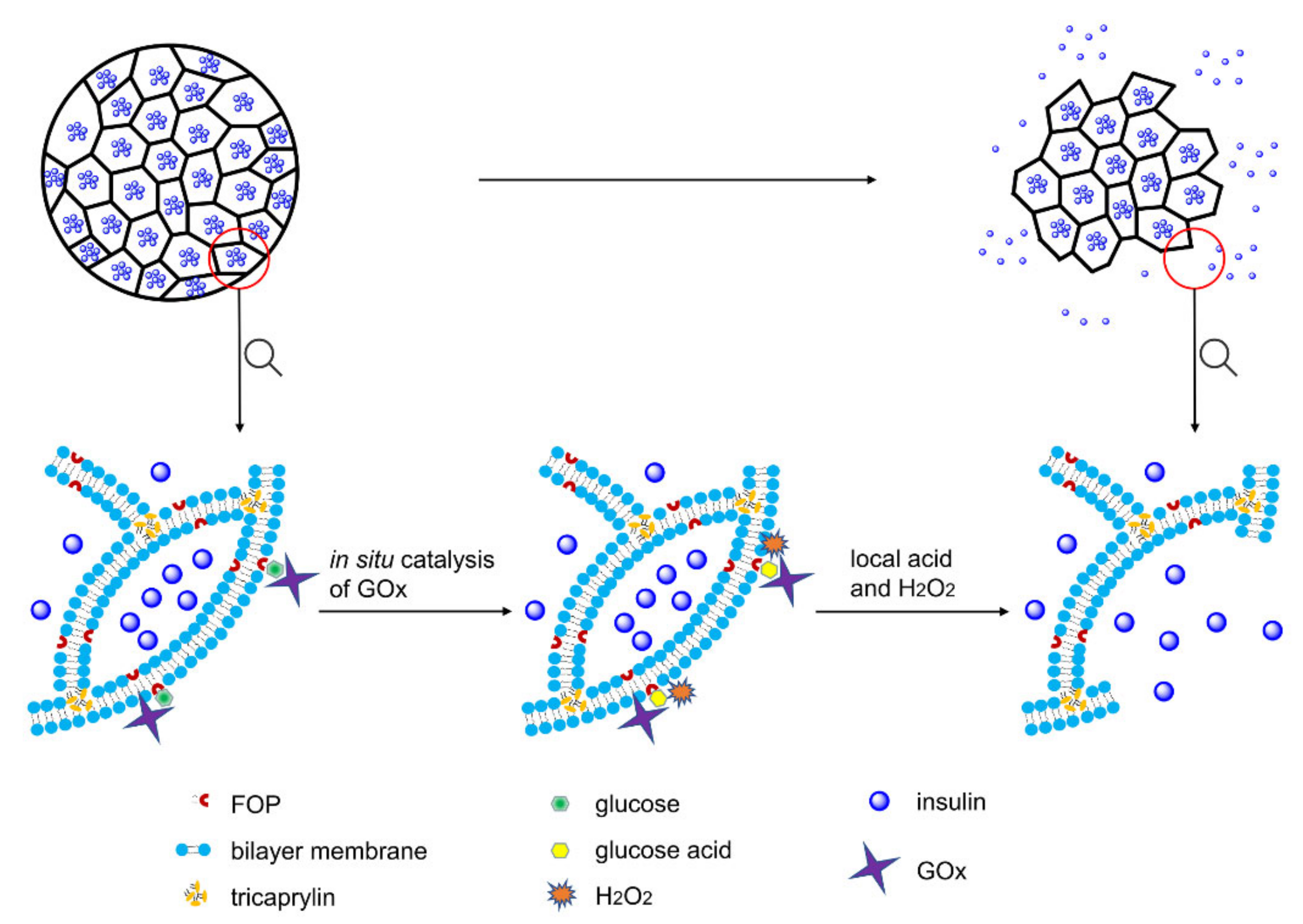
Herein, we formulated a pH and H
2O
2 dual-sensitive MVL using the double emulsion method. The liposomal membrane of MVLs encapsulates (3-fluoro-4-((octyloxy)carbonyl)phenyl)boronic acid (FOP) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), and the internal vesicles of MVLs encapsulate insulin, GOx, and catalase (CAT). Glucose enrichment on the MVL surface was achieved by the reversible combination of glucose and FOP. As shown in
Figure 1, on increasing the local concentration (400 mg/dL), glucose around the MVL membrane undergoes in situ catalyzation by GOx to form gluconic acid and H
2O
2, reducing local pH and generating local H
2O
2.
Then, DSPE protonation in the liposomal membrane due to pH decrease, as well as lipid peroxidation due to local H
2O
2, destroys the outer MVL membrane and, in turn, triggers the release of entrapped insulin. However, the structure of internal vesicles in MVLs remains stable, and the membrane destruction needs to be triggered again prior to insulin release. Under normal glucose conditions (100 mg/dL), the MVL structure can be kept intact, and only a small amount of insulin is released. Furthermore, CAT is beneficial for GOx catalysis as it scavenges H
2O
2 and provides oxygen (
Figure S1). In the present study, MVL as a drug carrier was used to construct a glucose-responsive system for the first time, and its multivesicular structure affords the basis for glucose-regulated insulin delivery. Moreover, low molecular weight FOP can be easily synthesized with a well-controlled quality; this PBA derivative can be encapsulated into the membrane to achieve glucose enrichment. In addition, the novel trigger mechanism based on in situ catalysis results in glucose-regulated release of insulin. Glucose-responsive MVLs are expected to provide a desirable and intelligent insulin delivery system with rapid response and good biocompatibility, presenting enormous potential in diabetes management.
2. Materials and Methods
2.1. Materials
Porcine insulin (27 IU/mg) was purchased from Wanbang Biochemical (Xuzhou, China). D(+)-glucose, Alizarin red, glycine, lysine, GOx, and CAT were purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG), cholesterol, tricaprylin (TC), and DSPE were purchased from Southeast Nano Materials Co., Ltd. (Huai’an, China). Streptozotocin was purchased from BioFroxx (Hesse, Germany). Insulin ELISA kit was purchased from SenBeiJia Biotechnology Co., Ltd. (Nanjing, China). The other chemicals were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China).
2.2. Synthesis and Characterization of PBA Derivatives
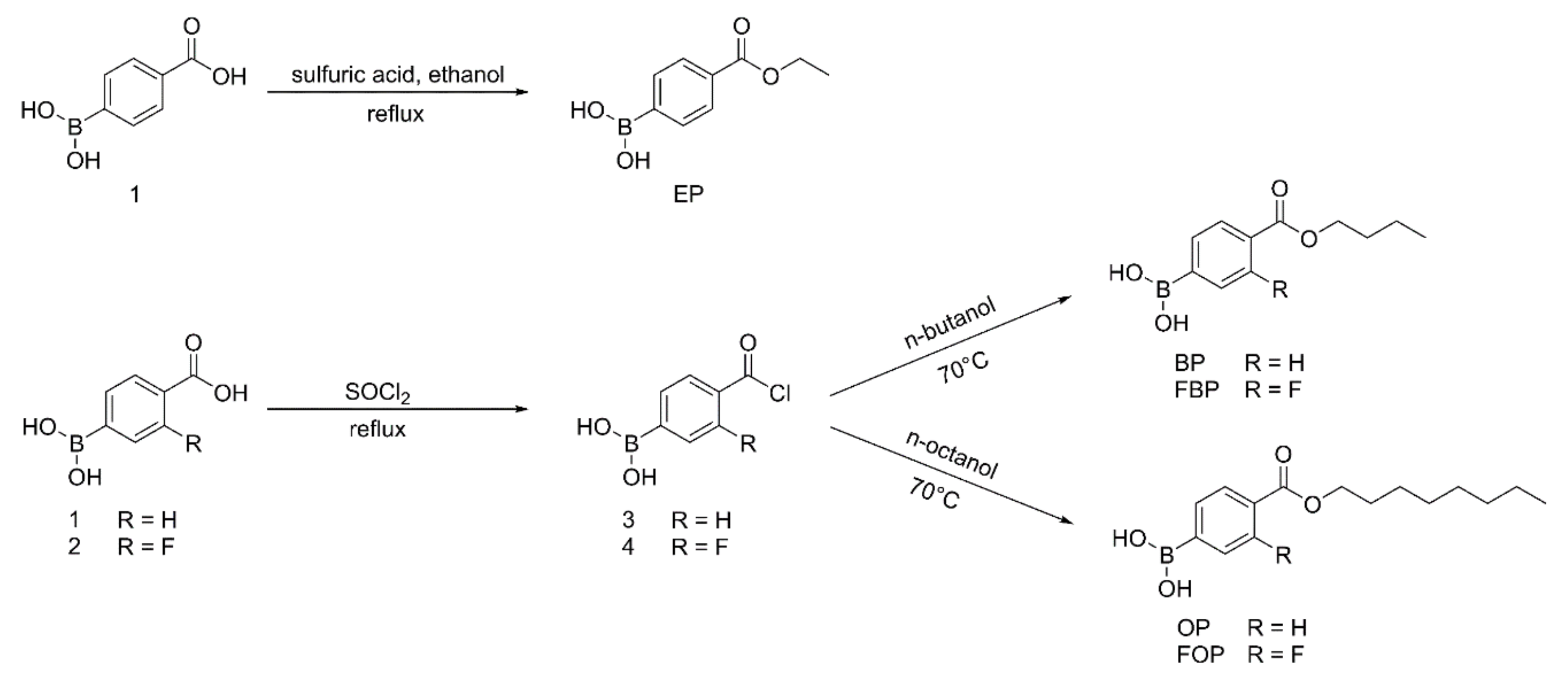
As shown in
Figure 2, (4-(ethoxycarbonyl)phenyl)boronic acid (EP) was obtained by refluxing compound 1 with sulfuric acid in absolute ethanol. Briefly, concentrated sulfuric acid (4 mL) was added to a solution of compound 1 (1.28 g, 0.078 mol) in absolute ethanol (160 mL). The reaction mixture was then refluxed for 12 h. After the reaction was completed, the reaction mixture was cooled to room temperature, and ethanol was removed under reduced pressure to obtain the crude product. The crude product was dispersed in water (20 mL). The resulting precipitate was filtered, washed with saturated sodium bicarbonate solution, washed with water, and dried to yield EP.
(4-(butoxycarbonyl)phenyl)boronic acid (BP), (4-(butoxycarbonyl)-3-fluorophenyl)boronic acid (FBP), (4-((octyloxy)carbonyl)phenyl)boronic acid (OP), and FOP were synthesized with modified benzoyl chloride and the corresponding
n-butanol or n-octanol (
Figure 2). Considering FOP as an example, a drop of N, N-dimethylformamide and compound 2 (2.0 g, 0.011 mol) were added to SOCl
2 (20 mL). The reaction mixture was refluxed for 8 h, and excess SOCl
2 was removed under reduced pressure to obtain compound 4. Under stirring, compound 4 (1.0 g, 0.005 mol) was added to
n-octanol (2 mL). The reaction mixture was stirred for 1 h at 70 °C. After completion of the reaction, n-octanol was removed under reduced pressure to obtain an oily liquid. A 10% ethanol solution was added to the oily liquid to precipitate the product. The resulting precipitate was filtered, washed with water, and dried to yield FOP.
The reaction progress was monitored by analytical thin-layer chromatography, and spots were observed under 254 nm ultraviolet (UV) light. The structures of target compounds were determined by high-resolution mass spectrometry (HRMS; Agilent 6520 Q-TOF MS, Santa Clara, CA, USA) and 1H nuclear magnetic resonance (1H NMR; Bruker AV-300, Bruker, Billerrica, MA, USA). The purity of target compounds was determined by high-performance liquid chromatography (HPLC; LC-20AT, Shimadzu, Kyoto, Japan). The pKa values of PBA derivatives were determined by the titration method.
2.3. Preparation of MVLs
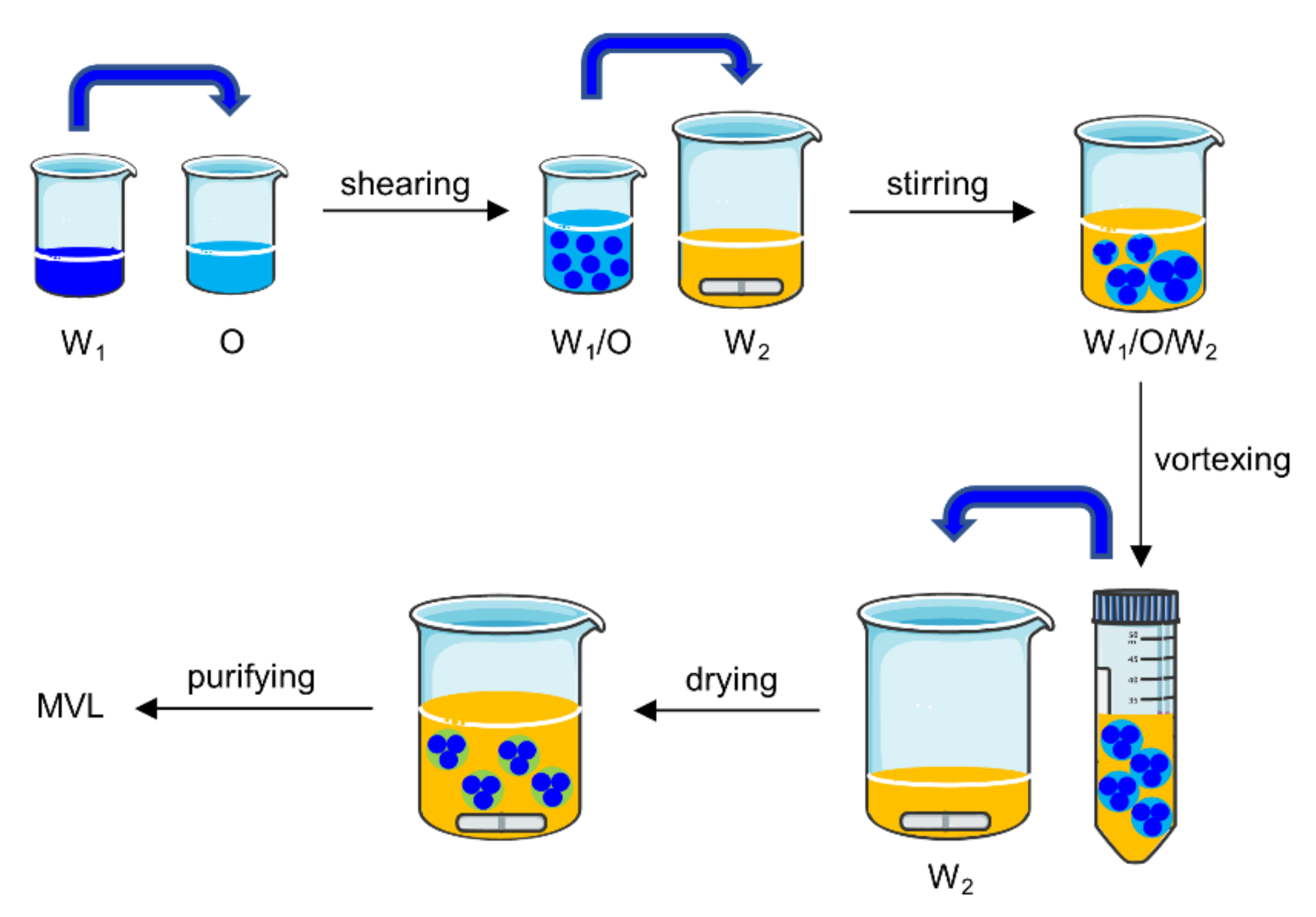
MVLs were prepared using the double emulsion method (
Figure 3).
The specific experiments were as follows: (1) 13 mg insulin was dissolved in 1.3 mL HCl solution (pH 2.0). The solution was adjusted to pH 7.0 using an amino acid buffer solution containing 1.2 M glycine and 65 mM lysine; then, 6 mg GOx and 1.5 mg CAT were dissolved in the above solution. The resulting solution was the inner water phase (W1). W1 was emulsified with 2 mL of a chloroform solution (O), which contained 20 mM neutral phospholipids (containing DOPC and DSPE), 4.2 mM DPPG, 10 mM FOP or FBP, 30 mM cholesterol, and 5 mM TC, by high-speed shearing to obtain a W1/O emulsion. To prepare the stable MVLs with pH sensitivity, the molar ratios of DOPC and DSPE were set at 0:1, 1;1, 3:1, and 4:1, respectively. (2) The W1/O emulsion was mixed with 16 mL of external water phase (W2), which was composed of 306 mM glycine and 1 mM lysine (pH 7.1), by magnetic stirring to obtain a W1/O/W2 emulsion. (3) The W1/O/W2 emulsion was further homogenized by vortexing for 30 s in a centrifuge tube. (4) The W1/O/W2 emulsion was transferred to a beaker containing 16 mL of W2; the organic solvent was removed by flushing nitrogen gas over the surface of the mixture at 37 °C at a flow rate of 3 L/min for 20 min. (5) The resulting MVL particles were harvested by centrifugation for 10 min at 600× g and washed three times by resuspending in W2. The resulting product was resuspended in W2 at 20% packed-particle volume per total volume, followed by storage at 2–8 °C for subsequent analysis.
2.4. Characterization of MVLs
2.4.1. EEs of Insulin and PBA Derivatives
The EE of insulin is defined as the ratio of the insulin content in MVL particles to the total amount of insulin in the MVL suspension before purification. To determine the EE of insulin, a 0.1 mL of MVL suspension, after nitrogen sweeping, was dissolved by adding 0.5 mL 10% Triton X-100 aqueous solution to detect the total amount of insulin. Next, 0.9 mL of 0.9% saline solution was mixed with another 0.1 mL of MVL suspension. The mixture was centrifuged for 10 min at 600× g, and the upper layer of clear liquid was collected to estimate the free insulin. The amount of insulin was detected by HPLC (LC-20AT, Shimadzu, Japan) using an Inertsil ODS-SP column (150 × 4.6 mm, 5 μm; Shimadzu, Kyoto, Japan). The mobile phase consisted of 28% acetonitrile and 72% water (containing 0.2 M Na2SO4 and 0.46 wt% phosphoric acid; pH was adjusted to 2.3 using triethanolamine), and the flow rate was 1.0 mL/min. The injection volume was 20 μL, and the detection wavelength was 214 nm. The EE was calculated using the following formula: EE (%) = (mtotal − mfree)/mtotal × 100%. Here, mtotal represents the total amount of insulin and mfree represents the free amount of insulin. For determining the EEs of FOP or FBP, 0.9% saline solution was replaced with an amino acid buffer solution containing 133 mM glycine and 150 mM lysine (pH 9.5) as the washing medium; all other steps were the same as for insulin. The leakage rate of insulin was determined using the method used for the EE of insulin.
2.4.2. EEs of GOx and CAT
The method used to determine the EE of GOx was the same as that of insulin. Enzyme activity was used as an index to determine the EE of GOx. The activities of free GOx and total GOx were determined and recorded as U
free and U
total, respectively. The EE of GOx was calculated using the following formula: EE (%) = (U
total − U
free)/U
total × 100%. GOx activity was determined using the titration method [
30]. Briefly, 1 mL of test solution was added to 25 mL of 60 mM sodium acetate buffer (pH 5.6) containing 2% glucose. The mixture was shaken for 1 h at 30 °C in a water bath with stirring at 200 cycles/min. The reaction was stopped by adding 20 mL of 0.1 M sodium hydroxide solution. The resulting mixture was titrated to a red endpoint using a 0.1 M standard HCl solution using phenolphthalein as an indicator. GOx activity was determined by the volume of the added standard HCl solution. The EE of CAT was determined using the method used for insulin. Enzyme activity was used as an index to determine the EE of CAT. CAT activity was determined using a CAT assay kit in accordance with the manufacturer’s instructions.
2.4.3. Stability of Entrapped Insulin
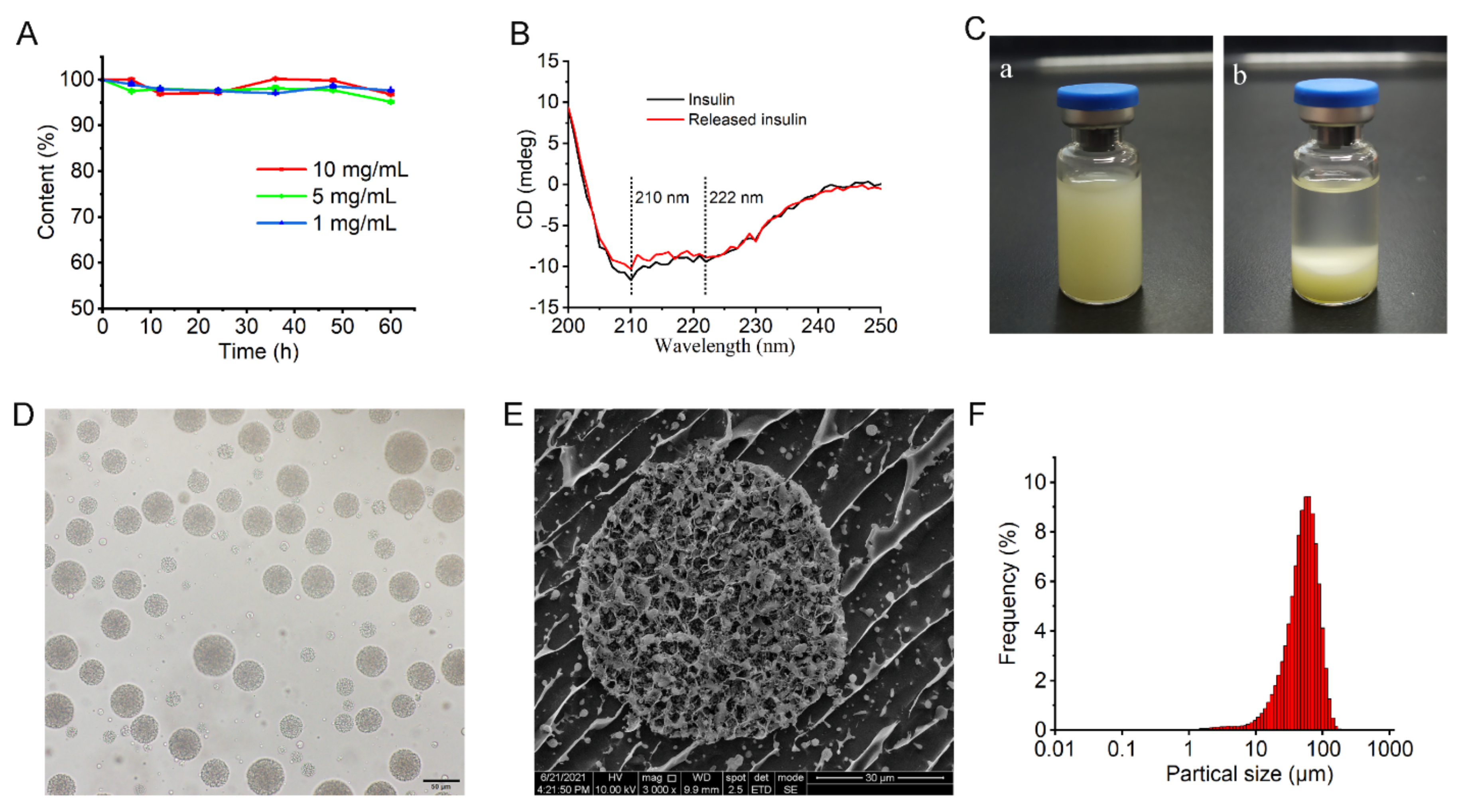
The stability of entrapped insulin was determined by HPLC and circular dichroism (CD) spectroscopy. In the HPLC method, the insulin solution was prepared according to the preparation method of W1 of MVLs. Briefly, insulin was dissolved in HCl solution (pH 2.0) at concentrations of 1, 5, and 10 mg/mL. The solution was adjusted to pH 7.0, with an amino acid buffer solution containing 1.2 M glycine and 65 mM lysine. The resulting solution was incubated at 37 °C in an orbital shaker stirred at 12 cycles/min. The insulin content of the incubation solution was determined by HPLC at each set time point and compared with that at 0 h.
The secondary structural change in insulin released from MVLs was evaluated as reported previously [
31]. The insulin solution (130 μg/mL) was used as the standard CD spectrum. The MVL suspension stored at 2–8 °C for 1 month was diluted in release media, and the mixture was incubated at 37 °C in a water bath. The released insulin was obtained by centrifugation, and the insulin concentration was adjusted to 130 μg/mL for CD measurement. Test solution spectra were recorded in the range of 200–250 nm at 25 °C using a CD spectrometer (J-800, JASCO, Tokyo, Japan).
2.4.4. Morphology and Particle Size of MVLs
The MVL morphology was examined using both optical microscopy (BX-53, Olympus, Tokyo, Japan) and cryogenic-scanning electron microscopy (cryo-SEM; Quanta 450, FEI, Hillsboro, OR, USA). The cross-section of MVLs could be observed by cryo-SEM. Briefly, conductive carbon adhesive was put on the sample table, and the diluted MVLs were placed on the conductive carbon adhesive. Then, the sample table with MVLs was rapidly frozen in liquid nitrogen for 30 s and transferred to the preparation chamber in a vacuum state for sublimation gold-coating. Finally, the sample was fractured and an approximately 5 nm of gold layer was sputter-coated on the exposed surface. The sample was imaged in the pre-cooled (~ −140) FEI Quanta450 SEM (FEI, Hillsboro, OR, USA) operated at 5 kV. The particle size distribution and average particle size of the MVLs were measured using a laser particle size analyzer (Bettersize2600, Better, Dandong, China).
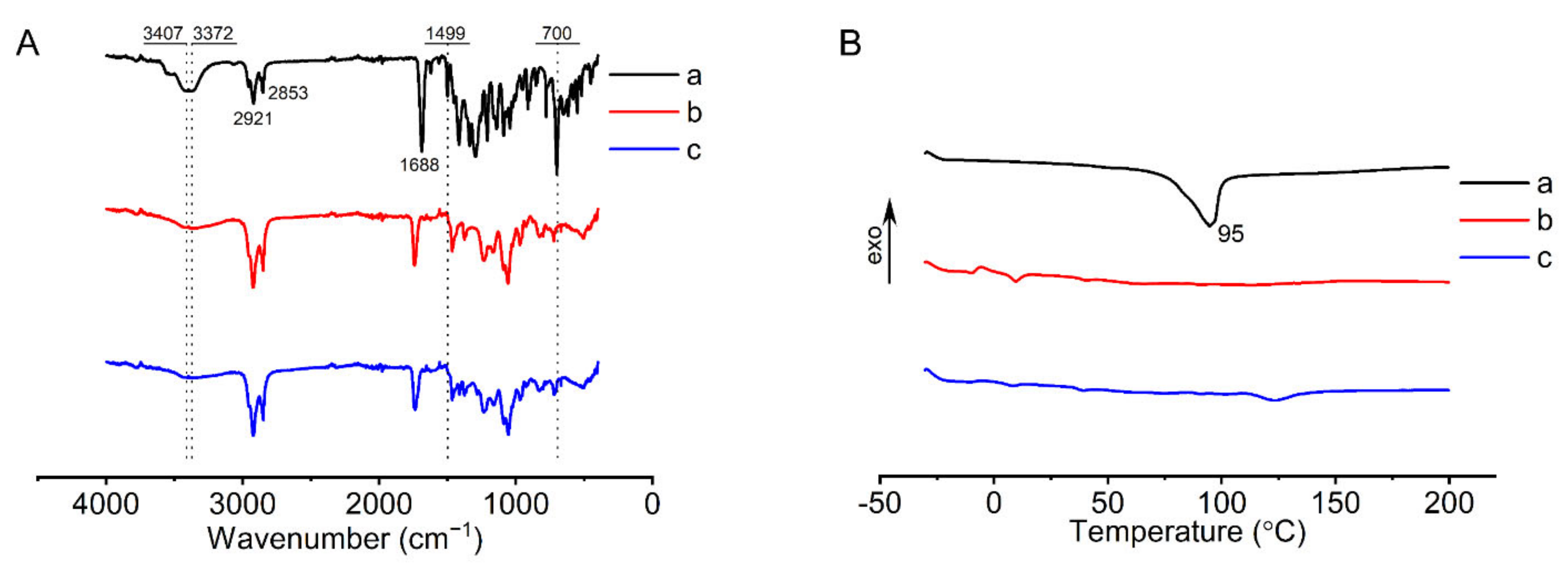
2.4.5. Fourier Transform Infrared (FTIR) Analysis
The FTIR spectra of FOP, liposomal membranes without FOP, and liposomal membranes with FOP were recorded using an FTIR spectrometer (Tensor 27, Bruker, Ettlingen, Germany). The liposomal membranes were prepared as follows: The membrane materials were dissolved in chloroform and dried under evaporation in a rotary evaporator (RV-10, IKA, Staufen, Germany) at 35 °C. The three samples were compressed in a potassium bromide pellet and scanned for spectra in the range of 4000–400 cm−1 at a resolution of 1 cm−1.
2.4.6. Differential Scanning Calorimetry (DSC) Analysis
The thermal properties of FOP, liposomal membranes without FOP, and liposomal membranes with FOP were investigated by DSC (DSC 3500, Netzsch, Selb, Germany). Liposomal membranes were prepared in the same way as described in
Section 2.4.5. The three samples were weighed and measured in a calorimeter pool. The temperature range was −30 to 200 °C, and the temperature rise rate was 10 °C/min.
2.5. In Vitro Insulin Release from MVLs
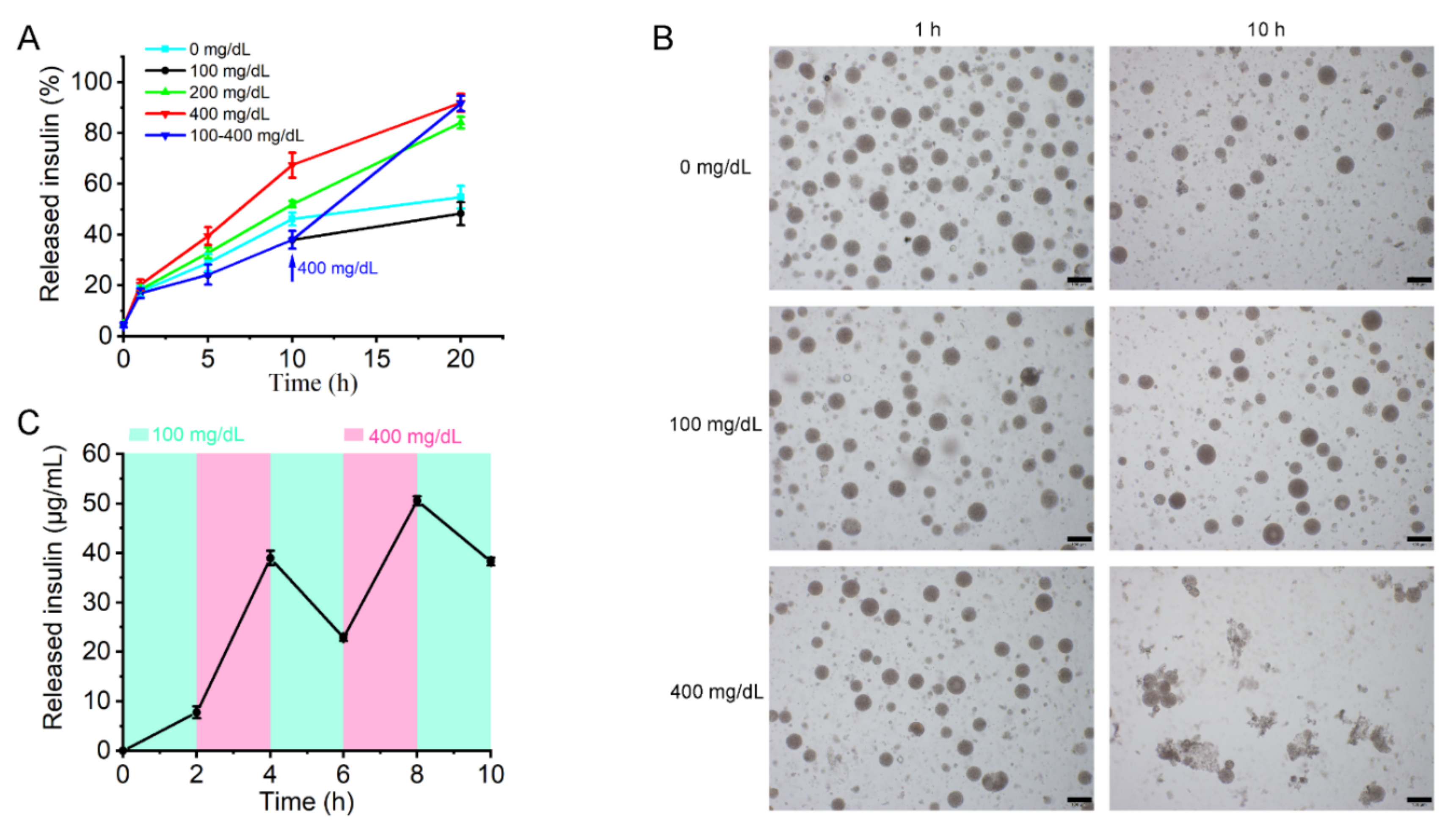
Briefly, 10 mM phosphate-buffered saline (PBS; pH 7.4) with different glucose concentrations (0, 100, or 400 mg/dL) was used to perform the in vitro drug release experiments. Aliquots of MVL suspensions were added to different Eppendorf tubes containing the release media. The mixture was incubated at 37 °C in a water bath stirred at 12 cycles/min. Additionally, insulin release from MVLs was assessed by changing the glucose concentration from 100 to 400 mg/dL in PBS (pH 7.4). The MVL particles were first incubated in PBS (pH 7.4) with 100 mg/dL glucose for 10 h, followed by the addition of glucose to achieve a concentration of 400 mg/dL. At indicated time points, tubes (three tubes per time point) were centrifuged at 600× g for 5 min after 3-fold PBS (pH 7.4) was added. The supernatant solution was removed. MVL particles were lysed with 10% Triton X-100 aqueous solution, and the amount of insulin in the MVL particles was detected by HPLC. The release rate was calculated using the following formula: release rate (%) = (mtotal − mparticle)/mtotal × 100%. Here, mparticle represents the amount of insulin in MVL particles and mtotal represents the total amount of insulin in the same amount of MVL suspension. Meanwhile, the average particle size of MVLs was measured during the release process using a laser particle size analyzer. The glucose-dependent pulsatile test was conducted to further access the glucose responsiveness of the MVLs. First, MVL particles were incubated in 100 mg/dL glucose solution for 2 h. The supernatant solution was removed after MVL particles were centrifuged, and the insulin concentration in the supernatant solution was detected by HPLC. The MVL particles were resuspended in 400 mg/dL glucose solution for another 2 h. The cycles were repeated three times.
2.6. Interaction between 1,2—Diols and FOP
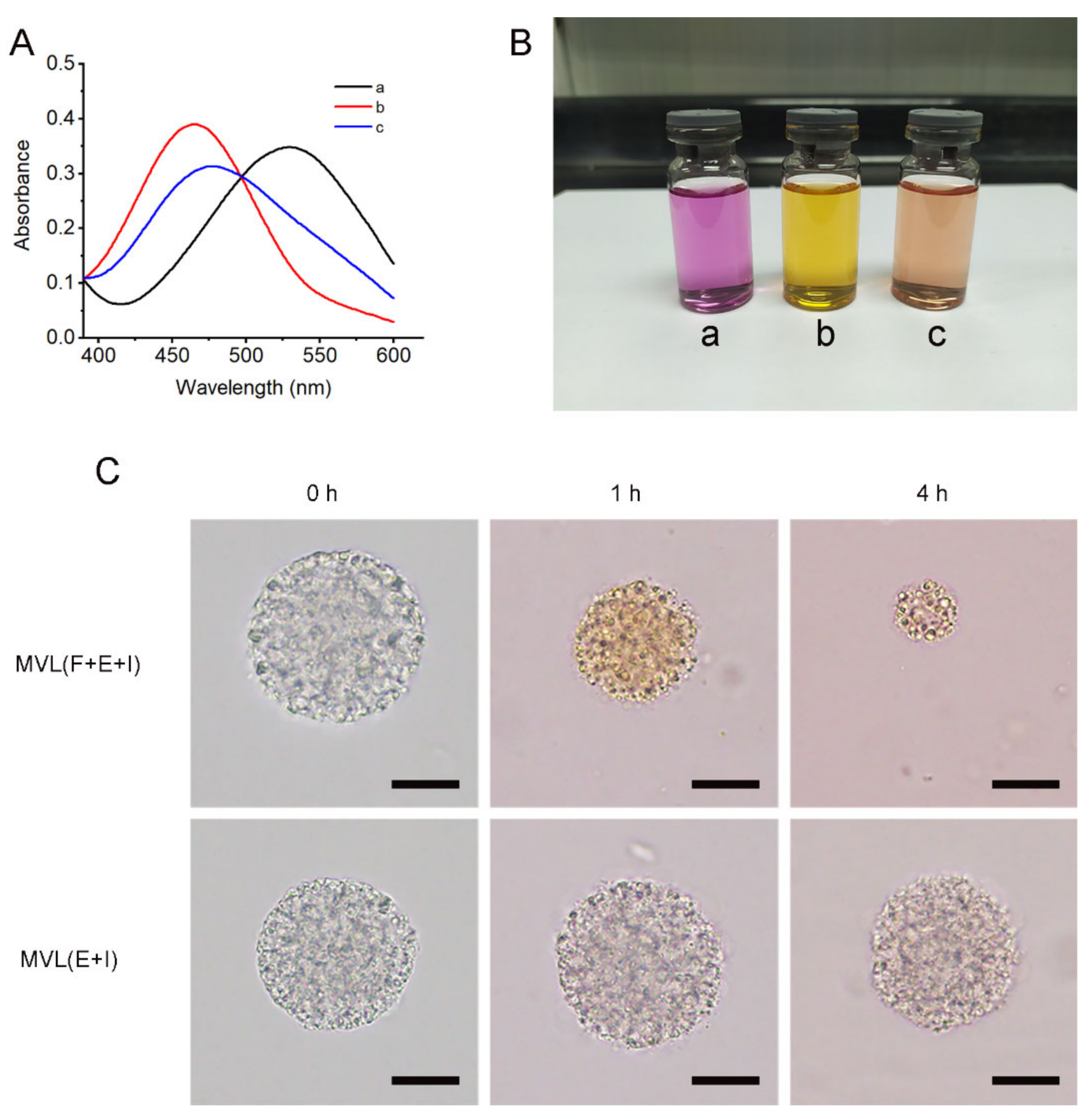
Alizarin red with an ortho-hydroxyl group was selected as the probe to examine the interaction between 1,2-diols and FOP. UV absorption spectra of 10−4 M Alizarin red (a), a mixture of 10−4 M Alizarin red and 10−3 M FOP (b), and a mixture of 10−4 M Alizarin red, 10−3 M FOP, and 10−1 M glucose (c) in PBS (pH 7.4) were determined in the range of 390–600 nm using a UV spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan). Ethanol was added to the PBS (pH 7.4) to improve FOP solubility in the solvent. The color of each solution was also determined.
2.7. Interaction between Alizarin Red and MVL Membrane
An appropriate amount of freshly prepared Alizarin red solution was added to MVL encapsulating enzymes and insulin (MVL(E+I)), or MVL encapsulating FOP, enzymes, and insulin (MVL(F+E+I)) suspensions, respectively, and the samples were incubated at 37 °C in a water bath. At indicated time points, MVL particles were washed with PBS (pH 7.4) and observed under an optical microscope. The morphology and color of the MVL particles were examined to analyze the interaction between Alizarin red and the MVL membrane.
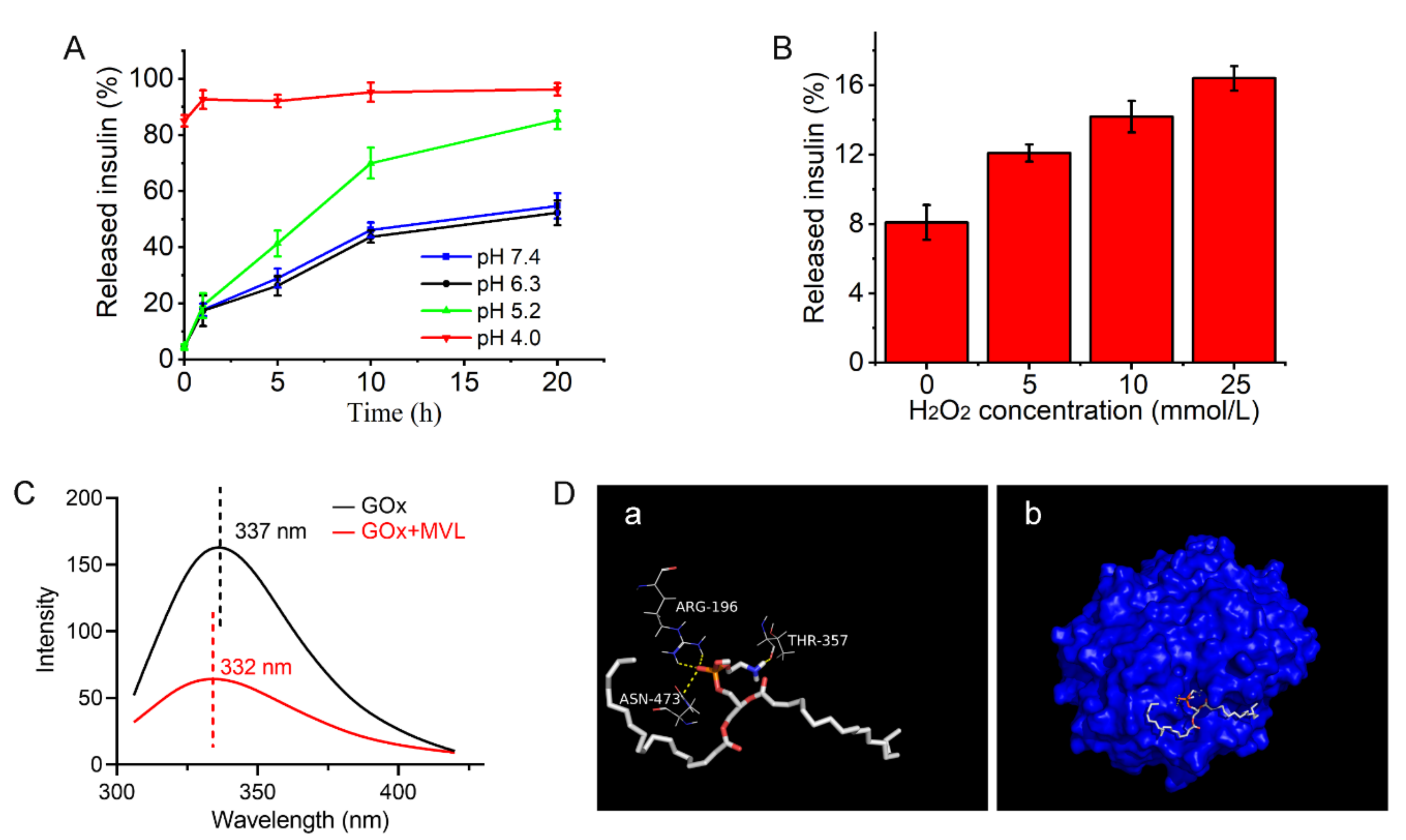
2.8. pH and H2O2 Sensitivities of MVLs
MVLs were incubated in PBS with different concentrations of gluconic acid or H
2O
2 to assess the pH or H
2O
2 sensitivities of MVLs, respectively. The concentrations of gluconic acid and H
2O
2 in the release media were determined according to the BGLs under physiological conditions [
32,
33]. The release media containing gluconic acid at different pH values (pH = 7.4, 6.3, 5.2, or 4.0) and the release media with different concentrations of H
2O
2 (0, 5, 10, or 25 mmol/L) were used for the in vitro experiments. The in vitro release method was the same as that described in
Section 2.5.
2.9. Interaction between GOx and MVL mMembrane
2.9.1. Fluorescence Spectroscopy
In brief, GOx was dissolved in PBS (pH 7.4) at a concentration of 4 mg/mL, and the fluorescence emission spectra of the GOx solutions, both in the absence and presence of blank MVLs, were collected using a fluorescence spectrometer (RF-5301PC, Shimadzu, Kyoto, Japan). Fluorescence emission spectra (excitation at 282 nm) were recorded in the range of 305–420 nm at 25 °C, with an excitation slit width of 20 nm and an emission slit width of 5 nm. The maximum emission wavelength of each spectrum was recorded, and the blue or red shift of the maximum emission wavelength reflected the interaction of GOx and the MVL membrane [
34].
2.9.2. Docking Study
The X-ray crystallographic structure of GOx (PDB code: 3QVR) was obtained from the Protein Data Bank. DSPE, DOPC, DPPG, FOP, and cholesterol were preprocessed using ChemBio3D Ultra 14.0 (PerkinElmer, Waltham, MA, USA) and ChemBioDraw Ultra 14.0 (PerkinElmer, Waltham, MA, USA). The interactions between GOx and membrane materials were performed using AutoDock Tools 1.5.6 (Scripps Research Institute, San Diego, CA, USA) and AutoDock Vina (Scripps Research Institute, San Diego, CA, USA). The docking results were analyzed using PyMOL (Schrodinger, New York, NY, USA) [
35]. Hydrogen bonds and docking diagram between GOx and membrane materials were recorded to analyze the interaction between GOx and the MVL membrane.
2.10. In Vivo Studies for Type 1 Diabetes Treatment
The diabetic rat (male Sprague-Dawley rat) model was established by streptozotocin administration according to a previously described method [
36,
37]. Fasting BGLs were measured using a blood glucose meter (5DM-2A, Yicheng Biotech. Co., Ltd., Beijing, China). Hyperglycemic rats that maintained stable BGLs (> 16.7 mmol/L) were randomly divided into four groups (five rats per group) and subcutaneously administered MVL(E+I), MVL(F+E+I), MVL encapsulating FOP and insulin (MVL(F+I)), and MVL encapsulating FOP and enzymes (MVL(F+E)), respectively. The insulin dosage was set at 12 IU/kg for MVL treatments. BGLs were measured using a blood glucose meter at specific time points after administration. Meanwhile, the concentration of serum insulin was determined using an insulin ELISA kit in accordance with the manufacturer’s instructions. Moreover, to perform an intraperitoneal glucose tolerance test (IPGTT), diabetic rats were administered a glucose injection (2 g/kg). Hyperglycemic rats were fasted for 6 h, with free access to water before IPGTT. A single IPGTT was performed 3 h after administering MVL(F+E+I) or insulin solution. The insulin dosage was set at 5 IU/kg for MVL(F+E+I) and the insulin solution. Multiple IPGTTs were performed 2 h post-MVL(F+E+I) administration, and the insulin dosage was set at 12 IU/kg. Glucose solution was injected every 2 h, thrice in total. Multiple IPGTTs in healthy rats were used as controls. In addition, the side effects were evaluated in healthy rats by administering MVL(F+E+I) and insulin solution.
2.11. Cytotoxicity Study
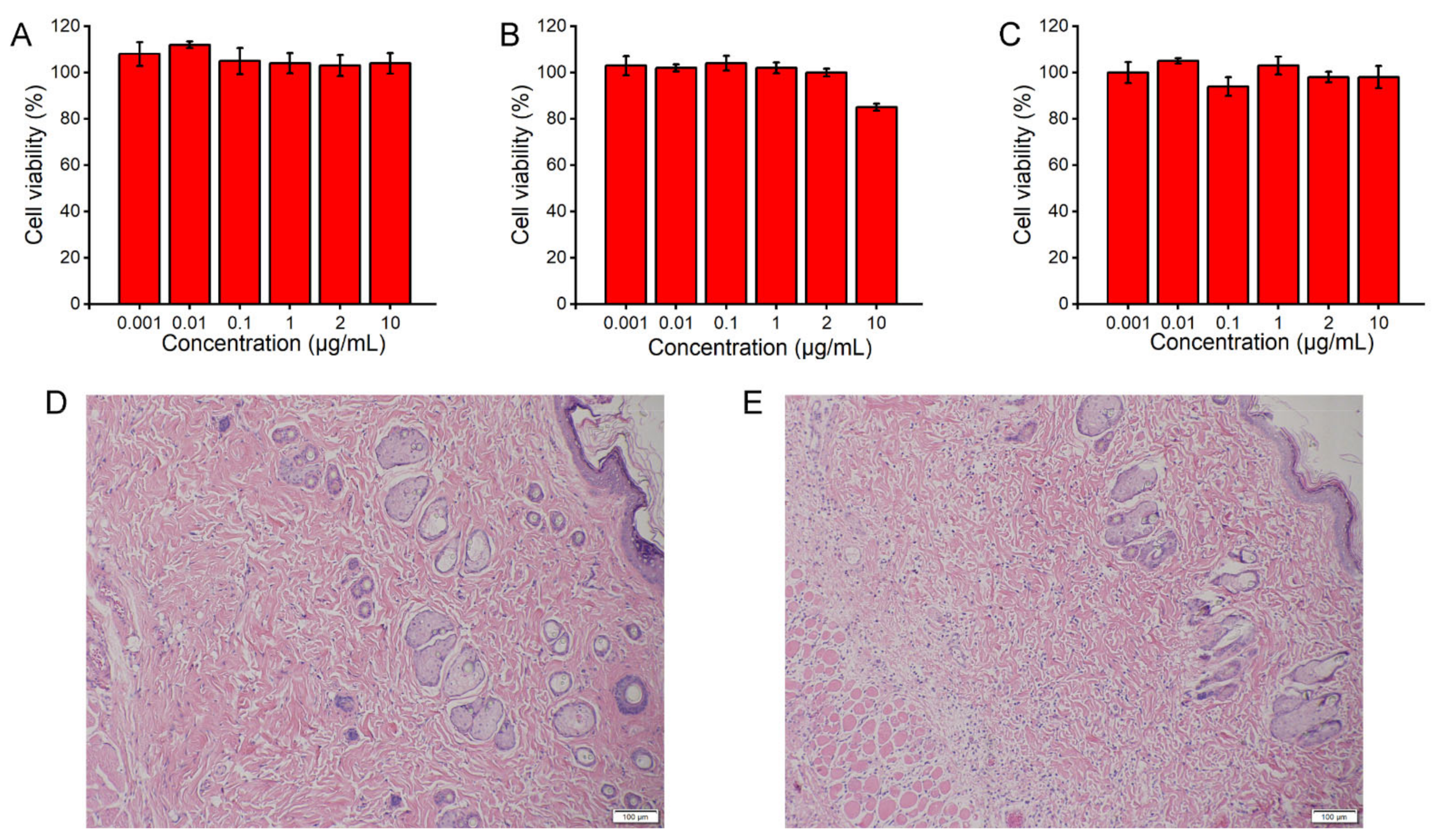
The cytotoxicity of PBA derivatives was examined in HeLa cells using the 3-(4,5)-dimethylthiahiazo(-z-yl)-3,5-di-phenytetrazoliumromide (MTT) assay [
38]. Briefly, HeLa cells were seeded in 96-well plates at a density of 6000 cells per well and cultured for 24 h. Then, serially diluted PBA derivatives (ranging from 0.001 to 10 μg/mL) were added to the wells. After 24 h of incubation at 37 °C, the cells were subjected to the MTT assay.
2.12. Histopathological Evaluation
Briefly, diabetic rats subcutaneously administered MVL(F+E+I) or the blank solution (306 mM glycine and 1 mM lysine; pH 7.1) were euthanized by CO2 asphyxiation; the insulin dosage of MVL(F+E+I) was set at 12 IU/kg. Then, 24 h after injection, the tissues around the injection site were excised, fixed in 10% formalin, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E) for histological analysis.
2.13. Statistical Analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA or unpaired t-test, and p < 0.05 was deemed a minimal level of significance.
4. Discussion
In patients with diabetes, the large fluctuation in BGLs has been associated with several complications, and the use of glucose-responsive insulin delivery systems is an important means to maintain blood glucose homeostasis. Previous studies examining potential glucose-responsive systems have largely focused on nanoparticles, microgels, and micelles [
42]; however, it is difficult to achieve good glucose control and biocompatibility for these delivery systems. In the present study, glucose-sensitive elements were introduced into MVLs to render formulated MVLs glucose-responsive. In the MVL preparation process (
Figure 3), W
2 was added twice. An appropriate volume of W
2 was added the first time to efficiently form a W
1/O/W
2 emulsion; it was then added a second time to accelerate the removal of the organic solvent. After the formation of the W
1/O/W
2 emulsion, further vortexing allowed the formation of uniformly sized MVL particles. In addition, W
1 and W
2 should have similar pH values, which is critical for preparing pH-sensitive MVLs. If the pH value of W
1 significantly differs from that of W
2, the flow of hydrogen ions between W
1 and W
2 can cause structural changes in the DSPE, decreasing the stability of the MVLs [
43]. The EE of insulin increased with a decrease in the DSPE ratio (
Table 1); this could be attributed to the rigidity of DSPE. The alkyl chain of DOPC contains two double bonds, whereas that of DSPE is saturated. On decreasing the DSPE ratio, the rigidity of liposomal membranes decreases to stabilize the structure of MVLs, thus increasing the EE of insulin. For the final MVLs, almost all the insulin was encapsulated in the internal vesicles; this affords the basis for sustained and controlled insulin release [
23]. Insulin encapsulated in the MVLs accumulates at the injection site, and released insulin is absorbed into the blood and exerts its pharmacological effect.
Given its large size, MVL(F+E+I) remains at the injection site to slowly release insulin following subcutaneous administration. Cryo-SEM image indicates that the multivesicular structure of MVL(F+E+I) was slightly destroyed (
Figure 4E); this was because ice crystals or mechanical forces led to the destruction of liposomal membranes during sample manipulation. This multivesicular structure plays a vital role in its hypoglycemic effect. Although the outer liposomal membrane is destroyed due to high glucose stimulation, the internal structure of MVLs can remain intact to preserve insulin. As described in the literature, MVLs present enormous potential in drug delivery based on their unique structure [
22,
24].
In the in vitro release experiments (
Figure 6), the insulin release rate from MVLs in the 100 mg/dL glucose solution was marginally reduced when compared with the glucose-free solution. The difference between the two release curves could be attributed to the protective effect of glucose enrichment. In the 100 mg/dL glucose solution, the generated gluconic acid and H
2O
2 are not sufficient to destroy liposomal membranes. The glucose enrichment on the surface of MVLs prevents insulin release. From 100 to 400 mg/dL, the insulin release rate increased with increasing glucose concentrations. In the 200 and 400 mg/dL glucose solutions, the local reduced pH and generated H
2O
2 destroyed liposomal membranes and triggered insulin release. This effect is strong when compared with the protective effect of glucose enrichment, thus accelerating insulin release. Notably, this insulin release behavior is of considerable significance for the effective regulation of BGLs.
The trigger mechanism based on in situ catalysis affords the basis for the controlled release of insulin in MVLs. The selection of PBA derivatives is a critical factor in achieving in situ catalysis. Following the screening process, FOP, possessing a fluorinated benzene ring and an eight-carbon aliphatic chain, was selected to participate in the construction of the glucose-responsive systems. In this work, the pKa value of FBP (6.79) was lower than that of BP (7.53), and the pKa value of FOP (6.90) was lower than that of OP (7.84). The pKa values of fluorinated compounds were lower than that of non-fluorinated compounds, consistent with previously reported findings in the literature [
44]. The introduction of an electron-withdrawing fluorine atom can reduce the pKa value of OP. Therefore, FOP has a higher affinity to glucose than OP. In addition, the EE of FOP (18.75 ± 2.50%) was higher than that of FBP (3.10 ± 0.52%); this could be explained by the lipid solubility of FOP when compared with FBP. The longer the alkyl chain of PBA derivatives, the better the lipid solubility of PBA derivatives [
45]. The alkyl chain of FOP has eight carbon atoms, whereas FBP has only four carbon atoms. Accordingly, the eight-carbon aliphatic chain confers high lipid solubility on FOP. FOP in the liposomal membrane binds with glucose through reversible boronate formation, resulting in glucose enrichment on the MVL surface (
Figure 7). Previous studies have confirmed that the ortho-hydroxyl group of glucose can form a complex with PBA [
46]. Glucose cannot be observed under an optical microscope; thus, the Alizarin red containing ortho-hydroxyl group was introduced to confirm the interaction between 1,2-diols and the MVL membrane. GOx outside MVLs is slowly metabolized by lymphatic capillaries owing to its high molecular weight (approximately 150 kDa) and can accumulate subcutaneously for a sufficient period to induce catalysis [
47,
48]. Moreover, we identified that GOx and MVLs interacted via hydrophobic interactions and hydrogen bonds, which were confirmed by fluorescence experiments and molecular docking, respectively. The interactions between GOx and MVLs are also beneficial for in situ catalysis. Notably, a molecular docking method for analyzing the interaction between GOx and liposomal membranes was proposed in the present study.
In situ, GOx catalyzes the rapid and efficient conversion of glucose into gluconic acid and H
2O
2, and the local acidic and H
2O
2-enriched microenvironment affords the basis for the glucose responsiveness of MVL(F+E+I). Therefore, the pH and H
2O
2 sensitivities of MVL(F+E+I) render it glucose-responsive. In the present study, the dual sensitivity of MVL(F+E+I) was confirmed by in vitro release tests. Almost all the insulin was released at pH 4.0 in 1 h, whereas only a small amount of insulin was released at pH 7.4 (
Figure 8A). The pH sensitivity of MVL(F+E+I) is conferred by DSPE in the membrane, and the protonation of DSPE leads to lipid membrane instability [
43,
49]. Notably, the pH-sensitive MVLs were obtained for the first time. This pH-sensitive MVL could be employed as a carrier to deliver various therapeutic agents for disease treatment. Moreover, the insulin release rate of MVL(F+E+I) increased with increasing H
2O
2 concentrations (
Figure 8B). The local H
2O
2-enriched environment can induce lipid peroxidation [
50,
51]. Then, the permeability and stability of liposomal membranes are modified, which leads to the destruction of liposomal structures.
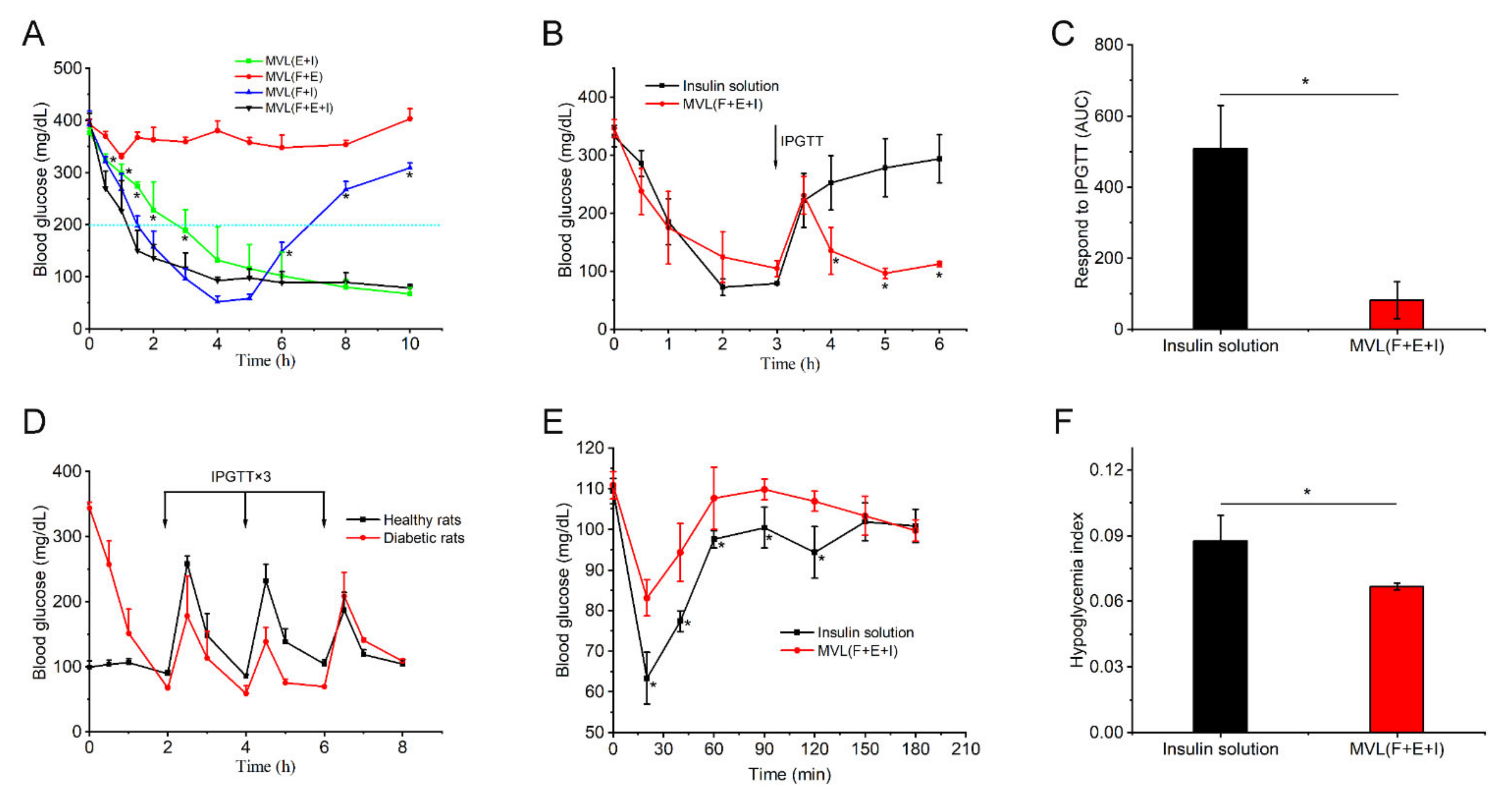
In animal studies, MVL(F+E+I) exhibited excellent regulation of BGLs, good resistance to glucose challenge, and low hypoglycemic index (
Figure 9). MVL(F+E+I) showed a better antidiabetic efficacy than MVL(F+I) or MVL(E+I) (
Figure 9A). BGLs were decreased gradually in the MVL(E+I) group when compared with the MVL(F+E+I) group; this can be explained by the lack of in situ catalysis of GOx based on FOP in the MVL(E+I) group. The addition of FOP induced glucose enrichment on the surface of MVL(F+E+I). Given the glucose enrichment, MVL(F+E+I) quickly responded to glucose to release insulin under hyperglycemic conditions. In the MVL(F+I) group, BGLs steadily increased after 4 h, as MVL(F+I) could not provide sufficient insulin after the initial burst release. In contrast, BGLs in the MVL(F+E+I) group were maintained within the normoglycemic range (< 200 mg/dL) after an initial reduction in BGLs. The acidic and H
2O
2-enriched microenvironment surrounding the MVL(F+E+I) membrane could be formed under hyperglycemic conditions due to the addition of enzymes, and BGLs could be reduced and maintained within the normal range. Therefore, FOP and biological enzymes play a crucial role in the glucose responsiveness of MVLs; this is in accordance with the original hypothesis. The peak serum insulin level could be achieved in less than 1 h following a subcutaneous insulin injection (
Figure 9E); our finding is consistent with previous studies [
32]. For MVL(F+E+I), the peak serum insulin level was reached in approximately 2 h (
Figure S17). Under hyperglycemic conditions, sufficient insulin released from MVL(F+E+I) reduced BGLs to the normoglycemic level. Accordingly, the peak serum insulin level was delayed following MVL(F+E+I) treatment when compared with the insulin solution. Moreover, when blood glucose decreased to the normal level, insulin release was reduced to maintain BGLs in the normoglycemic state. Finally, we determined the cytotoxicity of PBA derivatives and inflammatory reaction of MVL(F+E+I), and the results confirmed the safety of MVL(F+E+I) (
Figure 10). In comparison with other reported systems, composed of synthetic materials, MVLs are both biodegradable and biocompatible, since their components are mainly derived from naturally occurring lipids.
Our research still has several limitations. Further research is crucial for optimizing the sensitivity of the glucose response and establishing long-term animal experiments assessing MVLs. Moreover, the docking model of MVLs needs to be constructed and used to analyze the interaction of MVLs and proteins. Despite these limitations, this MVL with its modified membrane offers a clinical opportunity for glucose-responsive insulin delivery and is expected to maintain blood glucose homeostasis precisely to reduce the complications in patients with diabetes.