Development and Evaluation of an In Silico Dermal Absorption Model Relevant for Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dermal Absorption Modeling Preliminaries
2.2. Physiological and Anatomical Changes in Skin as a Function of Age
2.3. Development of an Age-Dependent Dermal Absorption Model
2.3.1. Development of Model Structure
2.3.2. Maturation Models for Age-Dependent Model Parameters
2.4. Age-Dependent Dermal Absorption Model Optimization and Evaluation
2.4.1. Dermal Absorption Model Sensitivity Analysis and Parameter Uncertainty
2.4.2. Model Optimization and Evaluation
- S1.
- S2.
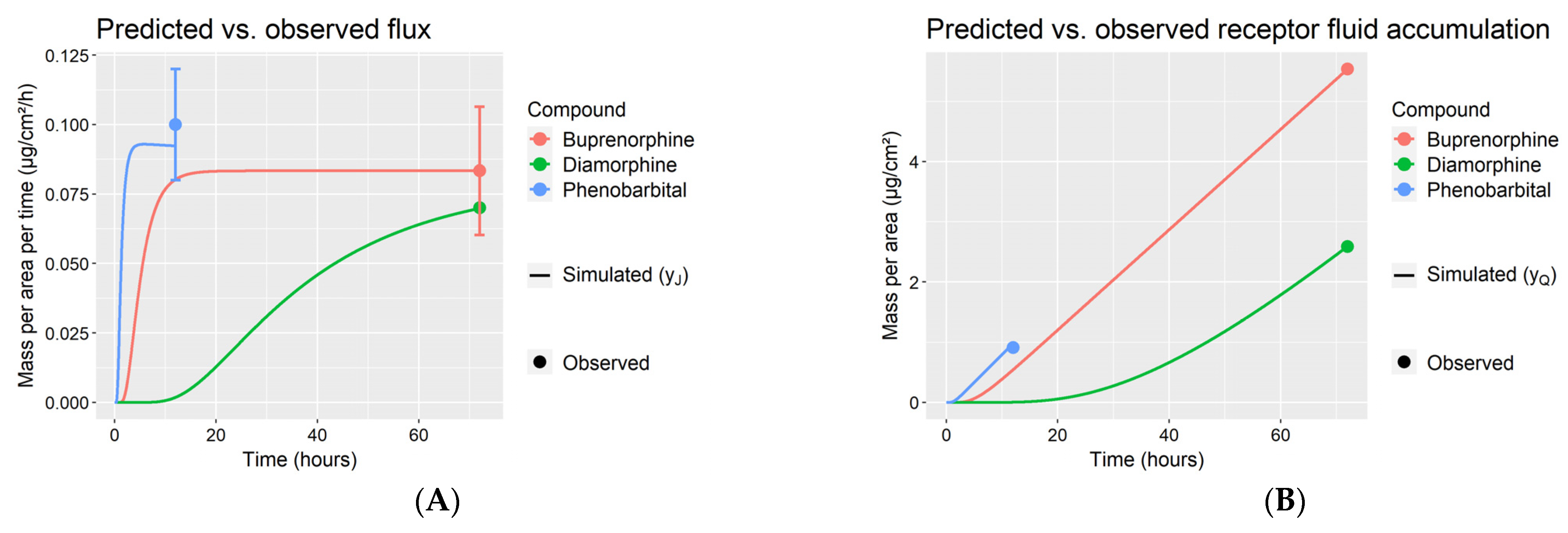
- The uncertain age-independent parameters , to which the model outputs (5) and (6) are sensitive, were estimated by fitting outputs and from the adult model generated in step S1 to the observed adult flux and receptor fluid accumulation in Table 2. Model fitting was performed via the Monte Carlo parameter identification algorithm in MoBi (Open Systems Pharmacology v.9.1), initiated from 10 randomly selected initial values.
- S3.
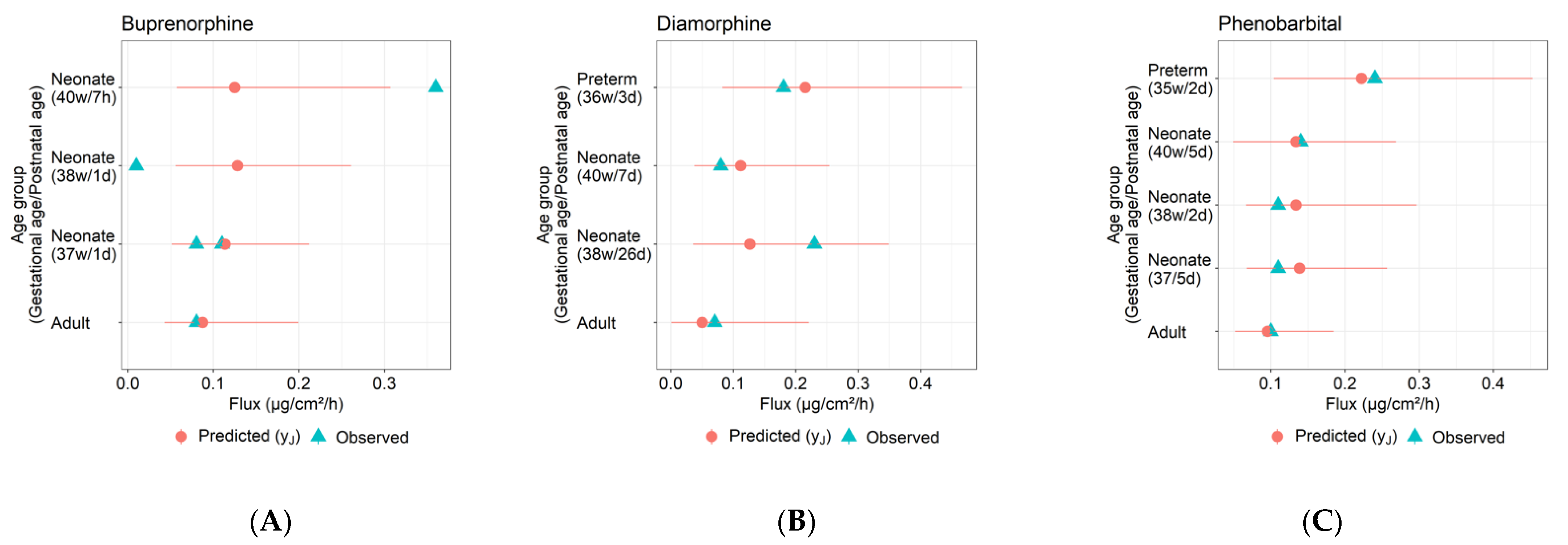
- Infant skin permeation by the compound was simulated assuming the experimental conditions and skin ages in Table 3 using the optimized parameter values obtained in step S2. A total of 100 simulations of the model were run for each infant skin on which the compound was experimentally tested. Each such simulation was run after updating the model with a new sample from the distribution of age-dependent parameters . For each experiment, the mean and 95% confidence intervals of the simulated permeant flux were evaluated and compared with the corresponding observed flux in Table 3.
2.5. Identification of Critical Input Parameter
3. Results
3.1. Physiological and Anatomical Changes in Skin as a Function of Age
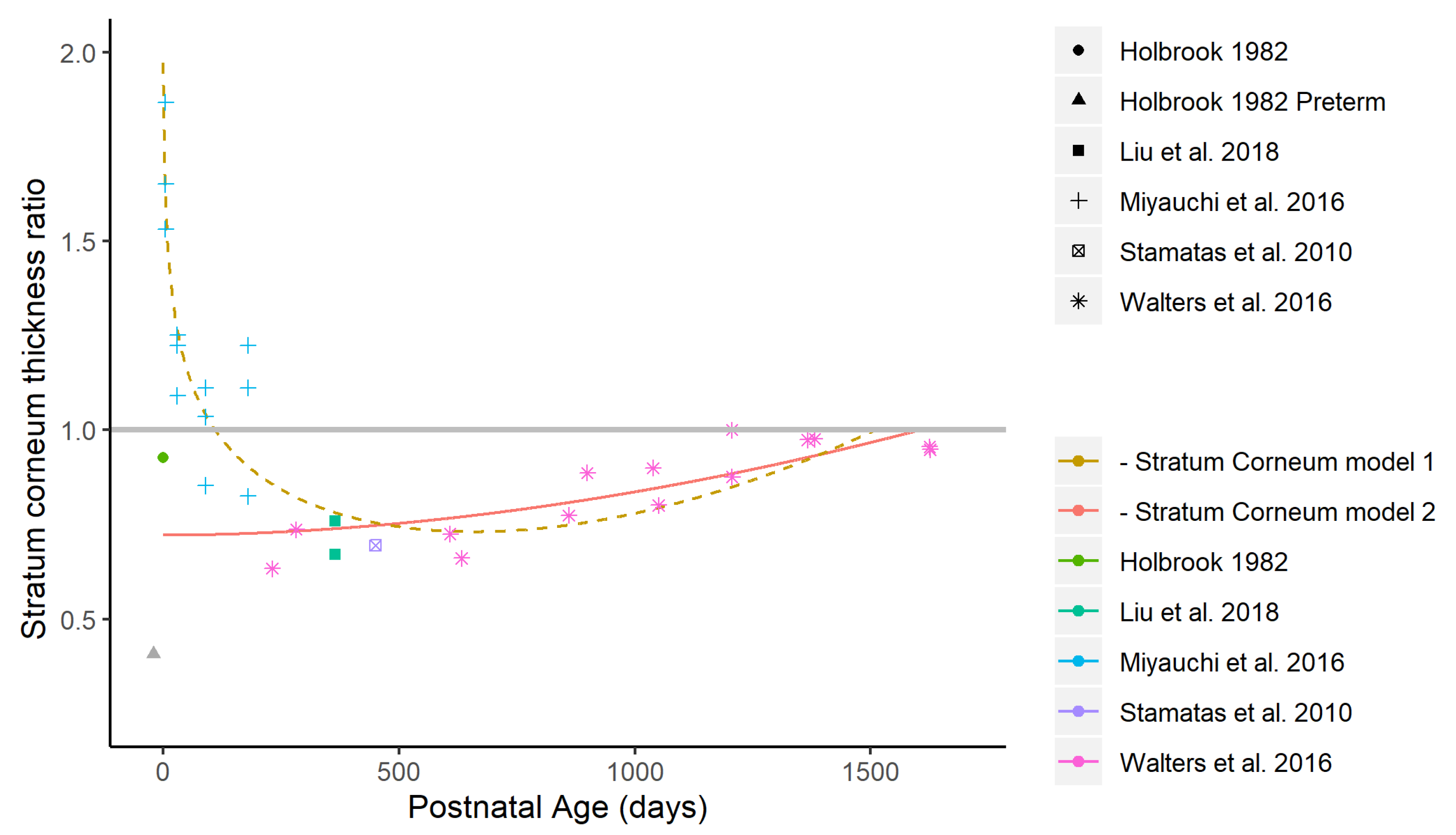
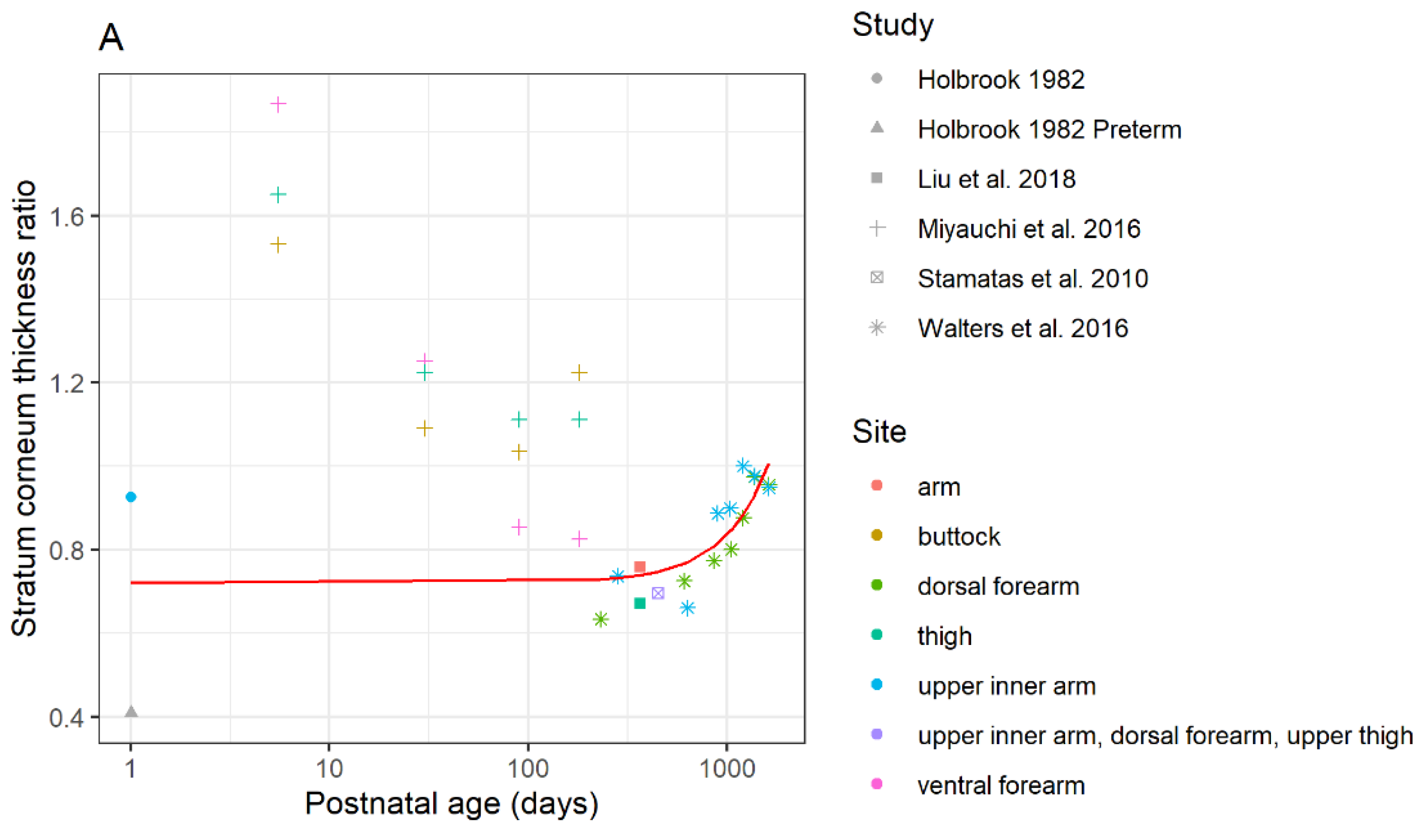
3.1.1. Differences in Stratum Corneum Thickness
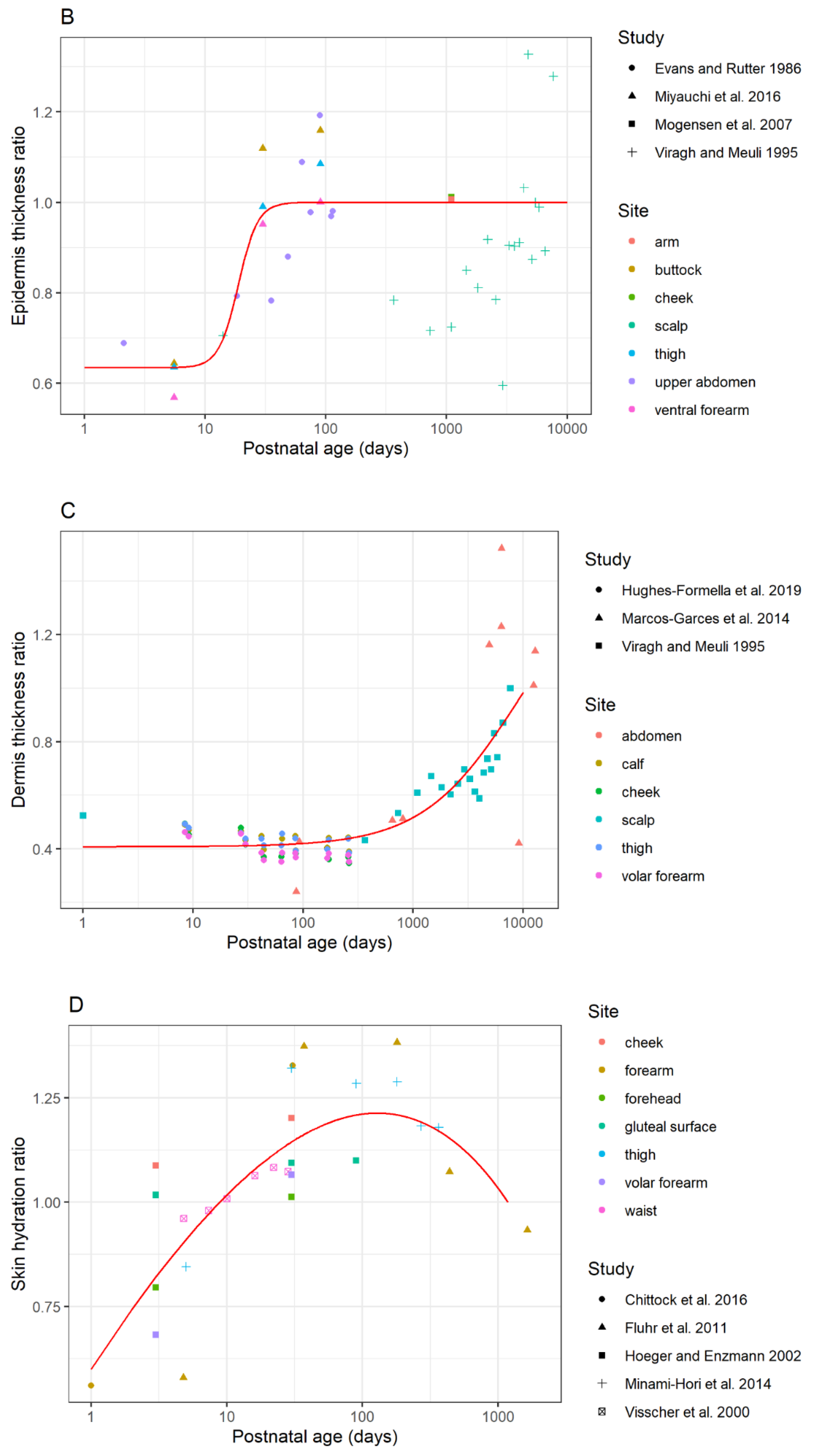
3.1.2. Differences in the Thickness of the Viable Epidermis between Children and Adults
3.1.3. Differences in the Dermal Thickness between Children and Adults
3.1.4. Difference in Skin Hydration of Child and Adult Epidermal Barrier
3.1.5. Differences in the Corneocyte Volume Fraction
3.1.6. Differences in the Lipid/Protein Ratio
3.1.7. Differences in Albumin Concentration
3.1.8. Differences in Skin Blood Flow in Children
3.1.9. Differences in Surface Skin pH and Follicle Density/Size of Children and Adults
3.2. Development of a Dermal Absorption Model (Age-Dependent)
Maturation Models for Age-Dependent Model Parameters
3.3. Age-Dependent Dermal Absorption Model Optimization and Evaluation
3.3.1. Dermal Absorption Model Sensitivity Analysis and Parameter Uncertainty
3.3.2. Model Optimization and Evaluation
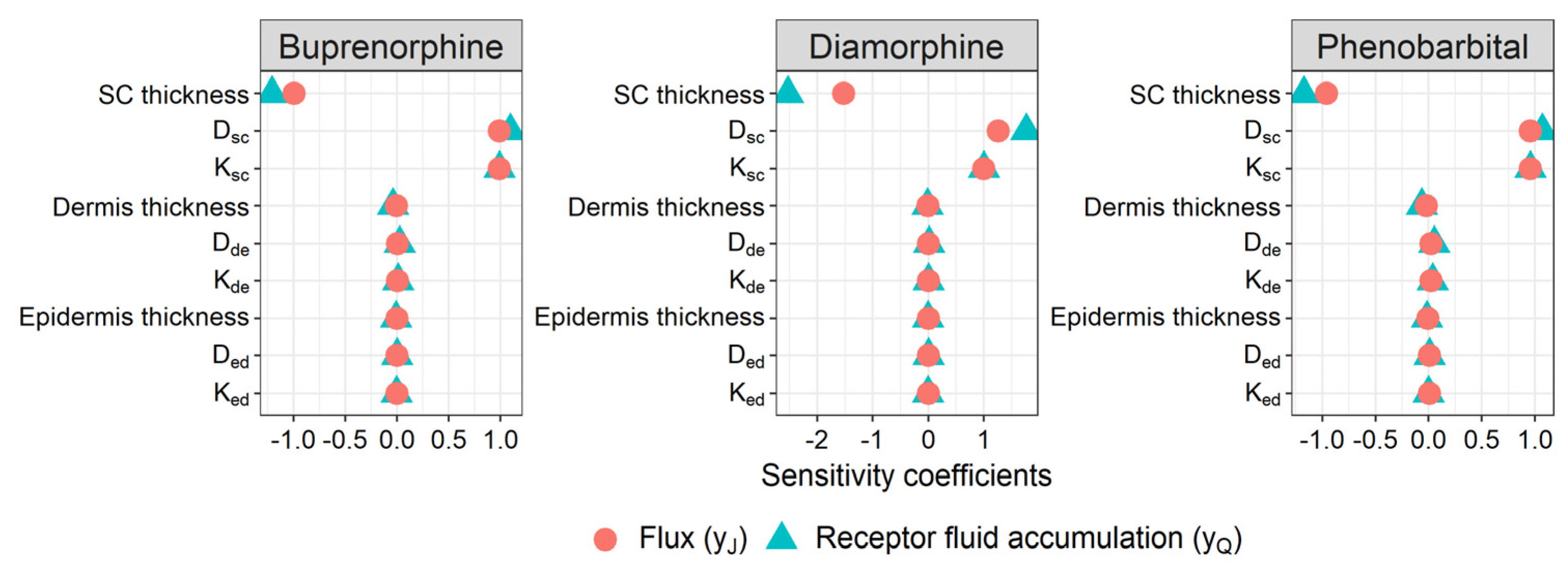
3.4. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Alternative Maturation Model
References
- Anissimov, Y.G. Mathematical models for skin toxicology. Expert Opin. Drug Metab. Toxicol. 2014, 10, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Dancik, Y.; Miller, M.A.; Jaworska, J.; Kasting, G.B. Design and performance of a spreadsheet-based model for estimating bioavailability of chemicals from dermal exposure. Adv. Drug Deliv. Rev. 2013, 65, 221–236. [Google Scholar] [CrossRef]
- Dancik, Y.; Bigliardi, P.L.; Bigliardi-Qi, M. What happens in the skin? Integrating skin permeation kinetics into studies of developmental and reproductive toxicity following topical exposure. Reprod. Toxicol. 2015, 58, 252–281. [Google Scholar] [CrossRef]
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Anissimov, Y.G.; Jepps, O.G.; Dancik, Y. Roberts MS: Mathematical and pharmacokinetic modelling of epidermal and dermal transport processes. Adv. Drug Deliv. Rev. 2013, 65, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Jepps, O.G.; Dancik, Y.; Anissimov, Y.G.; Roberts, M.S. Modeling the human skin barrier--towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013, 65, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Law, R.M.; Ngo, M.A.; Maibach, H.I. Twenty Clinically Pertinent Factors/Observations for Percutaneous Absorption in Humans. Am. J. Clin. Dermatol. 2020, 21, 85–95. [Google Scholar] [CrossRef]
- Roberts, M.S. Targeted drug delivery to the skin and deeper tissues: Role of physiology, solute structure and disease. Clin. Exp. Pharmacol. Physiol. 1997, 24, 874–879. [Google Scholar] [CrossRef]
- Debeer, S.; Le Luduec, J.B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.F.; Dubois, B.; Kanitakis, J. Comparative histology and immunohistochemistry of porcine versus human skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar] [PubMed]
- McLafferty, E.; Hendry, C.; Alistair, F. The integumentary system: Anatomy, physiology and function of skin. Nurs. Stand. 2012, 27, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K. New insights into skin structure: Scratching the surface. Adv. Drug Deliv. Rev. 2002, 54, S3–S17. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, A.B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 1999, 34, 777–797. [Google Scholar]
- Cices, A.; Bayers, S.; Verzì, A.E.; Schachner, L.A.; West, D.P.; Micali, G. Poisoning Through Pediatric Skin: Cases from the Literature. Am. J. Clin. Dermatol. 2017, 18, 391–403. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Alvarez-Rivera, G.; Lamas, J.P.; Llompart, M.; Lores, M.; Garcia-Jares, C. Content of suspected allergens and preservatives in marketed baby and child care products. Anal. Methods 2013, 5, 416–427. [Google Scholar] [CrossRef]
- Mathieu-Huart, A.; De Lentdecker, C.; Riviere, G.; Sissoke, F.; Rousselle, C. French Agency for Food, Environmental and Occupational Health and Safety (ANSES) health reference values (RV). Arch. Mal. Prof. Environ. 2014, 75, 29–301. [Google Scholar]
- Ginsberg, G.; Hattis, D.; Sonawane, B. Incorporating pharmacokinetic differences between children and adults in assessing children’s risks to environmental toxicants. Toxicol. Appl. Pharmacol. 2004, 198, 164–183. [Google Scholar] [CrossRef]
- Lansdown, A.B. Epidermal differentiation in normal and growth-retarded infants: Studies in two animal models and in human babies. Br. J. Dermatol. 1978, 99, 139–146. [Google Scholar] [CrossRef]
- Evans, N.J.; Rutter, N. Development of the epidermis in the newborn. Biol. Neonate 1986, 49, 74–80. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Nikolovski, J.; MacK, M.C.; Kollias, N. Infant skin physiology and development during the first years of life: A review of recent findings based on in vivo studies. Int. J. Cosmet. Sci. 2011, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, A.; Troutman, J.; Edginton, A.N. Assessment of Vehicle Volatility and Deposition Layer Thickness in Skin Penetration Models. Pharmaceutics 2021, 13, 807. [Google Scholar] [CrossRef]
- Hewitt, N.J.; Gregoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Genies, C.; et al. Measurement of the penetration of 56 cosmetic relevant chemicals into and through human skin using a standardized protocol. J. Appl. Toxicol. 2020, 40, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasting, G.B.; Miller, M.A. Kinetics of finite dose absorption through skin 2: Volatile compounds. J. Pharm. Sci. 2006, 95, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing; Vienna, Austria, 2021; Available online: http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 17 October 2021).
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013. [Google Scholar]
- Barrett, D.; Rutter, N.; Kurihara-Bergstrom, T.; Davies, S. Buprenorphine permeation through premature neonatal skin. Pharm. Sci. Commun. 1994, 4, 125. [Google Scholar]
- Barrett, D.A.; Rutter, N.; Davis, S.S. An in vitro study of diamorphine permeation through premature human neonatal skin. Pharm. Res. 1993, 10, 583–587. [Google Scholar] [CrossRef]
- Bonina, F.; Montenegro, L.; Micali, G.; West, D.; Palicharla, P.; Koch, R. In vitro percutaneous absorption evaluation of phenobarbital through hairless mouse, adult and premature human skin. Int. J. Pharm. 1993, 98, 93–99. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.J.; Gindulyte, A.; He, J.; He, S.Q.; Li, Q.L.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kushner, I.V.J.; Deen, W.; Blankschtein, D.; Langer, R. First-principles, structure-based transdermal transport model to evaluate lipid partition and diffusion coefficients of hydrophobic permeants solely from stratum corneum permeation experiments. J. Pharm. Sci. 2007, 96, 3236–3251. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E. Roberts MS: Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [PubMed] [Green Version]
- Stamatas, G.N.; Nikolovski, J.; Luedtke, M.A.; Kollias, N.; Wiegand, B.C. Infant Skin Microstructure Assessed In Vivo Differs from Adult Skin in Organization and at the Cellular Level. Pediatr. Dermatol. 2010, 27, 125–131. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Danby, S.G.; Cork, M.J.; Stamatas, G.N. Infant Skin Barrier, Structure, and Enzymatic Activity Differ from Those of Adult in an East Asian Cohort. Biomed Res. Int. 2018, 2018, 1302465. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, Y.; Shimaoka, Y.; Fujimura, T.; Koike, Y.; Yatabe, M.; Nishikawa, M.; Hayashi, M.; Sugata, K.; Moriwaki, S.; Hatamochi, A. Developmental Changes in Neonatal and Infant Skin Structures During the First 6 Months: In Vivo Observation. Pediatr. Dermatol. 2016, 33, 289–295. [Google Scholar] [CrossRef]
- Fairley, J.A.; Rasmussen, J.E. Comparison of stratum corneum thickness in children and adults. J. Am. Acad. Dermatol. 1983, 8, 652–654. [Google Scholar] [CrossRef]
- Tsugita, T.; Nishijima, T.; Kitahara, T.; Takema, Y. Positional differences and aging changes in Japanese woman epidermal thickness and corneous thickness determined by OCT (optical coherence tomography). Skin Res. Technol. 2013, 19, 242–250. [Google Scholar] [CrossRef]
- Anderson, R.L.; Cassidy, J.M. Variation in physical dimensions and chemical composition of human stratum corneum. J. Investig. Dermatol. 1973, 61, 30–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maibach, H.I.; Boisits, E.K. Neonatal Skin: Structure and Function; M. Dekker: New York, NY, USA, 1982. [Google Scholar]
- Walters, R.M.; Khanna, P.; Chu, M.; Mack, M.C. Developmental Changes in Skin Barrier and Structure during the First 5 Years of Life. Ski. Pharmacol. Physiol. 2016, 29, 111–118. [Google Scholar] [CrossRef]
- de Viragh, P.A.; Meuli, M. Human scalp hair follicle development from birth to adulthood: Statistical study with special regard to putative stem cells in the bulge and proliferating cells in the matrix. Arch. Dermatol. Res. 1995, 287, 279–284. [Google Scholar] [CrossRef]
- Mogensen, M.; Morsy, H.A.; Thrane, L.; Jemec, G.B. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology 2008, 217, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Roberts, M.S. Use of In Vitro Human Skin Membranes to Model and Predict the Effect of Changing Blood Flow on the Flux and Retention of Topically Applied Solutes. J. Pharm. Sci. 2008, 97, 3442–3450. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Kakasheva-Mazhenkovska, L.; Milenkova, L.; Gjokik, G.; Janevska, V. Variations of the histomorphological characteristics of human skin of different body regions in subjects of different age. Prilozi 2011, 32, 119–128. [Google Scholar] [PubMed]
- Hughes-Formella, B.; Wunderlich, O.; Williams, R.; Fernández, J.; Kim, Y.Z.; Wigger-Alberti, W.; Pecquet, S.; Moodycliffe, A. Comparison of Skin Structural and Functional Parameters in Well-Nourished and Moderately Undernourished Infants. Skin Pharmacol. Physiol. 2019, 32, 212–223. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing-a morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef]
- Ryatt, K.S.; Mobayen, M.; Stevenson, J.M.; Maibach, H.I.; Guy, R.H. Methodology to measure the transient effect of occlusion on skin penetration and stratum corneum hydration in vivo. Br. J. Dermatol. 1988, 119, 307–312. [Google Scholar] [CrossRef]
- Berardesca, E. European Group for Efficacy Measurements on C, Other Topical P: EEMCO guidance for the assessment of stratum corneum hydration: Electrical methods. Ski. Res. Technol. 1997, 3, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chittock, J.; Cooke, A.; Lavender, T.; Brown, K.; Wigley, A.; Victor, S.; Cork, M.J.; Danby, S.G. Development of stratum corneum chymotrypsin-like protease activity and natural moisturizing factors from birth to 4 weeks of age compared with adults. Br. J. Dermatol. 2016, 175, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Fluhr, J.W.; Darlenski, R.; Lachmann, N.; Baudouin, C.; Msika, P.; De Belilovsky, C.; Hachem, J.P. Infant epidermal skin physiology: Adaptation after birth. Br. J. Dermatol. 2012, 166, 483–490. [Google Scholar] [CrossRef]
- Garcia Bartels, N.; Mleczko, A.; Schink, T.; Proquitté, H.; Wauer, R.R.; Blume-Peytavi, U. Influence of bathing or washing on skin barrier function in newborns during the first four weeks of life. Skin Pharmacol. Physiol. 2009, 22, 248–257. [Google Scholar] [CrossRef]
- Garcia Bartels, N.; Scheufele, R.; Prosch, F.; Schink, T.; Proquitté, H.; Wauer, R.R.; Blume-Peytavi, U. Effect of Standardized Skin Care Regimens on Neonatal Skin Barrier Function in Different Body Areas. Pediatr. Dermatol. 2010, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, P.H.; Enzmann, C.C. Skin Physiology of the Neonate and Young Infant: A Prospective Study of Functional Skin Parameters During Early Infancy. Pediatr. Dermatol. 2002, 19, 256–262. [Google Scholar] [CrossRef]
- Minami-Hori, M.; Honma, M.; Fujii, M.; Nomura, W.; Kanno, K.; Hayashi, T.; Nakamura, E.; Nagaya, K.; Miyauchi, Y.; Fujimura, T.; et al. Developmental alterations of physical properties and components of neonatal-infantile stratum corneum of upper thighs and diaper-covered buttocks during the 1st year of life. J. Dermatol. Sci. 2014, 73, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Saijo, S.; Tagami, H. Dry skin of newborn infants: Functional analysis of the stratum corneum. Pediatr. Dermatol. 1991, 8, 155–159. [Google Scholar] [CrossRef]
- Visscher, M.O.; Chatterjee, R.; Munson, K.A.; Pickens, W.L.; Hoath, S.B. Changes in diapered and nondiapered infant skin over the first month of life. Pediatr. Dermatol. 2000, 17, 45–51. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Maayan-Metzger, A.; Merlob, P.; Sirota, L. Skin barrier properties in different body areas in neonates. Pediatrics 2000, 106, 105–108. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Pfisterer, S.; Gloor, M. Direct Comparison of Skin Physiology in Children and Adults with Bioengineering Methods. Pediatr. Dermatol. 2000, 17, 436–439. [Google Scholar] [CrossRef]
- Fujimura, T.; Miyauchi, Y.; Shima, K.; Hotta, M.; Tsujimura, H.; Kitahara, T.; Takema, Y.; Palungwachira, P.; Laohathai, D.; Chanthothai, J.; et al. Ethnic differences in stratum corneum functions between Chinese and Thai infants residing in Bangkok, Thailand. Pediatr. Dermatol. 2018, 35, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.; Boireau-Adamezyk, E.; Nikolovski, J. Assessment of infant stratum corneum composition and thickness with Raman spectroscopy. J. Am. Acad. Dermatol. 2012, 66, AB35. [Google Scholar]
- Stamatas, G.N.; Boireau, E.; Nikolovski, J. Compositional differences between infant and adult stratum corneum determined by in vivo Raman confocal microspectroscopy. J. Investig. Dermatol. 2010, 130, S69. [Google Scholar]
- Narendran, V.; Visscher, M.O.; Abril, I.; Hendrix, S.W.; Hoath, S.B. Biomarkers of Epidermal Innate Immunity in Premature and Full-Term Infants. Pediatr. Res. 2010, 67, 382–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretsos, K.; Kasting, G.B. Dermal capillary clearance: Physiology and modeling. Skin Pharmacol. Physiol. 2005, 18, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kretsos, K.; Kasting, G.B. A geometrical model of dermal capillary clearance. Math. Biosci. 2007, 208, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, J.; Weiss, T.; Diehm, C.; Linderkamp, O. Periodic variations in skin perfusion in full-term and preterm neonates using laser Doppler technique. Acta Pædiatri. 1991, 80, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R. Skin Surface pH in Newborns: Origin and Consequences. Curr. Probl. Dermatol. 2018, 54, 26–32. [Google Scholar]
- Fluhr, J.W.; Elias, P.M. Stratum corneum pH: Formation and Function of the ‘Acid Mantle’. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Lynde, C.; Tan, J.; Skotnicki, S.; Beecker, J.; Claveau, J.; Li, M.K.; Rao, J.; Salsberg, J.; Sauder, M.B.; Zip, C. Skin Surface pH. J. Drugs Dermatol. 2019, 18, 214. [Google Scholar]
- Giusti, F.; Martella, A.; Bertoni, L.; Seidenari, S. Skin barrier, hydration, and pH of the skin of infants under 2 years of age. Pediatr. Dermatol. 2001, 18, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Marchini, G.; Nelson, A.; Edner, J.; Lonne-Rahm, S.; Stavréus-Evers, A.; Hultenby, K. Erythema Toxicum Neonatorum Is an Innate Immune Response to Commensal Microbes Penetrated into the Skin of the Newborn Infant. Pediatr. Res. 2005, 58, 613–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakasheva-Mazhenkovska, L.; Milenkova, L.; Kostovska, N.; Gjokik, G. Histomorphometrical characteristics of human skin from capillitium in subjects of different age. Prilozi 2011, 32, 105–118. [Google Scholar] [PubMed]
- Wang, T.F.; Kasting, G.B.; Nitsche, J.M. A multiphase microscopic diffusion model for stratum corneum permeability. I. Formulation, solution, and illustrative results for representative compounds. J. Pharm. Sci. 2006, 95, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Raykar, P.V. Solute structure-permeability relationships in human stratum corneum. J. Investig. Dermatol. 1989, 93, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, J.M.; Wang, T.F.; Kasting, G.B. A two-phase analysis of solute partitioning into the stratum corneum. J. Pharm. Sci. 2006, 95, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and functional comparisons of four anatomical regions of porcine skin with human abdominal skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Human skin in vivo has a higher skin barrier function than porcine skin ex vivo-comprehensive Raman microscopic study of the stratum corneum. J. Biophotonics 2018, 11, e201700355. [Google Scholar] [CrossRef]
- Sauermann, K.; Clemann, S.; Jaspers, S.; Gambichler, T.; Altmeyer, P.; Hoffmann, K.; Ennen, J. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res. Technol. 2002, 8, 52–56. [Google Scholar] [CrossRef]
- Therkildsen, P.; Haedersdal, M.; Lock-Andersen, J.; de Fine Olivarius, F.; Poulsen, T.; Wulf, H.C. Epidermal thickness measured by light microscopy: A methodological study. Skin Res. Technol. 1998, 4, 174–179. [Google Scholar] [CrossRef]
- Sandby-Moller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef]
- Robertson, K.; Rees, J.L. Variation in epidermal morphology in human skin at different body sites as measured by reflectance confocal microscopy. Acta Derm. Venereol. 2010, 90, 368–373. [Google Scholar]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharm. 2006, 45, 1013–1034. [Google Scholar] [CrossRef]
- Yun, Y.E.; Edginton, A.N. Model qualification of the PK-Sim® pediatric module for pediatric exposure assessment of CYP450 metabolized compounds. J. Toxicol. Environ. Health A 2019, 82, 789–814. [Google Scholar] [CrossRef]
- Barrett, D.A.; Rutter, N. Percutaneous lignocaine absorption in newborn infants. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 71, F122–F124. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Guy, R.H. Effective use of transdermal drug delivery in children. Adv. Drug Del. Rev. 2014, 73, 63–82. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.; Guy, R.H.; Hadgraft, J.; Parr, G.D.; Rutter, N. Transdermal Drug Delivery to Neonates. Int. J. Pharm. 1985, 24, 259–265. [Google Scholar] [CrossRef]
- West, D.P.; Worobec, S.; Solomon, L.M. Pharmacology and toxicology of infant skin. J. Investig. Dermatol. 1981, 76, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Telofski, L.S.; Morello, A.P.; Mack Correa, M.C.; Stamatas, G.N. The infant skin barrier: Can we preserve, protect, and enhance the barrier? Dermatol. Res. Pract. 2012, 2012, 198789. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Food and Drug Administration: Code of Federal Regulations Title 21 PART 320 Sec. 320.23 Basis for Measuring In Vivo Bioavailability or Demonstrating Bioequivalence. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.23 (accessed on 1 November 2021).
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz Diffusion Cell Approach for Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage Forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration: Transdermal and Topical Delivery Systems-Product Development and Quality Considerations Draft Guidance. 2019. Available online: https://www.fda.gov/media/132674/download (accessed on 1 November 2021).

| Property | Buprenorphine | Diamorphine | Phenobarbital |
|---|---|---|---|
| Molecular formula | C29H41NO4 | C21H23NO5 | C12H12N2O3 |
| Molecular weight (g/mol) | 467.6 | 369.4 | 232.2 |
| Lipophilicity (Log P) | 4 | 1.5 | 1.47 |
| Boiling point (°C) | 578.7 | 272 | |
| Melting point (°C) | 217 | 173 | 174 |
| Water Solubility (mg/L) | 16.8 | 600 | 1110 |
| Solubility in ethanol (mg/mL) | N/A | N/A | 100 |
| pKa | 8.65 (basic) | 7.83 (basic) | 7.3 (acidic) |
| Vapor pressure (mmHg) | N/A | N/A | 1.4 × 10−11 |
| Compound (Reference) | Dose (µg/cm2) | Experiment Duration (h) | Solvent | Observed Steady-State Flux (µg/cm2/h) | Final Receptor Fluid Accumulation (µg/cm2) |
|---|---|---|---|---|---|
| Buprenorphine (Barret et al., 1994) | 2656 | 72 | 0.1 M acetate buffer, pH 4 | 0.08 ± 0.02 (mean ± SD) | 5.54 |
| Diamorphine (Barret et al., 1993) | 53,100 | 72 | 0.1 M acetate buffer, pH 4 | 0.07 | 2.59 |
| Phenobarbital (Bonina et al., 1993) | 78 | 12 | Ethanol | 0.1 ± 0.02 | 0.91 |
| Compound (Reference) | Gestational Age (Postnatal Age) | Dose (µg/cm2) | Experiment Duration (h) | Solvent | Observed Steady-State Flux (µg/cm2/h) |
|---|---|---|---|---|---|
| Buprenorphine (Barret et al., 1994) | 38 w (1 d) | 2656 | 72 | 0.1 M acetate buffer pH 4 | 0.01 |
| 40 w (7 h) | 0.36 | ||||
| 37 w (1 d) | 0.08 | ||||
| 37 w (1 d) | 0.11 | ||||
| Diamorphine (Barret et al., 1993) | 38 w (26 d) | 53,100 | 72 | 0.1 M acetate buffer pH 4 | 0.23 |
| 40 w (7 d) | 0.08 | ||||
| 36 w (3 d) | 0.18 | ||||
| Phenobarbital (Bonina et al., 1993) | 38 w (2 d) | 78 | 12 | Ethanol | 0.11 |
| 40 w (5 d) | 0.14 | ||||
| 37 w (5 d) | 0.11 | ||||
| 35 w (2 d) | 0.24 |
| Parameter | Equation | Coefficients |
|---|---|---|
| ) | SC Maturation Model 1 ≤ 1510 days for Age 1510 days | 2.401 × 10−7 2.000 −99.43 2.071 × 10−3 101.4 |
| SC Maturation Model 2 ≤ 1604 days 1604 days | 2.662 × 10−7 1.878 0.724 | |
| ) | 18.702 0.634 5.363 | |
| ) | ≤ 9883 days 9883 days | 8.974 × 103 0.407 |
| ) | ≤ 1182 days 1182 days | −0.344 −17.585 18.530 0.245 −0.0171 |
| Parameter (Units) | Nominal Value (Uncertainty Range) | Source |
|---|---|---|
| (cm/s) | Uncertainty range = Nominal value ± 1.26 | Wang et al., 2006 [76] |
Uncertainty range = Nominal value ± 0.32 | Anderson and Raykar 1989 [77] | |
Uncertainty range = Nominal value ± 0.434 | Nitsche et al., 2006 [78] |
| Mean (SD) SC Thickness (µm) | Skin Anatomical Site | Reference |
|---|---|---|
| 13.2 (2.3) | Abdomen | Khiao In et al., 2019 [79] |
| 21 (2.3) | Forearm | Choe et al., 2018 [80] |
| 19 (1.3) | Forearm | |
| 10.4 (3.2) | Forearm | Sauermann et al., 2002 [81] |
| 11.2 (1.9) | Forearm | |
| 13.3 | Buttock | Therkildsen et al., 1998 [82] |
| 18.3 (4.9) | Dorsal forearm | Sandby-Møller et al., 2003 [83] |
| 11 (2.2) | Shoulder | |
| 14.9 (3.4) | Buttock | |
| 9.3 | Back of hand | Robertson and Rees 2010 [84] |
| 8.7 | Centre of calf | |
| 10.9 | Outer forearm | |
| 6.2 | Inner forearm | |
| 6.4 | Inner upper arm | |
| 8.4 | Upper back | |
| 6.5 | Chest | |
| 6.3 | Abdomen | |
| 5.8 | Corner of eye | |
| 6.3 | Temple |
| Compound (Reference) | Gestational Age (Postnatal Age) | Observed Steady-State Flux (µg/cm2/h) | Predicted Terminal Flux (µm/cm2/h) | Predicted Geometric Mean/Observed Flux Ratio (Fold Error) | |
|---|---|---|---|---|---|
| Geometric Mean | 95% CI | ||||
| Buprenorphine (Barret et al., 1994) | 38 w (1 d) | 0.01 | 0.13 | 0.06–0.26 | 12.8 |
| 40 w (7 h) | 0.36 | 0.12 | 0.06–0.31 | 0.35 | |
| 37 w (1 d) | 0.08 | 0.11 | 0.05–0.21 | 1.37 | |
| 37 w (1 d) | 0.11 | 0.11 | 0.05–0.21 | 1.0 | |
| Diamorphine (Barret et al., 1993) | 38 w (26 d) | 0.23 | 0.13 | 0.04–0.35 | 0.55 |
| 40 w (7 d) | 0.08 | 0.11 | 0.04–0.25 | 1.4 | |
| 36 w (3 d) | 0.18 | 0.22 | 0.08–0.47 | 1.2 | |
| Phenobarbital (Bonina et al., 1993) | 38 w (2 d) | 0.11 | 0.13 | 0.07–0.3 | 1.22 |
| 40 w (5 d) | 0.14 | 0.13 | 0.05–0.27 | 0.96 | |
| 37 w (5 d) | 0.11 | 0.14 | 0.07–0.26 | 1.26 | |
| 35 w (2 d) | 0.24 | 0.22 | 0.1–0.45 | 0.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.E.; Calderon-Nieva, D.; Hamadeh, A.; Edginton, A.N. Development and Evaluation of an In Silico Dermal Absorption Model Relevant for Children. Pharmaceutics 2022, 14, 172. https://doi.org/10.3390/pharmaceutics14010172
Yun YE, Calderon-Nieva D, Hamadeh A, Edginton AN. Development and Evaluation of an In Silico Dermal Absorption Model Relevant for Children. Pharmaceutics. 2022; 14(1):172. https://doi.org/10.3390/pharmaceutics14010172
Chicago/Turabian StyleYun, Yejin Esther, Daniella Calderon-Nieva, Abdullah Hamadeh, and Andrea N. Edginton. 2022. "Development and Evaluation of an In Silico Dermal Absorption Model Relevant for Children" Pharmaceutics 14, no. 1: 172. https://doi.org/10.3390/pharmaceutics14010172
APA StyleYun, Y. E., Calderon-Nieva, D., Hamadeh, A., & Edginton, A. N. (2022). Development and Evaluation of an In Silico Dermal Absorption Model Relevant for Children. Pharmaceutics, 14(1), 172. https://doi.org/10.3390/pharmaceutics14010172






