Novel Use of Hypoxia-Inducible Polymerizable Protein to Augment Chemotherapy for Pancreatic Cancer
Abstract
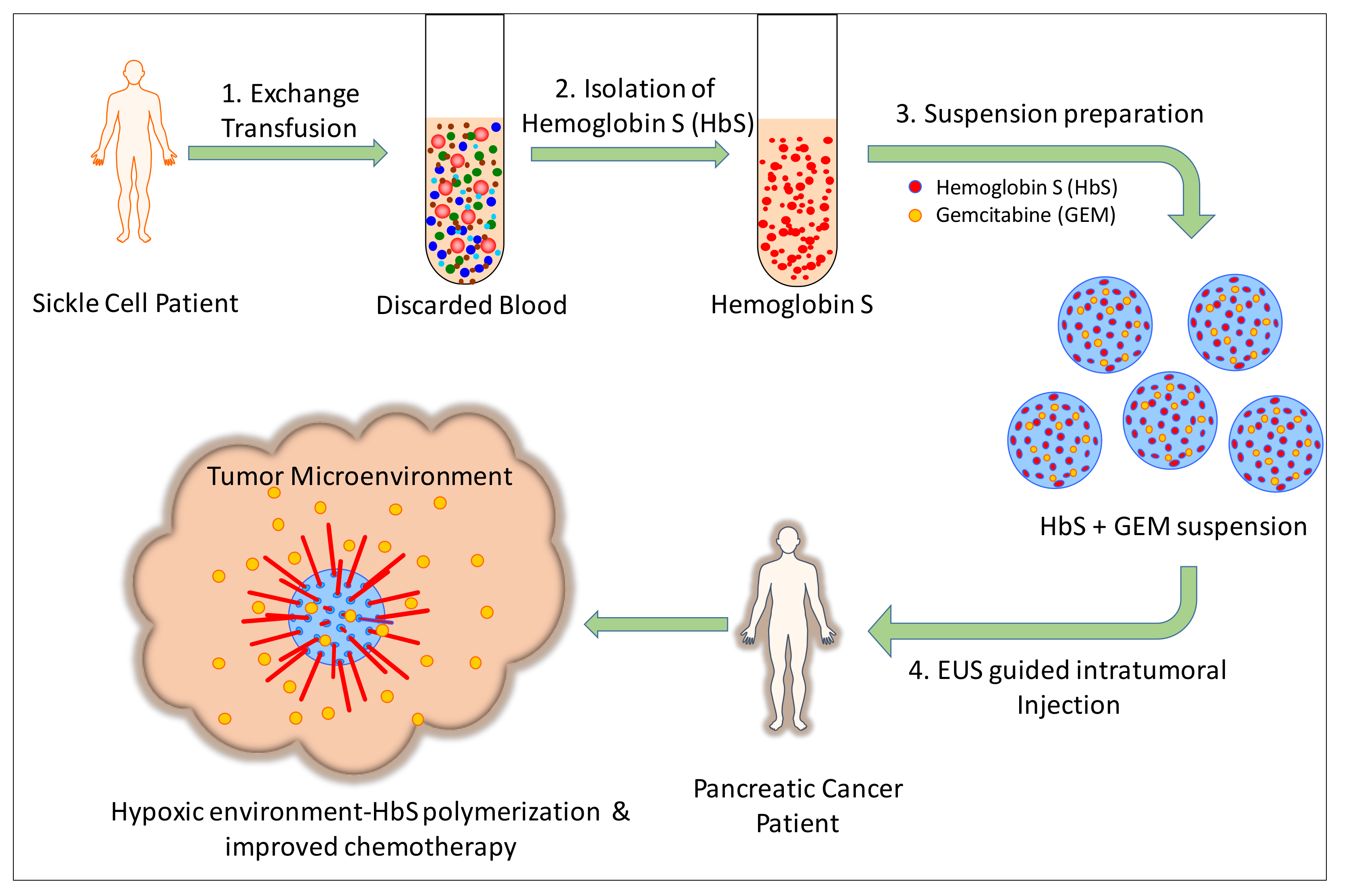
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Suspension Preparation
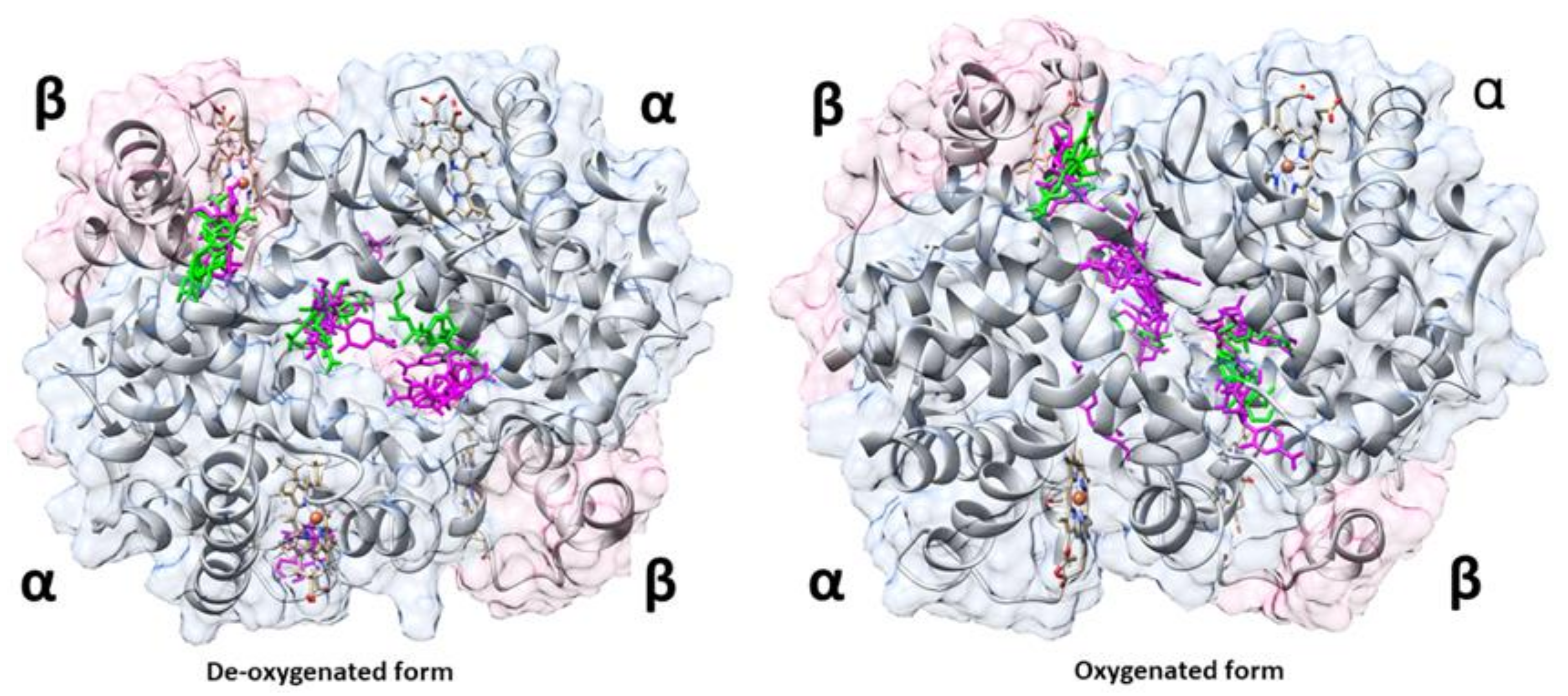
2.3. In Silico Molecular Docking of HbS and GEM
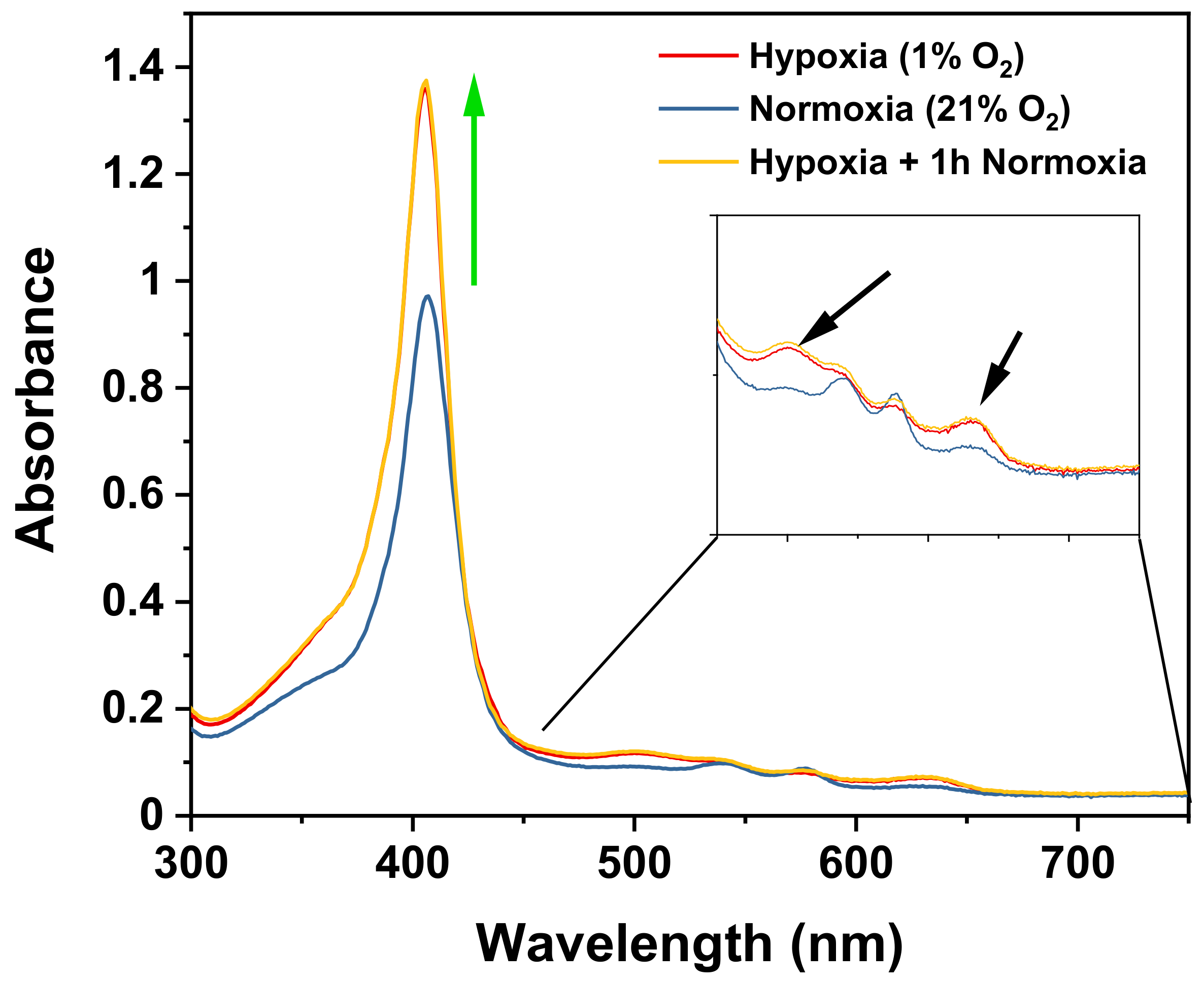
2.4. Absorption Spectrum Analysis of HbS
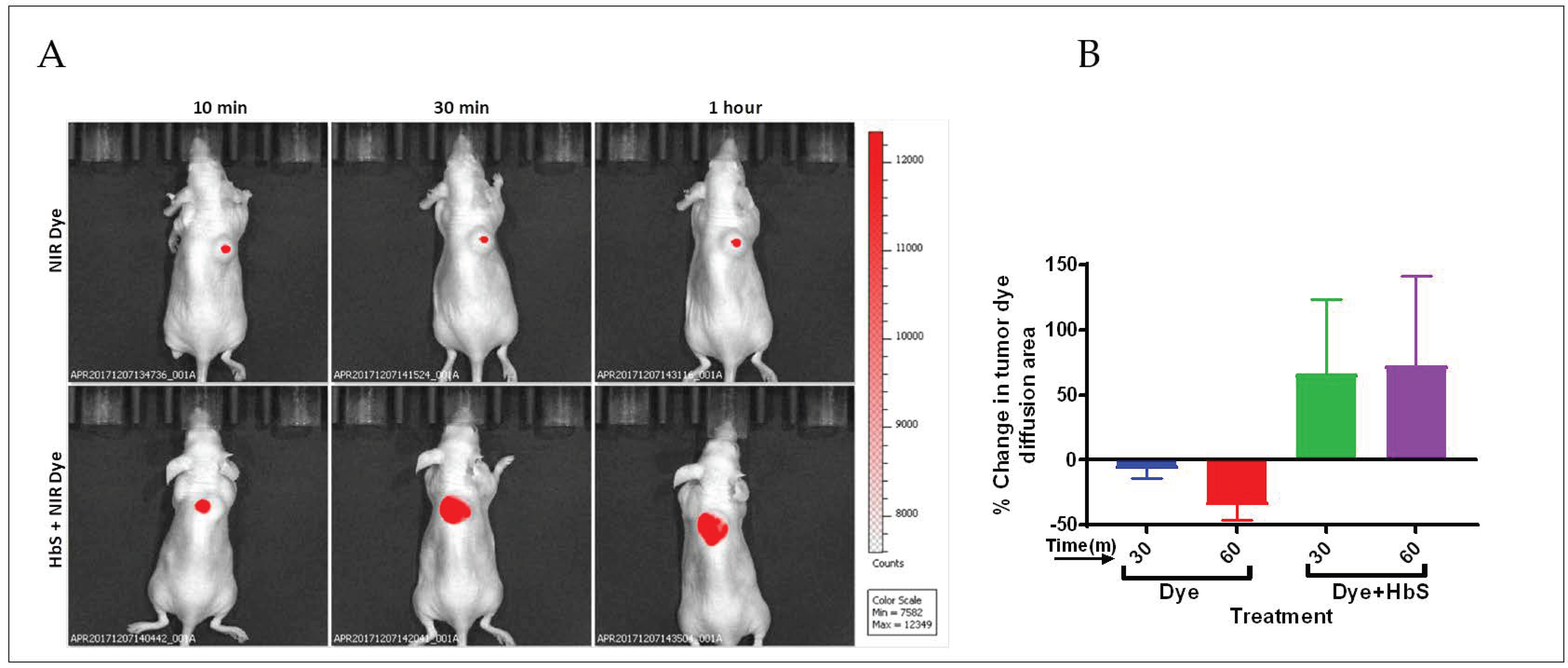
2.5. In Vivo Drug Diffusion Study
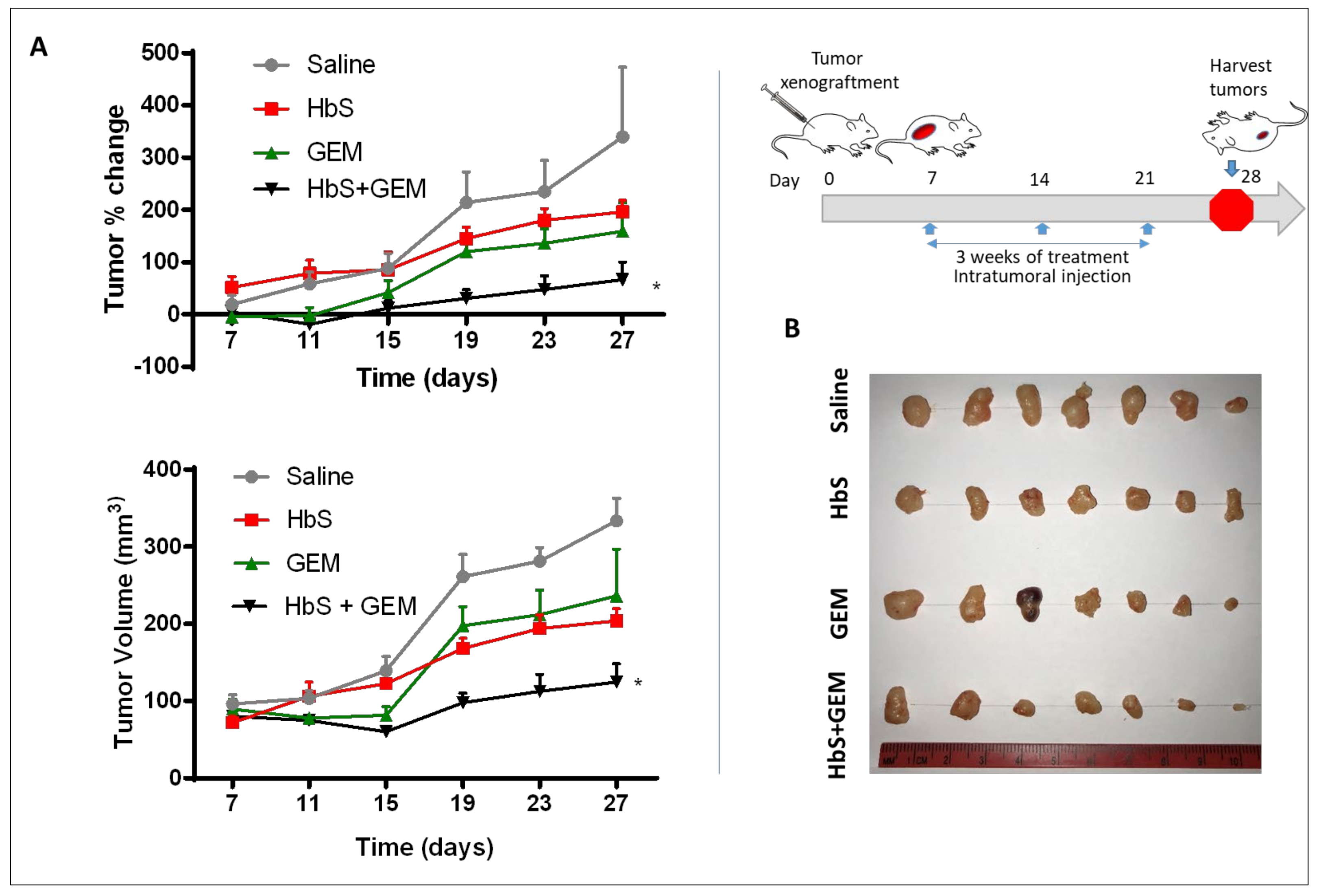
2.6. In Vivo Tumor Efficacy Study
3. Results
3.1. In Silico Interaction Analysis of HbS and GEM
3.2. Characterization of Polymerization of Hemoglobin S under Hypoxia
3.3. In Vivo Drug Diffusion Tumor Model
3.4. In Vivo Efficacy in Pancreatic Tumor Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G.; Czito, B.G.; Bendell, J.C.; Ryan, D.P. Locally Advanced Pancreatic Cancer. J. Clin. Oncol. 2005, 23, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, A.L.; Fernandez-del Castillo, C. Pancreatic Carcinoma. N. Engl. J. Med. 1992, 326, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Yang, L. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef]
- Ryan Heather, E.; Poloni, M.; McNulty, W.; Elson, D.; Gassmann, M.; Arbeit, J.M.; Johnson, R.S. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 2000, 60, 4010–4015. [Google Scholar]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mitra, A.; Das, R. Sickle Cell Hemoglobin, Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Springer: Berlin, Germany, 2020; pp. 297–322. [Google Scholar]
- Hebbel, R.P.; Vercellotti, G.M. Pathobiology of Sickle Cell Disease. In Hematology: Basic Principles and Practice, 7th ed.; Hoffman, R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 571–583. [Google Scholar]
- Takakura, K.; Koido, S. Direct therapeutic intervention for advanced pancreatic cancer. World J. Clin. Oncol. 2015, 6, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Patskovska, L.N.; Patskovsky, Y.V.; Almo, S.C.; Hirsch, R.E. COHbC and COHbS crystallize in the R2 quaternary state at neutral pH in the presence of PEG 4000. Acta Crystallogr. Sect. D 2005, 61, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.J.; Adachi, K.; Royer, W.E. The high resolution crystal structure of deoxyhemoglobin S11Edited by K. Nagai. J. Mol. Biol. 1997, 272, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Louderback, J.G.; Ballas, S.K.; Kim-Shapiro, D.B. Sickle Hemoglobin Polymer Melting in High Concentration Phosphate Buffer. Biophys. J. 1999, 76, 2216–2222. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Wei, W.; Qi, X.; Yu, H.; Feng, L.; Li, J.; Wang, S.; Zhang, J.; Dong, W. Preparation and characterization of a novel pH-sensitive Salecan-g-poly (acrylic acid) hydrogel for controlled release of doxorubicin. J. Mater. Chem. B3 2015, 3, 2685–2697. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, J.; Hu, L.; Wang, X.; Yang, G.; Fu, S.; Cheng, X.; Zhang, P.; Tang, R. Stepwise targeted drug delivery to liver cancer cells for enhanced therapeutic efficacy by galactose-grafted, ultra-pH-sensitive micelles. Acta Biomater. 2017, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, A.; Santi, M.; Capriotti, L.; Voliani, V.; Porciani, D.; Beltram, F.; Signore, G. Peptide-based stealth nanoparticles for targeted and pH-triggered delivery. Bioconjugate Chem. 2017, 28, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Y.; Cheng, R.; Deng, C.; Zhong, Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: Concept and recent advances. Nanomedicine 2014, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gdowski, A.; Hayatshahi, H.; Fudala, R.; Joshi, R.; Liu, J.; Vishwanatha, J.K.; Jeyarajah, R.; Guzik, P.; Ranjan, A.P. Novel Use of Hypoxia-Inducible Polymerizable Protein to Augment Chemotherapy for Pancreatic Cancer. Pharmaceutics 2022, 14, 128. https://doi.org/10.3390/pharmaceutics14010128
Gdowski A, Hayatshahi H, Fudala R, Joshi R, Liu J, Vishwanatha JK, Jeyarajah R, Guzik P, Ranjan AP. Novel Use of Hypoxia-Inducible Polymerizable Protein to Augment Chemotherapy for Pancreatic Cancer. Pharmaceutics. 2022; 14(1):128. https://doi.org/10.3390/pharmaceutics14010128
Chicago/Turabian StyleGdowski, Andrew, Hamed Hayatshahi, Rafal Fudala, Rohan Joshi, Jin Liu, Jamboor K. Vishwanatha, Rohan Jeyarajah, Paul Guzik, and Amalendu P. Ranjan. 2022. "Novel Use of Hypoxia-Inducible Polymerizable Protein to Augment Chemotherapy for Pancreatic Cancer" Pharmaceutics 14, no. 1: 128. https://doi.org/10.3390/pharmaceutics14010128
APA StyleGdowski, A., Hayatshahi, H., Fudala, R., Joshi, R., Liu, J., Vishwanatha, J. K., Jeyarajah, R., Guzik, P., & Ranjan, A. P. (2022). Novel Use of Hypoxia-Inducible Polymerizable Protein to Augment Chemotherapy for Pancreatic Cancer. Pharmaceutics, 14(1), 128. https://doi.org/10.3390/pharmaceutics14010128







