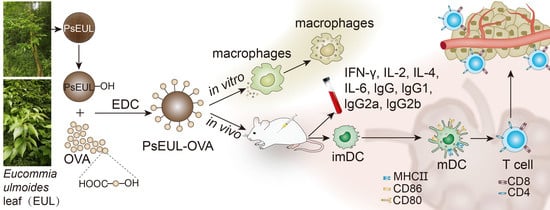
Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PsEUL
2.3. Coupling of Polysaccharides and Proteins
2.4. Structure Characterization
2.4.1. FT-IR Spectrum
2.4.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.3. SEM Analysis
2.4.4. Analysis Using Laser Particle-Size Analyzer
2.4.5. Zeta Potential Analysis
2.4.6. Polysaccharides and Protein Binding Degree
2.5. Macrophage Activity and Phagocytosis Test
2.6. Immunization Grouping and Processing of Serum and Immune Organs
2.7. Flow Cytometry (FCM) Analysis
2.8. Serum Antibody Level Determination
2.9. Serum Cytokine Analysis
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of PsEUL
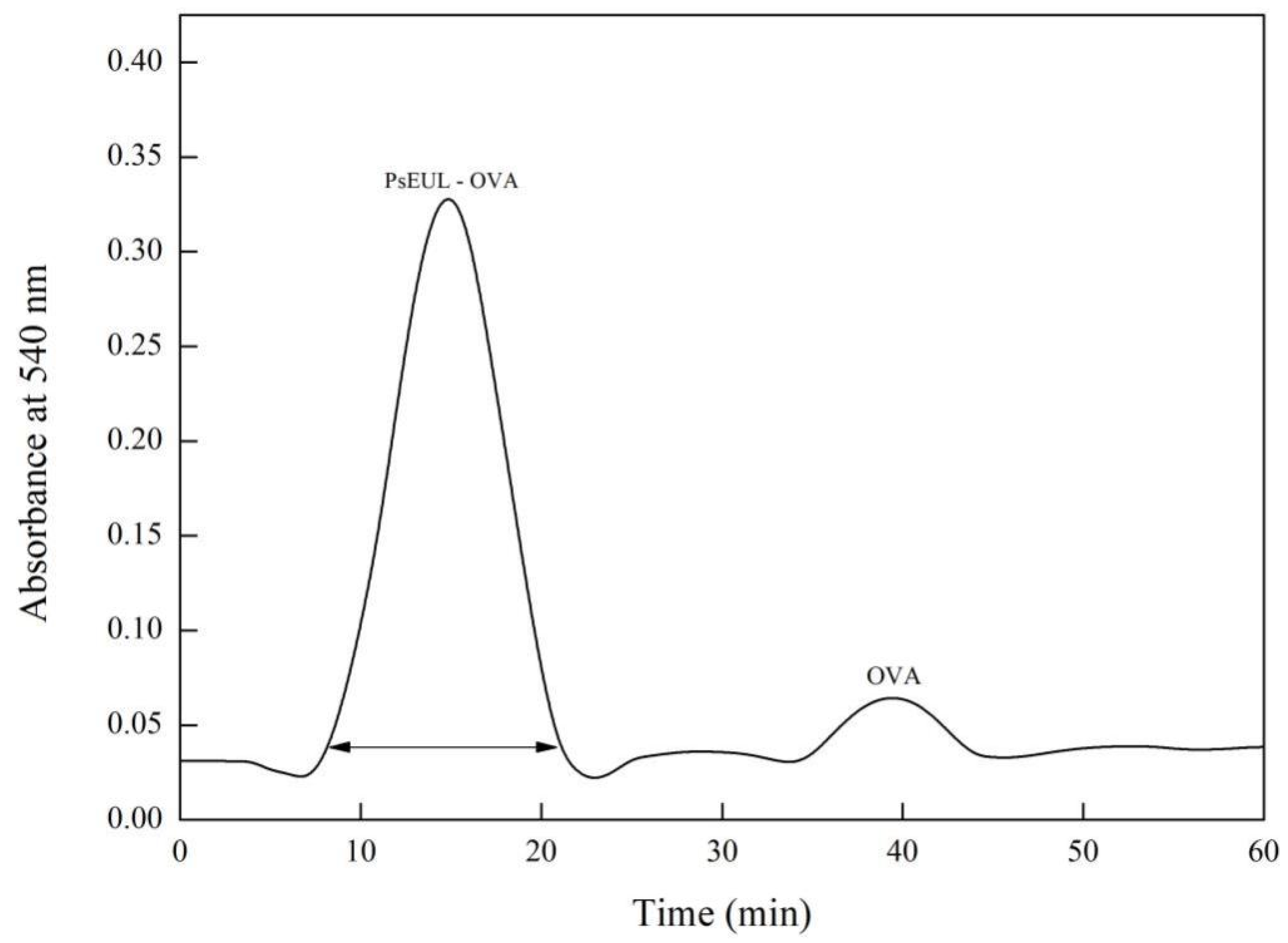
3.2. Separation and Purification of PsEUL-OVA
3.3. Structure Characterization
3.3.1. FT-IR Spectrum
3.3.2. SDS-PAGE
3.3.3. SEM Analysis
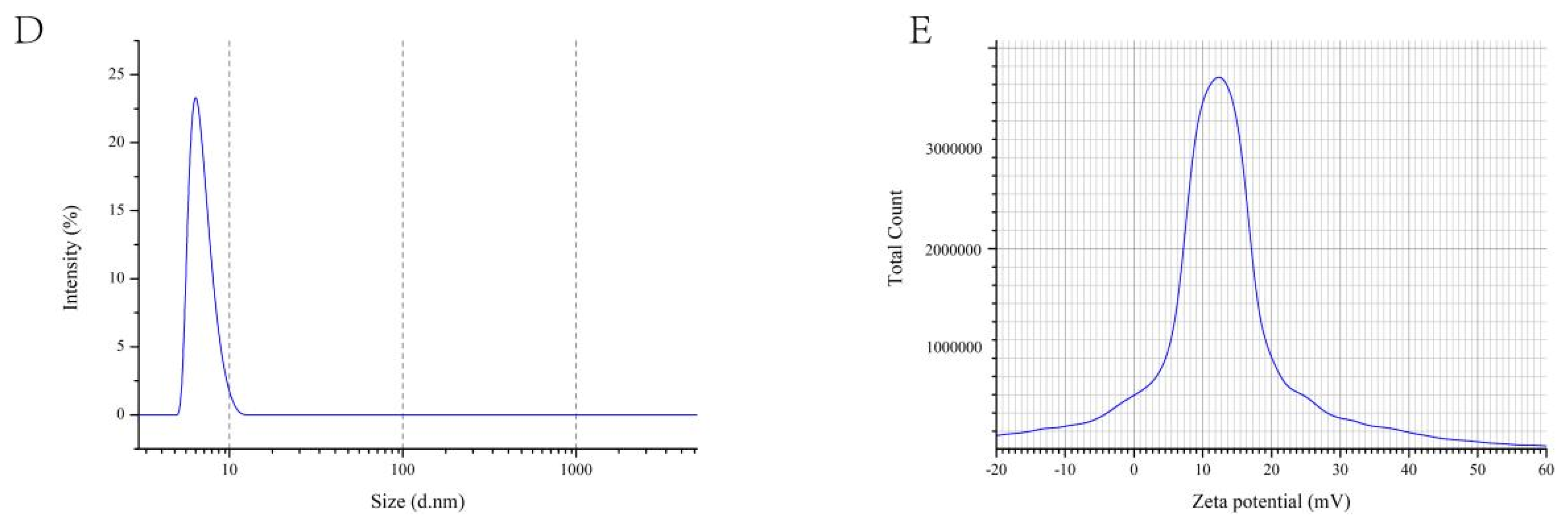
3.3.4. Laser Particle Size Tester
3.3.5. Polysaccharides and Protein Binding Degree
3.4. In Vitro Tests
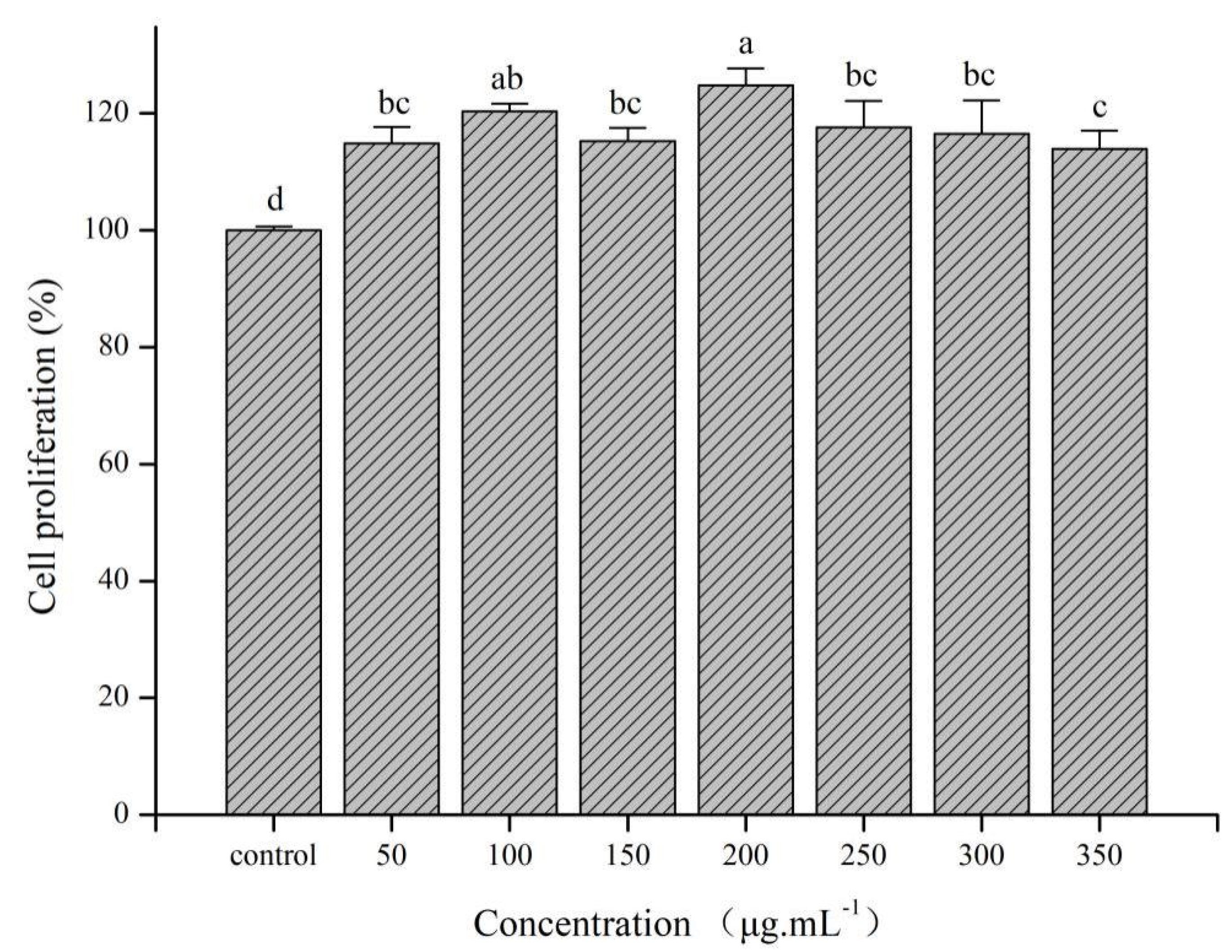
3.4.1. Cytotoxicity Analysis of PsEUL-OVA on RAW264.7
3.4.2. Effects of PsEUL-OVA on the Phagocytic Activity of Macrophages
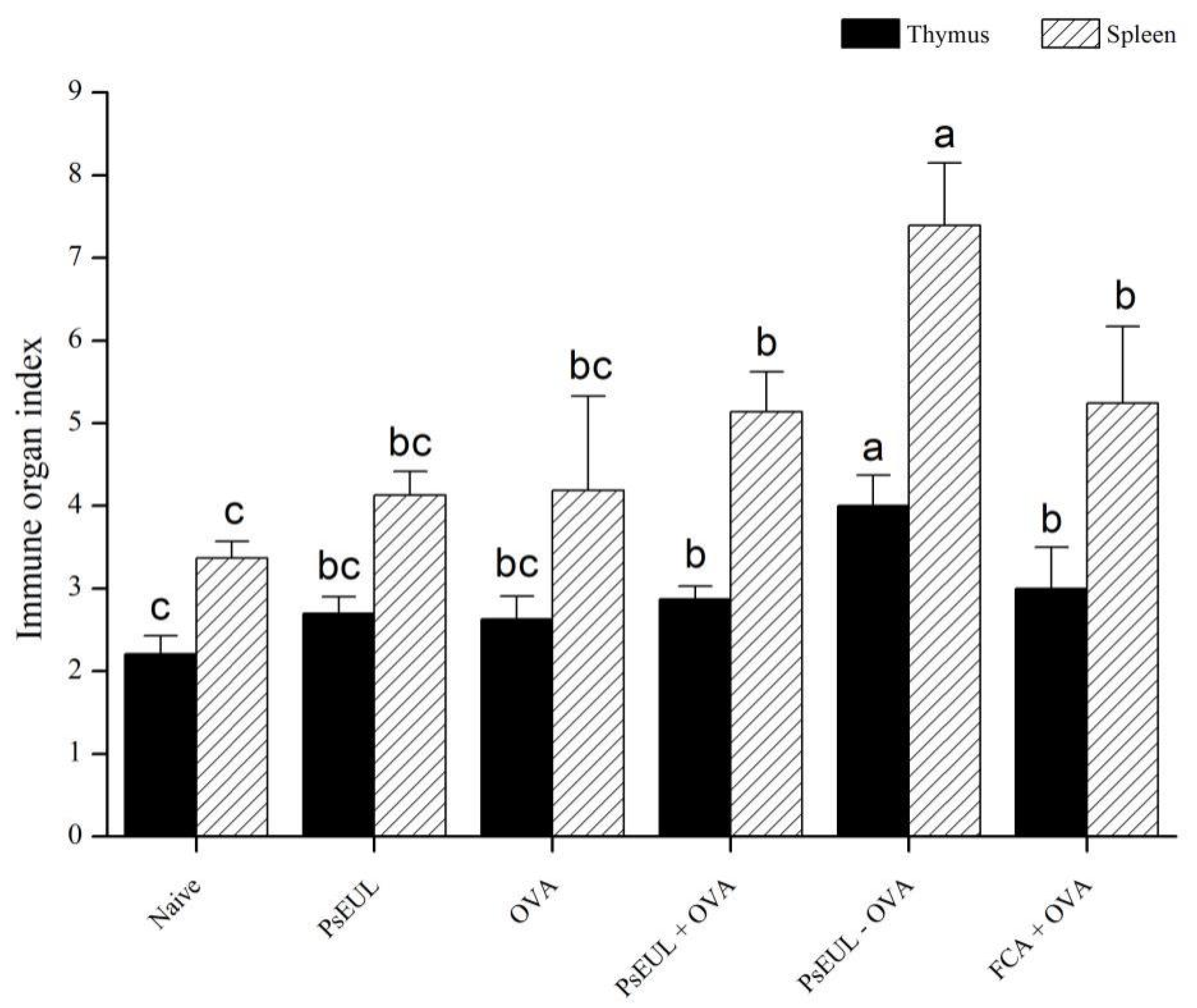
3.5. Effect of PsEUL-OVA on Immune Organ Index in Mice
3.6. Effect of PsEUL-OVA on the OVA-Specific IgG and the IgG Subclasses in Mouse Serum
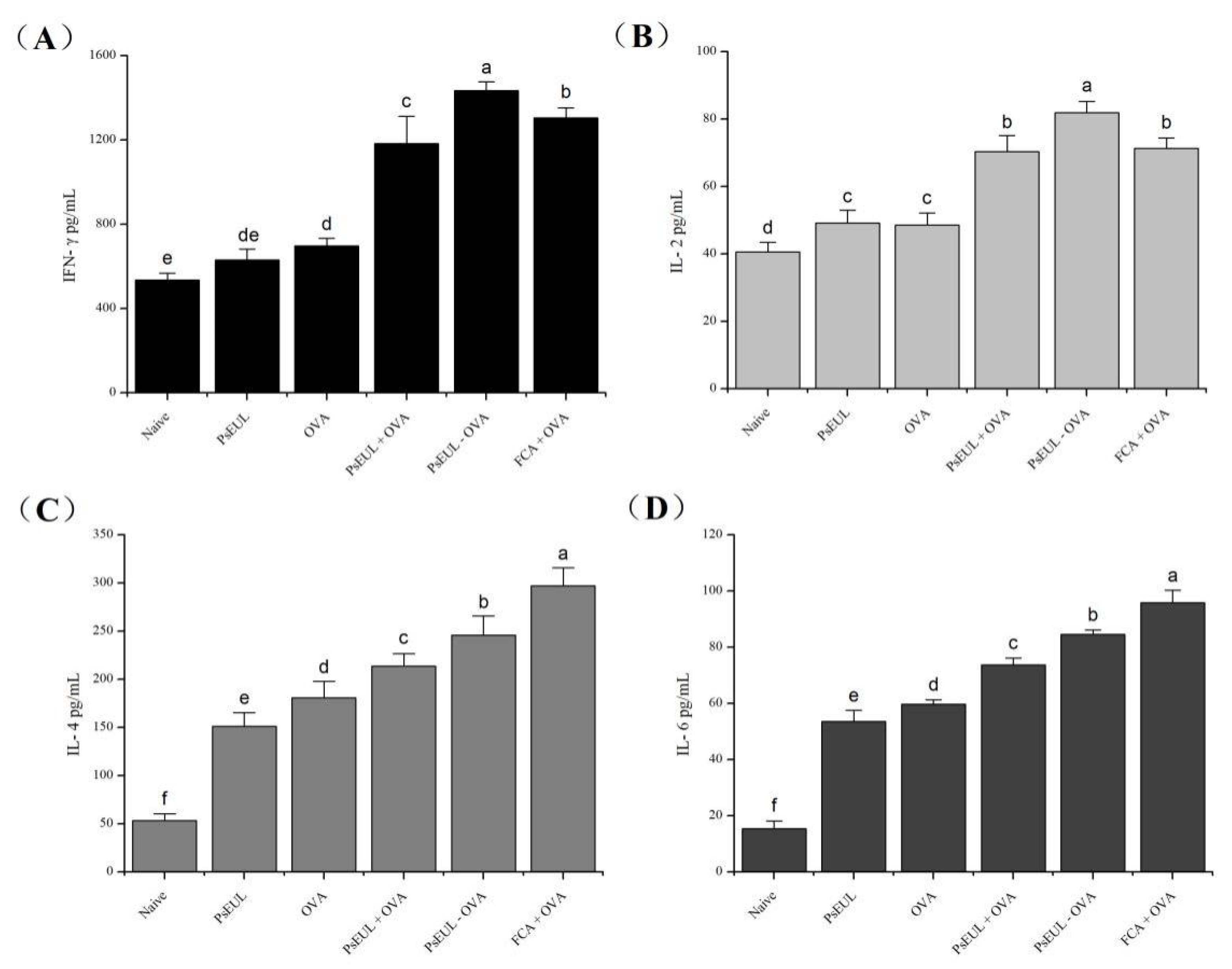
3.7. Effect of PsEUL-OVA on Serum Cytokines in Mice
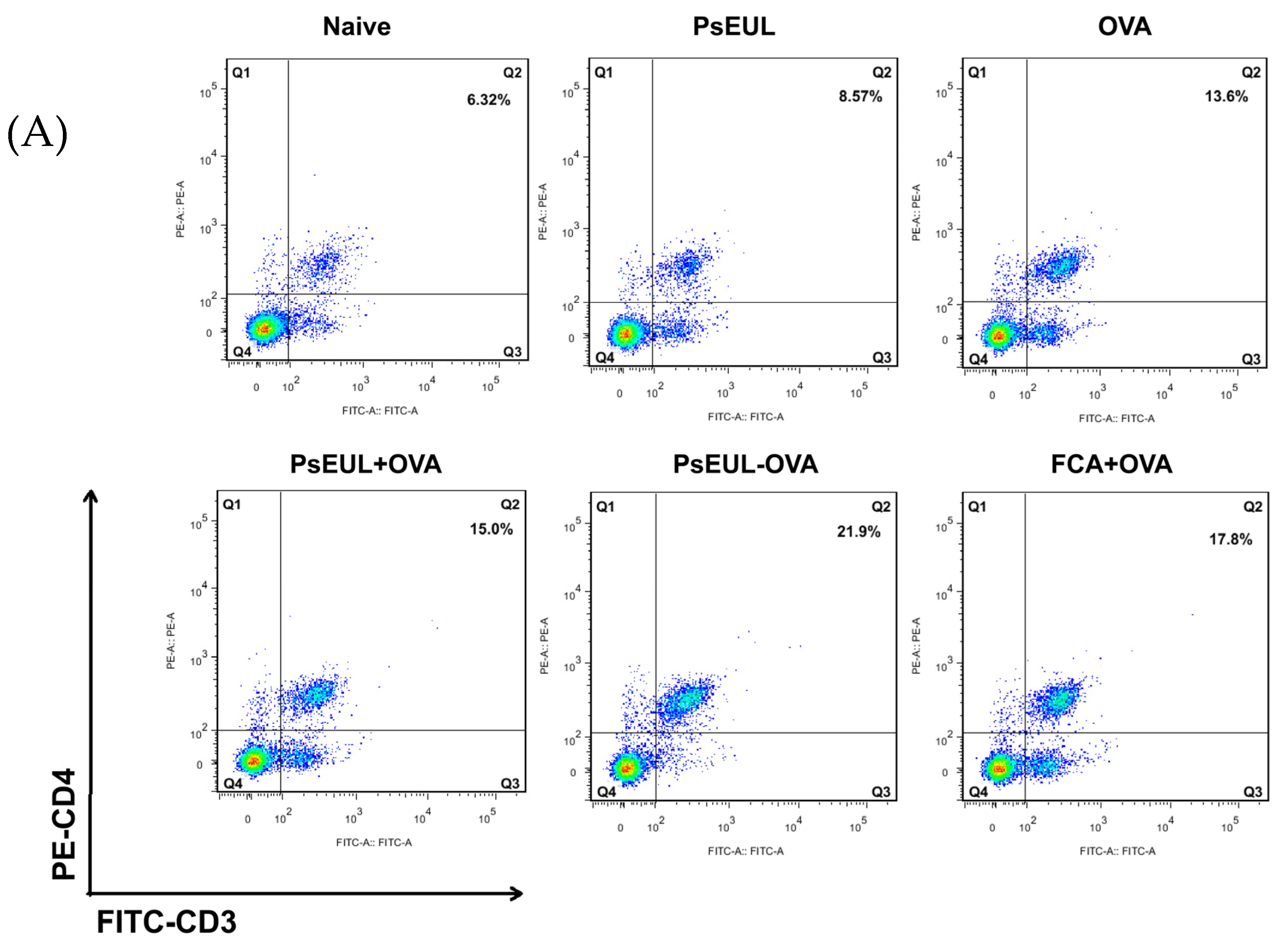
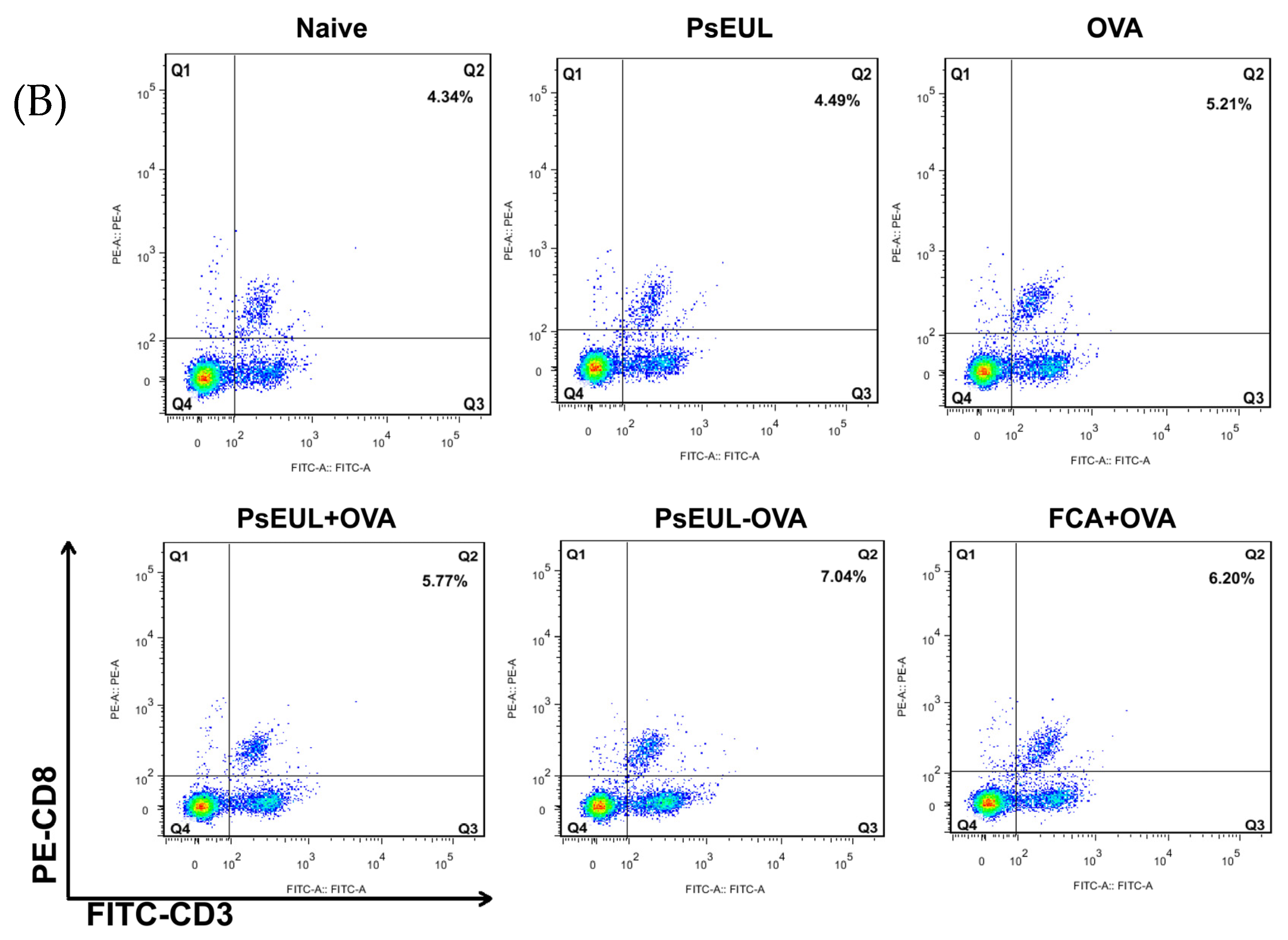
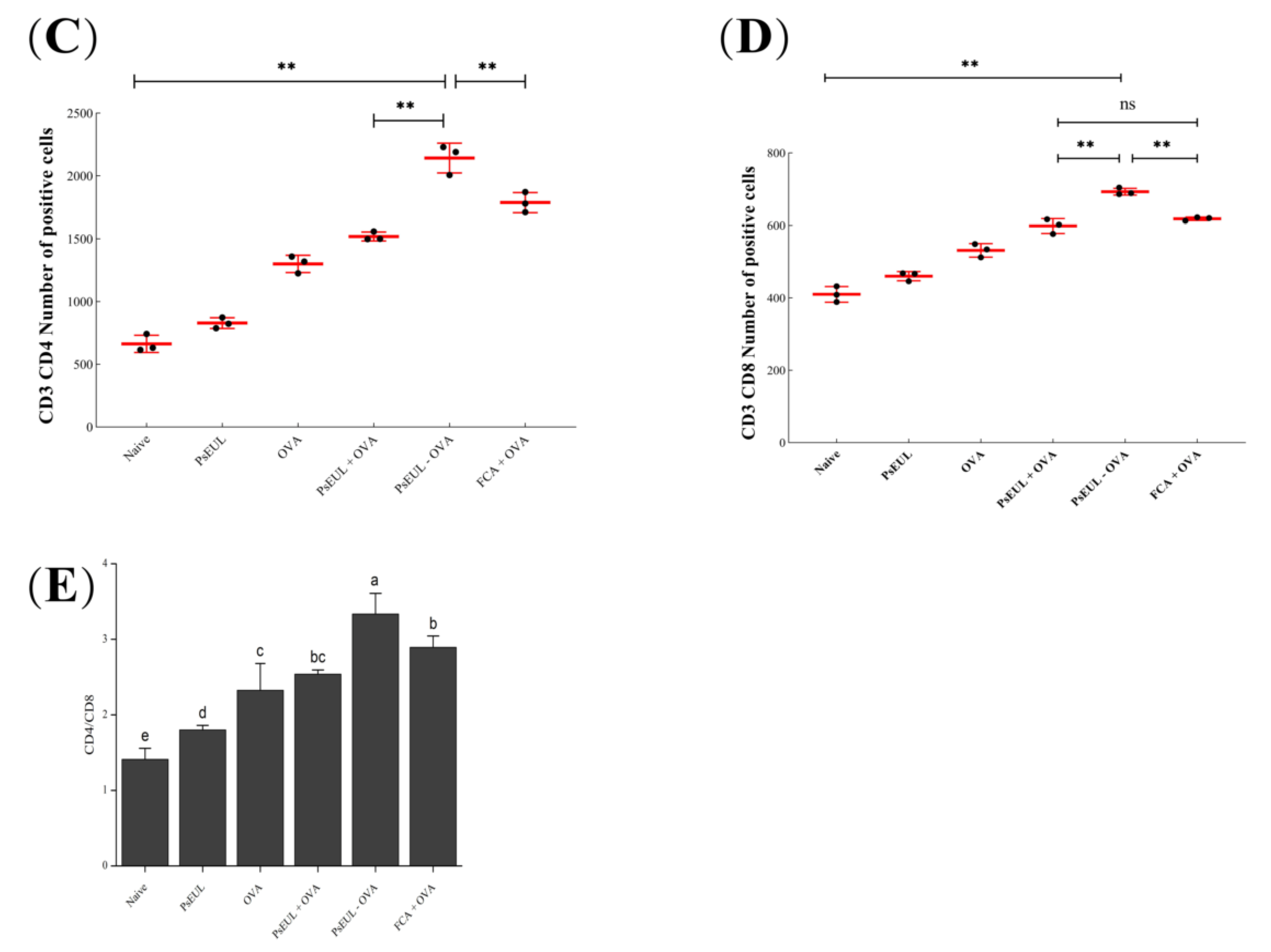
3.8. Effect of PsEUL-OVA on Mouse T lymphocytes
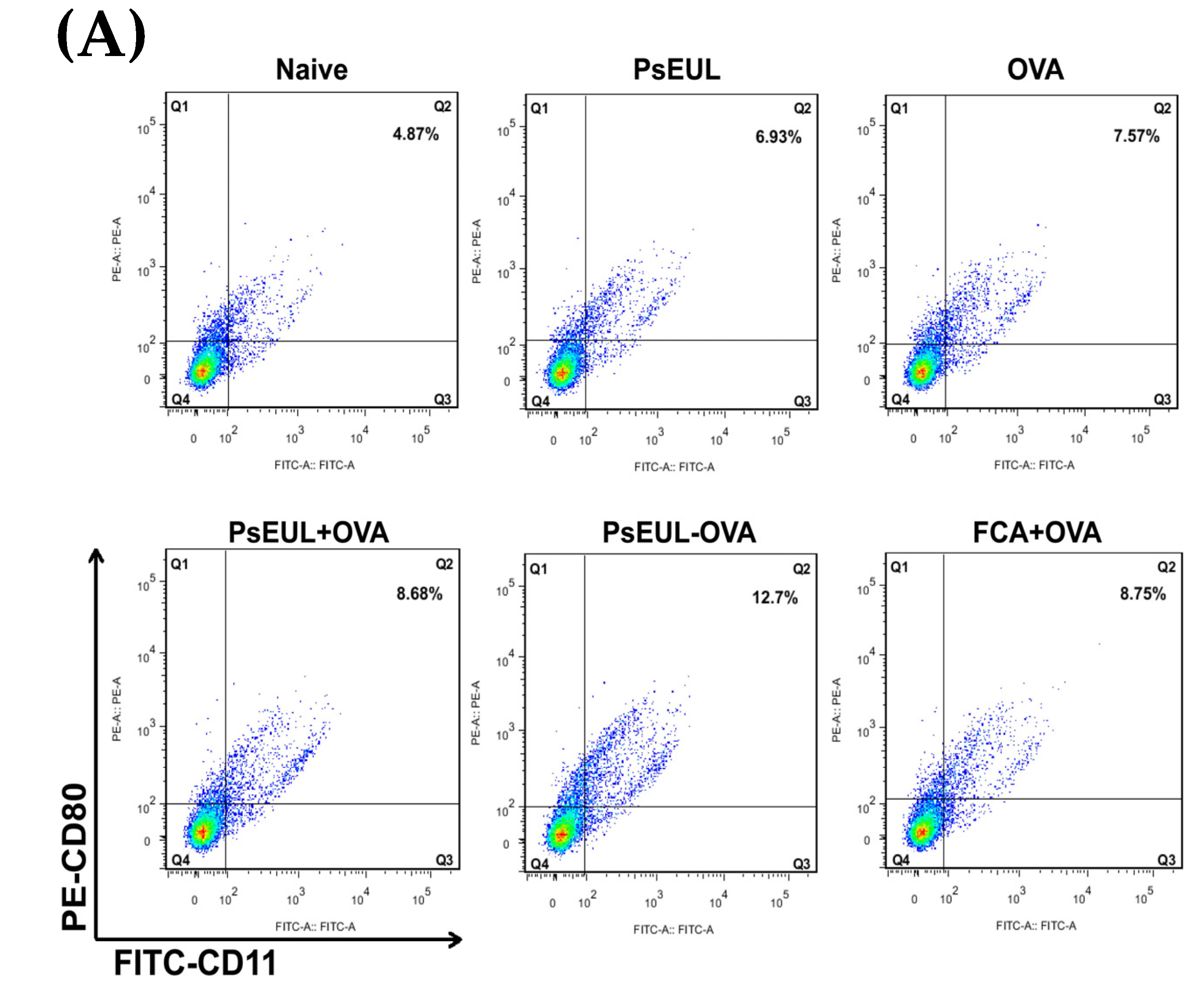
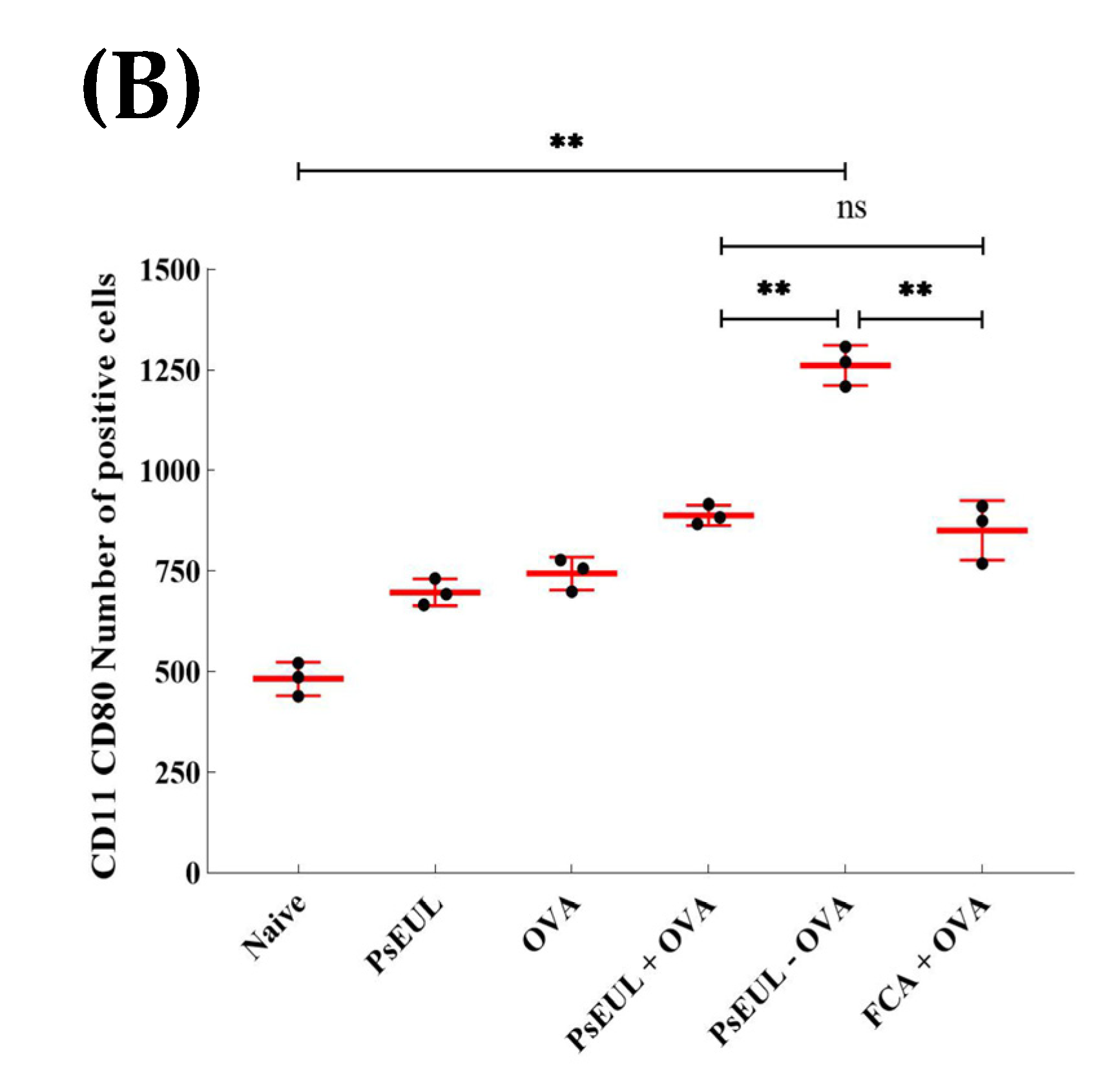
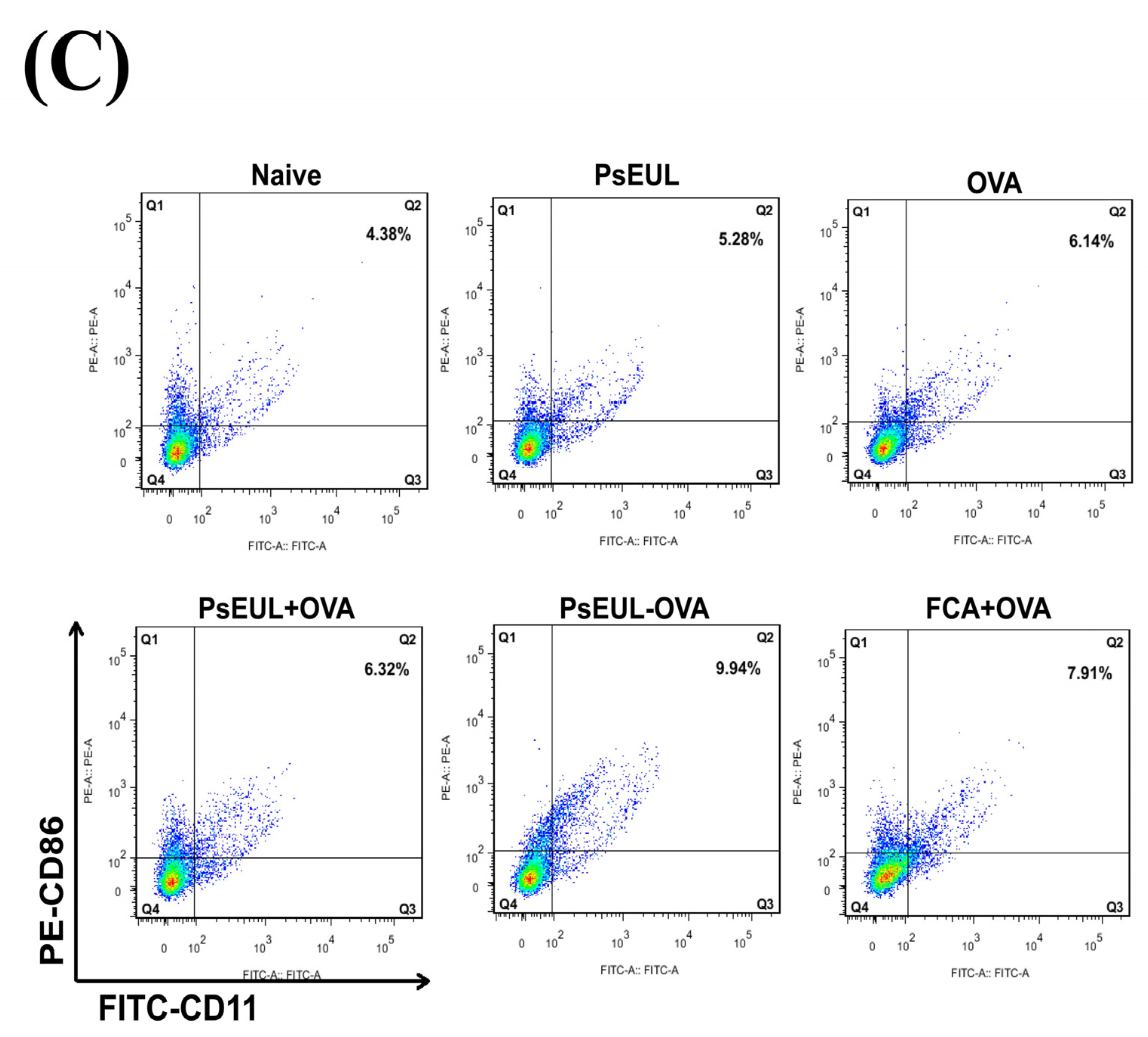
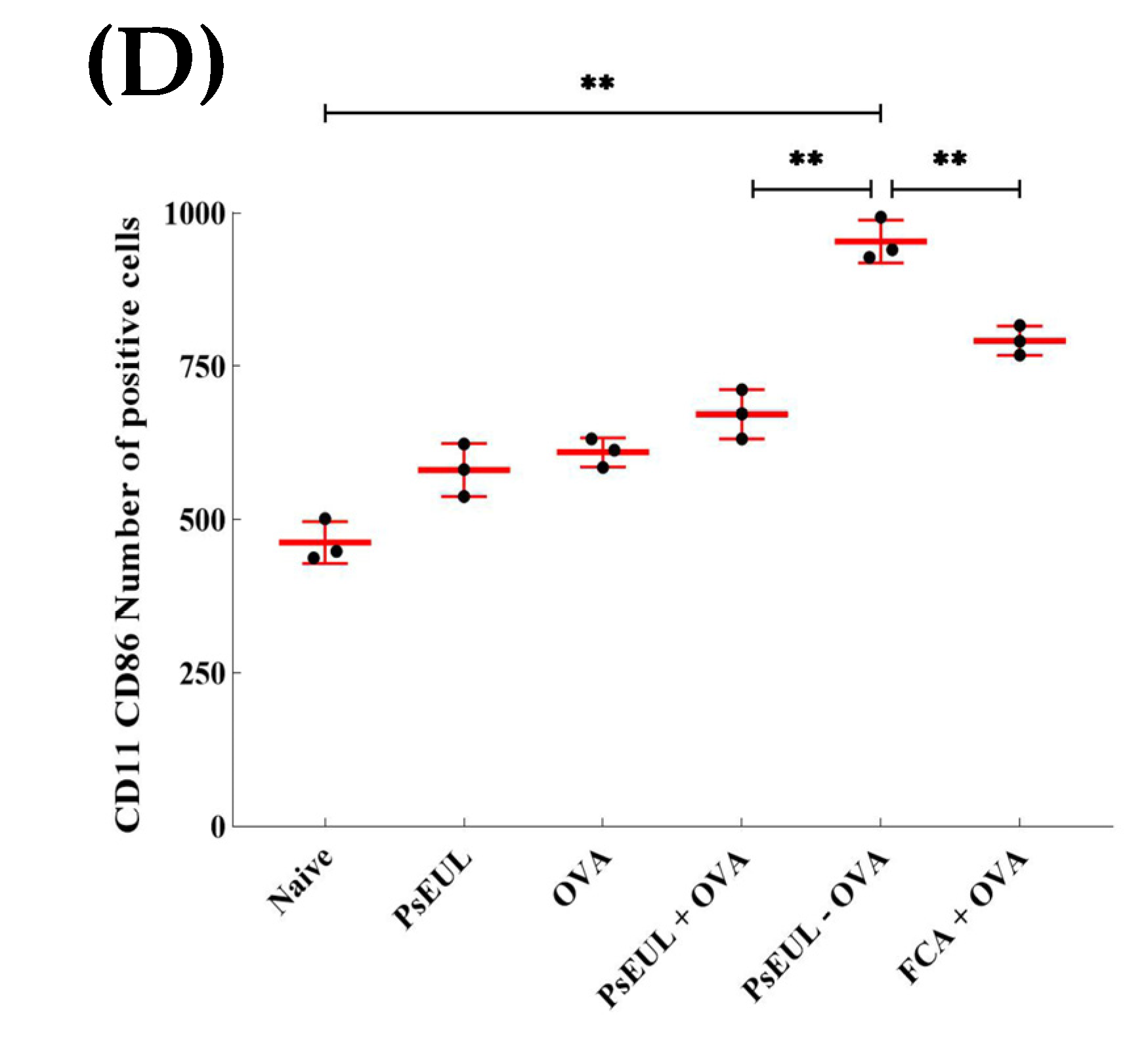
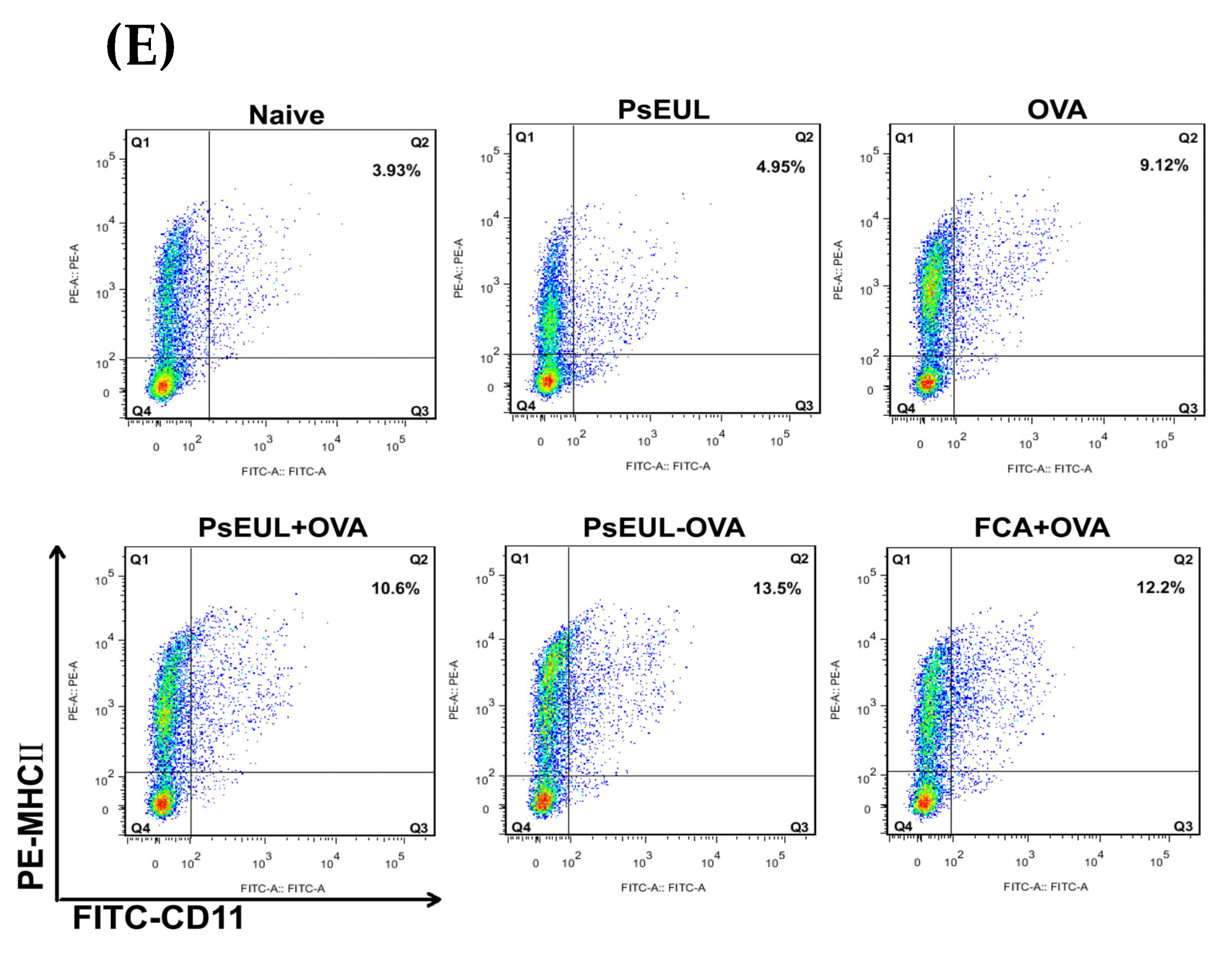
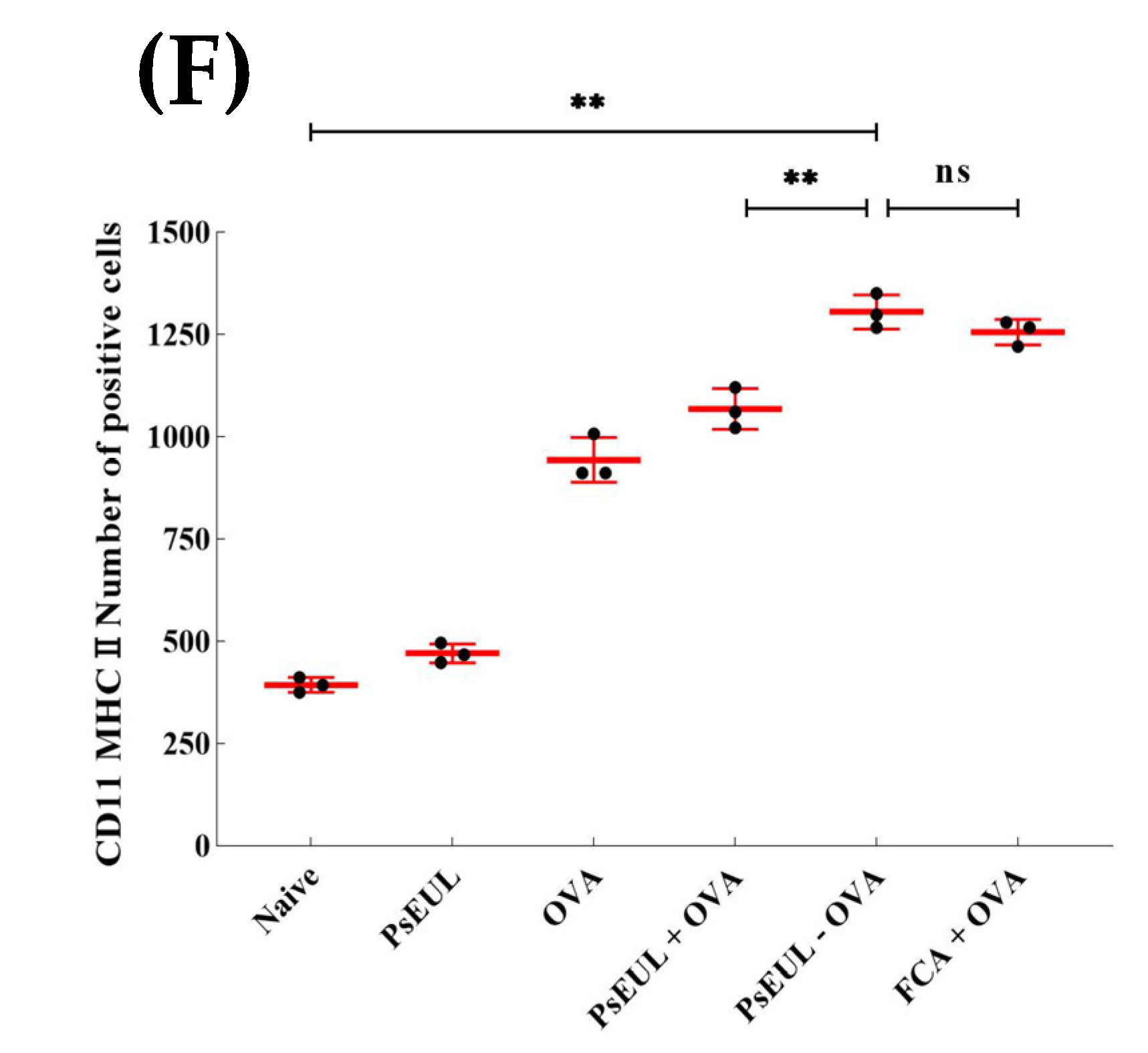
3.9. Effects of PsEUL-OVA on the Maturation of DCs in ICR Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.Y.; Tang, L.; He, J.W.; Li, J.; Wang, Y.Z. Ethnobotany, Phytochemistry and Pharmacological Properties of Eucommia ulmoides: A Review. Am. J. Chin. Med. 2019, 47, 259–300. [Google Scholar] [CrossRef]
- He, X.R.; Wang, J.H.; Li, M.X.; Hao, D.J.; Yang, Y.; Zhang, C.L.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Ma, F.B.; Ruiz-Ortega, L.I.; Peng, Y.; Tian, Y.; He, W.; Tang, B. Fabrication of strontium Eucommia ulmoides polysaccharides and in vitro evaluation of their osteoimmunomodulatory property. Int. J. Biol. Macromol. 2019, 140, 727–735. [Google Scholar] [CrossRef]
- Feng, H.B.; Fan, J.; Song, Z.H.; Du, X.G.; Chen, Y.; Wang, J.S.; Song, G.D. Characterization and immunoenhancement activities of Eucommia ulmoides polysaccharides. Carbohydr. Polym. 2016, 136, 803–811. [Google Scholar] [CrossRef]
- Ding, H.X.; Cao, A.Z.; Li, H.Y.; Zhao, Y.; Feng, J. Effects of Eucommia ulmoides leaf extracts on growth performance, antioxidant capacity and intestinal function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Carr, F. Pre-Clinical Considerations in the Assessment of Immunogenicity for Protein Therapeutics. Curr. Drug Saf. 2010, 5, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J. Introduction to current and future protein therapeutics: A protein engineering perspective. Exp. Cell Res. 2011, 317, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, A.R.S.; Gupta, S.; Jawalekar, S.; Pande, A.H. Protein Chimerization: A New Frontier for Engineering Protein Therapeutics with Improved Pharmacokinetics. J. Pharmacol. Exp. Ther. 2019, 370, 703–714. [Google Scholar] [CrossRef]
- Pecht, I. Immuno-receptors: From recognition to signaling and function. Eur. Biophys. J. 2018, 47, 363–371. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Lin, L.; Yang, J.; Yang, Y.; Zhi, H.; Hu, X.; Chai, D.K.; Liu, Y.J.; Shen, X.; Wang, J.; Song, Y.Q.; et al. Phosphorylation of Radix Cyathula officinalis polysaccharide improves its immune-enhancing activity. J. Carbohydr. Chem. 2020, 39, 50–62. [Google Scholar] [CrossRef]
- Yuan, B.; Han, J.N.; Cheng, Y.L.; Cheng, S.J.; Huang, D.C.; David, J.M.; Cao, C.J. Identification and characterization of antioxidant and immune-stimulatory polysaccharides in flaxseed hull. Food Chem. 2020, 315, 126266. [Google Scholar]
- Colombo, C.; Pitirollo, O.; Lay, L. Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development. Molecules 2018, 23, 1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Lu, C.Y.; Pi, C.C.; Zhuang, Y.J.; Chu, C.L.; Liu, W.H.; Chen, C.J. Extracellular polysaccharides produced by Ganoderma formosanum stimulate macrophage activation via multiple pattern-recognition receptors. BMC Complement. Altern. Med. 2012, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Mao, J.B.; Zhou, M.Q.; Jin, Y.W.; Lou, C.H.; Dong, Y.; Shou, D.; Hu, Y.; Yang, B.; Jin, C.Y.; et al. Polysaccharide from Phellinus igniarius activates TLR4-mediated signaling pathways in macrophages and shows immune adjuvant activity in mice. Int. J. Biol. Macromol. 2019, 123, 157–166. [Google Scholar] [CrossRef]
- Tae, H.; Lee, S.; Ki, C.S. β-Glucan hybridized poly(ethylene glycol) microgels for macrophage-targeted protein delivery. J. Ind. Eng. Chem. 2019, 75, 69–76. [Google Scholar] [CrossRef]
- Su, C.H.; Lu, M.K.; Lu, T.J.; Lai, M.N.; Ng, L.T. A (1→6)-Branched (1→4)-β-D-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264.7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef]
- Divya, M.; Govindarajan, M.; Karthikeyan, S.; Preetham, E.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. Antibiofilm and anticancer potential of β-glucan-binding protein-encrusted zinc oxide nanoparticles. Microb. Pathog. 2020, 141, 103992. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Muthana, S.M.; Freedberg, D.I.; Chen, X. Size-Controlled Chemoenzymatic Synthesis of Homogeneous Oligosaccharides of Neisseria meningitidis W Capsular Polysaccharide. ACS Catal. 2020, 10, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Y.; Caro-Aguilar, I.; Indrawati, L.; Smith, W.J.; Giovarelli, C.; Winters, M.A.; MacNair, J.; He, J.; Abeygunawardana, C.; et al. Immunogenicity Comparison of a Next Generation Pneumococcal Conjugate Vaccine in Animal Models and Human Infants. Pediatr. Infect. Dis. J. 2020, 39, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A. A new method of non-cross-linking conjugation of polysaccharides to proteins via thioether bonds for the preparation of saccharide? Protein conjugate vaccines. Vaccine 1999, 17, 1474–1483. [Google Scholar] [CrossRef]
- Thompson, A.; Lamberth, E.; Severs, J.; Scully, I.; Tarabar, S.; Ginis, J.; Jansen, K.U.; Gruber, W.C.; Scott, D.A.; Watson, W. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2019, 37, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Ravenscroft, N.; Kuttel, M.M. Modeling the conformations of Neisseria meningitidis serogroup a CPS and a carba-analogue: Implications for vaccine development. Carbohydr. Res. 2019, 486, 107838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Li, H.L.; Wan, A.J. EDC-Induced Self-Assembly of BSA-Au NCs. J. Fluoresc. 2019, 29, 627–630. [Google Scholar] [CrossRef]
- Han, U.; Choi, M.; Hong, J. Immobilization of basic fibroblast growth factor on heparin/EDC-methiodide nano-aggregates to maintain its continuous signaling. J. Ind. Eng. Chem. 2017, 53, 404–410. [Google Scholar] [CrossRef]
- Xu, J.K.; Hou, H.J.; Hu, J.P.; Liu, B.C. Optimized microwave extraction, characterization and antioxidant capacity of biological polysaccharides from Eucommia ulmoides Oliver leaf. Sci. Rep. 2018, 8, 6561. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Liu, H.M.; Yan, Y.Y.; Fan, L.Y.; Yang, J.N.; Wang, X.D.; Qin, G.Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. LWT 2020, 117, 108645. [Google Scholar] [CrossRef]
- Wu, Q.N.; Luo, M.; Yao, X.D.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydr. Polym. 2020, 236, 116020. [Google Scholar] [CrossRef] [PubMed]
- Kossaczka, Z.; Shiloach, J.; Johnson, Y.; Taylor, D.N.; Finkelstein, R.A.; Robbins, B.J.; Szu, S.C. Vibrio cholerae O139 conjugate vaccines: Synthesis and immunogenicity of V. cholerae O139 capsular polysaccharide conjugates with recombinant diphtheria toxin mutant in mice. Infect. Immun. 2000, 68, 5037–5043. [Google Scholar] [CrossRef][Green Version]
- Jin, Z.K.; Chu, C.Y.; Robbins, J.B.; Schneerson, R. Preparation and characterization of group A meningococcal capsular polysaccharide conjugates and evaluation of their immunogenicity in mice. Infect. Immun. 2003, 71, 5115–5120. [Google Scholar] [CrossRef]
- Xu, Z.P.; Tang, E.; Zhao, H.J. An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers 2019, 11, 1980. [Google Scholar] [CrossRef]
- Wang, W.D.; Li, C.; Zhang, B.; Huang, Q.; You, L.J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inul in conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Yang, X.N.; Zhang, L.Z.; Liu, Q.Q.; Feng, Y.Y.; Wu, D.Y.; Liu, Y.J.; Yang, J. Mannose-Modified Chitosan Poly (lactic-co-glycolicacid) Microspheres Actasa Mannose Receptor-Mediated Delivery System Enhancing the Immune Response. Polymers 2021, 13, 2208. [Google Scholar] [CrossRef]
- Feng, H.B.; Yang, X.N.; Fan, J.; Zhang, L.Z.; Liu, Q.Q.; Chai, D.K. DEC-205 receptor-mediated long-circling nanoliposome as an antigen and Eucommia ulmoides polysaccharide delivery system enhances the immune response via facilitatingdendritic cells maturation. Drug Deliv. 2020, 7, 1581–1596. [Google Scholar] [CrossRef]
- Bradley, J.S.C.; Olson, J.M. Assays for Determination of Protein Concentration. Curr. Protoc. Pharmacol. 2007, 73, A.3A.1–A.3A.32. [Google Scholar]
- Huang, W.; Wang, L.; Lu, M. Isolation, purification and structural characterization of an acidic polysaccharide fraction from leaves of Eucommia ulmoides oliver, named EOP-1. J. Funct. Mater. 2014, 45, 3047–3050. [Google Scholar]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Kanari, M. An acidic polysaccharide having activity on the reticuloendothelial system from the bark of Eucommia ulmoides. Chem. Pharm. Bull. 1990, 38, 1966–1969. [Google Scholar] [CrossRef]
- Wu, D.T.; Deng, Y.; Zhao, J.; Li, S.P. Molecular characterization of branched polysaccharides from Tremella fuciformis by asymmetrical flow field-flow fractionation and size exclusion chromatography. J. Sep. Sci. 2017, 40, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Kutzli, I.; Griener, D.; Gibis, M.; Schmid, C.; Dawid, C.; Baier, S.K.; Hofmann, T.; Weiss, J. Influence of Maillard reaction conditions on the formation and solubility of pea protein isolate-maltodextrin conjugates in electrospun fibers. Food Hydrocoll. 2020, 101, 105535. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, J. A practical method for extending the biuret assay to protein determination of corn-based products. Food Chem. 2017, 224, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Fan, J.; Qiu, H.; Wang, Z.Q.; Yan, Z.Q.; Yuan, L.; Guan, L.; Du, X.G.; Song, Z.H.; Han, X.F.; et al. Chuanminshen violaceum polysaccharides improve the immune responses of foot-and-mouth disease vaccine in mice. Int. J. Biol. Macromol. 2015, 78, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Han, Z.M.; Yang, L.M.; Hong, B.; Zhu, H. Extraction, characterization and antioxidant activity evaluation of polysaccharides from Smilacina japonica. Int. J. Biol. Macromol. 2020, 151, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, P.; Lin, P.; Zeng, X.; Brennan, M.A. Comparison of litchi polysaccharides extracted by four methods: Composition, structure and in vitro antioxidant activity. Int. J. Food Sci. Technol. 2020, 55, 1343–1350. [Google Scholar] [CrossRef]
- Qu, W.J.; Zhang, X.X.; Han, X.; Wang, Z.P.; He, R.H.; Ma, H.L. Structure and functional characteristics of rapeseed protein isolate-dextran conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Haque, S.; Sengupta, S.; Gupta, D.; Bhan, M.K.; Kumar, R.; Khan, A.; Jailkhani, B.S. Typhi derived OmpC peptide conjugated with Vi-polysaccharide evokes better immune response than free Vi-polysaccharide in mice. Biologicals 2019, 62, 50–56. [Google Scholar] [CrossRef]
- Bu, G.; Zhang, N.; Chen, F. The influence of glycosylation on the antigenicity, allergenicity, and structural properties of 11S-lactose conjugates. Food Res. Int. 2015, 76, 511–517. [Google Scholar] [CrossRef]
- Wang, P.P.; Wang, W.D.; Chen, C.; Fu, X.; Liu, R.H. Effect of Fructus Mori. bioactive polysaccharide conjugation on improving functional and antioxidant activity of whey protein. Int. J. Biol. Macromol. 2020, 148, 761–767. [Google Scholar]
- Nejati, S.; Vadeghani, E.M.; Khorshidi, S.; Karkhaneh, A. Role of particle shape on efficient and organ-based drug delivery. Eur. Polym. J. 2020, 122, 109353. [Google Scholar] [CrossRef]
- Goerdt, S. Alternative Aktivierung antigen presentierender Zellen Konzept und klinische Bedeutun. Hautarzt 2001, 52, 193–200. [Google Scholar]
- Chen, S.P.; Liu, C.C.; Huang, X.J.; Hu, L.Y.; Huang, Y.S.; Chen, H.Z.; Fang, Q.Y.; Dong, N.; Li, M.; Tang, W.; et al. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts. J. Funct. Foods 2020, 66, 103791. [Google Scholar] [CrossRef]
- Bo, R.N.; Sun, Y.Q.; Zhou, S.Z.; Ou, N.; Gu, P.F.; Liu, Z.G.; Hu, Y.L.; Liu, J.G.; Wang, D.Y. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int. J. Nanomed. 2017, 12, 6289–6301. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Gao, Z.L.; Zeng, D.Y.; Wang, J.P.; Baranenko, D.; Li, Y.Z.; Lu, W.H. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef]
- Chang, Y.; Lu, W.; Chu, Y.; Yan, J.K.; Wang, S.J.; Xu, H.L.; Ma, H.L.; Ma, J. Extraction of polysaccharides from maca: Characterization and immunoregulatory effects on CD4+ T cells. Int. J. Biol. Macromol. 2020, 154, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Fu, Y.J.; Luo, S.; Luo, X.; Wang, Q.; Hu, M.H.; Ma, F.L.; Ma, C.W.; Zhou, L. Polysaccharide from Radix codonopsis has beneficial effects on the maintenance of T-cell balance in mice. Biomed. Pharmacother. 2019, 112, 108682. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, A.; Xu, S.W.; Ni, H.Y.; Gu, P.F.; Liu, Z.G.; Zhang, Y.; Qiu, T.X.; Hu, Y.L.; Liu, J.G.; Wu, Y.; et al. Immunomodulatory effects of Alhagi honey polysaccharides encapsulated into PLGA nanoparticles. Carbohydr. Polym. 2019, 211, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Hao, C.F.; Zhai, R.N.; Bao, L.; Wang, D.; Li, Y.P.; Yu, X.H.; Huang, R.X.; Yao, W. Effects of cyclophosphamide on the phenotypes and functions of THP-1 cells. Environ. Toxicol. Pharmacol. 2019, 70, 103201. [Google Scholar] [CrossRef]
- Guo, M.Z.; Meng, M.; Zhao, J.H.; Wang, X.; Wang, C.L. Immunomodulatory effects of the polysaccharide from Craterellus cornucopioides via activating the TLR4-NFκB signaling pathway in peritoneal macrophages of BALB/c mice. Int. J. Biol. Macromol. 2020, 160, 871–879. [Google Scholar] [CrossRef]
- Qiao, W.L.; Ji, S.Y.; Zhao, Y.B.; Hu, T. Conjugation of β-glucan markedly increase the immunogencity of meningococcal group Y polysaccharide conjugate vaccine. Vaccine 2015, 33, 2066–2072. [Google Scholar] [CrossRef]
- Chaiyama, V.; Keawsompong, S.; LeBlanc, J.G.; de Moreno de LeBlanc, A.; Chatel, J.-M.; Chanput, W. Action modes of the immune modulating activities of crude mushroom polysaccharide from Phallus atrovolvatus, Bioact. Carbohydr. Diet. Fibre 2020, 23, 100216. [Google Scholar] [CrossRef]
- Cheung, J.K.H.; Li, J.; Cheung, A.W.H.; Zhu, Y.; Zheng, K.Y.Z.; Bi, C.W.C.; Duan, R.; Choi, R.C.Y.; Lau, D.T.W.; Dong, T.T.X.; et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J. Ethnopharmacol. 2009, 124, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Jung, J.H.; Kim, H.W.; Lee, S.J.; Chi, Y.M.; Jee, H.S.; Yoon, T.J.; Shin, K.S. Polysaccharide isolated from fermented barley activates innate immune system and anti-tumor metastasis in mice. J. Cereal Sci. 2020, 92, 102919. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, J.S.; Song, Y.R.; Rhee, Y.K.; Cho, C.W.; Ryu, J.H.; Inn, K.S.; Hong, H.D.; Lee, K.T. Immunostimulatory effects of polysaccharides isolated from young barley leaves (Hordeum vulgare L.) with dual activation of Th1 and Th2 in splenic T cells and cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Miao, J.F.; Zhang, Y.S.; Huang, G.Q.; Ma, H.T.; Zou, S.X.; Zhu, Y.M. Polysaccharide Nucleic Acid of Bacillus Calmette Guerin Modulates Th1/Th2 Cytokine Gene Expression in Lipopolysaccharide-Induced Mastitis in Rats. Agric. Sci. China 2009, 8, 1010–1018. [Google Scholar] [CrossRef]
- Huang, Q.R.; Yu, W.L.; Hu, T. Potent Antigen-Adjuvant Delivery System by Conjugation of Mycobacterium tuberculosis Ag85B-HspX Fusion Protein with Arabinogalactan-Poly(I:C) Conjugate. Bioconjug. Chem. 2016, 20, 1165–1174. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ni, H.Y.; Yu, L.; Xu, S.W.; Bo, R.N.; Qiu, T.X.; Gu, P.F.; Zhu, T.Y.; He, J.; Wusiman, A.; et al. Adjuvant activities of CTAB-modified Polygonatum sibiricum polysaccharide cubosomes on immune responses to ovalbumin in mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef]
- Huang, Q.R. Meningitis polysaccharide conjugate vaccine and Mycobacterium tuberculosis subunit vaccine based on chemical modification. Grad. Sch. Chin. Acad. Sci. 2016, 1, 131–134. [Google Scholar]
- Wang, H.; Yu, Q.; Ding, X.M.; Hu, X.Y.; Hou, K.Y.; Liu, X.Z.; Nie, S.P.; Xie, M.Y. RNA-seq based elucidation of mechanism underlying Ganoderma atrum polysaccharide induced immune activation of murine myeloid-derived dendritic cells. J. Funct. Foods. 2019, 55, 104–116. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef]
- Chen, X.Y.; Han, W.W.; Wang, G.X.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Yang, J.; Zhi, H.; Hu, X.; Yang, Y.; Zhang, L.; Liu, Q.; Feng, Y.; Wu, D.; Li, H. Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response. Pharmaceutics 2021, 13, 1384. https://doi.org/10.3390/pharmaceutics13091384
Feng H, Yang J, Zhi H, Hu X, Yang Y, Zhang L, Liu Q, Feng Y, Wu D, Li H. Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response. Pharmaceutics. 2021; 13(9):1384. https://doi.org/10.3390/pharmaceutics13091384
Chicago/Turabian StyleFeng, Haibo, Jie Yang, Hui Zhi, Xin Hu, Yan Yang, Linzi Zhang, Qianqian Liu, Yangyang Feng, Daiyan Wu, and Hangyu Li. 2021. "Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response" Pharmaceutics 13, no. 9: 1384. https://doi.org/10.3390/pharmaceutics13091384
APA StyleFeng, H., Yang, J., Zhi, H., Hu, X., Yang, Y., Zhang, L., Liu, Q., Feng, Y., Wu, D., & Li, H. (2021). Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response. Pharmaceutics, 13(9), 1384. https://doi.org/10.3390/pharmaceutics13091384