Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy
Abstract
:1. Introduction
2. Properties of Black Phosphorus Nanomaterials
2.1. Optical Properties
2.2. Biocompatibility and Biodegradability
2.3. High Loading Efficiency
3. Synthesis of Black Phosphorus Nanomaterials
3.1. Synthesis of Black Phosphorus Nanosheets
3.1.1. Mechanical Exfoliation Method
3.1.2. Ultrasonic-Assisted Liquid-Phase Exfoliation Method
3.1.3. Electrochemical Exfoliation Method
3.1.4. Chemical Vapor Deposition Method
3.2. Synthesis of Black Phosphorus Quantum Dots
3.2.1. Ultrasonic Exfoliation Method
3.2.2. Solvothermal Method
3.2.3. Blender Breaking Method
3.2.4. Pulsed Laser Ablation Method
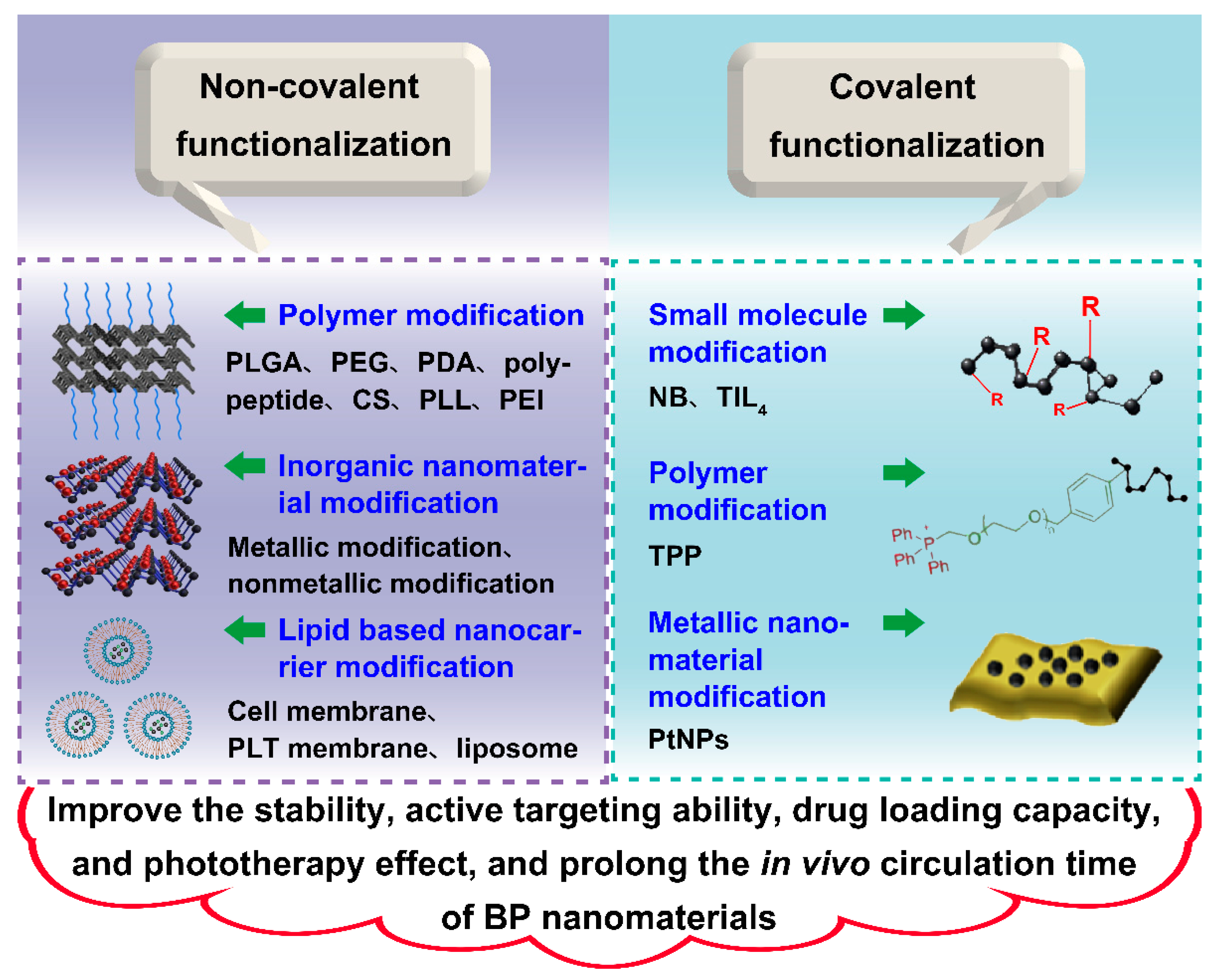
4. Surface Modification of Black Phosphorus Nanomaterials
4.1. Physical Modification
4.1.1. Modification Using Polymers
4.1.2. Modification Using Inorganic Nanomaterials
4.1.3. Modification Using Lipid Based Nanocarrier
4.2. Chemical Modification
4.2.1. Modification Using Small Molecule
4.2.2. Modification Using Polymer
4.2.3. Modification Using Metallic Nanomaterial
5. Delivery of Immunotherapeutic Agents for Cancer Immunotherapy
5.1. Checkpoint Inhibitors Delivery
5.2. Antigen Delivery
5.3. Immunoadjuvant Delivery
6. Synergistic Cancer Photoimmunotherapy Based on Black Phosphorus Nanomaterials
6.1. Synergistic Photothermal-Immuno Therapy
6.2. Synergistic Photodynamic-Immuno Therapy
6.3. Synergistic Photothermal-Photodynamic-Immuno Therapy
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| aCD47 | CD47 antibody |
| Adpgk | Adpgk neoantigen peptide |
| Ag+ | Argentum ion |
| aPD-1 | Programmed cell death protein 1 (PD-1) antibody |
| Au | Aurum |
| CCNV | Cancer cell membrane nanovesicle |
| CS | Chitosan |
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4 |
| FKF | Phenylalanine-lysine-phenylalanine |
| FKK | Fmoc-Lys-Lys-Phe peptide |
| hEX | Serum exosomes |
| Ir | Iridium |
| MFL | Multifunctional liposome |
| MS | Mesoporous silica |
| MSC | Mesenchymal stem cell |
| NB | Nile Blue |
| NG | Neutrophil membrane-derived nanoghost |
| NIPAM | N-isopropylacrylamide |
| NVs | Nanovesicles |
| OVAp | OVA257-264 peptide |
| PDA | Polydopamine |
| PEG | Polyethylene glycol |
| PEI | Polyetherimide |
| PL | Programmed death ligand 1 |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLL | Polylysine |
| PLT | Platelet |
| PLTm | Platelet membrane |
| PtNPs | Platinum nanoparticles |
| R837 | Imiquimod |
| RdB | Rhodamine B |
| RMNV | Erythrocyte membrane nanovesicle |
| TIL4 | Titanium sulfonate ligand |
| TPP | Triphenylphosphine |
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.; Tang, L.; Xiao, Q.; Zhang, Z.; Zhang, Z.; Zang, J.; Zhou, J.; Wang, Y.; Wang, W.; Ren, M. Reconstituted high density lipoprotein (rHDL), a versatile drug delivery nanoplatform for tumor targeted therapy. J. Mater. Chem. B 2021, 9, 612–633. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1590. [Google Scholar] [CrossRef]
- Li, J.; Han, M.; Li, J.; Ge, Z.; Wang, Q.; Zhou, K.; Yin, X. Sterically stabilized recombined HDL composed of modified apolipoprotein A-I for efficient targeting toward glioma cells. Drug Deliv. 2020, 27, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummerer, K.; Menz, J.; Schubert, T.; Thielemans, W. Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 2011, 82, 1387–1392. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Sun, K.; Yu, S.; Wang, R.; Xie, H. Electrochemical Activity of Black Phosphorus as an Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 14772–14779. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ponraj, J.; Fan, D.; Zhang, H. An overview of the optical properties and applications of black phosphorus. Nanoscale 2020, 12, 3513–3534. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ge, Y.; Wang, C.; Zhang, H. Mid-Infrared Photonics Using 2D Materials: Status and Challenges. Laser Photonics Rev. 2020, 14. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.; Jeong, T.; Won, M.; Park, G.; Sang, D.; Liu, L.; Zhang, H.; Kim, J. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Ouyang, J.; Chu, D.; Han, F.; Shi, L.; Liu, R.; Guo, Z.; Gu, G.; Tao, W.; et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021, 6, 4053–4064. [Google Scholar] [CrossRef]
- Lu, F.; Li, Z.; Kang, Y.; Su, Z.; Yu, R.; Zhang, S. Black phosphorus quantum dots encapsulated in anionic waterborne polyurethane nanoparticles for enhancing stability and reactive oxygen species generation for cancer PDT/PTT therapy. J. Mater. Chem. B 2020, 8, 10650–10661. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Liu, Y.; Qi, X.; Jin, H.; Yang, C.; Liu, J.; Li, G.; He, Q. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, Z.; Lan, S.; Sun, H.; Zeng, Y.; Liu, X. The design of Janus black phosphorus quantum dots@metal-organic nanoparticles for simultaneously enhancing environmental stability and photodynamic therapy efficiency. Mater. Chem. Front. 2019, 3, 656–663. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, Y. Surface modification of black phosphorus-based nanomaterials in biomedical applications: Strategies and recent advances. Acta Biomater. 2020, 118, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tao, Z.; Wang, D.; Liu, Q.; Wu, H.; Lan, S.; Dong, A. Engineering a black phosphorus-based magnetic nanosystem armed with antibacterial N-halamine polymer for recyclable blood disinfection. Chem. Eng. J. 2021, 415. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.; Nordquist, R.; Chen, W. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef]
- Sanmamed, M.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Beatty, G.; Gladney, W. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Singhal, A.; Liu, H.; Nordquist, R. Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res. 2001, 61, 459–461. [Google Scholar] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy (vol 10, pg 12431, 2018). ACS Appl. Mater. Interfaces 2019, 11, 43797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Lu, H.; Lee, R. Near Infrared Light Triggered Photo/Immuno-Therapy Toward Cancers. Front. Bioeng. Biotechnol. 2020, 8, 488. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, X.; Liu, Y.; Fan, T.; Wang, Q.; Zhang, H.; Chen, T. Black phosphorus as a versatile nanoplatform: From unique properties to biomedical applications. J. Innov. Opt. Health Sci. 2020, 13, 2030008. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Yong, K.; Choi, J.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106. [Google Scholar] [CrossRef]
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033. [Google Scholar] [CrossRef]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.; Berner, N.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef]
- Gao, N.; Xing, C.; Wang, H.; Feng, L.; Zeng, X.; Mei, L.; Peng, Z. pH-Responsive Dual Drug-Loaded Nanocarriers Based on Poly (2-Ethyl-2-Oxazoline) Modified Black Phosphorus Nanosheets for Cancer Chemo/Photothermal Therapy. Front. Pharmacol. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2020, 6, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, L.; Jiang, Y.; Guo, C. Phycocyanin-functionalized black phosphorus quantum dots enhance PDT/PTT therapy by inducing ROS and irreparable DNA damage. Biomater. Sci. 2021, 9, 5302–5318. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, K.; Li, X.; Xu, Q.; Weng, J.; Xu, J. Electron Matters: Recent Advances in Passivation and Applications of Black Phosphorus. Adv. Mater. 2021, 2005924. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Su, C.; Yang, B.; Fei, X.; Li, Y.; Hou, Y.; Zhao, H.; Guo, Y.; Zhuang, Z.; et al. Biodegradable Black Phosphorus-based Nanomaterials in Biomedicine: Theranostic Applications. Curr. Med. Chem. 2019, 26, 1788–1805. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.; Yu, X. Black Phosphorus: Bioactive Nanomaterials with Inherent and Selective Chemotherapeutic Effects. Angew. Chem. Int. Ed. 2019, 58, 769–774. [Google Scholar] [CrossRef]
- Abbaraju, P.; Meka, A.; Song, H.; Yang, Y.; Jambhrunkar, M.; Zhang, J.; Xu, C.; Yu, M.; Yu, C. Asymmetric Silica Nanoparticles with Tunable Head–Tail Structures Enhance Hemocompatibility and Maturation of Immune Cells. J. Am. Chem. Soc. 2017, 139, 6321–6328. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, D.; Fan, T.; Xing, C.; Li, Z.; Tao, W.; Liu, L.; Bao, S.; Fan, D.; Zhang, H. Black phosphorus analogue tin sulfide nanosheets: Synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B 2018, 6, 4747–4755. [Google Scholar] [CrossRef]
- Zhou, W.; Cui, H.; Ying, L.; Yu, X. Enhanced Cytosolic Delivery and Release of CRISPR/Cas9 by Black Phosphorus Nanosheets for Genome Editing. Angew. Chem. Int. Ed. 2018, 57, 10268–10272. [Google Scholar] [CrossRef]
- Guan, L.; Xing, B.; Niu, X.; Wang, D.; Yu, Y.; Zhang, S.; Yan, X.; Wang, Y.; Sha, J. Metal-assisted exfoliation of few-layer black phosphorus with high yield. Chem. Commun. 2018, 54, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, Y.; Xu, X.; Wang, S.; Zhang, X. Preparation of few-layer black phosphorus by wet ball milling exfoliation. J. Mater. Sci. 2020, 31, 9543–9549. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Kang, J.; Wells, S.; Wood, J.; Lee, J.; Liu, X.; Ryder, C.; Zhu, J.; Guest, J.; Husko, C.; Hersam, M. Stable aqueous dispersions of optically and electronically active phosphorene. Proc. Natl. Acad. Sci. USA 2016, 113, 11688–11693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Zhao, M.; Zhang, J.; Ma, X.; Zhang, J.; Hu, T.; Tang, T.; Jia, J.; Wu, H. Electrochemical cathode exfoliation of bulky black phosphorus into few-layer phosphorene nanosheets. Electrochem. Commun. 2018, 89, 10–13. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. Electrochemical Exfoliation of Layered Black Phosphorus into Phosphorene. Angew. Chem. Int. Ed. Engl. 2017, 56, 10443–10445. [Google Scholar] [CrossRef]
- Erande, M.; Pawar, M.; Late, D. Humidity Sensing and Photodetection Behavior of Electrochemically Exfoliated Atomically Thin-Layered Black Phosphorus Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef]
- Smith, J.; Hagaman, D.; Ji, H. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J.; Yuan, S.; Lin, S.; Yau, H.; Dai, J.; Lau, S.P. Field-effect transistors based on amorphous black phosphorus ultrathin films by pulsed laser deposition. Adv. Mater. 2015, 27, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liang, Q.; Xu, Y.; Fu, H.; Xiao, X. Facile Solvothermal Synthesis of Black Phosphorus Nanosheets from Red Phosphorus for Efficient Photocatalytic Hydrogen Evolution. Eur. J. Inorg. Chem. 2020, 2020, 773–779. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, T.; Shao, Y.; Wu, Y.; Huang, B.; Hao, X. A Novel Mild Phase-Transition to Prepare Black Phosphorus Nanosheets with Excellent Energy Applications. Small 2017, 13, 1602243. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. Engl. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, Y.; Liu, Y.; Ren, G.; Zhang, Z.; Wu, S.; Shen, J. Ultrasmall black phosphorus quantum dots: Synthesis, characterization, and application in cancer treatment. Analyst 2018, 143, 5822–5833. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wu, Y.; Lin, Y.; Xu, X.; Lian, H.; Huang, G.; Liu, J.Z.; Wu, X.; Yang, H. Black Phosphorus Quantum Dots with Renal Clearance Property for Efficient Photodynamic Therapy. Small 2018, 14, 1702815. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Zhang, X. Synthesis of Black Phosphorus Quantum Dots with High Quantum Yield by Pulsed Laser Ablation for Cell Bioimaging. Chem. Asian J. 2018, 13, 1842–1846. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, F.; Zhang, L.; Li, M.; Chen, J.; Xu, S.; Huang, G.; Chen, W.; Sun, L. Ultrafast Preparation of Black Phosphorus Quantum Dots for Efficient Humidity Sensing. Chemistry 2016, 22, 7357–7362. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Luo, Z.; Xu, X. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Onodera, M.; Masubuchi, S.; Moriya, R.; Machida, T. Assembly of van der Waals heterostructures: Exfoliation, searching, and stacking of 2D materials. Jpn. J. Appl. Phys. 2020, 59, 010101. [Google Scholar] [CrossRef]
- Gao, E.; Lin, S.; Qin, Z.; Buehler, M.; Feng, X.; Xu, Z. Mechanical exfoliation of two-dimensional materials. J. Mech. Phys. Solids 2018, 115, 248–262. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Xu, B.; Ding, J.; Yu, H. Large-yield exfoliation of few-layer black phosphorus nanosheets in liquid. New J. Chem. 2019, 43, 19365–19371. [Google Scholar] [CrossRef]
- Shen, J.; Liu, L.; Huang, W.; Wu, K. Polyvinylpyrrolidone-assisted solvent exfoliation of black phosphorus nanosheets and electrochemical sensing of p-nitrophenol. Anal. Chim. Acta 2021, 1167, 338594. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J. Electrochemical exfoliation of two-dimensional layered black phosphorus and applications. J. Energy Chem. 2020, 49, 365–374. [Google Scholar] [CrossRef]
- Suryawanshi, S.; More, M.; Late, D. Laser exfoliation of 2D black phosphorus nanosheets and their application as a field emitter. RSC Adv. 2016, 6, 112103–112108. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, X.; Zhang, Y.; Zhang, H.; Zhang, Q.; Huang, Z.; Xu, X.; Guo, J.; Zhang, H.; Sun, L.; et al. Epitaxial nucleation and lateral growth of high-crystalline black phosphorus films on silicon. Nat. Commun. 2020, 11, 1330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black phosphorus quantum dots. Angew. Chem. Int. Ed. Engl. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Han, S.; Hu, L.; Wang, X.; Zhou, Y.; Zeng, Y.; Ruan, S.; Pan, C.; Peng, Z. Black Phosphorus Quantum Dots with Tunable Memory Properties and Multilevel Resistive Switching Characteristics. Adv. Sci. 2017, 4, 1600435. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.; Lee, S.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.; Park, H.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Wang, Y.; Feng, X.; Liao, Z.; Su, Q.; Lin, X.; He, Z. Black Phosphorus Quantum Dots for Hole Extraction of Typical Planar Hybrid Perovskite Solar Cells. J. Phys. Chem. Lett. 2017, 8, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-functionalized black phosphorus quantum dots for cancer theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Guo, Z.; Huang, H.; Xiao, Q.; Zhang, H.; Yu, X. Solvothermal Synthesis and Ultrafast Photonics of Black Phosphorus Quantum Dots. Adv. Opt. Mater. 2016, 4, 1223–1229. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, S.; Wang, P.; Yang, Y.; Li, Z.; Chen, D.; Yu, Z.; Zou, Z. Bandgap-tunable black phosphorus quantum dots: Visible-light-active photocatalysts. Chem. Commun. 2018, 54, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, Y.; Wang, M.; Wang, H.; Lu, C.; Yang, H. Biodegradable Black-Phosphorus-Nanosheet-Based Nanoagent for Enhanced Chemo–Photothermal Therapy. Part. Part. Syst. Charact. 2020, 37, 2000243. [Google Scholar] [CrossRef]
- Wang, S.; Shao, J.; Li, Z.; Ren, Q.; Yu, X.F.; Liu, S. Black Phosphorus-Based Multimodal Nanoagent: Showing Targeted Combinatory Therapeutics against Cancer Metastasis. Nano Lett. 2019, 19, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, Y.; Gao, N.; Cheng, W.; Wang, X.; Cao, J.; Zeng, X.; Liu, G.; Mei, L. Mesenchymal stem cells transporting black phosphorus-based biocompatible nanospheres: Active trojan horse for enhanced photothermal cancer therapy. Chem. Eng. J. 2020, 385, 123942. [Google Scholar] [CrossRef]
- Chan, L.; Gao, P.; Zhou, W.; Mei, C.; Huang, Y.; Yu, X.; Chu, P.; Chen, T. Sequentially Triggered Delivery System of Black Phosphorus Quantum Dots with Surface Charge-Switching Ability for Precise Tumor Radiosensitization. ACS Nano 2018, 12, 12401–12415. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, B.; Li, S.; He, B.; Pu, Y. Combination of PEG-decorated black phosphorus nanosheets and immunoadjuvant for photoimmunotherapy of melanoma. J. Mater. Chem. B 2020, 8, 2805–2813. [Google Scholar] [CrossRef]
- Wu, R.; Lin, J.; Xing, Y.; Dai, Z.; Wang, L.; Zhang, X. pH-Sensitive Black Phosphorous-Incorporated Hydrogel as Novel Implant for Cancer Treatment. J. Pharm. Sci. 2019, 108, 2542–2551. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Zhu, J.; Xue, L.; Ou, C.; Wang, W.; Lu, M.; Song, X.; Dong, X. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem. Sci. 2019, 10, 3779–3785. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Nie, J.; Wang, H.; Xing, C.; Mei, L.; Xiong, W.; Zeng, X.; Peng, Z. A Versatile Platform Based on Black Phosphorus Nanosheets with Enhanced Stability for Cancer Synergistic Therapy. J. Biomed. Nanotechnol. 2018, 14, 1883–1897. [Google Scholar] [CrossRef]
- Wang, H.; Hu, K.; Li, Z.; Wang, C.; Yu, M.; Li, Z.; Li, Z. Black Phosphorus Nanosheets Passivation Using a Tripeptide. Small 2018, 14, e1801701. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Q.; Zhang, M.; Lv, X.; Li, Z.; Mohammadniaei, M.; Zhou, N.; Sun, Y. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr. Polym. 2021, 265, 118065. [Google Scholar] [CrossRef]
- Yue, H.; Huang, R.; Shan, Y.; Xing, D. Delivery of Cas13a/crRNA by self-degradable black phosphorus nanosheets to specifically inhibit Mcl-1 for breast cancer therapy. J. Mater. Chem. B 2020, 8, 11096–11106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, X.; Lan, S.; Sun, H.; Wang, X.; Liu, X.; Zhang, Y.; Zeng, Y. Localized Surface Plasmon Resonance Enhanced Singlet Oxygen Generation and Light Absorption Based on Black Phosphorus@AuNPs Nanosheet for Tumor Photodynamic/Thermal Therapy. Part. Part. Syst. Charact. 2018, 35, 1800010. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile synthesis of black phosphorus-Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qian, M.; Jiang, H.; Zhou, Y.; Du, Y.; Yang, Y.; Huo, T.; Huang, R.; Wang, Y. Multifunctional mesoporous black phosphorus-based nanosheet for enhanced tumor-targeted combined therapy with biodegradation-mediated metastasis inhibition. Biomaterials 2020, 236, 119770. [Google Scholar] [CrossRef]
- Chan, L.; Chen, X.; Gao, P.; Xie, J.; Zhang, Z.; Zhao, J.; Chen, T. Coordination-Driven Enhancement of Radiosensitization by Black Phosphorus via Regulating Tumor Metabolism. ACS Nano 2021, 15, 3047–3060. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, Q.; Wu, B.; Zhao, Q.; Li, J.; Huang, X.; Chen, W.; Gui, R. Platelet-Membrane-Camouflaged Black Phosphorus Quantum Dots Enhance Anticancer Effect Mediated by Apoptosis and Autophagy. ACS Appl. Mater. Interfaces 2019, 11, 28254–28266. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Zhang, A.; Wu, X.; Cheng, H.; He, D.; Wang, T.; He, X.; Wang, K. Liposome-Stabilized Black Phosphorus for Photothermal Drug Delivery and Oxygen Self-Enriched Photodynamic Therapy. ACS Appl. Nano Mater. 2019, 3, 563–575. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Xue, T.; Cheng, Q.; Ye, X.; Wang, C.; Yu, Y.; Ji, X.; Wu, M.; Zhang, X.; et al. Liposomes Encapsulating Neoantigens and Black Phosphorus Quantum Dots for Enhancing Photothermal Immunotherapy. J. Biomed. Nanotechnol. 2020, 16, 1394–1405. [Google Scholar] [CrossRef]
- Makadia, H.; Siegel, S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-Guided pH-Sensitive Photodynamic Therapy Using Charge Reversible Upconversion Nanoparticles under Near-Infrared Light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
- Prasad, R.; Aiyer, S.; Chauhan, D.; Srivastava, R.; Selvaraj, K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale 2016, 8, 4537–4546. [Google Scholar] [CrossRef] [Green Version]
- Alcantar, N.; Aydil, E.; Israelachvili, J. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.; Choi, S.; Song, I.; Kim, W.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, W.; Liu, D.; Wu, S.; Song, B.; Ma, J.; Chen, D.; Hu, H. Tumor Microenvironment Stimuli-Responsive Nanoparticles for Programmed Anticancer Drug Delivery. Mol. Pharm. 2020, 17, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.; Engbers, C.; Zalipsky, S. New amphipatic polymer lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjug. Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.; Sazanovich, I.; Baggaley, E.; Bonneau, M.; Guerchais, V.; Williams, J.; Weinstein, J.; Bryant, H. Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR Light. Chemistry 2017, 23, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Wang, S.; Duan, Y.; Zhang, Q.; Chen, M.; Gao, W.; Zhang, L. Emerging Approaches to Functionalizing Cell Membrane-Coated Nanoparticles. Biochemistry 2021, 60, 941–955. [Google Scholar] [CrossRef]
- Bhowmik, D.; Mote, K.; MacLaughlin, C.; Biswas, N.; Chandra, B.; Basu, J.; Walker, G.; Madhu, P.; Maiti, S. Cell-Membrane-Mimicking Lipid-Coated Nanoparticles Confer Raman Enhancement to Membrane Proteins and Reveal Membrane-Attached Amyloid-beta Conformation. ACS Nano 2015, 9, 9070–9077. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Ding, K.; Zheng, C.; Sun, L.; Liu, X.; Yin, Y.; Wang, L. NIR light-induced tumor phototherapy using ICG delivery system based on platelet-membrane-camouflaged hollow bismuth selenide nanoparticles. Chin. Chem. Lett. 2020, 31, 1168–1172. [Google Scholar] [CrossRef]
- Yin, X.; Luo, L.; Li, W.; Yang, J.; Zhu, C.; Jiang, M.; Qin, B.; Yuan, X.; Yin, H.; Lu, Y.; et al. A cabazitaxel liposome for increased solubility, enhanced antitumor effect and reduced systemic toxicity. Asian J. Pharm. Sci. 2019, 14, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Khan, T.; Callan, B. Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. Int. J. Pharm. 2020, 2, 100040. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gao, H.; Gao, L.; Li, F.; Xu, N.; Long, X.; Hu, Y.; Jin, J.; Ma, J. Covalent functionalization of black phosphorus nanoflakes by carbon free radicals for durable air and water stability. Nanoscale 2018, 10, 5834–5839. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Q.; Niu, X.; Zhao, Y.; Chen, Q.; Wang, J. Covalent Functionalization of Black Phosphorus from First-Principles. J. Phys. Chem. Lett. 2016, 7, 4540–4546. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, L.; Li, Z.; Yang, N.; Fu, H.; Wu, L.; Cui, H.; Zhou, W.; Wang, J.; Wang, H.; et al. Stable and Multifunctional Dye-Modified Black Phosphorus Nanosheets for Near-Infrared Imaging-Guided Photothermal Therapy. Chem. Mater. 2017, 29, 7131–7139. [Google Scholar] [CrossRef]
- Qu, G.; Liu, W.; Zhao, Y.; Gao, J.; Xia, T.; Shi, J.; Hu, L.; Zhou, W.; Gao, J.; Wang, H.; et al. Improved Biocompatibility of Black Phosphorus Nanosheets by Chemical Modification. Angew. Chem. Int. Ed. Engl. 2017, 56, 14488–14493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Xiong, Y.; Cheng, K.; Huang, Q.; Cao, J.; He, F.; Mei, L.; Liu, G.; Deng, W. Heterobifunctional PEG-grafted black phosphorus quantum dots: “Three-in-One” nano-platforms for mitochondria-targeted photothermal cancer therapy. Asian J. Pharm. Sci. 2021, 16, 222–235. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Zhong, Z.; Huang, H.; Shao, J.; Xie, X.; Zhang, Y.; Wang, W.; Dong, X. Platinum nanoenzyme functionalized black phosphorus nanosheets for photothermal and enhanced-photodynamic therapy. Chem. Eng. J. 2021, 409, 127381. [Google Scholar] [CrossRef]
- Rupakheti, C.; Virshup, A.; Yang, W.; Beratan, D. Strategy To Discover Diverse Optimal Molecules in the Small Molecule Universe. J. Chem. Inf. Model. 2015, 55, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, A.; Mohammadi, R.; Ahmadi, S.; Monfared, A.; Rahmani, Z. Arylhydrazines: Novel and versatile electrophilic partners in cross-coupling reactions. RSC Adv. 2018, 8, 33828–33844. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Huang, H.; Xiao, Q.; Xu, Y.; Guo, Z.; Xie, H.; Shao, J.; Sun, Z.; Han, W.; et al. Surface Coordination of Black Phosphorus for Robust Air and Water Stability. Angew. Chem. Int. Ed. Engl. 2016, 55, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, J.; Sun, D.; Li, Q.; Ma, J.; Chen, X.; Zhu, X.; Zheng, N. A Novel Theranostic Nanoplatform Based on Pd@Pt-PEG-Ce6 for Enhanced Photodynamic Therapy by Modulating Tumor Hypoxia Microenvironment. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- An, D.; Fu, J.; Xie, Z.; Xing, C.; Zhang, B.; Wang, B.; Qiu, M. Progress in the therapeutic applications of polymer-decorated black phosphorus and black phosphorus analog nanomaterials in biomedicine. J. Mater. Chem. B 2020, 8, 7076–7120. [Google Scholar] [CrossRef]
- Pandey, A.; Nikam, A.; Fernandes, G.; Kulkarni, S.; Padya, B.; Prassl, R.; Das, S.; Joseph, A.; Deshmukh, P.; Patil, P.; et al. Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials 2020, 11, 13. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, S.; Chatterjee, S.; Das, J.; Sil, P. Nanoparticles as Smart Carriers for Enhanced Cancer Immunotherapy. Front. Chem. 2020, 8, 597806. [Google Scholar] [CrossRef]
- Farshbafnadi, M.; Pastaki Khoshbin, A.; Rezaei, N. Immune checkpoint inhibitors for triple-negative breast cancer: From immunological mechanisms to clinical evidence. Int. Immunopharmacol. 2021, 98, 107876. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Sweeney, E.; Ramanujam, A.; Fernandes, R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int. J. Hyperth. 2020, 37, 34–49. [Google Scholar] [CrossRef]
- Cano-Mejia, J.; Burga, R.; Sweeney, E.; Fisher, J.; Bollard, C.; Sandler, A.; Cruz, C.; Fernandes, R. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine 2017, 13, 771–781. [Google Scholar] [CrossRef]
- Ou, W.; Byeon, J.; Thapa, R.; Ku, S.; Yong, C.; Kim, J. Plug-and-Play Nanorization of Coarse Black Phosphorus for Targeted Chemo-photoimmunotherapy of Colorectal Cancer. ACS Nano 2018, 12, 10061–10074. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, X.; Wu, Y.; Cao, P.; Movahedi, F.; Liu, J.; Wang, J.; Xu, Z.; Gu, W. Mannose-Functionalized Biodegradable Nanoparticles Efficiently Deliver DNA Vaccine and Promote Anti-tumor Immunity. ACS Appl. Mater. Interfaces 2021, 13, 14015–14027. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, Q.; Song, H.; Shi, X.; Zhang, Y.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. PolyTLR7/8a-conjugated, antigen-trapping gold nanorods elicit anticancer immunity against abscopal tumors by photothermal therapy-induced in situ vaccination. Biomaterials 2021, 275, 120921. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Morishita, H.; Kobiyama, K.; Aoshi, T.; Ishii, K.; Sakurai, K. Immunization with antigenic peptides complexed with β-glucan induces potent cytotoxic T-lymphocyte activity in combination with CpG-ODNs. J. Control. Release 2015, 220, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wu, J.; Wu, L.; Zhang, B.; Hu, H.; Zhao, L.; Li, Z.; Yu, X.; Li, Y. Black phosphorous nanosheet: A novel immune-potentiating nanoadjuvant for near-infrared-improved immunotherapy. Biomaterials 2021, 273, 120788. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.; McArdle, S. Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments. Front. Immunol. 2020, 11, 615240. [Google Scholar] [CrossRef]
- Abbasi, S.; Uchida, S. Multifunctional Immunoadjuvants for Use in Minimalist Nucleic Acid Vaccines. Pharmaceutics 2021, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, H.; Guo, Z.; Zhang, W.; Yu, H.; Zhuang, Z.; Zhong, H.; Liu, Z. In situ photothermal activation of necroptosis potentiates black phosphorus-mediated cancer photo-immunotherapy. Chem. Eng. J. 2020, 394, 124314. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Chen, W.; Li, K.; Chen, X.; Tang, X.; Xie, G.; Luo, X.; Wang, X.; Liang, H.; et al. Biodegradable Black Phosphorus Nanosheets Mediate Specific Delivery of hTERT siRNA for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21137–21148. [Google Scholar] [CrossRef]
- Liu, X.; Gaihre, B.; George, M.; Li, Y.; Tilton, M.; Yaszemski, M.; Lu, L. 2D phosphorene nanosheets, quantum dots, nanoribbons: Synthesis and biomedical applications. Biomater. Sci. 2021, 9, 2768–2803. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, T.; Zheng, Y.; Yang, S.; Yu, Z.; Duo, Y.; Zhang, Y.; Adah, D.; Shi, L.; Sun, Z.; et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale 2020, 12, 19939–19952. [Google Scholar] [CrossRef]
- Xie, Z.; Peng, M.; Lu, R.; Meng, X.; Liang, W.; Li, Z.; Qiu, M.; Zhang, B.; Nie, G.; Xie, N.; et al. Black phosphorus-based photothermal therapy with aCD47-mediated immune checkpoint blockade for enhanced cancer immunotherapy. Light Sci. Appl. 2020, 9, 161. [Google Scholar] [CrossRef]
- Shou, X.; Liu, Y.; Wu, D.; Zhang, H.; Zhao, Y.; Sun, W.; Shen, X. Black phosphorus quantum dots doped multifunctional hydrogel particles for cancer immunotherapy. Chem. Eng. J. 2021, 408, 127349. [Google Scholar] [CrossRef]
- Yao, X.; Yang, B.; Wang, S.; Dai, Z.; Zhang, D.; Zheng, X.; Liu, Q. A novel multifunctional FePt/BP nanoplatform for synergistic photothermal/photodynamic/chemodynamic cancer therapies and photothermally-enhanced immunotherapy. J. Mater. Chem. B 2020, 8, 8010–8021. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Q.; Ye, J.; Ge, X.; Wang, J.; Song, J.; Yang, H. Ag+-Coupled Black Phosphorus Vesicles with Emerging NIR-II Photoacoustic Imaging Performance for Cancer Immune-Dynamic Therapy and Fast Wound Healing. Angew. Chem. Int. Ed. 2020, 59, 22202–22209. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Fu, Q.; Liu, Y.; Wang, J.; Song, J.; Yang, H. NIR/ROS-Responsive Black Phosphorus QD Vesicles as Immunoadjuvant Carrier for Specific Cancer Photodynamic Immunotherapy. Adv. Funct. Mater. 2020, 30, 1905758. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Su, Y.; Li, M.; Zhou, J.; Zhang, W.; Wang, W. A neutrophil membrane-functionalized black phosphorus riding inflammatory signal for positive feedback and multimode cancer therapy. Mater. Horiz. 2020, 7, 574–585. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.; Yu, X. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Batts, A.J.; Mathew, A.S.; Thim, E.A.; Price, R.J. Focused Ultrasound Hyperthermia Augments Release of Glioma-derived Extracellular Vesicles with Differential Immunomodulatory Capacity. Theranostics 2020, 10, 7436–7447. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, W.; Zheng, Y.; Ding, Y.; Xiang, Y.; Liu, B.; Tong, A. Organic Nanoparticles with Persistent Luminescence for In Vivo Afterglow Imaging-Guided Photodynamic Therapy. Chemistry 2021, 27, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, X.; Hu, X.; Xiao, L.; Zhang, L.; Song, L.; Xu, M.; Zou, Y.; Chen, L.; Chen, Z.; et al. Simultaneous Application of Photothermal Therapy and an Anti-inflammatory Prodrug using Pyrene-Aspirin-Loaded Gold Nanorod Graphitic Nanocapsules. Angew. Chem. Int. Ed. Engl. 2018, 57, 177–181. [Google Scholar] [CrossRef] [PubMed]





| Classification of BP Nanomaterials | Synthesis Routes | Properties of the Prepared BP Nanomaterials | Ref. |
|---|---|---|---|
| BPNSs | Tape exfoliation method | The thickness is about 3 nm, and the lateral size is more than 50 μm | [46] |
| Wet ball milling exfoliation method | The lateral size is between 1 and 5 μm, and the flakes are generally 3–5 layers | [47] | |
| Ultrasonic-assisted liquid-phase exfoliation method | The thickness ranges from 2.06 to 9.4 nm with single or few layers | [33,48,49] | |
| Electrochemical exfoliation method | The lateral size ranges from 0.5 to 30 μm, and the thickness is from 1.4 to 10 nm with single or few layers | [50,51,52] | |
| Chemical vapor deposition method | Commonly with 4 layers’ nanoflakes | [53] | |
| Plus-laser deposition method | With the thickness from 2 to 8 nm | [54] | |
| Solvothermal method | The lateral size is between 0.8 and 2.0 μm, and the thickness is of about 4.7 nm with approximately 9 layers’ flakes | [55] | |
| Hydrothermal method | The lateral size is of approximately 5 μm, and the thickness is of around 3 nm with 1–2 layers’ flakes | [56] | |
| BPQDs | Ultrasonic exfoliation method | The average size is about 2.5 nm, and the average height is around 1.4 nm | [31,57] |
| Solvothermal method | The average size is about 2.1 nm, and the average height is around 3 nm | [58,59] | |
| Pulsed laser ablation method | The average size is about 6 nm, and the approximate average height is 1.1 nm | [60] | |
| Blender breaking method | The average size is about 2.2 nm, and the average height is between 0.58 and 1.45 nm | [61] |
| Modification Methods | Modifiers | Nanoagents | Modification Effects | Ref. |
|---|---|---|---|---|
| Modification Using Polymer | PLGA | BPNSs/PLGA/DOX | Ensure BPNSs’ PTT effect and biocompatibility, produced combined therapeutic effect | [78] |
| BPQDs/PLGA | Obtain controllable degradation rate of BPQDs, ensure BPQDs’ photothermal stability, biocompatibility, and long circulation in vivo | [38] | ||
| BPQDs/DTX@PLGA | Enhance BPQDs’ biocompatibility, promote chemophotothermal combinatory therapeutic efficacy, and tumor targeting effect through EPR mechanism | [79] | ||
| MSC@BPQDs/PLGA | Improve the uptake of the nanoagent by MSCs, enhance the nanoagent’s stability, and exhibit tumor specific tropism | [80] | ||
| PLGA-ss-D@BPQDs | Enhance radiotherapy efficacy, improve accurate tumor tissue localization through RGD targeting, surface charge switching, and bioresponsiveness, and reduce toxicity | [81] | ||
| PEG | BP-PEG NSs + R837 | Remarkably enhance photothermal stability, elicit a strong immune response through both PTT and R837 | [82] | |
| BP@PEG/Ce6 NSs | Improve biocompatibility, physiological stability, tumor-targeting property, and photothermal conversion efficiency (43.6%) | [24] | ||
| RdB/PEG-BPQDs | Enhance biocompatibility, physiological stability, and bioimaging property | [31] | ||
| DF-PEG-PAHy/BPNSs | Exhibit excellent gelation characteristics, pH sensitivity, NIR responsiveness, good biocompatibility, and outstanding photothermal characteristics | [83] | ||
| PDA | BP@PDA-Ce6 and TPP NSs | Improve the photothermal conversion efficiency and the stability of BPNSs, provide amine anchors for further functionalization by Ce6 | [84] | |
| BPQDs@PDA | Efficiently prevent the oxidation of BPQDs due to the enriched phenol groups on PDA, improve the photothermal conversion efficiency | [75] | ||
| BPNSs-DOX@PDA-PEG-FA | Enhance stability, photothermal efficiency, and targeting ability for cancer cells | [85] | ||
| BPNSs-DOX@PDA-PEOz-BTZ | Improve targeted long circulation and cellular uptake in vivo | [34] | ||
| Polypeptide | BPNSs@FKK | Exhibit excellent stability, favorable cell compatibility, enhance cellular uptake, and increase life span of the nanoagent | [86] | |
| CS | CS@BPNSs@CuNPs | Possess a remarkable temperature-sensitive spongy-like state, increase ROS production, improve postoperative therapy and multi-tumor treatments | [87] | |
| PLL | PLL/BPNSs/Cas13a/crRNA | Enhance cell adhesion and membrane penetration, improve stability in physiological solutions, enable the load of Cas13a/crRNA complexes | [88] | |
| PEI | BPNSs-PEI/AuNPs | Serve as ’bridge‘ to form BPNSs-PEI/AuNPs, increase 1O2 production, and enhanced light absorption | [89] | |
| Modification Using Inorganic Nanomaterials | Metallic Modification | BP-Au NSs | Enhance photothermal efficiency, serve as SERS substrates for Raman biodetection | [90] |
| Nonmetallic Modification | BPNSs@MS/PEG/TKD peptide/DOX | Enhance BPNSs’ dispersity, drug-loading efficiency, and post-modification feasibility | [91] | |
| RGD-Ir@BPNSs | Improve photoelectric properties, photo-induced charge carrier dynamics of BPNSs, and singlet oxygen generation after X-ray irradiation | [92] | ||
| Modification Using Lipid Based Nanocarrier | Cell Membrane | BPQD-RMNVs + aPD-1 | Improve long circulation time and tumor accumulation in vivo, prevent CD8+ T cells from exhaustion | [93] |
| Gel-BPQD-CCNVs+ aPD-1 | Serve as tumor vaccine, exhibit strong and durable PTT effect and immunological response, inhibit tumor recurrence and metastasis | [94] | ||
| PLT Membrane | PLTm@BPQDs-HED | Enhance tumor targeting ability, mitochondria-mediated cell apoptosis and autophagy in tumor cells, prolong in vivo circulation time | [95] | |
| Liposome | RV/CAT-BPNSs@MFL | Possess excellent stability, good photo-controlled release behavior of drug, high photothermal conversion efficiency, enhance singlet oxygen release efficiency, and outstanding tumor-targeting ability | [96] | |
| Adpgk-BPQDs-liposome@F127 gel | Enable the co-encapsulation of colon cancer cells derived neoantigen peptide Adpgk with BPQDs, enhance immunostimulatory effect, drug stability, and in vivo circulation time | [97] |
| Modification Methods | Modifiers | Nanoagents | Modification Effects | Ref. |
|---|---|---|---|---|
| Modification Using Small Molecule | NB | NB@BPNSs | Exhibit improved PTT and NIR imaging efficiency, possess good biocompatibility | [117] |
| TIL4 | TiL4@BPNSs | Improve biocompatibility, reduce cytotoxicity, proinflammation, and adverse immune responses, circumvent macrophage’s uptake | [118] | |
| Modification Using Polymer | TPP | BPQDs-PEG-TPP | Exhibit excellent mitochondria-targeted property, NIR photothermal properties, ROS production ability, stability, and dispersibility | [119] |
| Modification Using Metallic nanomaterial | PtNPs | BP/Pt-Ce6@PEG NSs | Ameliorate hypoxia and photodynamic effect, Improve the modifiability of nano DDS | [120] |
| Treatment Modalities | Nanoagents | Anticancer Efficacies | Ref. |
|---|---|---|---|
| PTT/IT | BPNSs-DcF@sPL | Circumvent PL pathway-regulated immune tolerance and suppression of CD8+ T cells, enhance the IFN-γ expression and promote the survival rate | [131] |
| FKF-OVAp@BPNSs | Enhance antigen uptake, activate systemic immunity and prolong the survival time | [135] | |
| BPNSs-bPEI-PEG-CpG | Induce necroptotic cell death, activate T lymphocytes, increase serum IL-2, TNF-α and IFN-γ level, suppress both primary and distal tumors | [138] | |
| hEX@BPNSs | Activate immune system, inhibit tumor progression and prolong survival | [141] | |
| BPNSs + aCD47 | Enhance the infiltration amount of CD8+ and CD4+ T cells in the tumor, increase the secretion of IL-6 and IFN-γ in the serum, suppress the tumor progression of both local and distal tumors | [142] | |
| BPNSs-PEG + R837 | Stimulate pro-inflammatory cytokine release, increase the percentage of CD8+ T cells in the spleen, inhibit tumor growth | [82] | |
| BPQD-RMNVs + aPD-1 | Increase the infiltration and activity of CD8+ T cells in the tumor, improve the serum IFN-γ levels, inhibit primary and secondary tumor growth | [93] | |
| Gel-BPQDs-CCNVs + aPD-1 | Upregulate the expression of Ki67 in the main immune cells, increase the level of IFN-γ and TNF-α, avoid cancer recurrence and metastasis | [94] | |
| Adpgk-BPQDs-liposome@F127 gel | Induce OVAp-specific splenocyte proliferation, increase the level of active CD8+ T cells in the spleen, prevent the tumor progress | [97] | |
| BPQDs@pNIPAM-zoledronate | Control the release of drug, promote γδ T cell proliferation and inhibit tumor growth | [143] | |
| FePt/BPNS-PEI-FA + CTLA-4 blockade | Induce DC maturation, upregulate DC-secreted immune-related cytokines (TNF-α, IL-12, IFN-γ), control the growth of residual and metastatic tumor | [144] | |
| PDT/IT | BPQD NVs-Ag+ | Upregulate the secretion of inflammatory-related cytokines (TLR4 and IL-1β) in the tumor microenvironment, activate anticancer IFN-γ+ CD8+ T cells to distal tumor and provoke T cell-mediated immunity, suppress tumor growth and metastasis | [145] |
| BPQD NVs-CpG | Increase serum levels of TNF-α, IL-6, and IL-12, enhance the tumor-infiltrating CD8+ T cells and the proliferation of T cells in the distal tumor, inhibit tumor growth and lung metastasis | [146] | |
| PTT/PDT/IT | NG/BPNSs-PEI-LY NPs | Produce acute inflammation in tumor in situ after PTT and PDT, improve the accumulation of NPs by NE membrane-mediated affinity based on positive feedback strategy, induce potent immune activation in tumor in situ and effectively inhibit lung metastasis through PTT and PDT combined with TGF-β inhibitor | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344. https://doi.org/10.3390/pharmaceutics13091344
Liu H, Mei Y, Zhao Q, Zhang A, Tang L, Gao H, Wang W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics. 2021; 13(9):1344. https://doi.org/10.3390/pharmaceutics13091344
Chicago/Turabian StyleLiu, Hao, Yijun Mei, Qingqing Zhao, Aining Zhang, Lu Tang, Hongbin Gao, and Wei Wang. 2021. "Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy" Pharmaceutics 13, no. 9: 1344. https://doi.org/10.3390/pharmaceutics13091344
APA StyleLiu, H., Mei, Y., Zhao, Q., Zhang, A., Tang, L., Gao, H., & Wang, W. (2021). Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics, 13(9), 1344. https://doi.org/10.3390/pharmaceutics13091344







