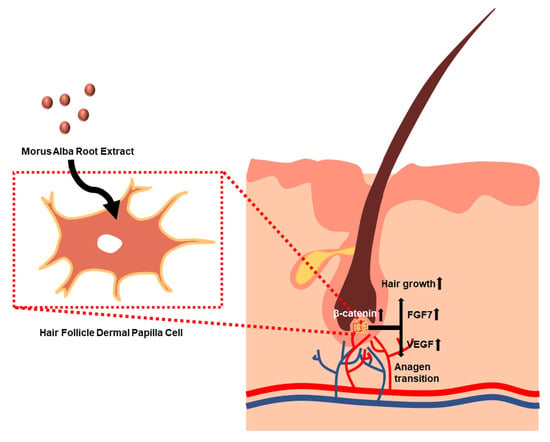
Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of M. alba Root Extract and Liquid Chromatography–Tandem Mass Spectrometry Analysis
2.2. Cell Culture
2.3. Cell Counting Kit-8 Assay
2.4. Live and Dead Assays
2.5. Cellular Morphology
2.6. Apoptotic Activity
2.7. Phalloidin Staining
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction
2.9. Western Blotting
2.10. Immunocytochemistry
2.11. Conditioned Medium Preparation
2.12. Tubular Formation Assay
2.13. Effect of Conditioned Medium Derived from Hair Follicle Dermal Papilla Cells on Proliferation and Involucrin Expression in Normal Human Epithelial Keratinocytes
2.14. Statistical Analyses
3. Results
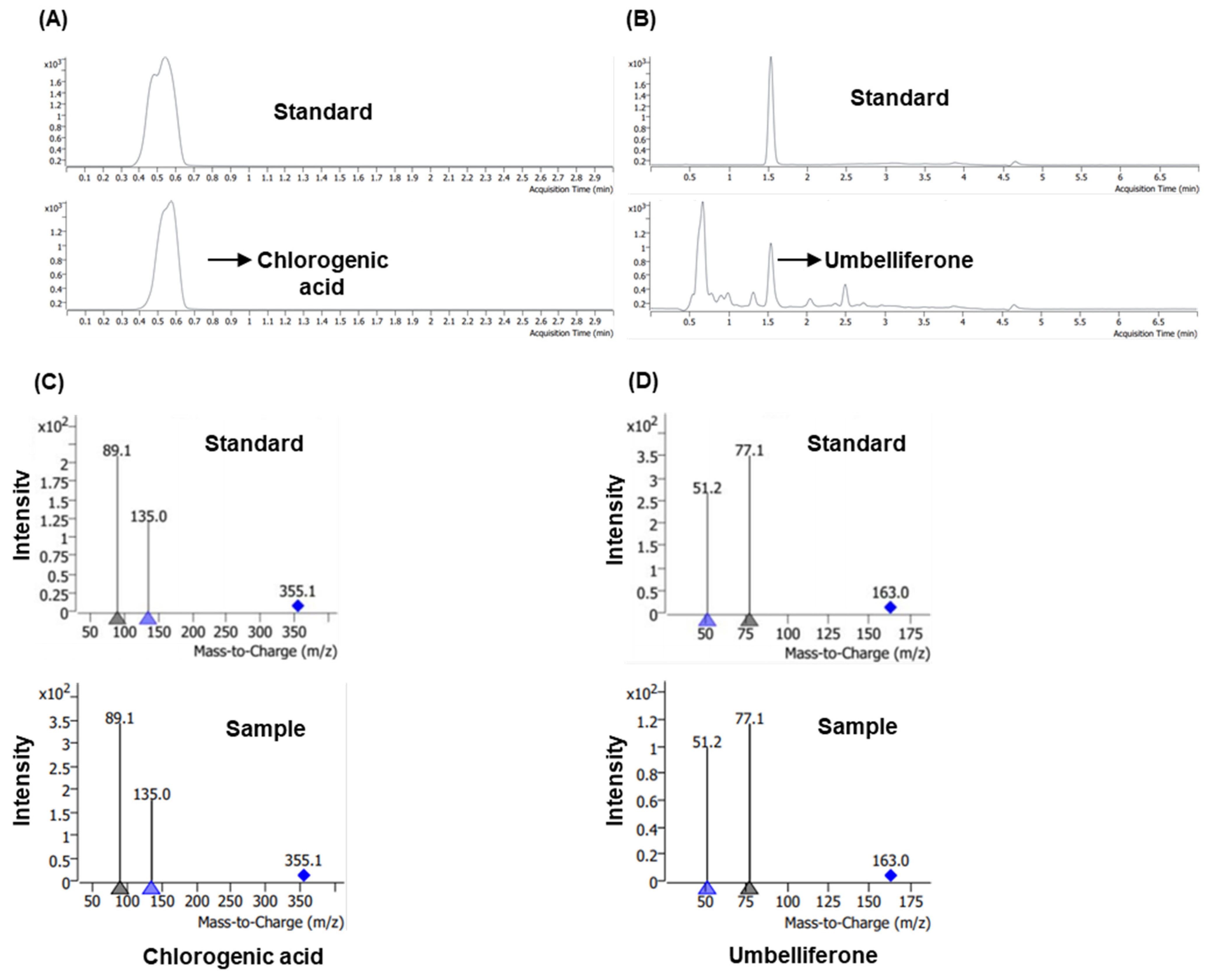
3.1. Liquid Chromatography–Tandeom Mass Spectrometry Profiling of Chlorogenic Acid and Umbelliferone in M. alba Root Extract
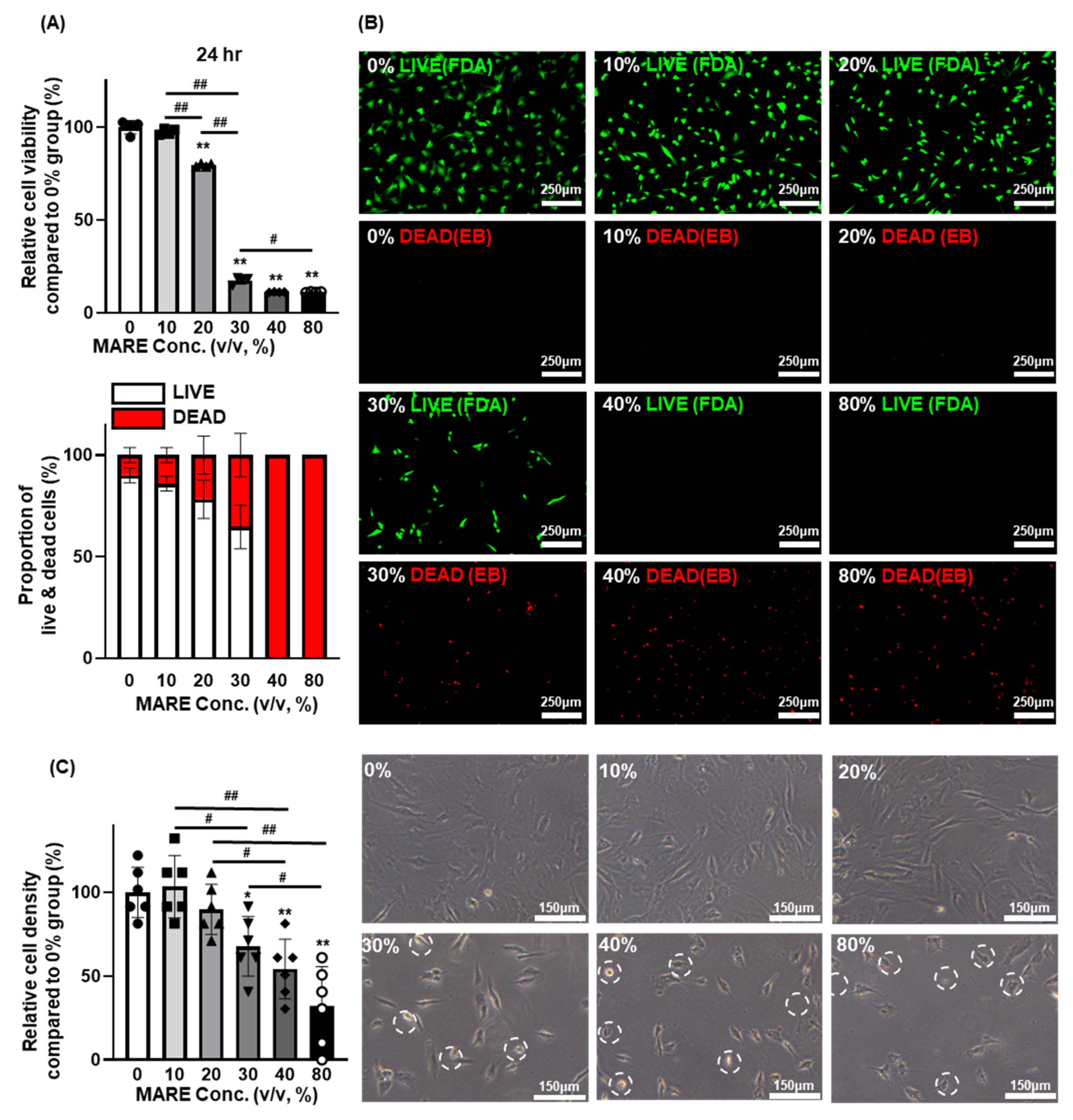
3.2. Optimizing the Concentration of M. alba Root Extract via the Viability of Hair Follicle Dermal Papilla Cells
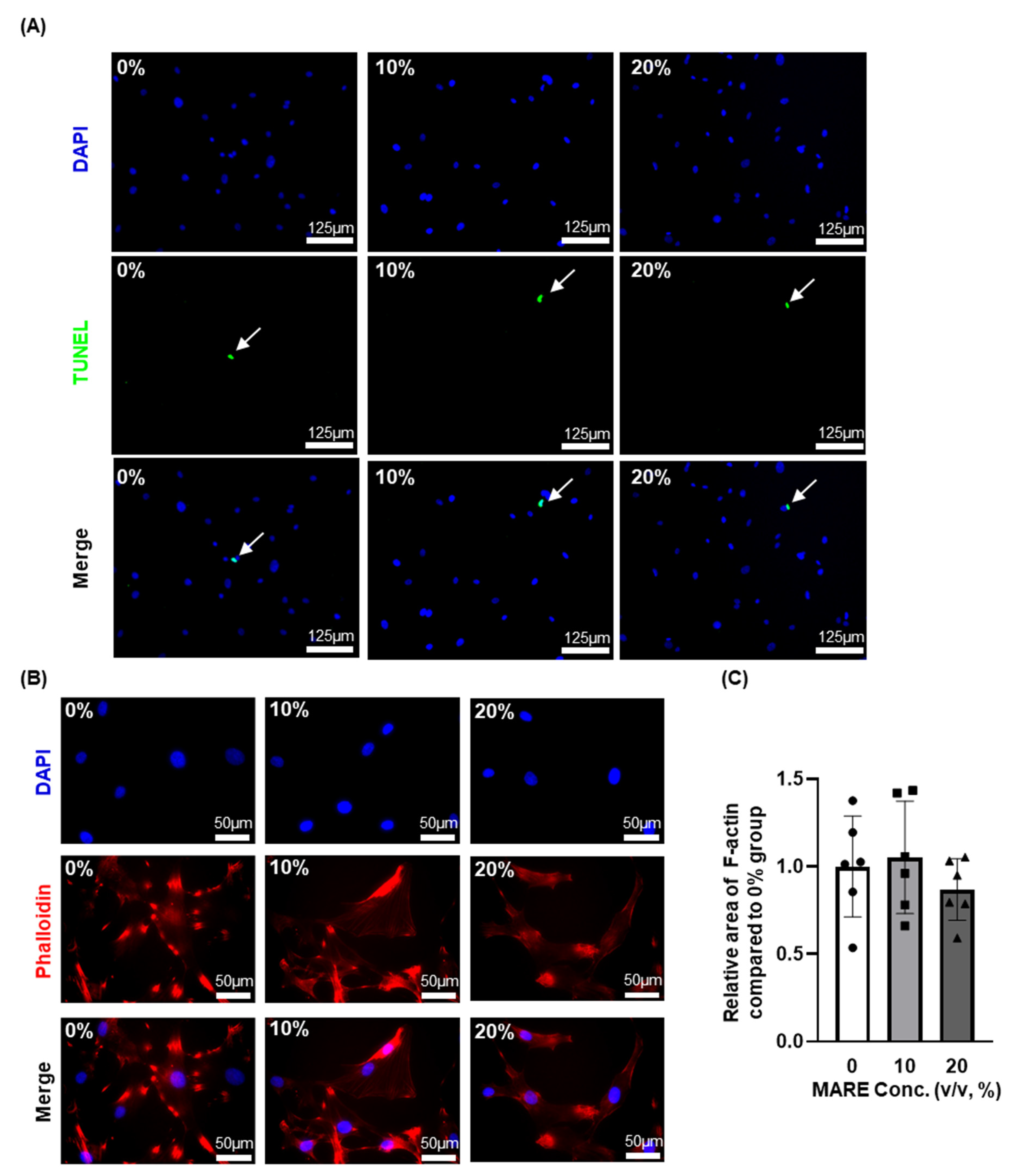
3.3. Cell Viability and Morphology Data Depend on M. alba Root Extract Concentration
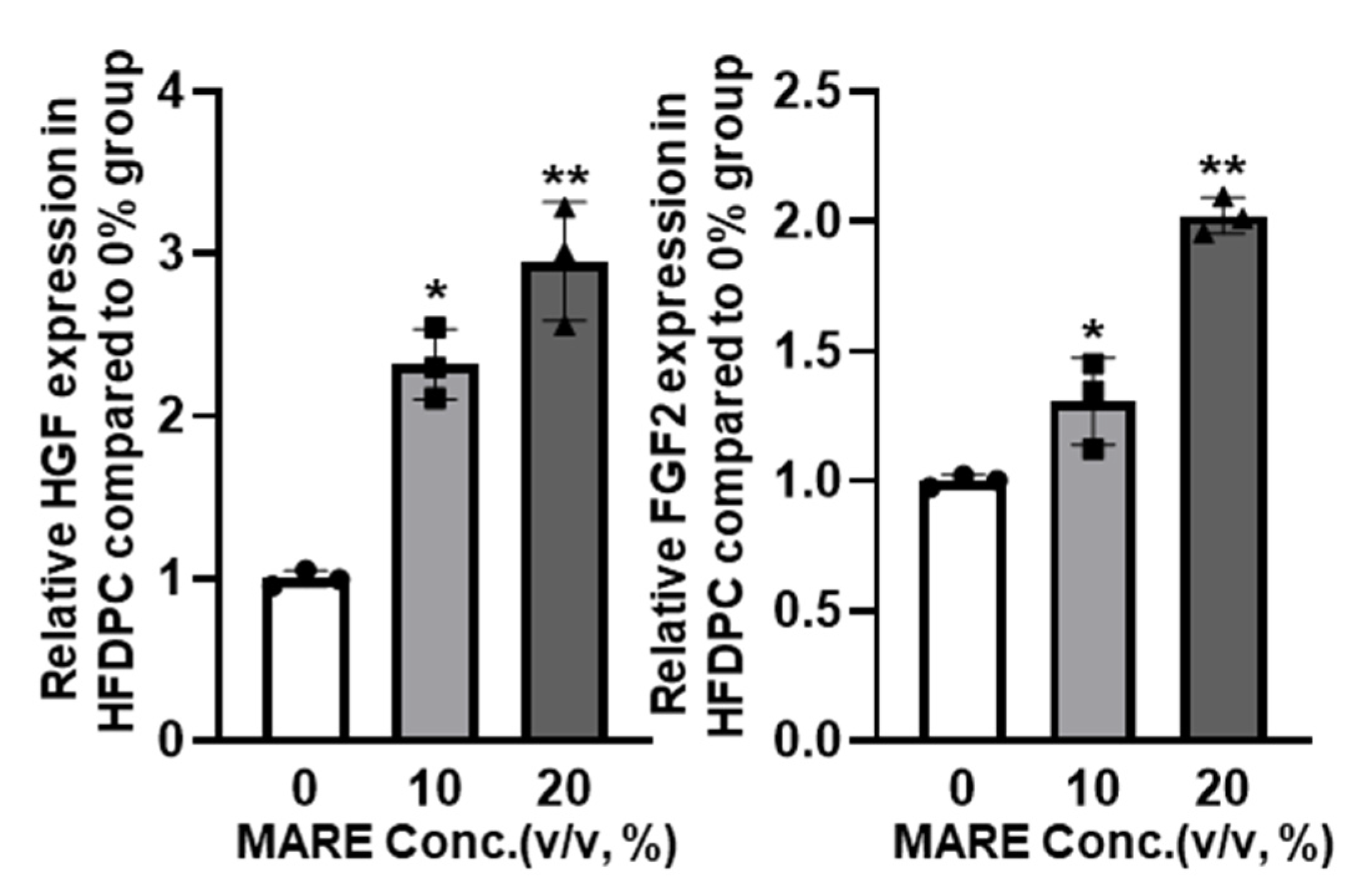
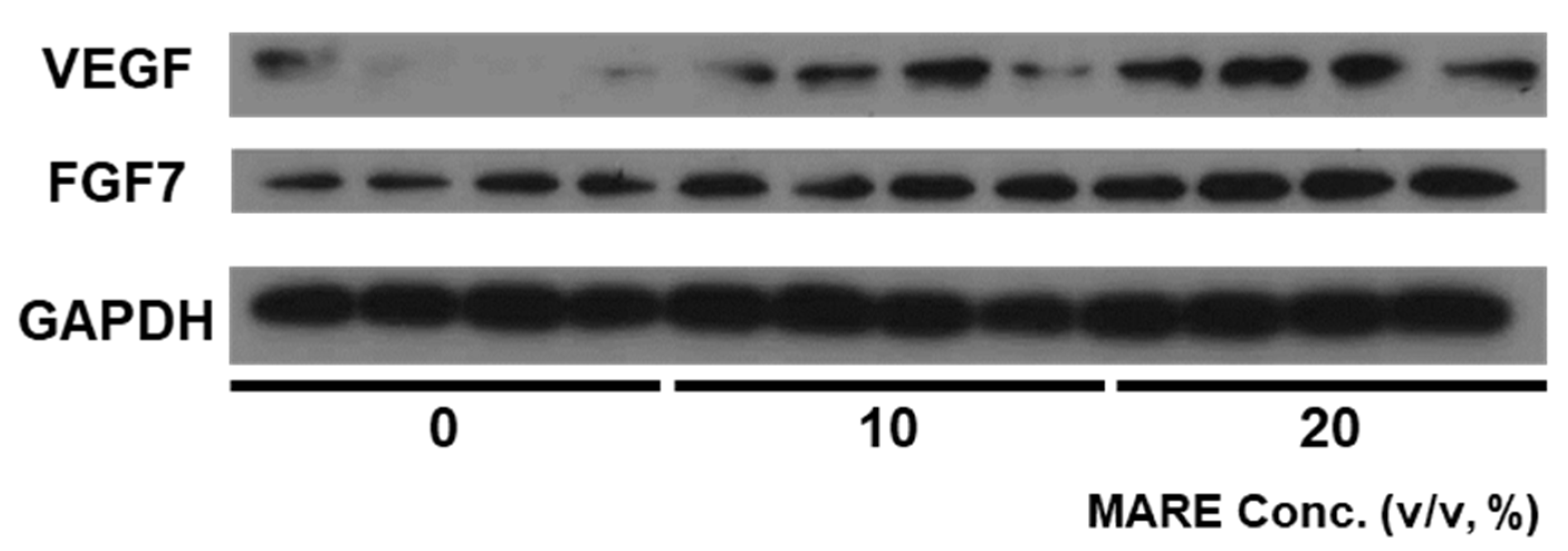
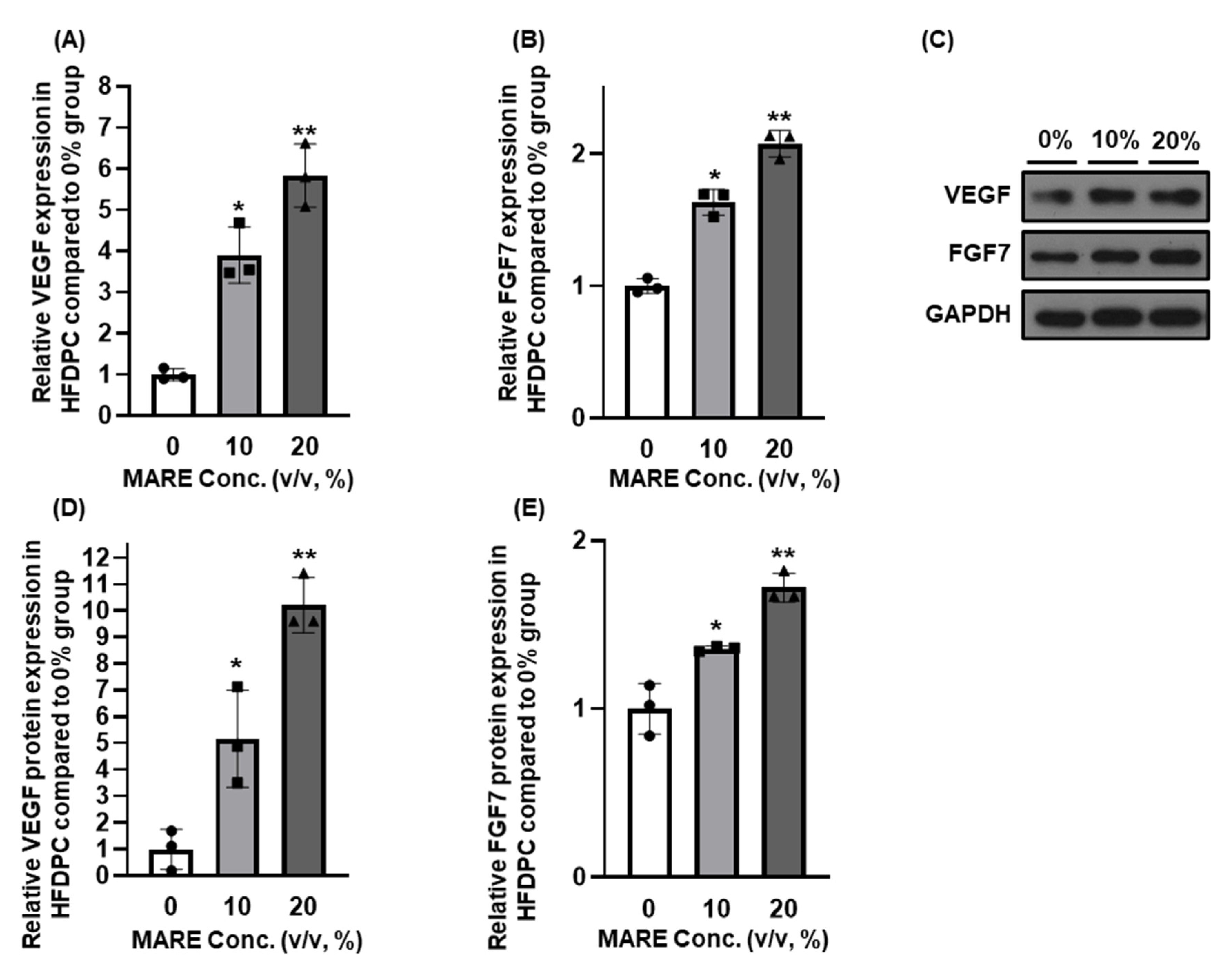
3.4. Growth Factor Expressions in M. alba Root Extract-Treated Hair Follicle Dermal Papilla Cells
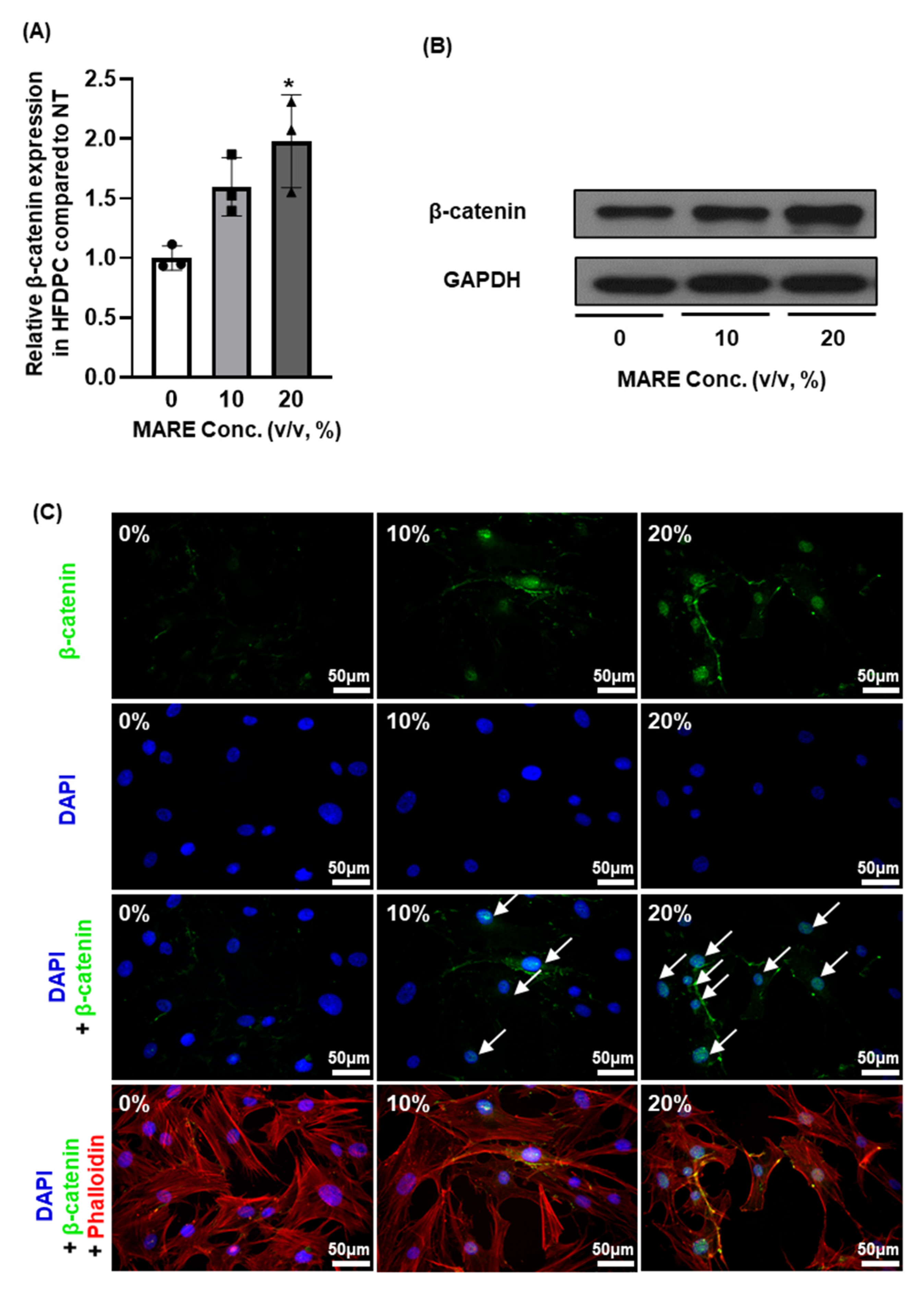
3.5. β-Catenin Expression in M. alba Root Extract-Treated Hair Follicle Dermal Papilla Cells
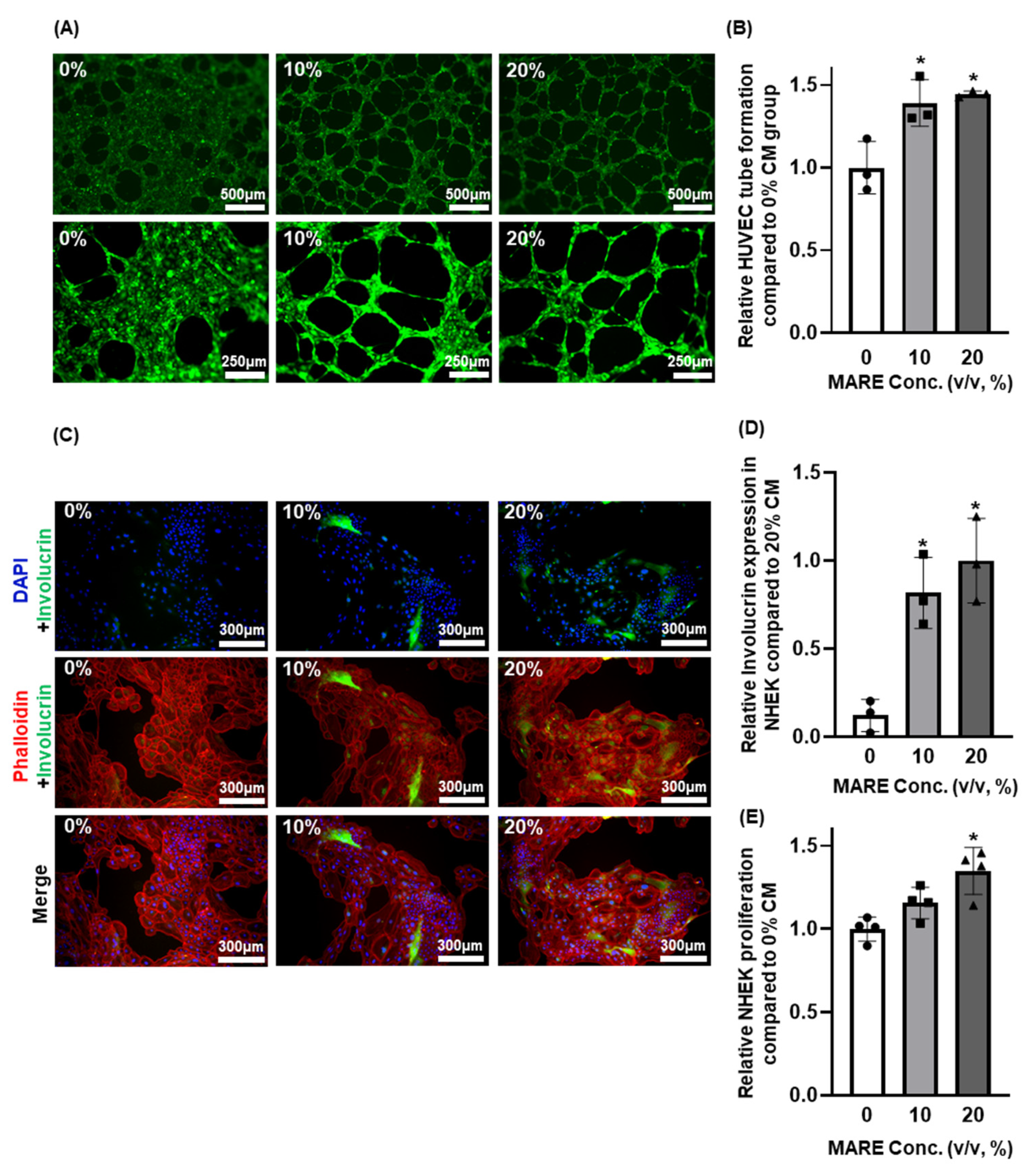
3.6. Effect of Conditioned Medium Derived from M. alba Root Extract-Treated Hair Follicle Dermal Papilla Cells on Human Umbilical Vein Endothelial Cells and Normal Human Epithelial Keratinocytes
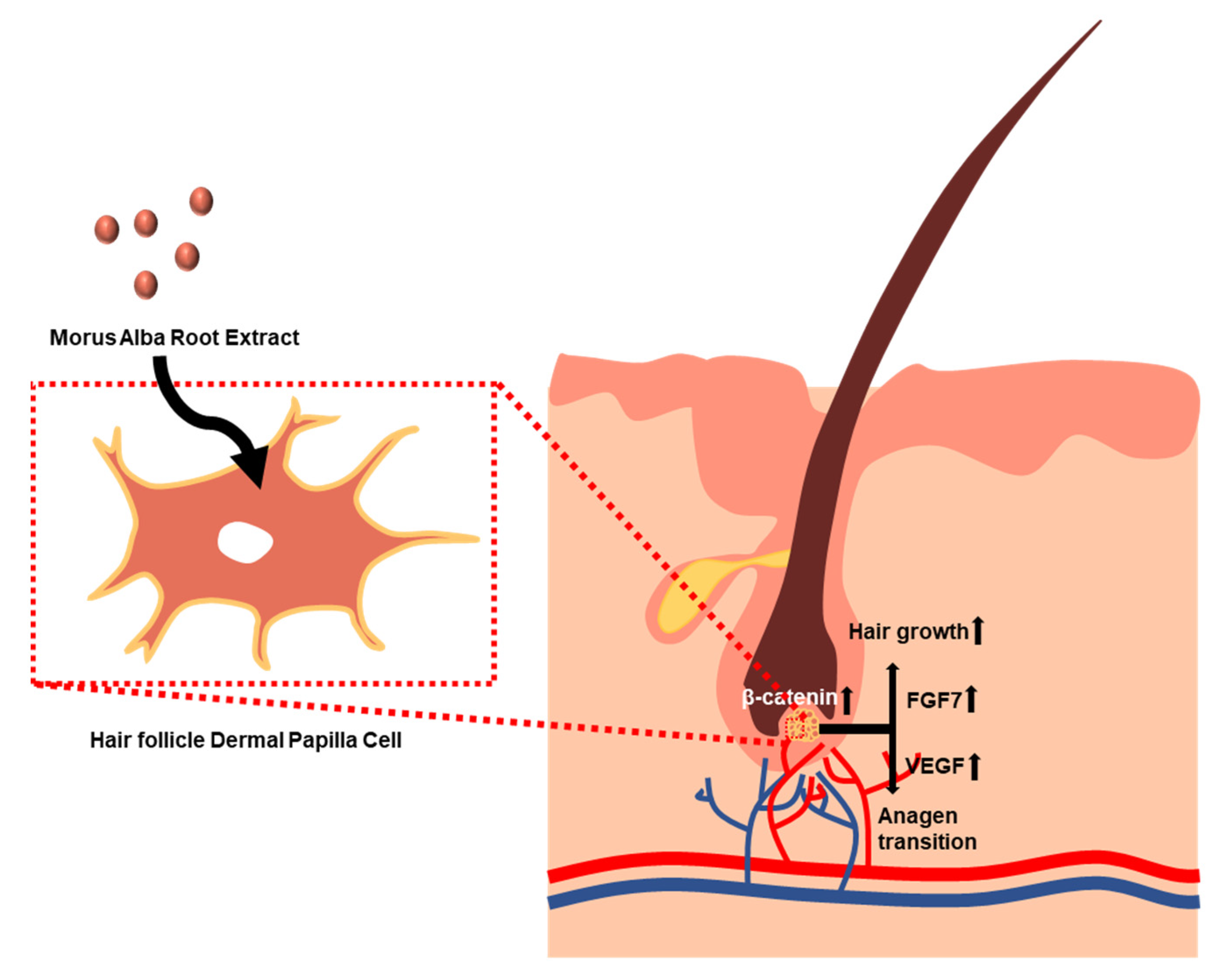
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Misciali, C.; Piraccini, B.M.; Peluso, A.M.; Bardazzi, F. Drug-induced hair loss and hair growth. Drug Saf. 1994, 10, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Sanders, D.; Westgate, G.E.; Kealey, T. Human hair growth In Vitro: A model for the study of hair follicle biology. J. Dermatol. Sci. 1994, 7, S55–S72. [Google Scholar] [CrossRef]
- Van der Veen, C.; Handjiski, B.; Paus, R.; Müller-Röver, S.; Maurer, M.; Eichmüller, S.; Ling, G.; Hofmann, U.; Foitzik, K.; Mecklenburg, L. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Investig. Dermatol. 1999, 113, 523–532. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Yoshizato, K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair Regen. 1998, 6, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair loss: Common causes and treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef]
- Lin, K.K.; Chudova, D.; Hatfield, G.W.; Smyth, P.; Andersen, B. Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance. Proc. Natl. Acad. Sci. USA 2004, 101, 15955–15960. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996, 10, 165–175. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by vegf-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Kim, M.J.; Lim, C.; Lee, J.Y.; Im, K.R.; Yoon, K.-S.; Song, J.M. Visible-to-near ir quantum dot–based hypermulticolor high-content screening of herbal medicines for the efficacy monitoring of hair growth promotion and hair loss inhibition. J. Biomol. Screen. 2013, 18, 462–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y. Extracellular vesicles derived from mscs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Habas, R.; Dawid, I.B.; He, X. Coactivation of rac and rho by wnt/frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003, 17, 295–309. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Friedman, E.S.; Friedman, P.M.; Cohen, D.E.; Washenik, K. Allergic contact dermatitis to topical minoxidil solution: Etiology and treatment. J. Am. Acad. Dermatol. 2002, 46, 309–312. [Google Scholar] [CrossRef]
- Ebner, H.; Muller, E. Allergic contact dermatitis from minoxidil. Contact Dermat. 1995, 32, 316–317. [Google Scholar] [CrossRef]
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): A systematic review. J. Clin. Aesthet. Dermatol. 2016, 9, 56. [Google Scholar]
- Kim, J.; Young Shin, J.; Choi, Y.-H.; Jang, M.; Nam, Y.J.; Lee, S.Y.; Jeon, J.; Jin, M.H.; Lee, S. Hair growth promoting effect of hottuynia cordata extract in cultured human hair follicle dermal papilla cells. Biol. Phar. Bull. 2019, 42, 1665–1673. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.-H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Singab, A.N.B.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of egyptian morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (morus alba l.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.J.; Park, J.H.; Park, G.H.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Moon, B.R.; Kim, J.E.; Kim, H.J.; Zhang, T. Aqueous extracts of morus alba root bark and cornus officinalis fruit protect against osteoarthritis symptoms in testosterone-deficient and osteoarthritis-induced rats. Pharmaceutics 2020, 12, 1245. [Google Scholar] [CrossRef]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Umbelliferone—An antioxidant isolated from Acacia nilotica (l.) Willd. ex. Del. Food Chem. 2010, 120, 825–830. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from prunus domestica (mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Oh, Y.-S.; Roh, S.-S.; Oh, M.-S. The experimental study on the effect of herbal exrtacts on hair growth and acnes. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2006, 19, 34–54. [Google Scholar]
- Choi, W.; Choi, J.-H.; Kim, J.-H. Studies on the effects of medicinal plant extracts on the hair growth stimulation. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2002, 15, 80–103. [Google Scholar]
- Kuwana, R.; Okada, A.; Morioka, M.; Date, A. Hair Growth Promoter Comprising Extract of Mulberry Root Bark and Persimmon and/or Paulownia, or Extract of Persimmon and Paulownia. U.S. Patent No. 5,674,497, 7 October 1997. [Google Scholar]
- Jung, J.; Park, J.; Cheong, H. Effect of morus alba extract for hair growth promotion in c57bl/6 mouse. J. Chosun Nat. Sci. 2008, 1, 19–23. [Google Scholar]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.; Pohl, S.; Moll, I.; Paus, R. Active hair growth (anagen) is associated with angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef]
- Alibardi, L. Ultrastructural immunolocalization of involucrin in the medulla and inner root sheath of the human hair. Ann. Anat. Anat. Anz. 2012, 194, 345–350. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. A necessary role for cell shrinkage in apoptosis. Biochem. Pharmacol. 1998, 56, 1549–1559. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A.J.R.m. Vegf and fgf-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Hwang, Y.-L.; Lee, M.-H.; Kim, N.-R.; Roh, S.-S.; Lee, Y.; Kim, C.D.; Lee, J.-H.; Choi, K.-C.J.I.j.o.m.m. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar] [PubMed]
- Ozeki, M.; Tabata, Y.J.B. Promoted growth of murine hair follicles through controlled release of vascular endothelial growth factor. Biomaterials 2002, 23, 2367–2373. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. Β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Sennett, R.; Rezza, A.; Clavel, C.; Grisanti, L.; Zemla, R.; Najam, S.; Rendl, M. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 2014, 385, 179–188. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, M.; Yang, Y.; Yang, K.; Wickett, R.R.; Andl, T.; Millar, S.E.; Zhang, Y. Activation of β-catenin signaling in cd133-positive dermal papilla cells drives postnatal hair growth. PLoS ONE 2016, 11, e0160425. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, S.; Sun, H.; Zhao, X.; Yang, L.; Hu, H.; Sun, J.; Jiang, W. Effects of wnt10b on dermal papilla cells via the canonical wnt/β-catenin signalling pathway in the angora rabbit. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1602–1609. [Google Scholar] [CrossRef]
- Wodarz, A.; Nusse, R. Mechanisms of wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998, 14, 59–88. [Google Scholar] [CrossRef]
- RA, E. Histology and cytochemistry of human skin. Xiii. The blood supply of the hair follicle. J. Natl. Cancer Inst. 1957, 19, 451–463. [Google Scholar]
- Castro, A.R.; Logarinho, E. Tissue engineering strategies for human hair follicle regeneration: How far from a hairy goal? Stem Cells Transl. Med. 2020, 9, 342–350. [Google Scholar] [CrossRef]
- Rogers, G.; Koike, K. Laser capture microscopy in a study of expression of structural proteins in the cuticle cells of human hair. Exp. Dermatol. 2009, 18, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H. Fundamental hair follicle biology and fine fibre production in animals. Animal 2010, 4, 1490–1509. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H. Treatment of mscs with wnt1a-conditioned medium activates dp cells and promotes hair follicle regrowth. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Shimizu, H.; Morgan, B.A. Wnt signaling through the β-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J. Investig. Dermatol. 2004, 122, 239–245. [Google Scholar] [CrossRef]
- Purba, T.S.; Brunken, L.; Peake, M.; Shahmalak, A.; Chaves, A.; Poblet, E.; Ceballos, L.; Gandarillas, A.; Paus, R. Characterisation of cell cycle arrest and terminal differentiation in a maximally proliferative human epithelial tissue: Lessons from the human hair follicle matrix. Eur. J. Cell Biol. 2017, 96, 632–641. [Google Scholar] [CrossRef]
- Purba, T.S.; Brunken, L.; Hawkshaw, N.J.; Peake, M.; Hardman, J.; Paus, R. A primer for studying cell cycle dynamics of the human hair follicle. Exp. Dermatol. 2016, 25, 663–668. [Google Scholar] [CrossRef]
- Semalty, M.; Semalty, A.; Joshi, G.P.; Rawat, M.S.M. Hair growth and rejuvenation: An overview. J. Dermatol. Treat. 2011, 22, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mamat, N.; Dou, J.; Lu, X.; Eblimit, A.; Haji Akber, A. Isochlorogenic acid a promotes melanin synthesis in b16 cell through the β-catenin signal pathway. Acta Biochim. Biophys. Sin. 2017, 49, 800–807. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145. [Google Scholar] [PubMed]
- Radek, K.A.; Taylor, K.R.; Gallo, R.L. Fgf-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen. 2009, 17, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Yamazaki, M.; Mitsui, S.; Tsuboi, R.; Ogawa, H. Hepatocyte growth factor (hgf) activator expressed in hair follicles is involved in in vitro hgf-dependent hair follicle elongation. J. Dermatol. Sci. 2001, 25, 156–163. [Google Scholar] [CrossRef]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1996, 205, 379–386. [Google Scholar] [CrossRef]
- Nicu, C.; O’Sullivan, J.D.; Ramos, R.; Timperi, L.; Lai, T.; Farjo, N.; Farjo, B.; Pople, J.; Bhogal, R.; Hardman, J.A. Dermal adipose tissue secretes hgf to promote human hair growth and pigmentation. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef]
- Barker, N. The canonical wnt/β-catenin signalling pathway. In Wnt Signaling; Humana Press: Totowa, NJ, USA, 2008; pp. 5–15. [Google Scholar]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, N.; Bodo, E.; Kromminga, A.; Gáspár, E.; Meyer, K.; Zmijewski, M.A.; Slominski, A.; Wenzel, B.E.; Paus, R. Thyroid hormones directly alter human hair follicle functions: Anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J. Clin. Endocrinol. Metab. 2008, 93, 4381–4388. [Google Scholar] [CrossRef] [PubMed]
- Ilan, N.; Mahooti, S.; Madri, J.A. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 1998, 111, 3621–3631. [Google Scholar] [CrossRef]
- Ansell, D.M.; Kloepper, J.E.; Thomason, H.A.; Paus, R.; Hardman, M.J. Exploring the “hair growth–wound healing connection”: Anagen phase promotes wound re-epithelialization. J. Investig. Dermatol. 2011, 131, 518–528. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| GAPDH | 5′-GTCGGAGTCAACGGATTTGG-3′ | 5′-GGGTGGAATCAATTGGAACAT-3′ |
| VEGF | 5′-GAGGGCAGAATCATCACGAAG T-3′ | 5′-CACCAGGGTCTCGATTGGAT-3′ |
| FGF7 | 5′-CGCAAATGGATACTGACACG-3′ | 5′-GGGCTGGAACAGTTCACACT-3′ |
| HGF | 5′-GATGGCCAGCCGAGGC-3′ | 5′-TCAGCCCATGTTTTAATTGCA-3′ |
| FGF2 | 5′-GACGGCAGAGTTGACGG-3′ | 5′-CTCTCTCTTCTGCTTGAAGTT-3′ |
| β-Catenin | 5′-TTGTGCGGCGCCATTTTAAG-3′ | 5′-TCCTCAGACCTTCCTCCGTC-3′ |
| Compounds | Regression Equation (x: Concentration, y: Peak Area) | Linear Range (ng/mL) | Correlation Coefficient (R2) | Dilution (mL/g) | Area of HPLC | Concentration (ng/mL) |
|---|---|---|---|---|---|---|
| Chlorogenic acid | y = 41,567.3320x + 625.4511 | 0.062–1.235 | 0.9991 | 200.0 | 8117 | 36.05 |
| Umbelliferone | y = 28,646.2256x + 915.3077 | 0.032–0.322 | 0.9931 | 2.000 | 2380 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, J.; Im, J.; Kim, S.-W.; Kim, H.Y.; Seo, I.; Bhang, S.H. Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells. Pharmaceutics 2021, 13, 1155. https://doi.org/10.3390/pharmaceutics13081155
Hyun J, Im J, Kim S-W, Kim HY, Seo I, Bhang SH. Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells. Pharmaceutics. 2021; 13(8):1155. https://doi.org/10.3390/pharmaceutics13081155
Chicago/Turabian StyleHyun, Jiyu, Jisoo Im, Sung-Won Kim, Han Young Kim, Inwoo Seo, and Suk Ho Bhang. 2021. "Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells" Pharmaceutics 13, no. 8: 1155. https://doi.org/10.3390/pharmaceutics13081155
APA StyleHyun, J., Im, J., Kim, S.-W., Kim, H. Y., Seo, I., & Bhang, S. H. (2021). Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells. Pharmaceutics, 13(8), 1155. https://doi.org/10.3390/pharmaceutics13081155